Abstract
Osteocytes play important roles in controlling bone quality as well as preferential alignment of biological apatite c-axis/collagen fibers. However, the relationship between osteocytes and mechanical stress remains unclear due to the difficulty of three-dimensional (3D) culture of osteocytes in vitro. The aim of this study was to investigate the effect of cyclic mechanical stretch on 3D-cultured osteocyte-like cells. Osteocyte-like cells were established using rat calvarial osteoblasts cultured in a 3D culture system. Cyclic mechanical stretch (8% amplitude at a rate of 2 cycles min−1) was applied for 24, 48 and 96 consecutive hours. Morphology, cell number and preferential cell alignment were evaluated. Apoptosis- and autophagy-related gene expression levels were measured using quantitative PCR. 3D-cultured osteoblasts became osteocyte-like cells that expressed osteocyte-specific genes such as Dmp1, Cx43, Sost, Fgf23 and RANKL, with morphological changes similar to osteocytes. Cell number was significantly decreased in a time-dependent manner under non-loaded conditions, whereas cyclic mechanical stretch significantly prevented decreased cell numbers with increased expression of anti-apoptosis-related genes. Moreover, cyclic mechanical stretch significantly decreased cell size and ellipticity with increased expression of autophagy-related genes, LC3b and atg7. Interestingly, preferential cell alignment did not occur, irrespective of mechanical stretch. These findings suggest that an anti-apoptotic effect contributes to network development of osteocyte-like cells under loaded condition. Spherical change of osteocyte-like cells induced by mechanical stretch may be associated with autophagy upregulation. Preferential alignment of osteocytes induced by mechanical load in vivo may be partially predetermined before osteoblasts differentiate into osteocytes and embed into bone matrix.
Keywords: Mechanical stimulation, Autophagy, Osteocyte-like cells, Cell morphology, Preferential cell alignment, Apoptosis
Highlights
-
•
Novel three-dimensional culture system for osteocyte-like cells were developed.
-
•
Mechanical stretch affected cell morphologies without preferential cell alignment.
-
•
Mechanical stretch upregulated expression of autophagy-related genes.
-
•
Mechanical stretch prevented cell decreases under non-loaded condition.
1. Introduction
Bone quality, which has been defined as “the sum of all characteristics of bone that influence the resistance to bone fracture [1],” is completely independent of bone mineral density (BMD). Bone quality plays a crucial role in determining bone strength with BMD. Particularly, both preferential alignment of biological apatite (BAp) c-axis/collagen fibers and osteocytes are key factors affecting bone quality [2]. Osteocytes, which reside within lacunae of bone matrix, play a central role in controlling bone quantity (bone volume). Mechanical load, in particular, is considered to be one of the essential factors regulating bone quantity, as mechanical unloading results in load-associated osteoporosis [3]. Mechanical load on bone tissue is converted to various mechanical stimuli such as fluid shear stress, hydrostatic pressure and direct deformation of osteocytes [4]. Recently, we reported that cyclic mechanical load on bone tissue via dental implants led to the development of osteocyte networks with preferential alignment of BAp c-axis/collagen fibers and increased bone volume around dental implants [5]. Moreover, it has been reported that preferential alignment of osteocytes occurred in response to mechanical stimulation [6]. However, when preferential alignment of osteocytes occurs, how osteocytes control bone quality and why osteocyte networks are improved in response to mechanical load remain unclear.
Autophagy and apoptosis, which are two distinct self-destructive processes, play an important role in degradation of cytoplasmic organelles and cell death, respectively. Autophagy is an intracellular degradation system that delivers cytoplasmic degraded components to lysosomes fused to autophagosomes [7]. Apoptosis is one of several types of programmed cell death that occurs by altering the balance between apoptosis-related proteins and anti-apoptosis-related proteins. Upregulation and downregulation of autophagy or apoptosis are influenced by multiple factors such as physiological stress, hormonal stimulation, drug exposure, bacterial infections, innate immune signals, senescence and several types of diseases including cancers and immune diseases [8], [9]. Recently, it has been reported that both autophagy and apoptosis are also influenced by mechanical stress in mammalian cells [10]. However, the effect of mechanical stimulation on autophagy and apoptosis in osteocytes has not been investigated due to the difficulty in monitoring osteocytes in vitro. Here, we developed a three-dimensional (3D) culture system in which rat calvarial osteoblasts were differentiated into osteocyte-like cells in vitro. The aim of this study was to investigate the effect of cyclic mechanical stretch on cultured osteocyte-like cells in rat.
2. Materials and methods
2.1. Establishment of osteocyte-like cells derived from rat calvarial osteoblasts in a 3D culture system
Three female Wistar rats (12-week-old) were purchased (Biotek Co. Ltd., Saga, Japan). Calvarial osteoblasts were isolated as previously described [11]. To establish osteocyte-like cells in vitro, a 3D culture system was developed with modification of previous studies [12], [13]. A silicone chamber (STREX Inc., Osaka, Japan) was coated with collagen type I rat tail (Corning, Inc., NY, USA) and 0.02 N acetic acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Gel constructs were made using a 1:1 ratio of Matrigel (BD Biosciences, NJ, USA) and a collagen solution consisting of 58% collagen type I tail, 26% 5× DMEM, 2.5% FBS, 2.5% fetal calf serum (Gibco by Life Technologies, CA, USA), 0.05 N sodium hydroxide (Wako Pure Chemical Industries, Ltd.) and a 5% cell suspension (1×106 cells/mL). The gel was transferred to the collagen-coated silicon chamber and incubated at 37 °C for 60 min. Osteoblasts were cultured in this 3D culture system to facilitate network formation with cell process-like structures. Osteoblasts cultured on collagen type I rat tail and 0.02 N acetic acid coated-silicon chambers were used as control (designated as 2D culture). All experiments were performed in accordance with the guidelines for the Animal Experimentation of Nagasaki University, and the current protocol was approved by the Ethics Committee for Animal Research of Nagasaki University.
