Abstract
In the eye, the retinal pigment epithelium (RPE) adheres to a complex protein matrix known as Bruch's membrane (BrM). The aim of this study was to provide enriched conditions for RPE cell culture through the production of a BrM-like matrix. Our hypothesis was that a human RPE cell line would deposit an extracellular matrix (ECM) resembling BrM. The composition and structure of ECM deposited by ARPE19 cells (ARPE19-ECM) was characterized. To produce ARPE19-ECM, ARPE19 cells were cultured in the presence dextran sulphate. ARPE19-ECM was decellularized using deoxycholate and characterized by immunostaining and western blot analysis. Primary human RPE and induced pluripotent stem cells were seeded onto ARPE19-ECM or geltrex coated surfaces and examined by microscopy or RT-PCR. Culture of ARPE19 cells with dextran sulphate promoted nuclear localization of SOX2, formation of tight junctions and deposition of ECM. ARPE19 cells deposited ECM proteins found in the inner layers of BrM, including fibronectin, vitronectin, collagens IV and V as well as laminin-alpha-5, but not those found in the middle elastic layer (elastin) or the outer layers (collagen VI). ARPE19-ECM promoted pigmentation in human RPE and pluripotent stem cell cultures. Expression of RPE65 was significantly increased on ARPE19-ECM compared with geltrex in differentiating pluripotent stem cell cultures. ARPE19 cells deposit ECM with a composition and structure similar to BrM in the retina. Molecular cues present in ARPE19-ECM promote the acquisition and maintenance of the RPE phenotype. Together, these results demonstrate a simple method for generating a BrM-like surface for enriched RPE cell cultures.
Abbreviations: AMD, Age-related macular degeneration; ARPE19-ECM, ARPE19 cell-derived extracellular matrix; BrM, Bruch's membrane; ECM, extracellular matrix; RPE, retinal pigment epithelium; ICL, inner collagenous layer; OCL, outer collagenous layer; RBL, retinal pigment epithelial basal lamina
Keywords: Bruch's membrane, Extracellular matrix, Retinal pigment epithelium, Macromolecular crowding, Decellularization, Induced pluripotent stem cells
Highlights
-
•
Macromolecular crowding promoted deposition of extracellular matrix by ARPE19 cells.
-
•
ARPE19 cells deposited matrix proteins found in the inner layers of Bruch's membrane.
-
•
ARPE19-ECM displayed similar microstructure to Bruch's membrane.
-
•
ARPE19-ECM promoted pigmentation in human retinal pigment epithelial cell cultures.
-
•
ARPE19-ECM promoted RPE differentiation from pluripotent stem cells.
1. Introduction
Somatic cells require a complex plethora of signals from their microenvironment to maintain their identity in the body. Disruption of these signals through injury or disease can cause pathological changes in cellular phenotypes, such as transdifferentiation or neoplastic transformation. Standard methods for primary cell culture involve removing cells from their tissue-specific microenvironments and introducing them into a new environment in which essential signals are provided by media supplemented with serum or growth factors. Since many cell types do not readily adhere to tissue culture plastic, culture surfaces often require coating with extracellular matrix (ECM) proteins to promote adherence and support cell growth. Standard cell culture conditions, in which cells are cultured on tissue culture plastic in a bath of cell culture media, have been likened to the physiological condition of oedema [5]. Under these conditions, many cell types undergo growth adaptation, resulting in non-physiological phenotypes that may influence experimental outcomes. Although these changes may be mitigated by providing the correct growth factors and ECM formulations, full recapitulation of complex tissue microenvironments in vitro remains a significant challenge for cell culture research.
An alternative approach to generating tissue-specific microenvironments involves the use of cultured cell lines to deposit ECM. Several authors have demonstrated that ECM is deposited by a number of different cultured cell lines, including fibroblasts, endothelial cells and retinal pigment epithelial cells (RPE) [10], [13], [3]. It has been shown that ECM deposition can be greatly enhanced by supplementation of media with macromolecular crowding agents, such as Ficoll or dextran sulphate [5]. In vivo, cells reside in a crowded microenvironment, surrounded with high densities of macromolecules. In a crowded environment, local concentrations of growth factors and ECM precursors are effectively increased by lower rates of diffusion away from the source, promoting autocrine signaling and ECM deposition. In contrast, standard cell culture conditions fail to provide a crowded microenvironment, resulting in diffusion of growth factors and ECM proteins away from their source and a reduction in the ability of cells to create their own niche. Supplementation of cell culture media with crowding agents such as dextran sulphate has been shown to recapitulate the crowded environment, promoting autocrine signaling and ECM deposition [5]. Using detergents, cell-deposited ECMs can be decellularized to produce surfaces that enable cell adhesion and promote cell growth [5]. Fibroblast-deposited ECM was previously shown to promote the growth of pluripotent stem cell cultures [1], while ECM derived from bone marrow stromal cells supported self-renewal and multipotency in human mesenchymal stem cell cultures [8]. In this study, ECM derived from the human retinal pigment epithelial (RPE) cell line, ARPE19, was characterized and evaluated as a surface for RPE cell culture and differentiation.
In the retina, the basal surface of the RPE is attached to a complex protein matrix, 2–5 µm thick, known as Bruch's membrane (BrM). BrM is comprised of 5 distinct layers, each containing a different array of extracellular matrix (ECM) proteins [7]. The outer and inner layers of BrM form the basal lamina of the choroid and RPE, respectively, and cover a middle elastic layer comprised of elastin. BrM provides structural support to the RPE cell layer and enables diffusion of nutrients and waste products into and out of the retina. The innermost layers of BrM are rich in ECM proteins such as fibronectin, vitronectin, laminin, and collagen IV, that bind to β1-integrins expressed on the basal surface of RPE cells [6].
Although commercial ECM coatings used in RPE cell culture, such as matrigel, laminin, vitronectin or fibronectin enable attachment of RPE cells to tissue culture plastic [11], [12], [14], they do not provide an organized, tissue-specific ECM that accurately recapitulates the structure and composition of BrM. Since the role of ECM in specifying and maintaining cellular phenotypes is becoming increasingly clear [2], the aim of this study was to produce a BrM-like matrix that can be used to recapitulate the native RPE microenvironment in vitro and provide enriched conditions for RPE cell culture. Our hypothesis was that the human RPE cell line ARPE19 would deposit an ECM resembling BrM. In this study, the composition and structure of ECM deposited by ARPE19 cells was characterized. The data presented demonstrate a simple method for producing decellularized ARPE19 cell-derived ECM surfaces (ARPE19-ECM) that promote an RPE phenotype in primary human RPE cells and pluripotent stem cells.
