Abstract
Ascorbic acid (AA) has been reported as a treatment for cancer patients. Intravenous (iv) administration of high-dose AA increases plasma AA levels to pharmacologic concentrations and generates reactive oxygen species (ROS) to exert anti-tumor activity via enhancement of oxidative stress. However, AA is very unstable in aqueous solutions and it is impossible to preserve AA for a long period in a solution. 2-O-α-D-Glucopyranosyl-l-ascorbic acid (AA-2G), which is a glucoside derivative of AA, has been found to exhibit much higher stability than AA in aqueous solutions and it shows vitamin C activity after enzymatic hydrolysis to AA. To evaluate the effectiveness of AA-2G for cancer treatment, we examined the antitumor activity of AA-2G to murine colon carcinoma (colon-26) cells and in tumor-bearing mice. AA-2G did not show cytotoxicity to colon-26 cells, whereas AA exhibited a significant cytotoxic effect in a concentration-dependent manner. In colon-26 tumor-bearing mice, iv administration of high-dose AA-2G as well as that of AA significantly inhibited tumor growth. Experiments on the biodistribution and clearance of AA-2G in tumor-bearing mice showed that AA-2G was rapidly hydrolyzed to AA and exhibited significant antitumor activity. Treatment of tumor-bearing mice with AA-2G tended to increase plasma malondialdehyde level. These results indicated that the antitumor activity of AA-2G was caused by ROS generated by AA released by rapid hydrolysis of AA-2G.
Keywords: 2-O-α-D-glucopyranosyl-L-ascorbic acid, Ascorbic acid, Cancer, Oxidative stress
Highlights
-
•
Tumor growth was inhibited by administration of high-dose ascorbic acid 2-glucoside in vivo.
-
•
High-dose ascorbic acid 2-glucoside showed oxidative stress-mediated antitumor activity.
-
•
Rapidly released ascorbic acid gave oxidative stress to tumors.
-
•
The antitumor activities of ascorbic acid 2-glucoside were the same as those of ascorbic acid.
-
•
Ascorbic acid 2-glucoside can be used as an agent in infusion therapy for cancer.
1. Introduction
Ascorbic acid (AA, Fig. 1A) is well known as vitamin C, and it is common knowledge that AA is an essential micronutrient for humans. AA has various physiological and pharmacological activities such as collagen synthesis [1], anti-oxidation [2], enhancement of iron absorption [3] and drug metabolism [4]. Recently, the effects of intravenous injection of high-dose AA as an anti-cancer drug have been reported, and high-dose AA has been used clinically [5], [6], [7], [8]. It has been reported that the tumoricidal action of AA occurs due to its prooxidant effect [8], [9], [10], [11], although the mechanism of AA on cancer cells has not been completely understood. Intravenous infusion of high-dose AA greatly increases plasma AA to a concentration higher than that achieved by oral administration [7], [8], [12], and AA acts as an excellent reducing agent. AA reduces Fe3+ to Fe2+, and Fe2+ donates an electron to O2, ultimately generating reactive oxygen species (ROS) including the highly reactive hydroxyl radical. Normal cells have antioxidant enzymes such as catalase and glutathione peroxidase, and ROS are thus scavenged by these enzymes. Many cancer cells, on the other hand, have lower levels of several antioxidant enzymes than those in normal cells [13], and cancer cells that have low anti-oxidant capacity are thus damaged by ROS, resulting in cell death. This hypothesis is supported by our previous findings that AA content in the tumor was consumed to resist oxidative stress caused by ROS that was generated by administered AA [14]. More recently, it was also reported that AA selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH [15]. The report showed that AA induced cell death in cancer cells triggered not by exogenous oxidative stress as generally known, but by endogenous oxidative stress. Therefore, therapy with AA exhibits cytotoxic activity selectively to cancer cells and has fewer adverse effects than those of other cancer treatments.