2.2. Mechanical stimulation of osteocyte-like cells in vitro
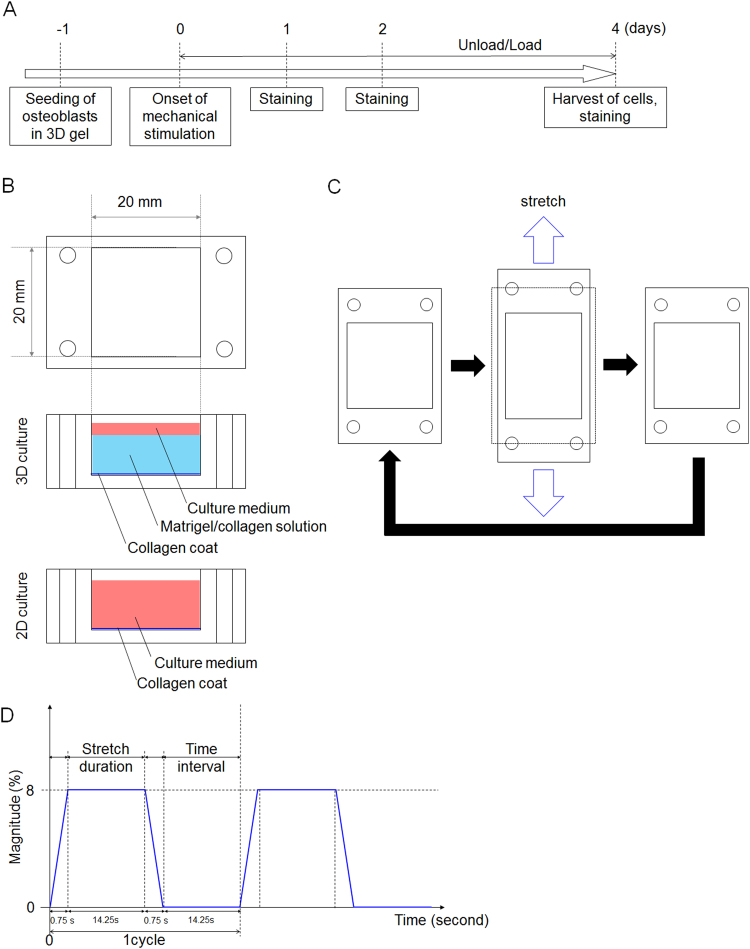
A loading device (ST-140, STREX Inc.) was used for mechanical stimulation. Silicon chambers were directly connected to the loading device. Cyclic mechanical stimulation was then initiated 24 hours after the start of 3D culture. Cells were cultured under continuous cyclic stretch of 8% amplitude at a rate of 2 cycles min-1. Cyclic stretch amplitude >8% was not used because the gel could not maintain its structure without tearing. Cyclic mechanical stimulation was applied for 24, 48 and 96 hours with medium changes every 2 days (Fig. 1A–D).
Fig. 1.
Experimental design. (A) Mechanical stimulation was started at 24 hours after seeding of osteoblasts. (B) Culture system used in this study. A Matrigel/collagen solution was used for the 3D culture system. Collagen alone was used for the 2D culture system. (C) Schematic illustration of the silicone chamber and cyclic mechanical stimulation. The dotted line indicates the shape of the culture chamber before stretching. (D) Schematic waveform of applied mechanical stimulation in this study. The waveform represents 8% amplitude and a rate of 2 cycles min-1.
2.3. Fluorescent staining and quantitative morphological analyses
Cells were fixed with culture gel and stained for phalloidin A (Cytoskeleton Inc., Denver, CO, USA) and DAPI (TAKARA Bio Inc., Tokyo, Japan) for 30 minutes at 37 °C. Fluorescent microscopy (BZ9000, Keyence, Osaka, Japan) and CellProfiler software (Broad Institute, Cambridge, MA, USA) were used to visualize cells and for morphological analyses [14]. The images at half thickness from the bottom area were used for all analyses. Cell number, cell area, cell perimeter, major axis length, minor axis length, cell eccentricity, cell solidity and cell orientation were automatically measured using CellProfiler. Each parameter was determined as follows: (1) cell number and area (pixel) = the actual number of pixels in the region; (2) cell perimeter (pixel) = the total number of pixels around the boundary of each region in the image; (3) cell eccentricity = the eccentricity of the ellipse that has the same second-moments as the region. Eccentricity is the ratio of the distance between the foci of the ellipse and its major axis length; (4) major axis length (pixel) = the length of the major axis of the ellipse that has the same normalized second central moments as the region; (5) minor axis of length (pixel) = the length of the minor axis of the ellipse that has the same normalized second central moments as the region; (6) cell solidity (ratio) = the proportion of pixels in the convex hull that are also in the object; and (7) cell orientation (degree) = the angle between the x-axis and the major axis of the ellipse that has the same second-moments as the region. Absolute values of the cell alignment angle were used in this study.