2. Methods
2.1. Production of ARPE19 cell-derived extracellular matrix
Unless otherwise specified, media, medium supplements and reagents used for cell cultures were purchased from Thermo Fisher Scientific, (Waltham, MA, USA). The human RPE cell line ARPE19 (American Type Culture Collection, Manassas, VA, USA) was cultured in DMEM supplemented with 10% fetal calf serum (DMEM/FCS). To generate cell-derived extracellular matrix (ECM) coatings, ARPE19 cells were seeded at 80–90% confluence in DMEM/FCS (uncrowded conditions). Upon reaching confluence, the media was changed to DMEM/FCS supplemented with the macromolecular crowder dextran sulphate (200 μg/ml) and ascorbic acid (30 μg/ml) (Sigma-Aldrich, St. Louis, MO, USA) (crowded conditions). Confluent ARPE19 monolayers were cultured for indicated times with half media changes twice per week. Wells were then decellularized by three successive incubations with 0.5% deoxycholate (Sigma) in distilled H2O for 15 min at room temperature, followed by three successive washes with PBS to remove cell debris and residual detergents. Wells then were treated for 20 min with 10 U/ml of TURBO DNase (Thermo Fisher Scientific, Invitrogen) in PBS to remove residual DNA then washed three times in PBS.
2.2. Dissection of human posterior eye cups and primary RPE culture
Human posterior eye cups were obtained from the Lions Eye Bank (WA, Australia, https://www.lei.org.au/services/lions-eye-bank/) after written informed consent was obtained from next of kin and within 12 h of enucleation. The use of cadaveric human tissues was approved by the Sir Charles Gardiner Hospital Human Research Ethics Committee (Approval number 2012-090). Uveal sacs containing choroid, retina and vitreous were dissected out of posterior cups, opened with relaxing cuts and the neuroretina and vitreous removed from the underlying RPE-choroid. Patches of RPE adhering to the retina were collected and pooled with RPE cells scraped from the inner aspect of the choroid. RPE-free retinal tissue was collected for protein harvesting (see Section 2.6). Isolated RPE was dissociated with TrypLE Express (Thermo Fisher Scientific) for 30 min at 37 °C, pelleted by centrifugation and resuspended in culture media. Live cell numbers were determined by Trypan blue exclusion before RPE cells were plated into 24-well plates (50,000 cells per well) and cultured in DMEM supplemented with 10% FCS. To promote RPE cell adherence, culture plates were coated with the commercial ECM formulations Geltrex (Thermo Fisher Scientific) and Vitronectin (Stem Cell Technologies, Vancouver, BC, Canada) or ARPE19-ECM.
2.3. Human induced pluripotent stem cell culture
A human episomal induced pluripotent stem cell (iPSC) line was obtained from Thermo Fisher Scientific (A18945). Human iPSC cells were cultured under feeder free conditions on geltrex-coated plates in TeSer-E8 media according to the manufacturer's recommendations (Stem Cell Technologies).
2.4. Preparation of decellularized of choroid tissues
Choroid-RPE tissues were dissected from human posterior eye cups as described above. After removal of the retina, choroid-RPE tissues was placed into 50 ml of 0.5% deoxycholate in H2O and incubated on a rocker at 4 °C. The dexoycholate solution was removed and replaced three times over a 48 h period and decellularized choroid-RPE tissues then transferred to a new tube containing 50 ml distilled H2O. Decellularized choroid-RPE tissues were washed three times in H20 at 4 °C over a 48 h period, then divided into pieces for wholemount immunostaining analysis. Decellularization was confirmed by the absence of DAPI staining (Supp Fig. 4).
2.5. Immunostaining
Cells or ECM were fixed with 4% Paraformaldehyde (PFA) in PBS for 15 min at 37 °C then washed three times with PBS. Cells were then incubated with chilled methanol (−20 °C) for 10 min at room temperature and washed in PBS three times. Cells were incubated with blocking buffer (PBS, 5% goat serum, 0.3% Triton-X100) for one hour at room temperature. Primary antibodies were diluted in blocking buffer and added to the cells for overnight incubation at 4 °C. Cells were then washed three times with PBS before the addition of fluorophore conjugated secondary antibodies and the nuclear stain DAPI (1 μg/ml). Primary mouse antibodies used in this study included: anti-MITF; anti-N-cadherin; anti-laminin-α5; anti-collagen VI; anti-pan-cytokeratin (Abcam, Cambridge, England, UK); and anti-βIII-tubulin (Promega, Fitchburg, WI, USA). Primary rabbit antibodies included: anti-SOX2; anti-ZO1; anti-Fibronectin; anti-Vitronectin; anti-collagen V; anti-collagen IV; anti-elastin (Abcam). Secondary antibodies used in this study were: Goat anti-Mouse-AlexaFluor546 and Goat anti-Rabbit-AlexaFluor488 (Thermo Fisher Scientific), both used at a dilution of 1:500. Fluorescent microscopy was performed on an Olympus IX70 inverted fluorescence microscope (for culture plates) or the Olympus BX60 fluorescence microscope (for slides) and imaged using Olympus DP-Controller 3.1.1.267 acquisition software (Olympus Corporation, Tokyo, Japan). Negative controls in which primary antibodies were omitted were routinely performed for each experiment.
2.6. Western blot
For protein extraction, retinal cells and tissue were lysed with RIPA buffer containing protease inhibitor cocktails (Sigma) for 20–30 min on ice. Samples were centrifuged to remove debris and the supernatant collected for determination of protein concentration using the Micro BCA™ Protein Assay Kit (Thermo Fisher Scientific). For collection of ECM proteins from 24 well plates, decellularized ARPE19-ECM was immersed in 0.2 ml of 5% acetic acid and incubated at 4 °C overnight. The acetic acid solution was collected and wells then incubated in lysis buffer (125 mM Tris-HCl, pH 6.8; 0.1% SDS; 10% glycerol; 1% dithiothreitol; Proteinase Inhibitor Cocktail) at 37 °C for one hour. Wells were then scraped and the lysis buffer with matrix proteins was collected. The lysis-scraping-collection step was performed twice and protein samples pooled. Acetic acid samples were combined with scraped lysis buffer samples and mixed with 5 volumes of acetone. The mixture was kept at −20 °C overnight then centrifuged at 8000g for 20 min at 4 °C. The protein pellet was resuspended with in buffer containing 20 mM Tris-HCl (pH 7.6), 100 mM NaCl, and 1 mM EDTA. Protein concentration was determined using Micro BCA™ Protein Assay Kit (Thermo Fisher Scientific) and samples stored at −20 °C.