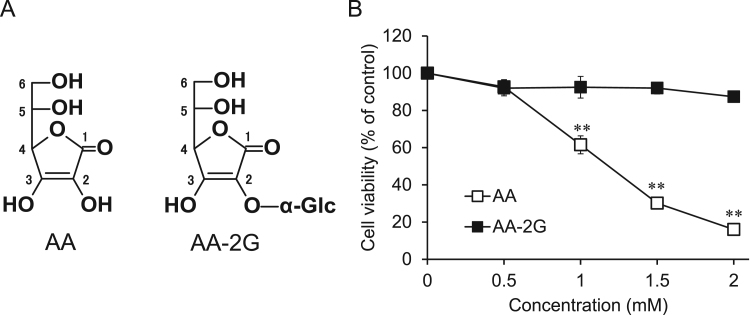
Fig. 1.
Structures and cytotoxic activities of AA and AA-2G. (A) Structures of AA and AA-2G. (B) In vitro cytotoxicities of AA and AA-2G. Colon-26 cells were incubated in a medium containing AA-2G or AA at indicated concentrations for 24 h. Vehicle-treated cells were arbitrarily set as 100% control viability. All data represent means ± SD of three independent cultures (**P<0.01, compared with the control).
One problem with AA infusion therapy is that AA is very unstable in aqueous solutions, especially under particular oxidative conditions such as high temperature and intense light conditions [16]. It is difficult to preserve AA for a long period in solutions because its activity is easily lost by oxidation. Sufficient care is needed in handling AA, i.e., it is necessary to keep an AA injection under refrigeration and to prepare the infusate from an AA injection returned to normal temperature just before use. 2-O-α-D-Glucopyranosyl-l-ascorbic acid (AA-2G, Fig. 1A) is a stable AA derivative. AA-2G is markedly stable under nonenzymatic conditions, while AA decomposes rapidly [17], [18], [19]. For example, AA-2G was scarcely degraded in 100 mM potassium phosphate buffer (pH 6.5) at 50 °C for 28 days, while the most of AA was decomposed for 2 weeks [19]. AA-2G exhibits vitamin C activities such as collagen synthesis [20], [21] and enhancement of antibody production after enzymatic hydrolysis to AA by α-glucosidase [22], [23]. AA-2G has been noted as a stable source for AA supply, and it has been widely used in the fields of cosmetics and is permitted as a food additive in Japan. These properties of AA-2G suggest that AA-2G could be used as an anti-cancer drug without the above-mentioned problem with the use of AA.
The aim of the present study was to assess the possibility of using AA-2G as an anti-cancer agent for infusion therapy instead of AA. For this purpose, we evaluated the effects of AA-2G on murine colon carcinoma (colon-26) cells and in tumor-bearing mice. We also investigated the biodistribution and clearance of AA-2G and plasma lipid peroxidation in the mice after administration AA-2G.
2. Materials and methods
2.1. Chemicals
AA-2G was provided by Hayashibara Biochemical Laboratories (Okayama, Japan). Sodium ascorbate (AA-Na), heparin and calcein-AM solution were obtained from Wako Pure Chemical Industries (Osaka, Japan). RPMI 1640 medium was purchased from Corning (NY, USA). Fetal bovine serum (FBS, heat-inactivated) was from Hyclone (Logan, UT, USA). Triton X-100 was from Sigma-Aldrich (St. Louis, MO, USA). Penicillin and streptomycin solution and dithiothreitol (DTT) were purchased from Nacalai Tesque (Kyoto, Japan). All of the chemicals used were of the highest grade commercially available.
2.2. Cell lines
Murine colon carcinoma (colon-26) cells were purchased from RIKEN BRC CELL BANK (Tsukuba, Japan). Colon-26 cells were grown in RPMI 1640 with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin at 37 °C in 5% CO2. Experiments were performed when cell growth was approximately 80% confluent.