2.4. Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was extracted using TRIZOL (Invitrogen, CA, USA) and the phenol/chloroform method [15]. First strand cDNA was synthesized using the Promega system (Promega, WI, USA). Quantitative real-time PCR (qPCR) was conducted using the Thermal Cycler Dice system (TAKARA, Shiga, Japan) with SYBR green (Invitrogen). Samples were run in triplicate. All results were normalized to β-actin expression. Relative quantification of data generated using this system was performed using the standard curve method. The following primer sets were used: osteoblast-specific genes (Runx2 and Opn), osteocyte-specific genes (Cx43, E11, Mepe, RANKL, Dmp1, Fgf23 and Sost), apoptosis-related genes (Bcl-XL, Bcl2, Bim, Bid, Bak1, Bax, PUMA, Casp3 and Casp7) and autophagy-related genes (Ulk1, LC3b, Becn1, AMPKα1, AMPKα2, Mtor, p62/sqstm1, p62, atg7 and p53) (Table 1).
Table 1.
Primer sets.
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | GTAGCCATCCAGGCTGTGTT | CCCTCATAGATGGGCACAGT |
| Runx2 | CGACAGCCCCAACTTCCT | ACGGTAACCACGGTCCCAT |
| Opn | AGACCATGCAGAGAGCGAG | ACGTCTGCTTGTGTGCTGG |
| Cx43 | CTTCCTGCTCATCCAGTGGT | GGACGTGAGAGGAAGCAGTC |
| E11 | ACCCCAATAGAGATAACGCAGG | CCAGGGTCACTACAGCCAAG |
| Mepe | AGGCTGTGTCTGTTGGACTG | AAGTGGATGTTGCCTTGGTT |
| RANKL | CATCGGGTTCCCATAAAG | GAAGCAAATGTTGGCGTA |
| Dmp1 | CGGCTGGTGGTCTCTCTAAG | CTGGACTGTGTGGTGTCTGC |
| Fgf23 | TTGGATCGTATCACTTCAGC | TGCTTCGGTGACAGGTAG |
| Sost | GGCAAGCCTTCAAGAATGATGCCA | TGTACTCGGACACGTCTTTGGTGT |
| Bcl-XL | TGCGTGGAAAGCGTAGACAAGGA | AAGGCTCTAGGTGGTCATTCAGGT |
| Bcl2 | TGGAAAGCGTAGACAAGGAGATGC | CAAGGCTCTAGGTGGTCATTCAGG |
| Bim | AAACGATTACCGAGAGGCGGAAGA | AATGCCTTCTCCATACCAGACGGA |
| Bid | ACGACAAGGCCATGCTGATA | AGGCACCCTCAGTCCATCTC |
| Bak1 | GAGTTTGCGTAGAGACCCCATCCT | CCACAAATTGGCCCAACAGAACCA |
| Bax | TGCTACAGGGTTTCATCCAG | CCAGTTCATCGCCAATTCG |
| PUMA | CGGAGACAAGAAGAGCAACA | TAGTTGGGCTCCATTTCTGG |
| Casp3 | AGAAATTCAAGGGACGGGTC | TGCGCGTACAGTTTCAGCA |
| Casp7 | TACAAGATCCCGGTGGAAGCT | CTGGGTTCCTCCACGAATAATAG |
| LC3b | CATGCCGTCCGAGAAGACCT | GATGAGCCGGACATCTTCCACT |
| Ulk1 | GCTGCTCTCTTCAGGCTTACAG | CCTGCTTCACAGTAGACGAAAG |
| Becn1(Atg6) | TTGGCCAATAAGATGGGTCTGAA | TGTCAGGGACTCCAGATACGAGTG |
| AMPKα2 | TGGAGGTGAATTGTTCGACTACAT | ACAGTAGTCCACGGCAGACAGA |
| AMPKα1 | TGTGGCTCGCCCAATTATG | GACCCCGCTGCTCCAGAT |
| Mtor | TGGAGGGAGAGCGTCTGAGA | TGATGTGCCGAGGCTTTGT |
| P62/sqstm1 | CCATGGGTTTCTCGGATGAA | GGAGGGTGCTTGGAATACTGG |
| p62 | GCTGCTCTCTTCAGGCTTACAG | CCTGCTTCACAGTAGACGAAAG |
| Atg7 | CGATGGCTTCCTACTGTTATT | CATGACAACAAAGGTGTCAAA |
| p53 | CATGAGCGTTGCTCTGATG | CAGATACTCAGCATACGGATTTCC |
2.5. Statistics
Statistical analyses were conducted in a blinded manner. The Shapiro-Wilk test was performed to test for normality. The independent t-test and one-way analysis of variance were used for parametric data. The Kruskal-Wallis test was used for non-parametric data. All statistical analyses were conducted using SYSTAT 12 (Systat Software, Chicago, IL, USA). An α-level of 0.05 was used for statistical significance. All data are represented as the mean ±SEM.
