Abstract
Background
Para-Dinitrobenzene (p-DNB) is one of the isomers of dinitrobenzene which have been detected as environmental toxicants. Skin irritation and organ toxicities are likely for industrial workers exposed to p-DNB. This study evaluated the effect of sub-chronic exposure of rats to p-DNB on cellular redox balance, hepatic and renal integrity.
Methods
Forty eight male Wistar rats weighing 160–180 g were administered 50, 75, 1000 and 2000 mg/kg b.wt (body weight) of p-DNB or an equivalent volume of vehicle (control) orally and topically for 14 days. After the period of treatment, the activities of kidney and liver catalase (CAT), alkaline phosphatase (ALP) and superoxide dismutase (SOD) as well as extent of renal and hepatic lipid peroxidation (LPO) were determined. Serum ALP activity and plasma urea concentration were also evaluated.
Results
Compared with control animals, p-DNB -administered rats showed decrease in the body and relative kidney and liver weights as well as increased renal and hepatic hydrogen peroxide and lipid peroxidation levels accompanied by decreased superoxide dismutase and catalase activities. However, p-DNB caused a significant increase in plasma urea concentration and serum, liver and kidney ALP activities relative to control. In addition, p-DNB caused periportal infiltration, severe macro vesicular steatosis and hepatic necrosis in the liver.
Conclusions
Our findings show that sub-chronic oral and sub-dermal administration of p-DNB may produce hepato-nephrotoxicity through oxidative stress.
List of abbreviations: p-DNB, para-dinitrobenzene; CAT, Catalase; ALP, alanine phosphatase; SOD, Superoxide dismutase; LPO, lipid peroxidation; o-DNB, ortho-dinitrobenzene, m-DNB, meta-dinitrobenzene; TNB, trinitrobenzene; GSH, glutathione; GST, glutathione –s –transferase, GPX, glutathione reductase, NIH, national institute of health; PHS, public health service; OECD, Organisation for economic co-operation and Development; TBA, thiobarbituric acid; H&E, hamatoxilin eosin; SPSS, Statistical Pucteage for Social Sciences; MDA, malodialdehyde
Keywords: Environmental toxicants, Kidney, Liver, p‐DNB, Oxidative stress, Sub-dermal
Highlights
-
•
Activities of kidney and liver catalase and superoxide dismutase were decreased by p-DNB.
-
•
p-DNB increased serum, liver and kidney activity of alkaline phosphatase.
-
•
Plasma urea concentration was increased by p-DNB.
-
•
Lipid peroxidation and H2O2 level were increased by p-DNB.
-
•
p-DNB caused histopathological changes in liver and kidney tissues.
1. Background
One of the global health problems is liver disorders and diseases which are due to exposure to various toxic chemicals. The major site of xenobiotic metabolism is the liver and it can be damaged by toxic chemicals, drugs and environmental agents which can lead to deleterious effect on the liver cells and functions. Furthermore, in preclinical toxicity studies, renal toxicity is one of the major concerns. Renal toxicity can be a result of hemodynamic changes, direct injury to cells and tissue, inflammatory tissue injury, and/or obstruction of renal excretion. The kidney has important roles in plasma filtration and maintenance of metabolic homeostasis. Toxic effects on the kidney as a result of environmental toxicants can impair these kidney roles and induce changes in kidney function and structure [1].
Dinitrobenzene (DNB) has been detected has environmental contaminants of ground water and soil near sites and at military munitions test grounds. Also, it has been characterized as occupational toxicants/pollutants, by its toxicity [2], [3], [4]. DNB production yields a mixture of the three isomers namely ortho-isomer (o-DNB), meta-isomer (m-DNB) and para-isomer (p-DNB) [5], [6]. Both DNB mixture and the individual isomers of DNB are available commercially. (p-DNB) or 1,4-DNB forms colourless to yellow monoclinic needles and is used in the synthesis of dyes, explosive and in plastics industry. DNB induces serious peroxidation in membrane structures.[5]. Many investigators have described testicular toxicity associated with an exposure of rats to 1,3-dinitrobenzene or trinitrobenzene (TNB) [7], [8], [9], [10]. It has been reported that in rats DNB cause encephalopathy, hematological alterations and toxicity to brain, testes, epididymis and spleen [11], [12], [13], [14], [54].
Free radical is a reactive atom or group of atoms that has one or more unpaired electrons. They are produced in the body by natural biological processes or introduced from an exogenous source such as drugs and environmental toxicants [15]. Excessive production of free radicals which are not neutralised can oxidize macromolecules, such as DNA, proteins, carbohydrates, and lipids and ultimately damage the cell [16]. Toxins, toxicants and free radicals are distributed to the liver and kidney, thus exposing them to a state of induced toxicities. The liver and kidney tissues have evolved an array of antioxidant defense systems to protect themselves against harmful effect of metabolites and free radicals [15].