2.3. Evaluation of in vitro cytotoxic activity of AA-2G
Cytotoxicity of AA-2G to colon-26 cells was assessed using calcein-AM. The cells were suspended in 10% FBS/RPMI 1640 medium and seeded in a 96-well microplate at a density of 1.0×104 cells/100 μL/well and then incubated for 24 h at 37 °C in 5% CO2. After incubation, each medium was replaced with 90 μL of fresh medium and then 10 μL each of AA-2G and AA-Na dissolved in the medium were added and the cells were incubated for 24 h. The cells were then washed with 100 μL of PBS (-), and 100 μL of calcein-AM solution (5 μM) was added. After 30 min of incubation, 20 μL of 0.6% Triton X-100 solution was added to each well to lyse the cells. The fluorescence intensity (FI) of the cell lysate was recorded on a microplate reader (Varioskan Flash from Thermo Scientific, Ex. 485 nm, Em. 527 nm). Cell viability (%) was caluculated as (FI of treated - FI of blank) /(FI of control - FI of blank) x 100. The difference in cell viability between the control and treatment was analyzed by Dunnett's test (**P<0.01).
2.4. Evaluation of in vivo antitumor activity of AA-2G
The antiproliferative activity of AA-2G was investigated in colon-26 xenograft mice. Balb/c mice were obtained from the CLEA Japan (Tokyo, Japan) and maintained at a room temperature of 20 ± 5 °C. Colon-26 cells were implanted at a density of 1.0×106 cells in five-week-old female Balb/c mice subcutaneously in the left and right dorsal areas. When transplanted tumor sizes were more than 10 mm in diameter, the tumors were excised and small pieces of the tumor (approximately 2-mm cubes) were engrafted subcutaneously into the left dorsal area of each five-week-old male CDF1 mouse (Japan SLC, Shizuoka, Japan). When tumor sizes had reached 8–10 mm in diameter after implantation, the mice were used for studies of antitumor activity, biodistribution and clearance of AA-2G. Each sample was dissolved in PBS (-) (equimolecular amount of 300 mg/kg of AA) and filtered. The pH of AA-2G solution was regulated to neutral. The solutions were then injected intravenously 4 times into colon-26 tumor-bearing mice on alternate days. Tumor size was calculated from caliper measurements using volume =(length) x (width)2 x 0.5 [24]. The differences in tumor size between the treatment groups and control group were analyzed by the dunnett's test (*P<0.05, **P<0.01). The experiments were approved by the Committee for Ethics in Animal Experiments of the Prefectural University of Hiroshima.
2.5. In vivo biodistribution and clearance of AA-2G
The mice that were injected intravenously with AA-2G were sacrificed under anesthesia by isoflurane. The liver, kidney, tumor and blood were collected at 15, 30, 60, 180 and 360 min after injection (n=4). A blood sample was collected by heart puncture into a heparin-coated syringe. Plasma was separated from whole blood by centrifugation at 10,000g for 10 min at 4 °C. The samples were stored at −80 °C until analysis. Samples were homogenized with 85% acetonitrile including 250 mg/L of DTT (100 mg wet tissue or 100 μL plasma/400 μL solution). Then all samples were centrifuged at 10,000g for 10 min at 4 °C and the supernatants were collected. The supernatants were analyzed by HPLC. Separation for AA-2G and AA was achieved by isocratic elution of an HILIC column (ϕ 4.6×250 mm, 5 µm, GL Sciences Inc., Tokyo) kept at 40 °C with 85% acetonitrile/66.7 mM ammonium acetate at a flow rate of 0.7 mL/min [25]. The absorbance at 260 nm was monitored. The mean concentration ± standard error of the mean (SE) was calculated for each time point. The difference in respective time between the control and treatment groups was analyzed by Dunnett's test (*P<0.05).
The blood glucose level was determined by Glucose CII-Test Wako (Wako Pure Chemical Industries) after injection of AA-2G into tumor-bearing mice.
2.6. Determination of lipid peroxidation in plasma
The levels of malondialdehyde (MDA) in plasma from mice after injection of AA-2G and AA were determined by using a thiobarbituric acid reactive substance assay kit (Cayman Chemical Company, MI, USA) following the manufacturer's instructions. Measurement of plasma MDA level enables determination of several products oxidative damage caused by ROS. The mean concentration ± standard error of the mean (SE) was calculated for each time point (n =4).