3. Results
3.1. Effect of 3D culture for 120 hours on rat calvarial osteoblasts
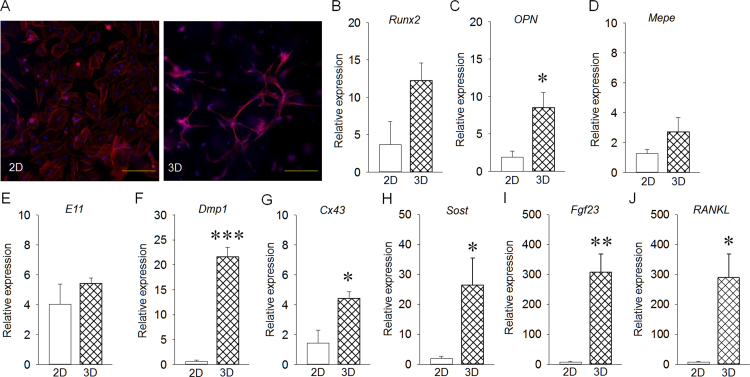
Representative fluorescent images of 2D and 3D culture are shown in Fig. 2A. 2D- and 3D-cultured cells exhibited distinct morphology. 3D-cultured cells appeared to have cell process-like structures that connected to adjacent cells. Runx2 expression was similar between 2D- and 3D- cultured cells (Fig. 2B). OPN expression in 3D-cultured cells was significantly higher than that in 2D-cultured cells (Fig. 2C). Mepe and E11 expression levels were almost the same between 2D- and 3D-cultured cells (Fig. 2D, E), whereas Dmp1, Cx43, Sost, Fgf23 and RANKL expression levels in 3D-cultured cells were significantly increased compared with those in 2D-cultured cells (Fig. 2F–J).
Fig. 2.
Effect of 3D culture on rat calvarial osteoblasts for 120 hours in vitro. (A) Representative images for 2D- and 3D-cultured cells (Bar: 100 µm). (B) Runx2 expression was similar in 2D- and 3D-cultured cells. (C) OPN expression in 3D-cultured cells was significantly increased compared with that in 2D-cultured cells. (D, E) Both Mepe and E11 expression levels were the same between groups. (F–J) Dmp1, Cx43, Sost, Fgf23 and RANKL expression levels in 3D-cultured cells were significantly increased compared with those in 2D-cultured cells. Results are expressed as relative gene expression after normalization to the house-keeping gene β-actin. *p<0.05, **p<0.01, ***p<0.001.
3.2. The effect of cyclic mechanical stretch on cell number and apoptosis
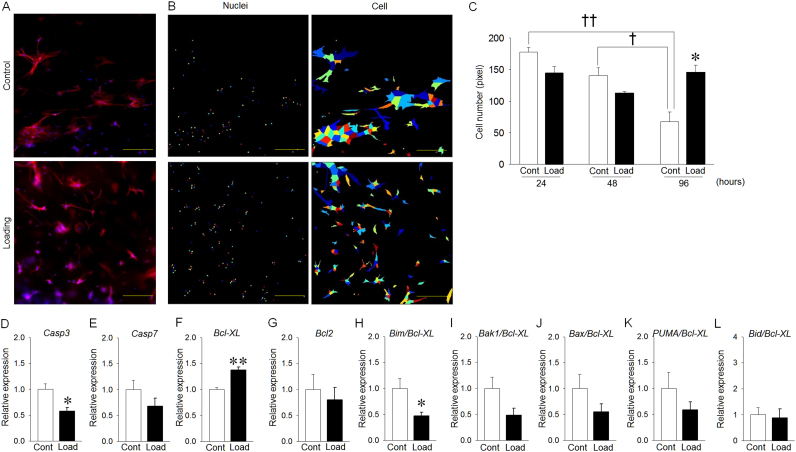
Cell numbers under loaded conditions at 96 hours after mechanical stretch appeared to be increased compared to that under non-loaded conditions (Fig. 3A, B). Cell number under non-loaded conditions was significantly decreased in a time-dependent manner, whereas it was not decreased at 48 and 96 hours after cyclic mechanical stretch (Fig. 3C). Cyclic mechanical stimulation significantly suppressed Casp3 expression (Fig. 3D), whereas Casp7 expression did not significantly change (Fig. 3E). Cyclic mechanical stimulation significantly increased Bcl-XL expression (Fig. 3F). Bcl2 expression did not change, regardless of mechanical stimulation (Fig. 3G). Additionally, cyclic mechanical stimulation significantly decreased the Bim/Bcl-XL ratio (Fig. 3H). The ratios of Bak1/Bcl-XL, Bax/Bcl-XL and PUMA/Bcl-XL under loaded conditions were smaller than those under non-loaded conditions, although the differences were not significant (Fig. 3I–K). The Bid/Bcl-XL ratio was the same between groups (Fig. 3L).
Fig. 3.