Oxidative stress occur when there is a relative imbalance between pro-oxidant and antioxidant molecules in the body thereby leading to an accumulation of reactive oxygen species [17], [18]. Reactive oxygen species are chemically active molecules that contain oxygen and are formed in normal physiology as by-products [19]. Oxidative stress can be linked to the pathophysiology of liver and kidney tissues such as inflammation, hypertrophy, apoptosis and fibrosis and it is characterized by a disruption of redox signaling and control. processes [20], [21], [22], [23] Substances that inhibit oxidation or reactions by oxygen, peroxides, or free radicals or their actions are called antioxidants [24]. They include both enzymatic and nonenzymatic antioxidants. Some examples are reduced glutathione (GSH), ascorbic acid, vitamin E, glutathione-S-transferase (GST), glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD), and catalase (CAT) [25], [26], [27].
One of the mechanisms of p-DNB is their ability to generate free radicals and trigger oxidative stress in vivo [28]. In Nigeria, the increasing use of dynamite, plastics, dyes, petrochemicals products and improperly managed waste products from manufacturing plants may contribute to p-DNB toxicity and possibly introduce high concentrations of this potential hepatorenal toxicant into the environment. Consequently, the present study was designed to investigate the effect of sub-chronic oral and dermal administration of p-DNB on target tissues (kidney and liver) and to evaluate the redox balance of p-DNB target tissues in male rats. For this purpose, we conducted oxidative stress biomarkers assays on rat kidney and liver treated with p-DNB to evaluate hepatotoxicity and nephrotoxicity through oxidative stress. In addition, we evaluate the kidney enzyme biomarkers for toxicity to evaluate p-DNB -type kidney toxicity.
2. Methods
2.1. Chemicals
p-DNB was purchased from Sigma Aldrich. All other chemicals used in the experiment were of analytical grade.
2.2. Animals and experimental design
Forty eight male Albino Wistar rats, weighing 150–170 g, were purchased from the Department of Biochemistry, Bingham University, Nigeria. They were housed in plastic cages placed in a well-ventilated rat house and allowed ad libitum access to rat chow (Vital Feeds Ltd, Nigeria) and water and subjected to natural 12-h light: dark cycle. All the animals received humane care according to the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Science, published by the National Institute of Health (NIH). Also, the ethic regulations have been followed in accordance with national and institutional guidelines for the protection of animal welfare during experiments (Public Health Service (PHS), 1996). After 1 week acclimation period, rats were randomly assigned to three groups of eight rats per group following oral administration. Group I rats received corn oil alone at 2 ml/kg bw. Group II were orally treated with 50 mg/kg bwt p-DNB dissolved in corn oil for 14 days While group III rats orally treated with 75 mg/kg bwt p-DNB dissolved in corn oil for 14 days of the experiment. Also, for sub-dermal administration, rats were randomly divided into three groups of eight rats per group. Before treatments, the dorsal fur of the rats where shaved at 2 cm by 2 cm length, then p-DNB dissolved in corn oil where applied topically to the skin of the animals. Group I rats were treated topically with corn oil alone at 2 ml/kg. Group II rats were treated with 1000 mg/kg p-DNB while Group III rats were treated with 2000 mg/kg for 14 days. The doses of p-DNB were chosen based on the Acute Oral Toxicity Up-and-Down-Procedure OECD 2008 described by Dixon and Mood [29], [30], [31], [32].
2.3. Serum collection and tissue preparation
At the end of experimental period approximately 24 h after the last p-DNB treatment, blood samples were collected by retro orbital bleeding. Blood samples were left to clot, centrifuged at 3000 rpm for 15 min, serum was collected and stored for further biochemical analysis. Afterwards, rats were sacrificed by cervical dislocation, liver and kidney were rapidly excised from each animal, connecting tissue and fats deposits were trimmed from the liver and kidney, and they were washed free of any blood and clots with ice cold 1.15% KCl solution. They were then blotted over a piece of filter paper.
2.4. Biochemical analyses
The liver and kidney were homogenised in 50 mM Tris–HCl buffer (pH 7.4) containing 1.15% potassium chloride and the homogenate was then centrifuged at 10 000g for 15 min at 4 °C. The supernatant was collected for the estimation of catalase (CAT) and Superoxide dismutase (SOD) activities. Serum was used for estimation of liver and renal injury according to manufacturer protocol.
2.4.1. Plasma biomarkers of Renal Toxicity assay
Plasma urea was determined with Randox diagnostic kits. Method for Plasma urea assays was based on the Fenton reaction [33] with the diazine chromogen formed absorbing strongly at 540 nm.
2.4.2. Renal and hepatic level of Lipid Peroxidation Assay
The extent of lipid peroxidation (LPO) in the kidney and liver was estimated by the method of Varshney and Kale [34]. The method involved the reaction between malondialdehyde (MDA) a product of lipid peroxidation) and thiobarbituric acid (TBA) to yield a stable pink chromophore with maximum absorption at 532 nm.
2.4.3. Renal and hepatic Antioxidant Enzymes Assay
Oxidative stress markers were assessed such as SOD and CAT. Renal and hepatic superoxide dismutase (SOD) activity was determined by measuring the inhibition of autooxidation of epinephrine at pH 10.2 and 30 °C by the method of Misra and Fridovich, (1972), [35]. Renal and hepatic catalase activity was determined by the method described by Sinha [36]. The method was based on the reduction of dichromate in acetic acid to chromic acetate when heated in the presence of hydrogen peroxide (H2O2). The chromic acetate produced is measured spectrophotometrically at 570 nm.