Unless otherwise stated, all reagents, pre-cast polyacrylamide gels and membranes used for protein electrophoresis and western blot analysis system were purchased from Thermo Fisher Scientific. For Western blot analysis, 20 μg of total protein was mixed with 1×NuPAGE LDS Sample Buffer, 1×NuPAGE® Sample Reducing Agent containing 50 mM dithiothreitol, then heated at 70 °C for 10 min. Denatured protein samples were loaded onto a NuPAGE 4–12% Bis-Tris Gels and electrophoretically separated at 200 V for 30 min in 1×NuPAGE MES SDS Running Buffer. Separated proteins in the gels were transferred with Original iBlot Gel Transfer Device onto nitrocellulose membranes at 20 V for 6 min. Blotted membranes were washed with TPBS (0.05% Tween20, 50 mM Tris-HCl, 150 mM NaCl), and incubated with blocking buffer (2% BSA in TPBS) for 60 min. Membranes were then incubated with primary antibodies in blocking buffer overnight at 4 °C. Membranes were then washed five times in TPBS and incubated for 1 h with Goat Anti-Rabbit Poly-HRP secondary antibody or Goat Anti-Mouse Poly-HRP secondary antibody (1:20,000). Protein bands were detected using the SuperSignal West Pico Chemiluminescent Substrate according to the manufacturer's instruction. Membranes were imaged using the Bio-Rad ChemiDoc XRS+ system. Primary antibodies used in this study included: Rabbit anti-SOX2, Mouse anti-GAPDH, Rabbit anti-Fibronectin and Rabbit anti-Vitronectin (Abcam).
2.7. Quantitative PCR
Total RNA was extracted from cells using the RNeasy Plus Micro Kit (Qiagen, Hilden, Germany). Total RNA was then quantified using the Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific). First-strand cDNA was synthesized using 0.5–1 μg of RNA and RT2 First Strand Kit (Qiagen) according to the supplier's protocol. RT-PCR reactions for human PAX6, MITF, OTX2, RPE65 and GAPDH were performed in a 25 μl mixture containing 1 μl (15-20 ng) of cDNA, primers (0.4 μM) and RT2 SYBR-Green qRT-PCR Master Mix (Qiagen) in the CFX Connect Real-Time PCR thermocycler (Bio-Rad, Hercules, CA, USA). Gene expression values for each mRNA species were determined using the ΔΔCt method [9] normalized against the GAPDH expression value from each mRNA sample. Sequences for primers used in this study are listed in Supplementary Table 1.
3. Results
3.1. Macromolecular crowding enhances nuclear localization of SOX2 and tight junction formation in ARPE19 cells
ARPE19 cells were cultured under control conditions or in the presence of dextran sulphate and ascorbic acid (crowded conditions). Immunostaining for the transcription factor SOX2 revealed nuclear localization in crowded cultures, while control cultures displayed only cytoplasmic immunoreactivity (Supp Fig. 1A). In contrast, immunostaining for the transcription factor MITF was similar in both control and crowded cultures, with nuclear localization observed under both conditions (Supp Fig. 1B).
In crowded cultures, N-cadherin immunostaining revealed confluent monolayers consisting of polygonal cells with tight cell-cell junctions. In comparison, monolayers cultured under control conditions contained elongated cells and N-cadherin immunoreactivity was associated with an interdigitated cell-cell interface (Supp Fig. 1A). Similarly, immunostaining for the tight junction protein ZO1 revealed tight junctions in crowded cultures, while few ZO1 labeled tight junctions were observed in control cultures. ZO1 immunolabelling was also detected in the nuclei of ARPE19 cells in both control and crowded cultures (Supp Fig. 1B). Together, these results demonstrate that macromolecular crowding can partially reverse the growth adapted phenotype of cultured ARPE19 cells.
3.2. Deposition of extracellular matrix by ARPE19 cells
To examine the deposition of extracellular matrix in vitro, ARPE19 cells were cultured in crowded conditions for 1–4 weeks. Culture wells were then decellularized using deoxycholate to produce cell-free ARPE19 cell-derived ECM surfaces. Immunostaining of ARPE19-ECM demonstrated robust deposition of fibronectin in crowded cultures, while control cultures deposited little fibronectin (Supp Fig. 1C). Decellularization was assessed by microscopy and βIII-tubulin immunostaining. No intact cells or βIII-tubulin immunopositive cell fragments were detected on decellularized ARPE19-ECM (Supp Fig. 2).
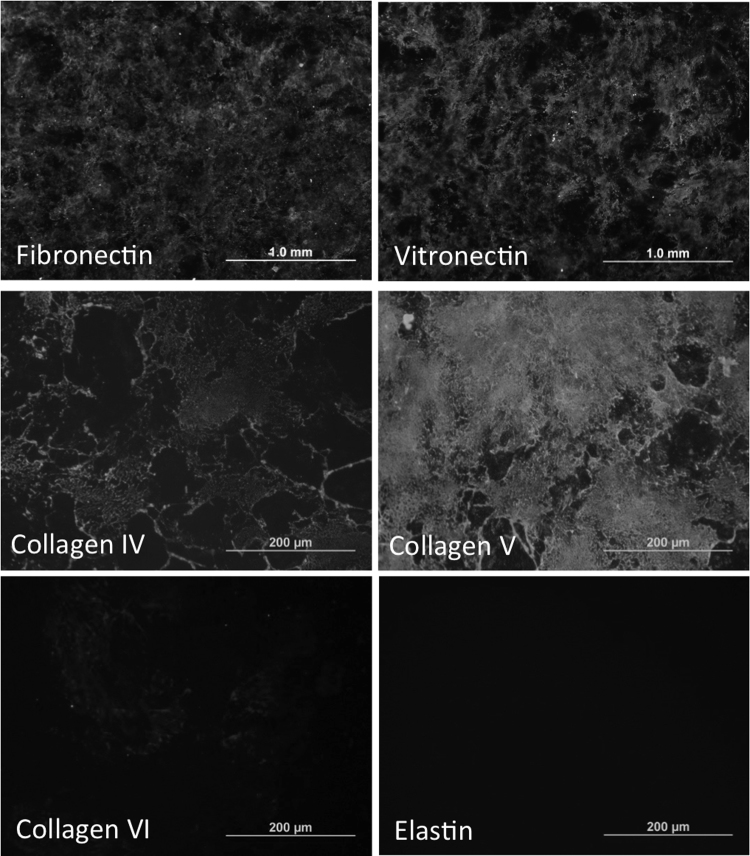
To examine the composition of ARPE19-ECM, decellularized matrices were subjected to immunostaining analysis using a panel of antibodies targeting ECM proteins. Decellularized ARPE19-ECM displayed immunoreactivity for proteins found in the inner layers of Bruch's membrane, including fibronectin, vitronectin, collagens IV, and V (Fig. 1) as well as laminin-α5 (Supp Fig. 3). ECM was deposited across the entire culture well floor within one week of culture and continued to accumulate for up to one month. Fibronectin immunoreactivity accumulated more rapidly than immunoreactivity to vitronectin and laminin-α5 (Supp Fig. 3). ARPE19-ECM displayed no immunoreactivity for collagen VI or elastin (Fig. 1), which are present in the outer and middle layers of BrM, respectively.