Effect of mechanical stretch on cell number and apoptosis-related genes of osteocyte-like cells. (A) Representative fluorescent images at 96 hours after mechanical stretch (Bar: 100 µm). (B) Each color represents cell shapes and nuclei. (C) The cell number in the control group was significantly decreased in a time-dependent manner, whereas the cell number in the loading group was not significantly decreased. (D) Casp3 expression in the loading group was significantly decreased compared with that in the control group. (E) Casp7 expression was the same between groups. (F) Bcl-XL expression in the loading group was significantly increased compared with that in the control group. (G) Bcl2 expression was the almost same between groups. (H) Bim/Bcl-XL expression in the loading group was significantly decreased compared with that in the control group. (I-K) Bak1/Bcl-XL, Bax/Bcl-XL and PUMA/Bcl-XL expression levels in the loading group were decreased compared with those in the control group, although the differences were not significant. (L) Bid/Bcl-XL expression was the same between the loading and control groups. Results are expressed as relative gene expression after normalization to the housekeeping gene β-actin. *p<0.05, **p<0.01, † p<0.05, †† p<0.01.
3.3. Effect of cyclic mechanical stimulation on autophagy
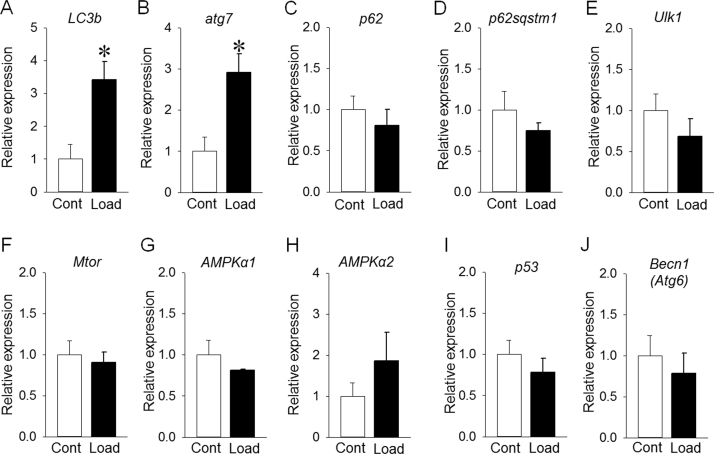
Cyclic mechanical stimulation for 96 hours significantly increased LC3b and atg7 expression levels (Fig. 4A, B). However, p62, p62/spstm1, Ulk1, Mtor, AMPKα1, AMPKα2, p53 and Becn1 expression levels were similar under loaded and non-loaded conditions (Fig. 4C-J).
Fig. 4.
Effect of mechanical stimulation for 96 hours on autophagy-related genes. (A) LC3b expression in the loading group was significantly increased compared with that in the control group. (B) atg7 expression in the loading group was significantly increased compared with that in the control group. (C-J) p62, p62sqstm1, ULK1, Mtor, AMPKα−1, AMPKα−2, p53 and Becn1 expression levels were almost the same between the loading and control groups. Results are expressed as relative gene expression after normalization to the housekeeping gene β-actin. *p<0.05.
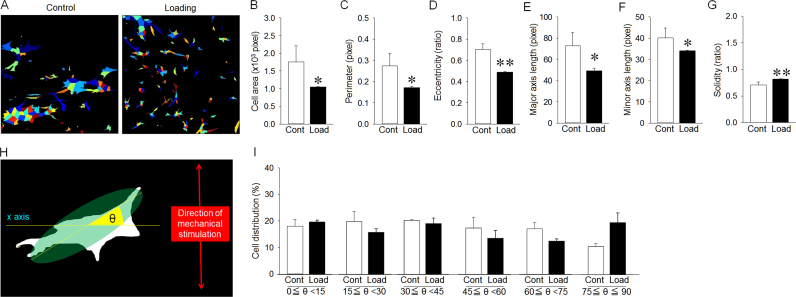
3.4. Effect of cyclic mechanical stimulation on morphology and preferential alignment of osteocyte-like cells
Cell morphology under loaded conditions for 96 hours appeared smaller than that under non-loaded conditions (Fig. 5A). Cyclic mechanical stimulation significantly decreased the cell area, perimeter, eccentricity, major axis length and minor axis length (Fig. 5B–F). Additionally, cyclic mechanical stimulation significantly increased cell solidity (Fig. 5G). The angle (θ) between the x-axis and long axis of cells was also measured (Fig. 5H). Cell distributions at each angle was almost the same, regardless of cyclic mechanical stimulation (Fig. 5I).
Fig. 5.
Effect of mechanical stimulation for 96 hours on morphology and preferential alignment of cells in vitro. (A) Analyzed diagrams of cell outlines using CellProfiler software. Each color represents cell nuclei. (B) Cell area in the loading group was significantly smaller than that in the control group. (C) Cell perimeter in the loading group was significantly smaller than that in the control group. (D) Cell eccentricity in the loading group was significantly shorter than that in the control group. (E) Major axis length in the loading group was significantly shorter than that in the control group. (F) Minor axis length in the loading group was significantly smaller than that in the control group. (G) Cell solidity in the loading group was significantly larger than that in the control group. (H) Schematic illustration indicates cell alignment to the direction of mechanical stimulation. The x-axis was orthogonal to the direction of mechanical stimulation. The angle (θ) between the x-axis and long axis of cells was measured. (I) Cell orientation did not change between the loading and control groups, irrespective of the angle between the x-axis and long axis of cells. *p<0.05, **p<0.01.