2.4.4. Determination of kidney, liver and plasma alkaline phosphatase
Plasma alkaline phosphatase (ALP) activities were determined using Randox diagnostic kits. ALP activity was determined in accordance with the principles of Tietz et al. [37]. The p-nitrophenol formed by the hydrolysis of p-nitrophenyl phosphate confers yellowish colour on the reaction mixture and its intensity can be monitored at 405 nm to give a measure of enzyme activity.
2.5. Histopathological examination
Liver sections were taken immediately from the liver, fixed in 10% buffered formalin, cleared in xylene, and embedded in paraffin. Sections (4–5 mm thick) were prepared and then stained with hematoxilin and eosin (H&E). The sections were examined for the pathological findings of hepatic changes.
2.6. Statistical analysis
All data were expressed as means ±standard deviation and statistically analyzed using SPSS (Statical Pucteage for Social Science) version 16.0 for Windows (SPSS Inc, Chicago, IL). Statistical significance of differences among different study groups was evaluated by one way analysis of variance (ANOVA) (P<0.05). Duncan's multiple range tests was used to differentiate between means (to determine differences between means of treatments at significance rates of 0.05).
3. Results
Effect of doses at 50 and 75 mg/kg of p-DNB on body weight of animals before and after oral administration was determined following treatment period. The data presented on weight of animals in Table 1 showed there was no significant differences observed in the oral administration of p-DNB induced rats and those of the control group. Also, dermal administration of p-DNB in rats at 1000 and 2000 mg/kg bwt (Table 2) on initial and final body weight of the induced rats was not significant when compared with the control. Table 2 showed the effect of doses at 50 and 75 mg/kg of p-DNB on the absolute weight of liver and kidney following oral administration. The data presented on absolute weight of organs in Table 2 showed there was no significant difference observed in the p-DNB -treated rats and those of the control group in the liver and kidney at all doses. However, the effect of dose at 1000 and 2000 mg/kg bwt of p-DNB on the absolute weights of liver and kidney following dermal administration showed no significant difference when compared to the control group.
Table 1.
Weight of animals (g) before and after oral and dermal administration.
| Oral administration | Dermal administration | |||||
|---|---|---|---|---|---|---|
| Parameters | Control | 50 mg/kg | 75 mg/kg | Control | 1000 mg/kg | 2000 mg/kg |
| FW(g) | 172.2 ± 5.49 | 167.2 ± 8.97 | 167.0 ± 8.20 | 188.4 ±1.52 | 175.0 ± 6.60 | 170.2 ± 5.90 |
| IW(g) | 121.2 ± 2.13 | 146.0 ± 3.34 | 154.6 ± 4.03 | 120.2 ± 2.10 | 123.4 ± 4.58 | 140.4 ± 9.40 |
FW: Final body weight, IW: Initial body weight. Values with asterisks were significantly different from control (*p<0.05). Values are expressed as mean ±standard deviation and n=8 (number of animals per group)
Table 2.
Absolute Weight of Organs (g) after oral and dermal administration.
| Oral administration | Dermal administration | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Control | 50 mg/kg | 75 mg/kg | Control | 1000 mg/kg | 2000 mg/kg | |
| Liver (g) | 4.03 ± 0.33 | 2.92 ± 0.30 | 2.87 ± 0.29 | 4.00 ± 0.30 | 3.15 ± 0.05 | 2.93 ±0.44 | |