3. Results
3.1. In vitro cytotoxic activity of AA-2G
First, we examined the effect of AA-2G on colon-26 cells. Colon-26 cells were exposed to various concentrations of AA-2G. Cell viability was measured 24 h later using the calcein-AM assay. AA-2G did not show significant cytotoxic activity to colon-26 cells, whereas AA exhibited cytotoxic activity in a concentration-dependent manner (Fig. 1B). AA showed a significant toxic effect at a concentration of more than 1 mM, but AA-2G did not show significant cytotoxic activity even at 2 mM. In addition, whereas AA exerted potent antitumor activity to rat basophilic leukemia (RBL-2H3) cells, AA-2G did not show the activity to the cells as well as colon-26 cells (data not shown).
3.2. In vivo antitumor activity of AA-2G
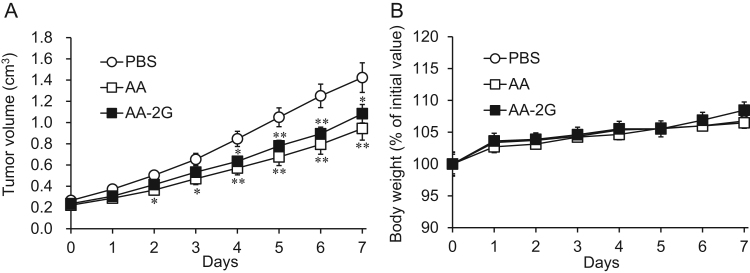
AA-2G did not have significant cytotoxic activity to colon-26 cells. We then assessed the antitumor activity by intravenous (iv) administration of AA-2G in tumor-bearing mice (Fig. 2). The model mice were treated 4 times with a placebo (phosphate buffered saline: PBS), AA-2G and AA-Na (equimolecular amount of 300 mg/kg of AA) on alternate days. As shown in Fig. 2A, AA-2G inhibited tumor growth significantly unlike in the in vitro experiment. The difference between tumor growth in the placebo group and that in the AA-2G-treated group was statistically significant at day 3. In the AA-treated group, a significant difference was observed in tumor growth at day 2. There is no significant difference between AA- and AA-2G-treated groups. The difference in the body weight between the two treatment groups was not statistically significant (Fig. 2B). The results suggested that the antitumor activity of high-dose iv AA-2G is weaker than or the same as that of AA in vivo.
Fig. 2.
Tumor growth in colon-26 tumor-bearing CDF1 mice treated with AA or AA-2G (equimolecular amount of 300 mg/kg of AA). Tumor sizes (A) and body weight (B) were measured for 7 days during treatment. All data represent means ± SE (n =16). (*P<0.05; **P<0.01, compared with the control).
3.3. In vivo biodistribution and clearance of AA-2G
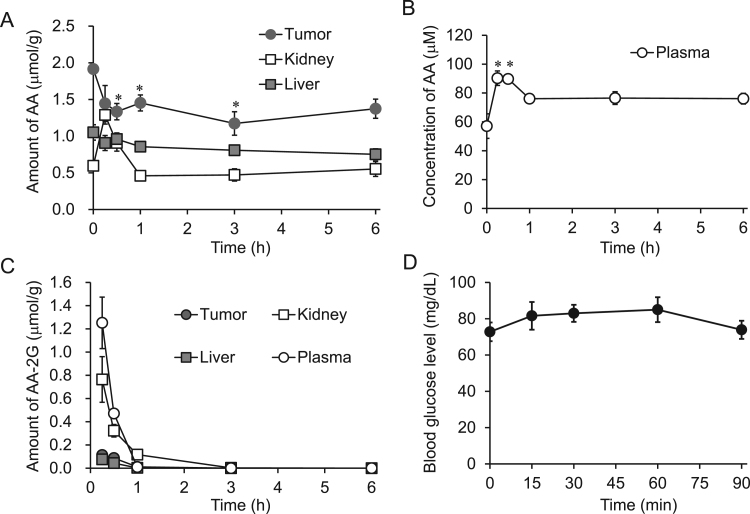
To investigate the biodistribution and clearance of AA-2G, liver, kidney, tumor and plasma levels of AA and AA-2G were determined by HPLC after iv administration of AA-2G to tumor-bearing mice. Fig. 3A and B show the time courses of AA level. AA levels in the kidney and plasma increased after injection of AA-2G. The maximum values of AA in the kidney and plasma were 1.29 μmol/g and 90.3 μM, respectively, at 15 min. At 1 h, AA in the kidney had decreased to the baseline level. AA content in plasma decreased slowly, and plasma AA was maintained from 1 to 6 h at a concentration slightly above the initial value (about 76 μM). On the other hand, AA levels in the tumor and liver decreased after injection of AA-2G. AA level in the tumor was significantly decreased. The time course of AA-2G level is shown in Fig. 3C. AA-2G level in the kidney was high in comparison with the levels in the tumor and liver. After 1–3 h, AA-2G disappeared from the kidney, liver and tumor. The plasma AA-2G level was 1.25 μmol/g at 15 min and rapidly decreased to the baseline level within 1 h.