4. Discussion
Osteocytes exist in a 3D environment, however, most in vitro studies have been performed in a 2D environment. MLO-Y4, a mouse osteocyte-like cell line characterized by cellular proliferation, dendrite processes and osteopontin and Cx43 expression, is the most widely used cell line to investigate osteocyte function. However, MLO-Y4 expresses undetectable levels of Dmp1 and Sost [16], although osteocytes highly express these genes in 3D environment. Thus, understanding osteocyte function has been challenging even when using MLO-Y4 cells. Some groups have used osteocyte-like cells developed in a 3D culture system to overcome this problem [12], [17], [18]. A recent study indicated that the function of 2D-cultured osteocyte-like cells was quite distinct from that of 3D-cultured osteocyte-like cells in vitro [13], indicating that a 3D environment is required to evaluate the function of osteocyte-like cells in in vitro studies. In the current study, the morphology of 3D-cultured osteoblasts was markedly changed with cell process-like structures that connected to adjacent cells compared with 2D-cultured osteoblasts. As expected, expression of Dmp1, Cx43, Sost, Fgf23 and RANKL, but not Mepe and E11, which are recognized as osteocyte-specific genes, was significantly increased in 3D-cultured osteoblasts compared with 2D-cultured osteoblasts. An in vitro study using human osteocyte-like cells established from osteoblasts in a unique 3D culture system also examined osteoblast- and osteocyte-specific gene expression levels to evaluate whether osteoblasts grown in 3D culture could differentiate into osteocyte-like cells [12]. Hence, osteoblasts cultured for 120 hours in the 3D culture system were designated as osteocyte-like cells and used to investigate the effect of mechanical stimulation in this study.
Long-term cell culture in a 3D system under cyclic mechanical load is challenging. Too strong cyclic mechanical stretch can rupture gels containing cells, while too weak cyclic mechanical stretch does not induce cell responses. It has been reported that the threshold of stress amplitude was 4–8% for osteoblasts in a 2D culture system [19]. In this study, amplitude >8% tore the gels within 2–3 days. Moreover, 3D-cultured cells indirectly received mechanical stimuli via gels, resulting in reduced stress amplitude transmitted to osteocyte-like cells. Hence, 8% stress amplitude was selected to compensate for cell responses to cyclic mechanical stretch. Additionally, a rate of 2 cycles min-1 was applied according to a previous study that demonstrated preferential alignment of osteoblasts by cyclic mechanical stretch in a 2D culture system [14].
It has been demonstrated that mechanical unloading significantly reduced density of osteocyte lacunae compared with loading in rat tibiae [20], and osteocyte density in peri-implant bone under loaded conditions was significantly increased compared with non-loaded conditions in a human study [21]. Moreover, we demonstrated that cyclic mechanical load via dental implants significantly increased osteocyte density around the implant neck in vivo [5], [22]. These findings suggest that the osteocyte network is upregulated by mechanical stimuli in both jaw and skeletal bones. However, clarification of the mechanisms by which mechanical stimuli alter osteocyte density have been challenging because many studies that examined osteocyte density were 2D-in vitro studies. Thus, we developed a 3D culture system to elucidate the mechanism. Cyclic mechanical stimulation significantly increased the number of osteocyte-like cells. This was in accordance with previous in vivo studies [20], [21], [22]. A recent study indicated that stretch force had an anti-apoptotic effect on 2D-cultured MLO-Y4 cells by suppressing caspase-3 activity and enhancing translocation of Src kinases [23]. In the current study, Casp3 expression significantly decreased under mechanical load. Moreover, anti-apoptotic gene (Bcl-XL but not Bcl2) expression significantly increased, and the ratios of pro-apoptotic genes to Bcl-XL, especially Bim/Bcl-XL, were decreased. It has been suggested that cell phenotypes and force types influence the expression patterns of apoptotic- and anti-apoptotic-related genes [24]. For instance, high shear stress increases Bcl-XL production in endothelial cells [25], whereas mechanical stretch induces Bad and Bax production in cardiomyocytes, but not in fibroblasts [26]. Our findings suggest that mechanical stimulation could have an anti-apoptotic effect on 3D-cultured osteocyte-like cells. Osteocyte-like cells survived but did not increase under cyclic loaded conditions, indicating that development of osteocyte networks by mechanical stimulation may contribute to decreased apoptosis of osteocytes.
There are no reports that investigated the effect of mechanical stimuli on autophagy in osteocytes in vitro and in vivo. To our knowledge, this is the first in vitro study to demonstrate that cyclic mechanical stretch significantly increased LC3b and atg7 expression levels with decreased cell area and ellipticity, indicating the shape of 3D-cultured osteocyte-like cells became spherical. Both LC3b and Atg7 are requisite for autophagosome formation [27]. In vivo studies have reported that a decline in autophagy-related gene atg7 from osteocytes decreased bone mass with decreased osteoclast number, osteoblast number and bone formation rate. Moreover, atg7 deletion in osteocytes significantly increased the diameter of mouse osteocytes and changed their shape [28], [29]. Therefore, mechanical stimuli could trigger autophagy. Our findings also suggest that osteocyte-associated bone quality is controlled by changing the osteocyte network through changes in cell number, shape and size via expression of autophagy-related genes, especially atg7, in osteocytes under loaded conditions.