| Kidney (g) | 1.12 ± 0.24 | 0.99 ± 0.05 | 0.96 ± 0.03 | 1.10 ± 0.20 | 1.03 ± 0.08 | 0.98 ± 0.06 | |
Values with asterisks were significantly different from control (*p<0.05). Values are expressed as mean ±standard deviation and n=8 (number of animals per group)
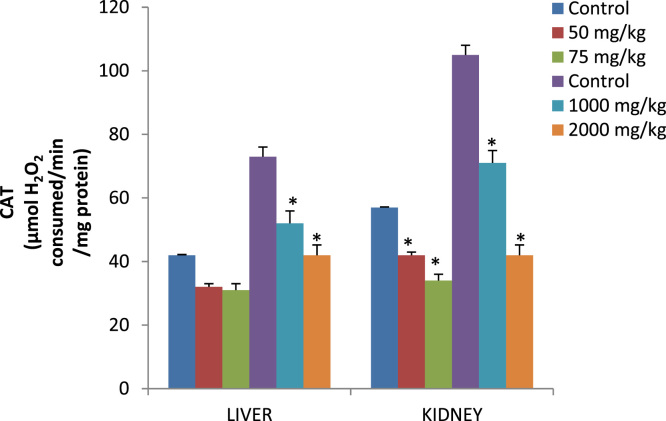
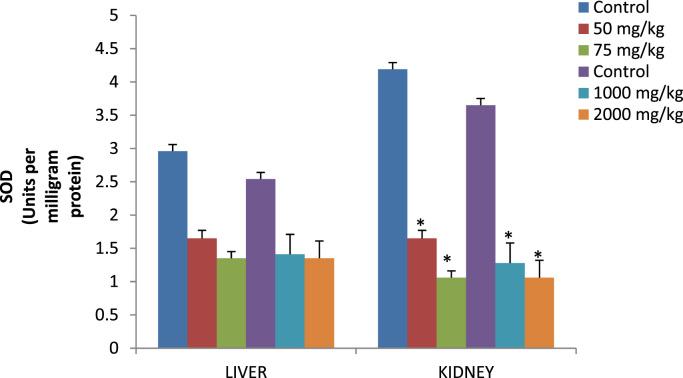
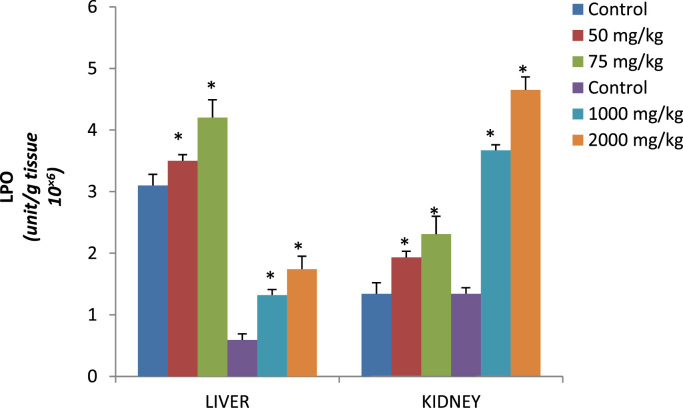
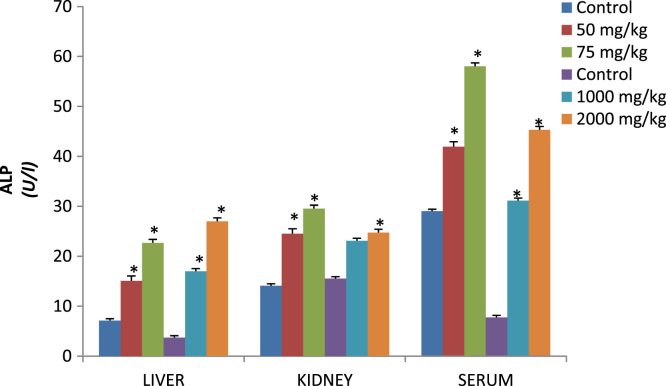
Effect of dose 50, 75 and 2000 mg/kg of p-DNB on relative weights of liver (Table 3) showed significant differences when compared to those of the control group. Conversely, kidney showed significant differences 2000 mg/kg when compared to control following dermal administration of p-DNB (Table 3) Fig. 1 reveals the activities of antioxidant enzymes namely superoxide dismutase (SOD), in the liver and kidney following oral and dermal administration of p-DNB in rats after 14 days. Acute oral and dermal administration of p-DNB caused a significant decrease in the kidney at all doses. Moreover, the activities of the antioxidant enzyme remain unaffected in the liver of rats administered with 50, 75, 1000, 2000 mg/kg bwt of p-DNB. The effects of p-DNB mediated changes in catalase status of liver and kidney as presented in Fig. 2, p-DNB treatment caused a significant decrease in the activities of CAT following oral and dermal administration when compared to control. The results presented in Fig. 3 showed that oral and dermal administration of p-DNB for 14 days significantly alter the levels of MDA in liver and kidney of the treated rats at all doses when compared to control. Fig. 4 reveals the effects of p-DNB on renal, hepatic and serum activities of ALP following oral administration in rats compared to control. Significant increases in renal ALP activities were observed in rats administered. Moreover, dermal administration of p-DNB in rats significantly increases in renal, hepatic and serum ALP activities when compared to the control groups. Table 4 shows the effect of p-DNB in plasma urea in rats. Plasma urea was significantly increased in the p-DNB – treated rats when compared to control (p<0.05) (Fig. 5, Fig. 6).
Table 3.
Relative weight of organs after oral and dermal administration.
| Oral administration | Dermal administration | |||||
|---|---|---|---|---|---|---|
| Parameters | Control | 50 mg/kg | 75 mg/kg | Control | 1000 mg/kg | 2000 mg/kg |
| Liver (g) | 2.34 ± 0.24 | 1.70 ± 0.28* | 1.56 ± 0.14* | 2.00± 0.24 | 1.93 ±0.19 | 1.80 ± 0.09* |
| Kidney (g) | 0.65 ± 0.14 | 0.55 ± 0.02 | 0.53 ± 0.02 | 0.68 ± 0.06 | 0.64 ± 0.14 | 0.56 ± 0.02* |
Values with asterisks were significantly different from control (*p<0.05). Values are expressed as mean ±standard deviation and n=8 (number of animals per group).
Fig. 1.
Catalase activity (µmol H2O2 consumed/min/mg protein) in the liver and kidney of rats after 14 days of oral and dermal administration.
Fig. 2.
Superoxide dismutase activity (Units per milligram protein) in liver and kidney of rats treated with p-DNB for 14 days after oral and dermal administration.
Fig. 3.
Lipid peroxidation levels (unit/g tissue 10×6) in liver and kidney of rats after 14 days of oral and dermal administration of p-DNB.
Fig. 4.
Effects of p-DNB on activities of ALP (U/l) in Kidney, Serum and liver of treated rats following oral and dermal administration.
Table 4.