Fig. 3.
In vivo biodistribution and clearance of AA-2G in colon-26 tumor-bearing CDF1 mice. AA-2G was injected intravenously into colon-26 tumor-bearing CDF1 mice. AA levels in the liver, kidney and tumor (A), AA level in plasma (B), AA-2G levels in tissues and plasma (C), and blood glucose level (D) after administration of AA-2G. All data represent means ± SE (n =4). A significant difference was found between kidney AA levels at 15 min and 0 min (A; P<0.05). Other significant differences are indicated in the figure (*P<0.05, each time point compared with that at 0 min).
Next, we examined the blood glucose level after an iv injection of AA-2G (Fig. 3D). Since plasma AA-2G had almost disappeared at 1 h (Fig. 3C), blood glucose level was measured for 90 min. At 15 min, blood glucose level after injection of high-dose AA-2G had increased to 81.6 mg/dL from the baseline level of 73 mg/dL, and the maximum concentration was 85 mg/dL at 60 min. At 90 min, glucose level had decreased to the baseline level. There was no significant difference, and the increase was within the normal range.
3.4. Effects of AA-2G on lipid peroxidation in plasma
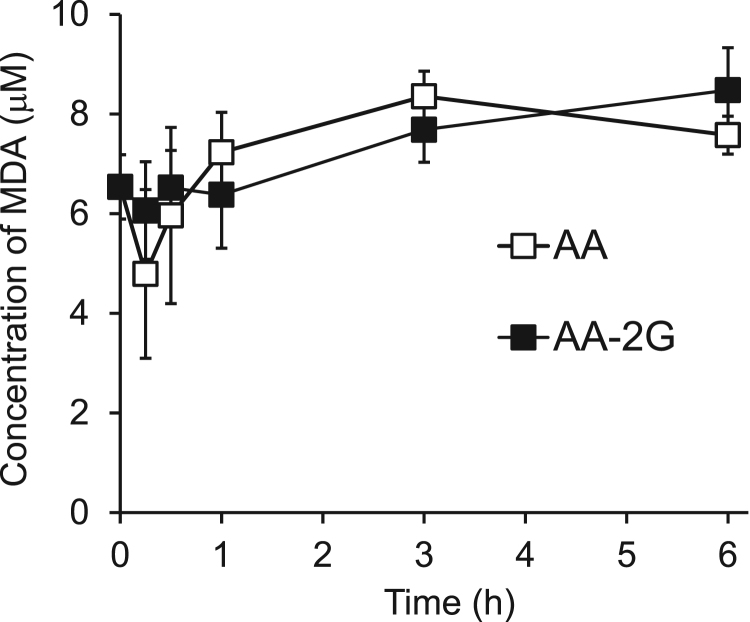
The tumoricidal action of AA occurs due to ROS generated by the prooxidant effect of AA itself [9], [10], [11]. Thus, we examined the level of malondialdehyde (MDA), which is a lipid peroxidation marker, in plasma after iv administration of AA-2G. The MDA level showed a tendency for continuous elevation during a period of 6 h (8.5 μM) after administration of AA-2G (Fig. 4). After administration of AA, the MDA level decreased to below the initial value within 15 min and then AA increased to the maximum value of 8.4 μM at 3 h. The points indicating maximum level of MDA were different between AA-2G and AA.
Fig. 4.