Preferential alignment of osteocytes has been demonstrated to be parallel to the direction of principal stress within the 60°-clockwise-grooves relative to a plane perpendicular to the long axis of hip implants in beagle dog [6]. Another study indicated that alignment of the long axis of osteocytes was parallel to the principal mechanical load direction in mouse fibulae, whereas osteocytes were randomly aligned in mouse calvariae [30]. Moreover, it has been demonstrated that osteocyte lacunae in human osteons are mainly located in longitudinally structured lamellae with an angle of 26–27° from the osteon axis which is parallel to the principal mechanical loading axis in long bones [31], [32]. The shape of osteocyte lacunae corresponds with osteocyte shape in human long bones [33]. From these previous findings, preferential alignment of osteocytes is thought to range from parallel to approximately 30° to principal mechanical load directions in a site-specific manner. However, our present data were not consistent with these in vivo findings; our osteocyte-like cells did not have preferential alignment along the force direction under loaded conditions. Experimental conditions such as types of mechanical stimuli, animal species, differences between in vivo and in vitro cultures and observation methods would affect the preferential alignment of osteocyte-like cells. To our knowledge, there have been no in vitro studies investigating the relationship between osteocyte alignment and mechanical load.
In contrast, a previous report showed that specific cyclic stretch significantly induced preferential alignment of osteoblastic cell line MC3T3-E1 with approximately 60° of orientation angle to the principal load direction [34]. It has also been reported that cyclic mechanical stretch induced preferential alignment of 2D-cultured osteoblasts at approximately 60° to the force direction in vitro [14]. The definitive reason why osteoblast alignment did not correspond with the force direction remains unclear. However, the authors concluded that elastic properties of poly-dimethylpolysiloxane, which was used as a cell culture substrate may be the cause of cell alignment at 60° to the principal mechanical load direction because the strain of poly-dimethylpolysiloxane was approximately 60° to the stretch direction. Accordingly, various factors including use of poly-dimethylpolysiloxane and thick gels may change principal mechanical load direction, resulting in the absence of the preferential alignment of osteocyte-like cells in this study.
Our current findings and previous data suggest that preferential alignment of osteocytes along the principal mechanical load direction is partially predetermined (e.g. approximately 60° to the principal force direction) before differentiation into osteocytes that embed into bone matrix. Osteocytes can access perilacunar calcium (osteocytic osteolysis) during periods of calcium depletion and synthesize new matrix upon calcium repletion [35], which suggests that preferential alignment and osteocytes shape are modified by various factors such as mechanical stimuli and osteocytic osteolysis.
In summary, cyclic mechanical stretch contributed to network development of osteocyte-like cells by anti-apoptotic effect. Cyclic mechanical stretch also changed the size and shape of osteocyte-like cells without preferential cell alignment via upregulation of LC3b and atg7. Our 3D culture system is a useful tool to determine the original function of osteocyte-like cells, although more in vitro studies that mimic in vivo 3D environment are required. Both osteoblasts and osteocytes may be key regulators controlling bone quality when mechanical stimuli are applied to bone tissue.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 25670824.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.04.018.
Contributor Information
Nao Inaba, Email: bb55313205@ms.nagasaki-u.ac.jp.
Shinichiro Kuroshima, Email: kuroshima@nagasaki-u.ac.jp.
Yusuke Uto, Email: y-uto@nagasaki-u.ac.jp.
Muneteru Sasaki, Email: m-sasaki@nagasaki-u.ac.jp.
Takashi Sawase, Email: sawase@nagasaki-u.ac.jp.
Appendix A. Transparency document
Supplementary material
.
References
- 1.Fyhrie D.P. Summary--measuring "bone quality". J. Musculoskelet. Neuron. Interact. 2005;5:318–320. [PubMed] [Google Scholar]
- 2.Nakano T., Kaibara K., Tabata Y., Nagata N., Enomoto S., Marukawa E., Umakoshi Y. Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam X-ray diffractometer system. Bone. 2002;31:479–487. doi: 10.1016/s8756-3282(02)00850-5. [DOI] [PubMed] [Google Scholar]
- 3.Bikle D.D., Halloran B.P. The response of bone to unloading. J. Bone Miner. Metab. 1999;17:233–244. doi: 10.1007/s007740050090. [DOI] [PubMed] [Google Scholar]
- 4.Klein-Nulend J., Bakker A.D., Bacabac R.G., Vatsa A., Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54:182–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Kuroshima S., Nakano T., Ishimoto T., Sasaki M., Inoue M., Yasutake M., Sawase T. Optimally oriented grooves on dental implants improve bone quality around implants under repetitive mechanical loading. Acta Biomater. 2016 doi: 10.1016/j.actbio.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Noyama Y., Nakano T., Ishimoto T., Sakai T., Yoshikawa H. Design and optimization of the oriented groove on the hip implant surface to promote bone microstructure integrity. Bone. 2013;52:659–667. doi: 10.1016/j.bone.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Mizushima N., Levine B., Cuervo A.M., Klionsky D.J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariño G., Niso-Santano M., Baehrecke E.H., Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizushima N., Yoshimori T., Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King J.S., Veltman D.M., Insall R.H. The induction of autophagy by mechanical stress. Autophagy. 2011;7:1490–1499. doi: 10.4161/auto.7.12.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor S.E., Shah M., Orriss I.R. Generation of rodent and human osteoblasts. Bone. Rep. 2014;3:585. doi: 10.1038/bonekey.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boukhechba F., Balaguer T., Michiels J.F., Ackermann K., Quincey D., Bouler J.M., Pyerin W., Carle G.F., Rochet N. Human primary osteocyte differentiation in a 3D culture system. J. Bone Miner. Res. 2009;24:1927–1935. doi: 10.1359/jbmr.090517. [DOI] [PubMed] [Google Scholar]