Fig. 1.
Composition of decellularized ARPE19-ECM. Immunostaining of ARPE19-ECM demonstrated the presence of fibronectin, vitronectin, collagens IV, V and VI and elastin.
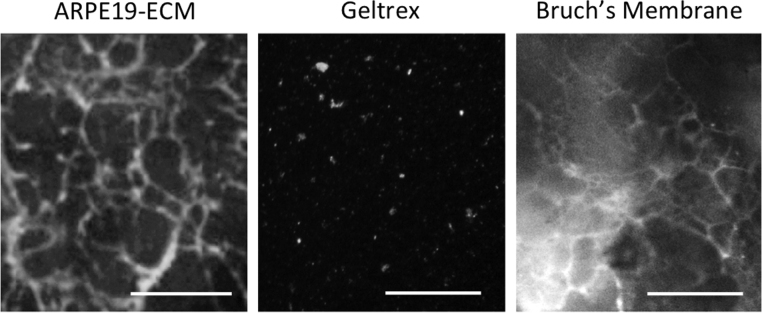
In decellularized choroid-BrM whole mounts, double immunolabeling for fibronectin and laminin-α5 revealed a polygonal network of fibronectin fibrils surrounding discrete laminin-α5 deposits (Supp Fig. 4). Similarly, fibronectin immunolabeling of ARPE19-ECM revealed a dense polygonal network of fibrils. In contrast, the commercial ECM geltrex displayed sparse, punctate fibronectin immunoreactivity (Fig. 2). These results show that ECM deposited by ARPE19 cells mimics the composition and microstructure of BrM deposited by RPE cells in the eye.
Fig. 2.
Comparison of ECM surface structure. Immunostaining of ARPE19-ECM (left panels) or geltrex coated tissue culture plastic (middle panels) or decellularized choroid-BrM wholemounts (right panels) using antibodies for fibronectin demonstrated networks of fibrils in ARPE19-ECM and BrM, but not in geltrex coated surfaces. Scale bars indicate 50 µm.
3.3. Post-translational modification of ECM proteins by ARPE19 cells
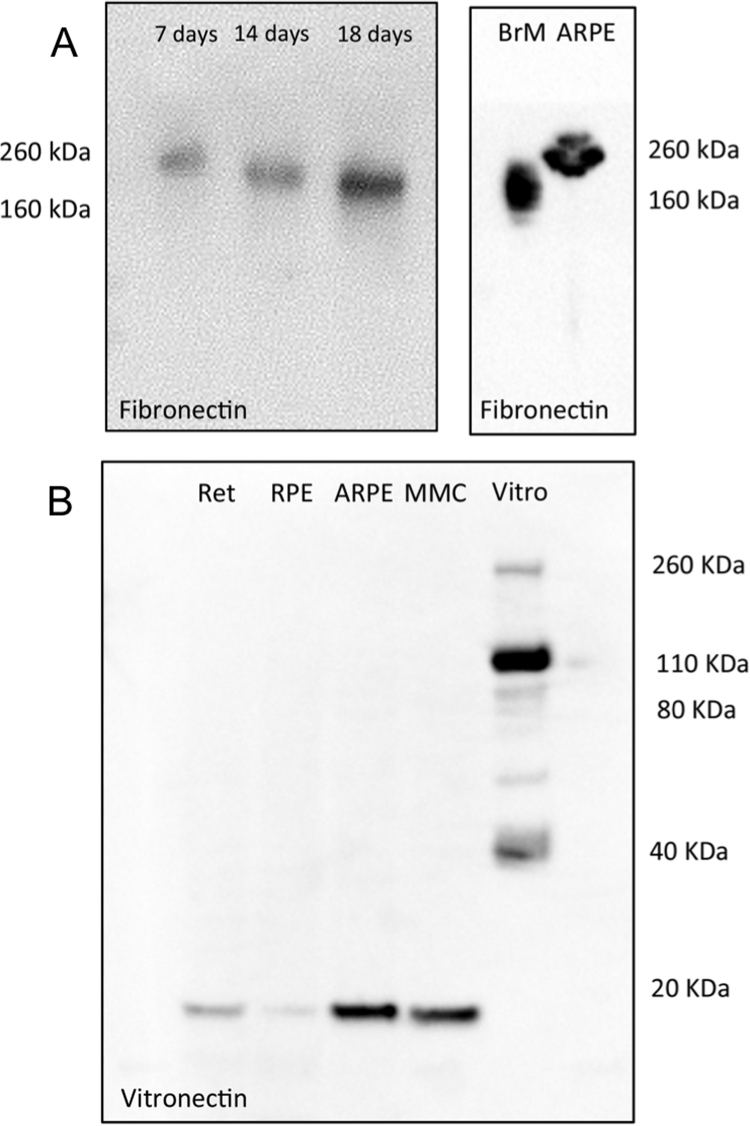
Analysis of protein lysates from decellularized ARPE19-ECM by Western blot demonstrated increasing fibronectin deposition from 7 to 18 days in culture. After 7 days, fibronectin immunoreactivity was associated with a 260 kDa band, however the mobility of the band increased at later timepoints, indicating post-translational modifications of the fibronectin protein occur during matrix development in vitro (Fig. 3A, left panel). Interestingly, the 260 kDa fibronectin-immunopositive band was present in protein lysates from cultured ARPE19 cells while protein lysates from freshly isolated BrM contained only the smaller band, which was approximately 160–180 kDa in size (Fig. 3A, right panel).