Time course of plasma MDA level in colon-26 tumor-bearing CDF1 mice after injection of AA-2G. MDA levels in plasma of tumor-bearing mice after injection of AA-2G and AA were determined. All data represent mean concentration ± SE (n =4).
4. Discussion
Our results showed that AA-2G can be used as a stable AA source in high-dose iv AA therapy. It is known that AA at a pharmacologic concentration is cytotoxic to various cancer cell lines [9], [26] and that AA exhibits significant cytotoxicity to colon-26 cells in a concentration-dependent manner [14]. AA-2G is known as a stable AA source under culture conditions [20], [21]; however, AA-2G had no effect on colon-26 cells even at a concentration of 2 mM (Fig. 1B). AA causes injury to cancer cells by ROS generated extracellularly [9], [10], [11], [26]. For generation of ROS, it seems to be necessary to rapidly increase AA concentration in the medium by hydrolyzing AA-2G. However, it has been reported that AA-2G is >90% of the intact form remained even after 60-h incubation in the presence of fibroblasts [21], suggesting that AA-2G very slowly releases AA in vitro. Based on these results, the difference between cytotoxicities of AA-2G and AA may be explained by the difference of AA concentrations in the medium.
We compared the effects of AA-2G and AA in tumor-bearing mice. AA-2G showed antitumor activity equivalent to that of AA (Fig. 2). The results suggested that AA-2G was hydrolyzed more rapidly in vivo than in vitro. Thus, it is thought that iv injection of AA-2G may increase AA in the plasma of mice to a very high level. Contrary to expectation, plasma AA in the model mice increased to a level only 1.5-times higher than the initial level (Fig. 3B). In a clinical setting, high-dose AA temporarily increased the plasma concentration of AA to a level 100–1000-times higher than normal levels [7], [8]. The plasma AA level did not reach the level at which AA showed significant cytotoxicity in vitro (Fig. 1B). However, AA-2G showed significant antitumor activity as well as AA in tumor-bearing mice (Fig. 2). AA level in the kidney substantially increased in the first 15 min and then decreased to baseline levels after 1 h (Fig. 3A). In addition, the kidney AA-2G level was high in comparison with levels in the tumor and liver at 15 min (Fig. 3C). After 1–3 h, AA-2G disappeared from the liver, kidney, tumor and plasma. The blood glucose level also tended to be slightly increased from the initial value at 15 min (Fig. 3D). It seems that a part of AA-2G was excreted as the intact form and that the remaining AA-2G immediately hydrolyzed to AA, the plasma AA level reached the pharmacological concentration within 15 min and then the excess AA was mostly excreted at 15 min. Our previous report has showed that only 1.59 mM of AA was observed in the plasma at 15 min after administration of AA (300 mg/kg) to tumor-bearing mice and the AA level further decreased to 0.35 mM at 30 min [14]. The results suggested that the plasma AA concentration was rapidly reduced to baseline levels even when high-dose AA was administrated. These might be the reason why little increment of plasma AA was observed after injection of AA-2G. It has been reported that plasma AA-2G level decreased sharply within 15 min after injection, and then decreased gradually until the complete disappearance at 3 h [27]. The drastic reduction of AA-2G from the plasma at an early stage after injection is in agreement with our results. Therefore, it was thought that AA-2G was rapidly hydrolyzed by α-glucosidase after administration and increased blood AA concentration to a pharmacologic level within 15 min.
AA-2G is remarkably stable in aqueous solutions, whereas AA is easily decomposed [17], [18], [19]. AA-2G is promising as a stable AA source for cancer therapy. In general, cancer cells need more glucose for energy than do normal cells. Thus, there is a possibility that the use of high-dose iv AA-2G for cancer treatment will be a problem, because glucose released from AA-2G might promote tumor growth. The rise in glucose level may also have bad effect the diabetic patients. Our results showed that there was little increment of glucose in plasma after injection of high-dose AA-2G (Fig. 3D). The results suggested that the increment of glucose would not be a problem for cancer therapy. Therefore, high-dose iv AA-2G can be used as cancer therapy for various patients.