Effect of oral administration of p-DNB in plasma urea (mg/dl) in rats after 14 days.
| Oral administration |
Dermal administration |
||
|---|---|---|---|
| Groups | UREA (mg/dl) | Groups | UREA (mg/dl) |
| Control | 13.78 ± 2.52 | Control | 16.78 ± 3.20 |
| 50 mg/kg | 20.10 ± 1.90* | 1000 mg/kg | 19.59 ± 0.87* |
| 75 mg/kg | 24.64 ± 3.49* | 2000 mg/kg | 24.64 ± 5.02* |
Values with asterisks were significantly different from control (*p<0.05). Values are expressed as mean ±standard deviation and n=8 (number of animals per group).
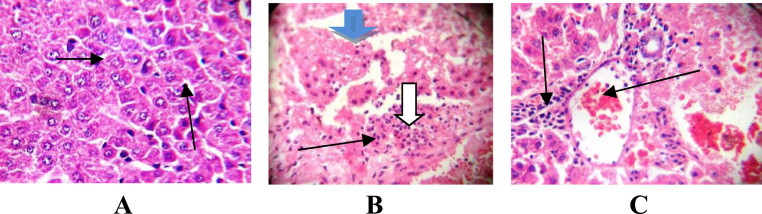
Fig. 5.
Photomicrograph of liver section after oral administration showing A(control): normal liver architecture, central venules, sinusoids without infiltration of inflammatory cells and hepatocytes morphology. B,C(50,75 mg/kg bwt p-DNB): poor liver architecture, moderate periportal infiltration, moderate peri vascular infiltration of inflammatory cells, tissue degeneration with loss of liver plates, severe macro vesicular steatosis with the fat fully infiltrating the cytoplasms of the liver cells and few apoptotic cells (X400).
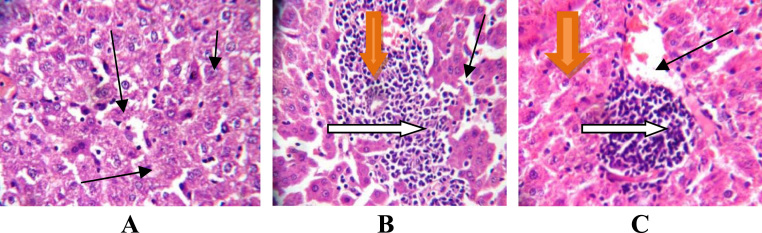
Fig. 6.
Photomicrograph of liver section after dermal administration showing (A control): normal liver architecture, hepatocytes, central venules, sinusoids without infiltration of inflammatory cells. B,C (1000, 2000 mg/kg bwt p-DNB): poor liver architecture, aggregating inflammatory cells, liver parenchyma with fibrosis and hepatic necrosis, severe micro vesicular steatosis with the fat infiltration within the cytoplasms. (X400).
4. Discussions
The mechanism responsible for the hepatic and renal toxic effect of p-DNB is not being well understood yet. In the present study, we hypothesized an oxidative stress-like as a potential mechanism inductor of renal and hepatic injury. ROS are capable of initiating and promoting oxidative damage as lipid peroxidation. [38]. Liver is a major organ attacked by ROS [39], [40]. Parenchymal, Kupffer, hepatic stellate and endothelial cells are potentially exposed to oxidative stress-related substances and caused oxidative damage to the cells. Hepatic stellate cells are triggered by lipid peroxidation caused by oxidative stress [41], [42], [43]. The kidney cells undergoes nitro-reduction caused by free radicals leading to renal toxicity and thereby compromising the cells mitochondrial antioxidant capacity [44]. The kidney acquires a number of balance mechanisms to deal with an excess amount of ROS.
Several studies have established the role of free radicals on chemical-induced toxicity and deduced a connection between toxicity and oxidative stress [45], [46], this initiates our findings on the effect of p-DNB-induced toxicity in the liver and kidney of male rats on enzymatic and non-enzymatic antioxidants systems. Examples of enzymatic antioxidants determined in this research are SOD and CAT. In the present investigation, exposure of male rats to p-DNB (50, 75,1000 and 2000 mg/kg bwt for 14 days) resulted in significant decrease in renal activities of SOD and CAT at all doses. Moreover, hepatic activities of CAT was significantly increased at 1000 and 2000 mg/kg. SOD and CAT are vital and crucial to the maintenance of the cellular redox balance [47]. There is a relationship between SOD and CAT against accumulation of free radicals thereby inactivates the superoxide anion and peroxide radicals by converting them into water and oxygen. Our results suggest an inhibition of renal antioxidants enzymes that are involved in antioxidant defence mechanism against free radicals generated by p-DNB in rats.
The imbalance between increased production of free radicals, and the decreased antioxidant capacity, results in a persistent lipid peroxidation (LPO) which is known to cause cellular injury by inactivation of membrane enzymes, depolymerisation of polysaccharide, protein fragmentation and cross-linking [48], [49]. One of the markers of oxidative stress in tissue is MDA (from oxidation of unsaturated fatty acids), an index of lipid peroxidation [15]. Lipid peroxidation is initiated by the attack of a free radical on fatty acid [26] leading to tissue damage. Oral and dermal administration of p-DNB caused a significant increase in the levels MDA at all doses in the liver and kidney of rats in the present study. According to the observation in this research, p-DNB has shown to cause an impairment of antioxidant enzymes activities resulting in excessive hepatic and renal ROS leading to increased lipid peroxidation indicating that p-DNB has lipid peroxidative properties.