Fig. 3.
Analysis of ECM proteins by Western Blot. A: Western blot analysis for fibronectin in ARPE19-ECM protein lysates produced after 7, 14 or 18 days of ARPE19 cell culture under crowded conditions (left panel) or from protein lysates harvested from adult human Bruch's membrane (BrM) and ARPE19 cells cultured without crowders (right panel). B: Western blot analysis for vitronectin in protein lysates harvested from fresh human retina (Ret) and RPE cells (RPE), ARPE19 cells cultured under non-crowded (ARPE) or crowded (MMC) conditions, and a commercial vitronectin solution (Vitro).
Western blot analysis of human retinal and RPE protein lysates for vitronectin revealed a single band approximately 10 kDa in size, which was also detected in ARPE19 cells grown under crowded and control conditions. In comparison, a commercial vitronectin sample contained at least 5 anti-vitronectin immunoreactive bands, with the majority of vitronectin immunolabelling attributed to a single band approximately 100 kDa in size. Minor vitronectin immunoreactivity was also observed in 4 other bands, 260, 75, 55 and 40 kDa in size, however the smaller 10 kDa band was not detected in the commercial sample (Fig. 3B). These results indicate that in the human retina and ARPE19 cells, vitronectin is produced in its ‘clipped’ form, in which the 75 kDa propeptide has undergone cleavage into two fragments (65 kDa and 10 kDa in size) held together by a disulphide bridge. [4] No single chain (75 kDa) vitronectin was detected in protein extracts from RPE, retina or ARPE19 cells (Fig. 3B). Together, these results demonstrate that ECM proteins produced by ARPE19 cells undergo similar post-translational modifications to those found in the retina.
3.4. ARPE19-ECM promotes RPE differentiation
Primary human RPE cells isolated from the same donor eyes attached to surfaces coated with geltrex, vitronectin or ARPE19-ECM, but not to uncoated tissue culture plastic. Culture of primary human RPE cells for one month on either geltrex or vitronectin matrices resulted in the dedifferentiation of RPE cells, indicated by cell elongation and loss of pigmentation. RPE cells grown on vitronectin failed to achieve confluence, while those cultured on geltrex or ARPE19-ECM formed confluent monolayers. In comparison with monolayers formed on geltrex, RPE cells cultured on ARPE19-ECM displayed a more differentiated phenotype, retaining a pigmented phenotype for over 1 month in culture (Fig. 4). These results indicate that ARPE19-ECM contains molecular cues that promote the maintenance of the RPE cell phenotype.
Fig. 4.
Culture of adult human RPE cells on ARPE19-ECM. Primary human RPE cells were seeded into wells coated with geltrex, vitronectin or ARPE19-ECM. One month after seeding, only cells cultured on ARPE19-ECM retained a pigmented phenotype.
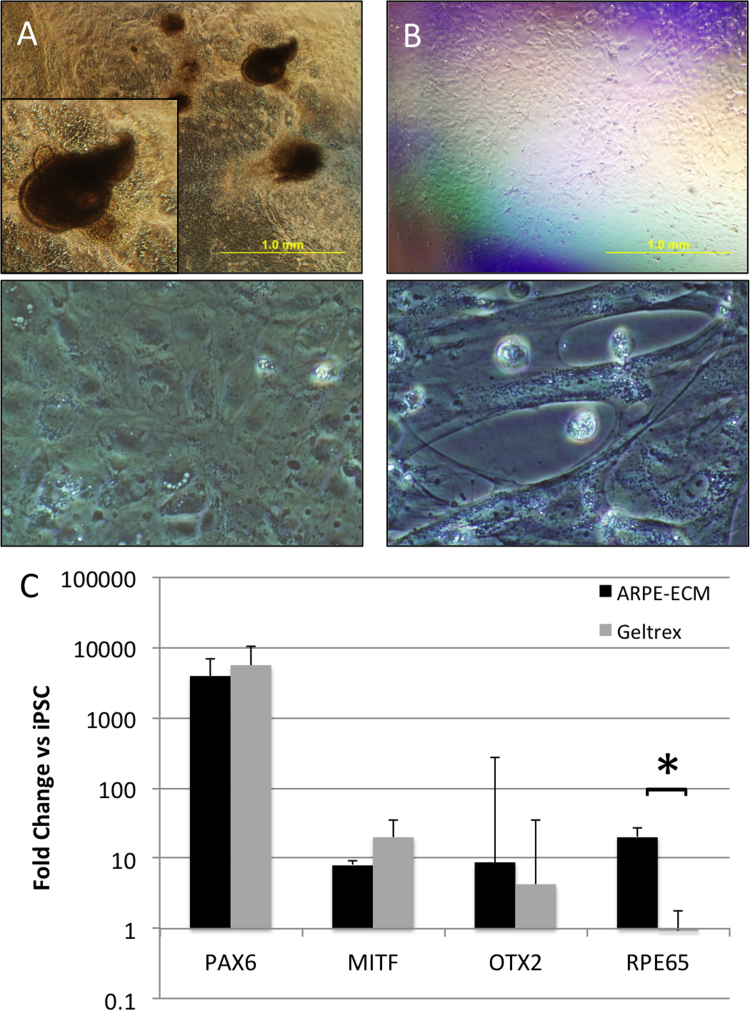
To further examine the RPE promoting properties of ARPE19-ECM, RPE differentiation from human induced pluripotent stem cells (iPSC) was compared on ARPE19-ECM and geltrex surfaces. Human iPSC cultured on ARPE19-ECM formed large pigmented colonies that were surrounded by non-pigmented, polygonal epithelial cells (Fig. 5A). In contrast, iPSC cultured on geltrex formed a thin monolayer consisting of non-pigmented fibroblast-like cells (Fig. 5B). Expression of the ocular transcription factors PAX6, MITF and OTX2 was similar in iPSC cultured on ARPE19-ECM or geltrex. In contrast, the RPE marker RPE65 was significantly increased in iPSC cultured on ARPE19-ECM, compared with geltrex (Fig. 5C). Together, these results indicate ARPE19-ECM contains signals that promote the differentiation of RPE cells from pluripotent stem cells.
Fig. 5.