We recently reported that AA content in the tumor was consumed to resist oxidative stress caused by ROS generated by high-dose iv AA [14]. Administration of high-dose AA temporarily decreases the AA level in the tumor at 15 min after injection. In the present study, although the maximum decrease in the level of AA in the tumor occurred at 30 min after injection of AA-2G, AA-2G administration decreased the tumor AA level significantly as did as AA administration (Fig. 3A). The delay in decrement of tumor AA level after AA-2G treatment compared with that after AA treatment suggested that AA-2G exhibited antitumor activity after being hydrolyzed to AA. Furthermore, the plasma level of malondialdehyde (MDA), a lipid peroxidation marker, slowly increased after injection of high-dose AA and AA-2G (Fig. 4). At 15 min, no significant differences were observed between treatments group and non-treatment group, but there was a tendency for AA to decrease the plasma MDA level temporarily. It was thought that AA might momentarily act as an antioxidant. A similar tendency was seen with AA-2G, though the magnitude was smaller than that of AA. Maximum levels of MDA were observed at 6 h after injection of AA-2G, and at 3 h after injection of AA. It took more time for AA-2G than for AA to induce oxidative stress, suggesting that ROS were generated by hydrolyzation of AA-2G to AA. These results suggested that the antitumor activity of high-dose AA-2G occurs due to ROS generated by AA released by rapid hydrolysis of AA-2G.
High-dose iv AA has been described as a treatment for cancer patients. However, AA is unstable in aqueous solutions. AA-2G, which is a glucoside derivative of AA, has been found to exhibit much higher stability than AA in aqueous solutions, and it shows vitamin C activity after enzymatic hydrolysis to AA. In the present study, we examined the effectiveness of AA-2G as a cancer treatment. The results suggested that AA-2G itself has no antitumor activity but that AA released by rapid hydrolysis of AA-2G depresses tumor growth by oxidative stress as does treatment with AA. AA-2G will be useful for cancer therapy as a stable AA source.
Footnotes
Transparency document associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2017.04.014.
Appendix A. Transparency document
Transperancy document
References
- 1.Tajima S., Pinnell S.R. Regulation of collagen synthesis by ascorbic acid. Ascorbic acid increases type I procollagen mRNA. Biochem. Biophys. Res. Commun. 1982;106:632–637. doi: 10.1016/0006-291x(82)91157-3. [DOI] [PubMed] [Google Scholar]
- 2.Rose R.C., Bode A.M. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J. 1993;7:1135–1142. [PubMed] [Google Scholar]
- 3.Hallberg L., Brune M., Rossander L. Effect of ascorbic acid on iron absorption from different types of meals. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum. Nutr. Appl. Nutr. 1986;40:97–113. [PubMed] [Google Scholar]
- 4.Sato P.H., Zannoni V.G. Ascorbic acid and hepatic drug metabolism. J. Pharmacol. Exp. Ther. 1976;198:295–307. [PubMed] [Google Scholar]
- 5.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta. 1826;2012:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA. 1978;75:4535–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffer L.J., Levine M., Assouline S., Melnychuk D., Padayatty S.J., Rosadiuk K., Rousseau C., Robitaille L., Miller W.H., Jr. Phase I clinical trial of iv ascorbic acid in advanced malignancy. Ann. Oncol. 2008;19:1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 8.Parrow N.L., Leshin J.A., Levine M. Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics. Antioxid. Redox Signal. 2013;19:2141–2156. doi: 10.1089/ars.2013.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R., Shacter E., Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q., Espey M.G., Sun A.Y., Lee J.H., Krishna M.C., Shacter E., Choyke P.L., Pooput C., Kirk K.L., Buettner G.R., Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q., Espey M.G., Sun A.Y., Pooput C., Kirk K.L., Krishna M.C., Khosh D.B., Drisko J., Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padayatty S.J., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A., Wesley R.A., Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 13.Oberley T.D., Oberley L.W. Antioxidant enzyme levels in cancer. Histol. Histopathol. 1997;12:525–535. [PubMed] [Google Scholar]