Urea is a metabolic product which may be detrimental to the body if accumulated and it can be removed from circulation by the kidney to prevent their accumulation. The plasma biomarkers of renal function urea were determined in our present finding. There was a significant increase in the plasma urea in this present study. Increase in plasma level of urea substances is regarded as an indication of loss of renal function [50], [51]. This may suggest that p-DNB caused loss of renal function in the treated male rats due to increase in the plasma level of urea. Alkaline phosphatase (ALP) is associated with the cell membrane of the liver and kidney and the activities of ALP are found to increase in conditions associated with hepato-biliary injury and overproduction or leakage of ALP [52]. In this study, we found out that there was a significant increase in the activities serum and kidney ALP when compared to the control. This indicates that p-DNB may have caused injury to the serum and kidney due to the increase in serum and kidney ALP activities. Hepatic damage induced by administration of p-DNB resulted in elevated levels of liver maker enzyme (ALP) which reflect liver damage. Elevated levels of this enzyme is a sign of cellular leakage and loss of functional integrity of the cell membrane in the liver which is reflected in the histopathological results [53].
It was observed in the present study that the weight of animals, absolute organ weight as well as the relative organ weights of liver and kidney was reduced in p-DNB -induced rats (50, 75, 1000 and 2000 mg/kg). This decrease in weight of animals in p-DNB -treated rats following oral and dermal administration indicates general metabolic dysfunctions in the rats. Moreover, the decrease in kidney weight has observed in our present findings may suggest a reduction in structure of nephron in the kidney caused by p-DNB. The reduction of kidney weight is a primary indicator of possible alteration in kidney functions. In addition, the decrease in the liver weight might be due to the mechanism of action of p-DNB thereby disrupting the structure and function of the organ. In our findings, we noted from the photomicrograph of the liver sections following oral and dermal administration, poor liver architecture, moderate periportal infiltration, vessels with moderate peri-vascular infiltration of inflammatory cells, the hepatocytes show severe macro vesicular steatosis with the fat fully infiltrating the cytoplasm of the liver cells and few apoptotic cells in p-DNB -treated rats at all doses. However, following dermal administration photomicrograph revealed liver parenchyma with fibrosis and hepatic necrosis, aggregating inflammatory cells, severe micro vesicular steatosis with the fat infiltration within the cytoplasm in at 1000 and 2000 mg/kg groups. The histological changes observed from the photomicrograph under this experimental condition represent the microscopic features of a sub-chronic event during p-DNB exposure, which may be attributed to the direct or indirect effect of increased ROS which consequently induces lipid peroxidation by p-DNB.
5. Conclusion
p-DNB treated animals revealed a significant increase in serum biochemical parameters as well as hepatic and renal lipid peroxidation but caused an inhibition in antioxidant biomarkers. In conclusion, our findings suggest that sub-chronic oral and dermal administration of p-DNB can produce hepato-nephrotoxicity through oxidative stress. Hence, the increasing use of dynamite, plastics, dyes, petrochemicals products and improperly managed waste products from manufacturing plants may contribute to p-DNB toxicity and possibly introduce high concentrations of this potential hepatorenal toxicant to factory and quarry workers and people living around ammunition sites in Nigeria.
Declarations
Ethics approval
All the animals received humane care according to the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Science, published by the National Institute of Health (NIH). Federal University of Technology, Akure Animal Care approved this research. No specific permission were required for these location, the study was carried out in our department. This study did not involve endangered or protected species. Also, the ethic regulations have been followed in accordance with national and institutional guidelines for the protection of animal welfare during experiments (Public Health Service (PHS), 1996).
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to plagiarism, unauthorised and illicit use of the data generated in this study but are available from the corresponding author on reasonable request.
Competing interest
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors.
Author Contributions
JOS and ACA conceived and designed the experiments, JOS performed the experiments, JOS ACA analyzed the data, JOS, ACA and MTO contributed reagents/materials/analysis tools, JOS treated the animals. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge the services of Mr Peter Otegbade for the technical assistances.
Footnotes
Transparency document associated with this article can be found in the online version at doi: 10.1016/j.bbrep.2017.04.017.
Appendix A. Transparency document
Transparency document
Transparency document
Transparency document
Transparency document
References
- 1.Palm F., Nordquist L. Renal oxidative stress, oxygenation, and hypertension. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011;301(5):R1229–R1241. doi: 10.1152/ajpregu.00720.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garman J.R., Freund T., Lawless E.W. Testing for groundwater contamination at hazardous waste sites. J. Chromatogr. Sci. 1987;25(8):328–337. [Google Scholar]
- 3.Reddy G., Reddy T.V., Choudhury H., Bernard Daniel F., Leach G.J. Assessment of environmental hazards of 1, 3, 5-trinitrobenzene. J. Toxicol. Environ. Health. 1997;52(5):447–460. doi: 10.1080/00984109708984075. [DOI] [PubMed] [Google Scholar]
- 4.M.E. Walsh, T.F. Jenkins, Identification of TNT transformation products in soil. In.: DTIC Document, 1992.