Differentiation of pluripotent stem cells on ARPE19-ECM. A: Human iPSC cultured on ARPE19-ECM produced pigmented spheres (upper panel, inset shows high magnification view) and polygonal epithelial monolayers (lower panel) after 4 weeks. B: In contrast, human iPSC cultured on geltrex produced non-pigmented monolayers (upper panel) consisting of elongated fibroblastic cells (lower panel). C: Quantitative RT-PCR demonstrated similar induction of PAX6, MITF and OTX2 mRNA expression in iPSC cultured on ARPE19-ECM (black bars) or geltrex (grey bars) for 2 weeks. In contrast, RPE65 expression was significantly increased in iPSC cultured on ARPE19-ECM compared with iPSC cultured on geltrex (p=0.018). Gene expression values were normalized against GAPDH and expressed as a fold change relative to undifferentiated iPSC. Each bar represents the mean of 3 independent experiments. Error bars indicate standard deviation.
4. Discussion
ECMs throughout the body display high degrees of tissue specificity, supplying complex chemical and physical cues and constraints that work together to specify cellular phenotypes. The presence of an appropriate ECM is critical for maintaining these phenotypes in vivo and in vitro [2]. While commercial ECM formulations provide permissive surfaces for the attachment of a wide variety of cells, they remain a poor substitute for the native basement membranes present in vivo. In this study, a simple method for generating BrM-like surfaces in vitro was shown to enrich RPE cell culture conditions and promote RPE cell differentiation from pluripotent stem cells.
4.1. Use of macromolecular crowding for ECM production
Removal of RPE cells from their subretinal niche and expansion in culture is often accompanied by a loss of mature RPE characteristics. Growth adapted RPE cells, such as the ARPE19 cell line and long-term primary cultures, cease production of melanin, lose their apical-basal polarity and adopt hypertrophic, elongated cell morphologies (Supp Fig. 1). In this study, ARPE19 cells retained their growth-adapted phenotype when cultured under standard conditions employing DMEM media with serum supplementation, indicated by elongated cell morphologies with interdigitated cell-cell junctions containing N-cadherin but little ZO1. Under standard conditions, expression of the transcription factor SOX2 was exclusively cytoplasmic (Supp Fig. 1). Addition of the macromolecular crowder dextran sulphate resulted in dramatic changes in ARPE19 cell morphology. Under crowded culture conditions, ARPE19 monolayers contained tightly packed polygonal cells with tight junctions containing ZO1 and N-cadherin, as well as SOX2 immunopositive nuclei (Supp Fig. 1). These data indicate that macromolecular crowders such as dextran sulphate can partially reverse the growth adaptations observed in cultured RPE cells.
4.2. Composition and microstructure of ARPE19-ECM
In addition to inducing changes in cell morphology and protein localization, dextran sulphate also promoted the deposition of ECM (Fig. 1C). The assembly of ECM under crowded culture conditions allows cells to manufacture their own microenvironments. In the retina, BrM forms a major component of the RPE microenvironment. During development, human BrM initially forms around 6–7 weeks of gestation at the interface between the RPE layer and the extraocular mesenchyme. Early BrM consists of two basal lamina layers; the outer layer, derived from mesenchymal cells, and the inner layer, which is the basement membrane produced by RPE cells. By 11–12 weeks of gestation, collagen and elastin secretion by fibroblasts and endothelial cells leads to the formation of the inner and outer collagenous layers (ICL and OCL, respectively) on either side of the middle elastic layer [6]. After 2 weeks of culture in crowded conditions, ARPE19-ECM contained a number of proteins found in the ICL and RPE basal lamina (RBL) of BrM, including fibronectin, vitronectin, collagens IV and V as well as laminin- α5 (Fig. 1, Supp Fig. 3).
Fibronectin is a common component of basement membranes and forms a dense meshwork in the ICL of BrM [6], [7]. In decellularized choroidal-BrM wholemounts, fibronectin immunostaining revealed a network of fibrils (Fig. 2) that surrounded discrete spots of laminin-α5 deposition (Supp Fig. 4). Fibronectin immunoreactivity in ARPE19-ECM was localized to a similar network of fibrils (Fig. 2), however laminin-α5 immunoreactivity was found to be sparse and punctate (Fig. 1). Since laminin-α5 is localized to the RBL of BrM, these results suggest the RBL may be partially removed by the decellularization process. Supporting this hypothesis, discrete spots of laminin-α5 immunoreactivity were observed when ARPE19 cells were immunostained prior to decellularization (Supp Fig. 4E).
Although fibronectin and laminin are found in both inner and outer layers of BrM, vitronectin has been localized to the ICL [6] suggesting this ECM protein may be supplied by RPE cells. In this study, abundant vitronectin deposition was observed in ARPE19-ECM (Supp Fig. 3). In contrast, collagen VI, which is localized to the OCL and elastic layer of BrM, and elastin, which forms the middle elastic layer of BrM [7], were not detected in ARPE19-ECM (Fig. 1). Collagen IV and V, which are found in the collagenous layers and basal lamina on both sides of the elastic layer [6], were both present in ARPE19-ECM (Fig. 1). The presence of vitronectin and the absence of collagen VI and elastin suggest that ARPE19 cells deposit a matrix resembling the inner layers (ICL-RBL) of BrM. Additionally, both vitronectin and fibronectin proteins were shown to undergo retinal specific post-translational modifications during ARPE19-ECM deposition (Fig. 3). Together, these results demonstrate that ARPE19-ECM provides a surface substrate that mimics the structural and molecular properties of BrM in the retina.
4.3. ARPE19-ECM promotes RPE differentiation
To examine the effect of ARPE19-ECM on primary human RPE cells, freshly isolated human RPE cells were seeded onto geltrex, vitronectin or ARPE19-ECM surfaces and cultured them for 1 month in DMEM with 10% FCS. Under these conditions, adult human RPE cells adhered to surfaces coated with geltrex or recombinant vitronectin, however long term culture on these substrates led to the loss of pigmentation in monolayers (Fig. 4). RPE cell survival on vitronectin was particularly poor, with cells failing to achieve confluence despite high cell seeding densities. It is notable that although the vitronectin used for coating plates in this study contained numerous vitronectin immunoreactive bands, the 10 kDa clipped vitronectin peptide was not present (Fig. 3B). In retinal, RPE and ARPE19 cell extracts only the 10 kDa vitronectin band was detected, indicating the clipped isoform represents a retinal specific post-translational modification of this protein. In contrast with geltrex or vitronectin, human primary RPE cells seeded onto ARPE19-ECM maintained a mature, pigmented phenotype for over 1 month (Fig. 4). Together, these results demonstrate that ARPE19-ECM provides a tissue-specific microenvironment that promotes pigmentation during primary RPE cell culture.