- 14.Miura K., Yazama F., Tai A. Oxidative stress-mediated antitumor activity of erythorbic acid in high doses. Biochem. Biophys. Rep. 2015;3:117–122. doi: 10.1016/j.bbrep.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun J., Mullarky E., Lu C., Bosch K.N., Kavalier A., Rivera K., Roper J., Chio I.I.C., Giannopoulou E.G., Rago C., Muley A., Asara J.M., Paik J., Elemento O., Chen Z., Pappin D.J., Dow L.E., Papadopoulos N., Gross S.S., Cantley L.C. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolbert B.M., Downing M., Carlson R.W., Knight M.K., Baker E.M. Chemistry and metabolism of ascorbic acid and ascorbate sulfate. Ann. N. Y. Acad. Sci. 1975;258:48–69. doi: 10.1111/j.1749-6632.1975.tb29267.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto I., Muto N., Murakami K., Suga S., Yamaguchi H. l-Ascorbic acid α-glucoside formed by regioselective transglucosylation with rat intestinal and rice seed α-glucosidases: its improved stability and structure determination. Chem. Pharm. Bull. 1990;38:3020–3023. doi: 10.1248/cpb.38.3020. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto I., Tai A., Fujinami Y., Sasaki K., Okazaki S. Synthesis and characterization of a series of novel monoacylated ascorbic acid derivatives, 6-O-acyl-2-O-α-D-glucopyranosyl-L-ascorbic acids, as akin antioxidants. J. Med. Chem. 2002;45:462–468. doi: 10.1021/jm010379f. [DOI] [PubMed] [Google Scholar]
- 19.Tai A., Kawasaki D., Sasaki K., Gohda E., Yamamoto I. Synthesis and characterization of 6-O-acyl-2-O-α-D-glucopyranosyl-L-ascorbic acids with a branched-acyl chain. Chem. Pharm. Bull. 2003;51:175–180. doi: 10.1248/cpb.51.175. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto I., Suga S., Mitoh Y., Tanaka M., Muto N. Antiscorbutic activity of L-ascorbic acid 2-glucoside and its availability as a vitamin C supplement in normal rats and guinea pigs. J. Pharm.-Dyn. 1990;13:688–695. doi: 10.1248/bpb1978.13.688. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto I., Muto N., Murakami K., Akiyama J. Collagen synthesis in human skin fibroblasts is stimulated by a stable form of ascorbate, 2-O-α-D-glucopyranosyl-L-ascorbic acid. J. Nutr. 1992;122:871–877. doi: 10.1093/jn/122.4.871. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto I., Tanaka M., Muto N. Enhancement of in vitro antibody production of murine splenocytes by ascorbic acid 2-O-α-glucoside. Int. J. Immunopharmacol. 1993;15:319–325. doi: 10.1016/0192-0561(93)90042-w. [DOI] [PubMed] [Google Scholar]
- 23.Ichiyama K., Mitsuzumi H., Zhong M., Tai A., Tsuchioka A., Kawai S., Yamamoto I., Gohda E. Promotion of IL-4-and IL-5-dependent differentiation of anti-μ-primed B cells by ascorbic acid 2-glucoside. Immunol. Lett. 2009;122:219–226. doi: 10.1016/j.imlet.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Haranaka K. Antitumor activity of murine tumor necrosis factor (TNF) against transplanted murine tumors and heterotransplanted human tumors in nude mice. Int. J. Cancer. 1984;34:263–267. doi: 10.1002/ijc.2910340219. [DOI] [PubMed] [Google Scholar]
- 25.Tai A., Gohda E. Determination of ascorbic acid and its related compounds in foods and beverages by hydrophilic interaction liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;853:214–220. doi: 10.1016/j.jchromb.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Verrax J., Calderon P.B. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 2009;47:32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Tai A., Fujinami Y., Matsumoto K., Kawasaki D., Yamamoto I. Bioavailability of a series of novel acylated ascorbic acid derivatives, 6-O-acyl-2-O-α-D-glucopyranosyl-L-ascorbic acids, as an ascorbic acid supplement in rats and guinea pigs. Biosci. Biotechnol. Biochem. 2002;66:1628–1634. doi: 10.1271/bbb.66.1628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transperancy document