- 5.Blackburn D.M., Gray A.J., Lloyd S.C., Sheard C.M., Foster P.M. A comparison of the effects of the three isomers of dinitrobenzene on the testis in the rat. Toxicol. Appl. Pharmacol. 1988;92(1):54–64. doi: 10.1016/0041-008x(88)90227-x. [DOI] [PubMed] [Google Scholar]
- 6.Cody T., Witherup S., Hastings L., Stemmer K., Christian R. 1, 3‐Dinitrobenzene: toxic effects in vivo and in vitro. J. Toxicol. Environ. Health, Part A Curr. Issues. 1981;7(5):829–847. doi: 10.1080/15287398109530024. [DOI] [PubMed] [Google Scholar]
- 7.Brown C.D., Forman C.L., McEuen S.F., Miller M.G. Metabolism and testicular toxicity of 1, 3-dinitrobenzene in rats of different ages. Toxicol. Sci. 1994;23(3):439–446. doi: 10.1006/faat.1994.1126. [DOI] [PubMed] [Google Scholar]
- 8.Kinkead E., Wolfe R., Flemming C., Caldwell D., Miller C., Marit G. Reproductive toxicity screen of 1, 3, 5-trinitrobenzene administered in the diet of Sprague-Dawley rats. Toxicol. Ind. Health. 1995;11(3):309–323. doi: 10.1177/074823379501100302. [DOI] [PubMed] [Google Scholar]
- 9.Chandra A.S., Qualls C.W., Jr, Reddy G. Testicular effects of 1, 3, 5-trinitrobenzene (TNB). I. dose response and reversibility studies. J. Toxicol. Environ. Health Part A. 1997;50(4):365–378. doi: 10.1080/009841097160401. [DOI] [PubMed] [Google Scholar]
- 10.Chandra A.S., Qualls C.W., Jr Reddy GACG: testicular effects of 1, 3, 5-trinitrobenzene (TNB). II. Immunolocalization of germ cells using proliferating cell nuclear antigen (PCNA) as an endogenous marker. J. Toxicol. Environ. Health Part A. 1997;50(4):379–388. [PubMed] [Google Scholar]
- 11.Reddy T.V., Olson G.R., Wiechman B., Reddy G., Robinson M., Torsella J.A., Daniel F.B. Fourteen‐day Toxicity Study of 1, 3, 5‐Trinitrobenzene in Fischer 344 Rats. J. Appl. Toxicol. 1996;16(4):289–295. doi: 10.1002/(SICI)1099-1263(199607)16:4<289::AID-JAT349>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Kim S., Qualls C.W., Reddy G., Stair E.L. 1, 3, 5-Trinitrobenzene-induced alpha-2u-globulin nephropathy. Toxicol. Pathol. 1997;25(2):195–201. doi: 10.1177/019262339702500209. [DOI] [PubMed] [Google Scholar]
- 13.Chandra A.S., Qualls C.W., Jr, Reddy G., Meinkoth J.H. Hematological effects of 1, 3, 5‐trinitrobenzene (TNB) in rats in vivo and in vitro. J. Toxicol. Environ. Health, Part A Curr. Issues. 1995;46(1):57–72. doi: 10.1080/15287399509532018. [DOI] [PubMed] [Google Scholar]
- 14.Vásquez G.B., Reddy G., Gilliland G.L., Stevens W.J. Dinitrobenzene induces methemoglobin formation from deoxyhemoglobin in vitro. Chem.-Biol. Interact. 1995;96(2):157–171. doi: 10.1016/0009-2797(94)03590-5. [DOI] [PubMed] [Google Scholar]
- 15.Gutteridge J. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995;41(12):1819–1828. [PubMed] [Google Scholar]
- 16.Uddin S., Ahmad S. Dietary antioxidants protection against oxidative stress. Biochem. Educ. 1995;23(1):2–7. [Google Scholar]
- 17.Puddu P., Puddu G.M., Cravero E., Rosati M., Muscari A. The molecular sources of reactive oxygen species in hypertension. Blood Press. 2008;17(2):70–77. doi: 10.1080/08037050802029954. [DOI] [PubMed] [Google Scholar]
- 18.Forman H.J., Fukuto J.M., Miller T., Zhang H., Rinna A., Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch. Biochem. Biophys. 2008;477(2):183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(6):338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 20.Traber M.G., Stevens J.F. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011;51(5):1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox C.S. Asymmetric dimethylarginine and reactive oxygen species unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension. 2012;59(2):375–381. doi: 10.1161/HYPERTENSIONAHA.111.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox C.S. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005;289(4):R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 23.Touyz R.M. reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid. Redox Signal. 2005;7(9–10):1302–1314. doi: 10.1089/ars.2005.7.1302. [DOI] [PubMed] [Google Scholar]
- 24.Gongora M.C., Qin Z., Laude K., Kim H.W., McCann L., Folz J.R., Dikalov S., Fukai T., Harrison D.G. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48(3):473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 25.Chen F., Haigh S., Barman S.A., Fulton D. From form to function: the role of Nox4 in the cardiovascular system. Front. Physiol. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valko M., Rhodes C., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Devasagayam T., Tilak J., Boloor K., Sane K.S., Ghaskadbi S.S., Lele R. Free radicals and antioxidants in human health: current status and future prospects. JAPI. 2004;52(794804):4. [PubMed] [Google Scholar]