Interestingly, previous studies demonstrated enhanced RPE differentiation on ECM derived from bovine corneal endothelial cells (BCEC) compared with RPE cell-derived ECM. [3] In this study, ECM was deposited over 1 week of culture and BCECs deposited more ECM protein during this period than RPE cells. Here the deposition of ECM proteins by ARPE19 cells was shown to increase over 1–4 weeks in culture (Supp Fig. 3). Fibronectin was rapidly deposited on the cell culture floor, while other ECM proteins, such as laminin-α5 and vitronectin took longer to accumulate in the matrix. In addition, fibronectin underwent post-translational modifications that increased its mobility during SDS-PAGE, suggesting maturation of the ARPE19-ECM occurs with extended culture times (Fig. 3A). These results suggest that ARPE19 cells require at least 2–3 weeks to accumulate ECM proteins and develop a mature basement membrane. Therefore, it is possible that the differences in RPE cell differentiation previously reported on BCEC and RPE cell-derived ECM reflect differences in the rates of ECM protein production and deposition between the two cells lines. In the present study, laminin-α5 was not detected in ARPE19-ECM after 1 week of culture (Supp Fig. 3), therefore ARPE19-ECM surfaces were produced over 2 weeks to allow accumulation of this and other ECM proteins in the matrix.
To further examine the RPE promoting properties of ARPE19-ECM, human iPSC were seeded onto geltrex or ARPE19-ECM and cultured in DMEM with 10% FCS to induce spontaneous differentiation. Expression of the ocular transcription factors PAX6, MITF and OTX2 was induced on both surfaces after 2 weeks of culture, however expression of RPE65, a marker of RPE cells, was significantly increased when cells were cultured on ARPE19-ECM compared with geltrex (Fig. 5C). After 4 weeks, iPSC cultured on geltrex adopted a fibroblastic, non-pigmented phenotype, while iPSC cultured on ARPE19-ECM formed three dimensional, pigmented spheres and polygonal RPE-like monolayers (Fig. 5A). These results indicate ARPE19-ECM contains signals that promote RPE cell differentiation from pluripotent stem cells. Together, the results presented here indicate that the manufacture of retinal-specific microenvironments using cultured cell lines provides an efficient and inexpensive method for inducing RPE cell differentiation and enriching RPE cell cultures.
4.4. Conclusions
In summary, our results demonstrate a method for producing a BrM-like surface for the culture of RPE cells. ARPE19-ECM contains physical and molecular cues found in BrM and promotes the acquisition and maintenance of the RPE phenotype. ARPE19-ECM could be produced under serum-free conditions and on alternative substrates, including fibrin gels and silk fibroin (Supp Fig. 5), demonstrating the utility of this method for surfacing substrates with the potential for use in RPE cell replacement therapies.
Acknowledgements
This work was supported by the Raine Medical Research Foundation (Raine Priming Grant 13/2014, University of Western Australia) and the Lions Eye Institute (Western Australia). We thank the Lions Eye Bank of Western Australia for providing human ocular tissues and acknowledge the generosity of the donor families for providing consent for ongoing ophthalmic disease research. We thank the Saleeba, Constantine and Miocevich Families for their generous donations to the Ocular Tissue Engineering Laboratory.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.03.008.
Appendix A. Supplementary material
Supplementary material
Supp Fig. 1: Effect of Macromolecular Crowders on ARPE19 Cells, A-B: ARPE19 cell monolayers were cultured under control conditions (left panels) or crowded conditions (with dextran sulphate and ascorbic acid, supplementation, right panels). After one week, cells were fixed and immunostained for SOX2 and N-cadherin (A) or ZO1 and MITF (B). C: Decellularized ARPE19-ECM was prepared after 2 weeks culture under control (left panel) or crowded (right panel) conditions. Fibronectin content in ARPE19-ECM was assessed by immunostaining, demonstrating increased ECM deposition in crowded cultures. Scale bars indicate 50 µm.
Supplementary Fig. 2: Evidence for ARPE19-ECM Decelluarization, Fixed ARPE19 cell monolayers (left panels) were compared with decellularized ARPE19-ECM (right panels) by brightfield microscopy (upper panels) or immunostaining for the cytoskeletal marker βIII-tubulin (lower panels). After decellularization no intact cells or βIII-tubulin immuopositive cell fragments remained on the culture dish surface.
Supp Fig. 3: Time Course of ARPE19-ECM Deposition, Immunostaining of decellularized ARPE19-ECM produced over 1, 2 or 4 weeks of culture under crowded conditions using antibodies for fibronectin (top panels), vitronectin (middle panels) or laminin-α5 (lower panels).
Supplementary Fig. 4: Immunostaining of Choroid-BrM Wholemounts, A-B: Fluorescence imaging of the same area in a decellularized choroid-BrM wholemount stained with anti-laminin-α5 antibodies (A) and the nuclear stain DAPI (B). Inset in B shows DAPI staining of a choroid-BrM wholemount without decellularization. Scale bars indicate 200 µm. C-D: Fluorescence imaging of the same area in a decellularized choroid-BrM wholemount double immunostained for laminin-α5 (red) and fibronectin (green). Images were taken on different focal planes, revealing a regular array of discrete laminin-α5 deposits surrounded by fibronectin fibrils in BrM (C, inset shows high magnification view) and localization of each protein to vascular structures in the underlying choroid (D). Scale bars indicate 100 µm. E: ARPE19 cells were cultured for 2 weeks in media supplemented with dextran sulphate and ascorbic acid and fixed for immunostaining prior to decellularization. Similar to BrM, double immunostaining for fibronectin (green) and laminin-α5 (red) revealed networks of fibronectin fibrils surrounding discrete laminin-α5 deposits (inset shows high magnification view). Scale bars indicate 50 µm.
Supplementary Fig. 5: Production of ARPE19-ECM on Substrates for RPE Patch Engineering, A: Fibronectin immunostaining of ARPE19-ECM produced using defined, serum- and xeno-free neural stem cell media supplemented with dextran sulphate. B-E: ARPE19 cells were cultured on the surface of a fibrin gel to produce a layer of ARPE19-ECM containing vitronectin (B, D) and fibronectin (C, E). Panels show sections of fibrin gels stained before (B-C) or after (D-E) decellularization. F: Fibronectin immunostaining of ARPE19-ECM produced on a silk fibroin membrane.
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Abraham S., Sheridan S.D., Miller B., Rao R.R. Stable propagation of human embryonic and induced pluripotent stem cells on decellularized human substrates. Biotechnol. Progress. 2010;26:1126–1134. doi: 10.1002/btpr.412. [DOI] [PubMed] [Google Scholar]
- 2.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campochiaro P.A., Hackett S.F. Corneal endothelial cell matrix promotes expression of differentiated features of retinal pigmented epithelial cells: implication of laminin and basic fibroblast growth factor as active components. Exp. Eye Res. 1993;57:539–547. doi: 10.1006/exer.1993.1158. [DOI] [PubMed] [Google Scholar]
- 4.Chain D., Kreizman T., Shapira H., Shaltiel S. Plasmin cleavage of vitronectin. Identification of the site and consequent attenuation in binding plasminogen activator inhibitor-1. FEBS Lett. 1991;285:251–256. doi: 10.1016/0014-5793(91)80810-p. [DOI] [PubMed] [Google Scholar]
- 5.Chen C., Loe F., Blocki A., Peng Y., Raghunath M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv. Drug Deliv. Rev. 2011;63:277–290. doi: 10.1016/j.addr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Curcio C.A., Johnson M. Structure, function, and pathology of Bruch's membrane. Elastic. 2013;146:210–213. [Google Scholar]
- 7.Das A., Frank R.N., Zhang N.L., Turczyn T.J. Ultrastructural localization of extracellular matrix components in human retinal vessels and Bruch's membrane. Arch. Ophthalmol. 1990;108:421–429. doi: 10.1001/archopht.1990.01070050119045. [DOI] [PubMed] [Google Scholar]
- 8.Lai Y., Sun Y., Skinner C.M., Son E.L., Lu Z., Tuan R.S., Jilka R.L., Ling J., Chen X.D. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 2010;19:1095–1107. doi: 10.1089/scd.2009.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Peng Y., Bocker M.T., Holm J., Toh W.S., Hughes C.S., Kidwai F., Lajoie G.A., Cao T., Lyko F., Raghunath M. Human fibroblast matrices bio-assembled under macromolecular crowding support stable propagation of human embryonic stem cells. J. Tissue Eng. Regen. Med. 2012;6:e74–86. doi: 10.1002/term.1560. [DOI] [PubMed] [Google Scholar]
- 11.Rowland T.J., Blaschke A.J., Buchholz D.E., Hikita S.T., Johnson L.V., Clegg D.O. Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J. Tissue Eng. Regen. Med. 2013;7:642–653. doi: 10.1002/term.1458. [DOI] [PubMed] [Google Scholar]
- 12.Sorkio A., Hongisto H., Kaarniranta K., Uusitalo H., Juuti-Uusitalo K., Skottman H. Structure and barrier properties of human embryonic stem cell-derived retinal pigment epithelial cells are affected by extracellular matrix protein coating. Tissue Eng. Part A. 2014;20:622–634. doi: 10.1089/ten.tea.2013.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugino I.K., Gullapalli V.K., Sun Q., Wang J., Nunes C.F., Cheewatrakoolpong N., Johnson A.C., Degner B.C., Hua J., Liu T., Chen W., Li H., Zarbin M.A. Cell-deposited matrix improves retinal pigment epithelium survival on aged submacular human Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 2011;52:1345–1358. doi: 10.1167/iovs.10-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian J., Ishibashi K., Handa J.T. The expression of native and cultured RPE grown on different matrices. Physiol. Genom. 2004;17:170–182. doi: 10.1152/physiolgenomics.00179.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supp Fig. 1: Effect of Macromolecular Crowders on ARPE19 Cells, A-B: ARPE19 cell monolayers were cultured under control conditions (left panels) or crowded conditions (with dextran sulphate and ascorbic acid, supplementation, right panels). After one week, cells were fixed and immunostained for SOX2 and N-cadherin (A) or ZO1 and MITF (B). C: Decellularized ARPE19-ECM was prepared after 2 weeks culture under control (left panel) or crowded (right panel) conditions. Fibronectin content in ARPE19-ECM was assessed by immunostaining, demonstrating increased ECM deposition in crowded cultures. Scale bars indicate 50 µm.
Supplementary Fig. 2: Evidence for ARPE19-ECM Decelluarization, Fixed ARPE19 cell monolayers (left panels) were compared with decellularized ARPE19-ECM (right panels) by brightfield microscopy (upper panels) or immunostaining for the cytoskeletal marker βIII-tubulin (lower panels). After decellularization no intact cells or βIII-tubulin immuopositive cell fragments remained on the culture dish surface.
Supp Fig. 3: Time Course of ARPE19-ECM Deposition, Immunostaining of decellularized ARPE19-ECM produced over 1, 2 or 4 weeks of culture under crowded conditions using antibodies for fibronectin (top panels), vitronectin (middle panels) or laminin-α5 (lower panels).
Supplementary Fig. 4: Immunostaining of Choroid-BrM Wholemounts, A-B: Fluorescence imaging of the same area in a decellularized choroid-BrM wholemount stained with anti-laminin-α5 antibodies (A) and the nuclear stain DAPI (B). Inset in B shows DAPI staining of a choroid-BrM wholemount without decellularization. Scale bars indicate 200 µm. C-D: Fluorescence imaging of the same area in a decellularized choroid-BrM wholemount double immunostained for laminin-α5 (red) and fibronectin (green). Images were taken on different focal planes, revealing a regular array of discrete laminin-α5 deposits surrounded by fibronectin fibrils in BrM (C, inset shows high magnification view) and localization of each protein to vascular structures in the underlying choroid (D). Scale bars indicate 100 µm. E: ARPE19 cells were cultured for 2 weeks in media supplemented with dextran sulphate and ascorbic acid and fixed for immunostaining prior to decellularization. Similar to BrM, double immunostaining for fibronectin (green) and laminin-α5 (red) revealed networks of fibronectin fibrils surrounding discrete laminin-α5 deposits (inset shows high magnification view). Scale bars indicate 50 µm.
Supplementary Fig. 5: Production of ARPE19-ECM on Substrates for RPE Patch Engineering, A: Fibronectin immunostaining of ARPE19-ECM produced using defined, serum- and xeno-free neural stem cell media supplemented with dextran sulphate. B-E: ARPE19 cells were cultured on the surface of a fibrin gel to produce a layer of ARPE19-ECM containing vitronectin (B, D) and fibronectin (C, E). Panels show sections of fibrin gels stained before (B-C) or after (D-E) decellularization. F: Fibronectin immunostaining of ARPE19-ECM produced on a silk fibroin membrane.
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material