- 28.Sajan M., Reddy G., Kulkarni A.P. In vitro inhibition of mammalian glutathione transferases by selected nitrobenzenes. Int. J. Toxicol. 2000;19(4):285–292. [Google Scholar]
- 29.Dixon W.J., Mood A.M. A method for obtaining and analyzing sensitivity data. J. Am. Stat. Assoc. 1948;43(241):109–126. [Google Scholar]
- 30.Dixon W. The up-and-down method for small samples. J. Am. Stat. Assoc. 1965;60(312):967–978. [Google Scholar]
- 31.Dixon W. Staircase bioassay: the up-and-down method. Neurosci. Biobehav. Rev. 1991;15(1):47–50. doi: 10.1016/s0149-7634(05)80090-9. [DOI] [PubMed] [Google Scholar]
- 32.Dixon W. Dixon Statistical Associates; Los Angeles CA, USA: 1991. Design and Analysis of Quantal Dose-Response Experiments (with Emphasis on Staircase Designs) [Google Scholar]
- 33.Tietz N.W. WB Saunders Co; 1995. Clinical Guide to Laboratory Tests. [Google Scholar]
- 34.Varshney R., Kale R. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 1990;58(5):733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 35.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 36.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 37.Tietz N., Pruden E., Siggaard-Andersen O. 1994. Liver function Tietz Textbook of Clinical Chemistry; pp. 1354–1374. [Google Scholar]
- 38.Kovacic P., Cooksy A.L. Unifying mechanism for toxicity and addiction by abused drugs: electron transfer and reactive oxygen species. Med. Hypotheses. 2005;64(2):357–366. doi: 10.1016/j.mehy.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez-Valle V., C Chavez-Tapia N., Uribe M., Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr. Med. Chem. 2012;19(28):4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 40.Bando I., Reus M.I.S., Andrés D., Cascales M. Endogenous antioxidant defence system in rat liver following mercury chloride oral intoxication. J. Biochem. Mol. Toxicol. 2005;19(3):154–161. doi: 10.1002/jbt.20067. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi S., Takahashi S., Sasaki T., Kumagai T., Nagata K. Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab. Pharmacokinet. 2011;26(1):30–46. doi: 10.2133/dmpk.dmpk-10-rv-087. [DOI] [PubMed] [Google Scholar]
- 42.Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol.: WJG. 2014;20(25):8082. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D. Wu, A.I. Cederbaum, Oxidative stress and alcoholic liver disease. in: Seminars in liver disease: 2009: © Thieme Medical Publishers: 141–154, 2009. [DOI] [PubMed]
- 44.Jacobson C.F., Miller M.G. Species difference in 1, 3-dinitrobenzene testicular toxicity: in vitro correlation with glutathione status. Reprod. Toxicol. 1998;12(1):49–56. doi: 10.1016/s0890-6238(97)00099-3. [DOI] [PubMed] [Google Scholar]
- 45.X-y Guo, G-f Sun, Y-c Sun. Oxidative stress from fluoride-induced hepatotoxicity in rats. Fluoride. 2003;36(1):25–29. [Google Scholar]
- 46.Yamamoto T., Kikkawa R., Yamada H., Horii I. Identification of oxidative stress-related proteins for predictive screening of hepatotoxicity using a proteomic approach. J. Toxicol. Sci. 2005;30(3):213–227. doi: 10.2131/jts.30.213. [DOI] [PubMed] [Google Scholar]
- 47.Notas G., Koutroubakis I.E., Kouroumalis E.A., Panglossi H. Oxidants and antioxidants in liver disease. Antioxid.: New Res. 2006:2–48. [Google Scholar]
- 48.Rizvi S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006;20(4):303–306. doi: 10.1002/ptr.1861. [DOI] [PubMed] [Google Scholar]
- 49.Reiter R.J., Acuña‐Castroviejo D., Tan Dx, Burkhardt S. Free radical‐mediated molecular damage. Ann. New Y. Acad. Sci. 2001;939(1):200–215. [PubMed] [Google Scholar]
- 50.Han W.K., Bonventre J.V. Biologic markers for the early detection of acute kidney injury. Curr. Opin. Crit. care. 2004;10(6):476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- 51.George G., Wakasi M., Egoro E. Creatinine and urea levels as critical markers in end-stage renal failure. Res. Rev.: J. Med. Health Sci. 2014;3(1):41–44. [Google Scholar]
- 52.Ramaiah S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007;45(9):1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 53.You Y., Yoo S., Yoon H.-G., Park J., Lee Y.-H., Kim S., Oh K.-T., Lee J., Cho H.-Y., Jun W. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010;48(6):1632–1637. doi: 10.1016/j.fct.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 54.Sangodele J.O. Redox status and sperm characteristics in 1, 4-dinitrobenzene-induced reproductive toxicity in Wistar rats. Toxicol. Environ. Health Sci. 2017;9(1):12–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document
Transparency document
Transparency document
Transparency document
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to plagiarism, unauthorised and illicit use of the data generated in this study but are available from the corresponding author on reasonable request.