- 13.Mulcahy L.E., Taylor D., Lee T.C., Duffy G.P. RANKL and OPG activity is regulated by injury size in networks of osteocyte-like cells. Bone. 2011;48:182–188. doi: 10.1016/j.bone.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Matsugaki A., Fujiwara N., Nakano T. Continuous cyclic stretch induces osteoblast alignment and formation of anisotropic collagen fiber matrix. Acta Biomater. 2013;9:7227–7235. doi: 10.1016/j.actbio.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Kuroshima S., Al-Salihi Z., Yamashita J. Mouse anti-RANKL antibody delays oral wound healing and increases TRAP-positive mononuclear cells in bone marrow. Clin. Oral. Investig. 2016;20:727–736. doi: 10.1007/s00784-015-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W., Harris M.A., Heinrich J.G., Guo D., Bonewald L.F., Harris S.E. Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO-Y4 osteocyte cell model. Bone. 2009;44:32–45. doi: 10.1016/j.bone.2008.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spatz J.M., Wein M.N., Gooi J.H., Qu Y., Garr J.L., Liu S., Barry K.J., Uda Y., Lai F., Dedic C., Balcells-Camps M., Kronenberg H.M., Babij P., Pajevic P.D. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J. Biol. Chem. 2015;290:16744–16758. doi: 10.1074/jbc.M114.628313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchihashi K., Aoki S., Matsunobu A., Toda S. Osteoblast migration into type I collagen gel and differentiation to osteocyte-like cells within a self-produced mineralized matrix: a novel system for analyzing differentiation from osteoblast to osteocyte. Bone. 2013;52:102–110. doi: 10.1016/j.bone.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Neidlinger-Wilke C., Grood E.S., Wang J.H.-C., Brand R.A., Claes L. Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. J. Orthop. Res. 2001;19:286–293. doi: 10.1016/S0736-0266(00)00029-2. [DOI] [PubMed] [Google Scholar]
- 20.Britz H.M., Carter Y., Jokihaara J., Leppänen O.V., Järvinen T.L., Belev G., Cooper D.M. Prolonged unloading in growing rats reduces cortical osteocyte lacunar density and volume in the distal tibia. Bone. 2012;51:913–919. doi: 10.1016/j.bone.2012.08.112. [DOI] [PubMed] [Google Scholar]
- 21.Barros R.R., Degidi M., Novaes A.B., Piattelli A., Shibli J.A., Iezzi G. Osteocyte density in the peri-implant bone of immediately loaded and submerged dental implants. J. Periodontol. 2009;80:499–504. doi: 10.1902/jop.2009.080484. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki M., Kuroshima S., Aoki Y., Inaba N., Sawase T. Ultrastructural alterations of osteocyte morphology via loaded implants in rabbit tibiae. J. Biomech. 2015;48:4130–4141. doi: 10.1016/j.jbiomech.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Plotkin L.I., Mathov I., Aguirre J.I., Parfitt A.M., Manolagas S.C., Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. Cell Physiol. 2005;289:C633–643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 24.Chan D.D., Van Dyke W.S., Bahls M., Connell S.D., Critser P., Kelleher J.E., Kramer M.A., Pearce S.M., Sharma S., Neu C.P. Mechanostasis in apoptosis and medicine. Prog. Biophys. Mol. Biol. 2011;106:517–524. doi: 10.1016/j.pbiomolbio.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Bartling B., Tostlebe H., Darmer D., Holtz J., Silber R.E., Morawietz H. Shear stress-dependent expression of apoptosis-regulating genes in endothelial cells. Biochem. Biophys. Res. Commun. 2000;278:740–746. doi: 10.1006/bbrc.2000.3873. [DOI] [PubMed] [Google Scholar]
- 26.Liao X.D., Wang X.H., Jin H.J., Chen L.Y., Chen Q. Mechanical stretch induces mitochondria-dependent apoptosis in neonatal rat cardiomyocytes and G2/M accumulation in cardiac fibroblasts. Cell Res. 2004;14:16–26. doi: 10.1038/sj.cr.7290198. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y., Kominami E., Tanaka K., Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piemontese M., Onal M., Xiong J., Han L., Thostenson J.D., Almeida M., O'Brien C.A. Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci. Rep. 2016;6:24262. doi: 10.1038/srep24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onal M., Piemontese M., Xiong J., Wang Y., Han L., Ye S., Komatsu M., Selig M., Weinstein R.S., Zhao H., Jilka R.L., Almeida M., Manolagas S.C., O'Brien C.A. Suppression of autophagy in osteocytes mimics skeletal aging. J. Biol. Chem. 2013;288:17432–17440. doi: 10.1074/jbc.M112.444190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vatsa A., Breuls R.G., Semeins C.M., Salmon P.L., Smit T.H., Klein-Nulend J. Osteocyte morphology in fibula and calvaria --- is there a role for mechanosensing? Bone. 2008;43:452–458. doi: 10.1016/j.bone.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Marotti G., Muglia M.A., Zaffe D. A SEM study of osteocyte orientation inalternately structured osteons. Bone. 1985;6:331–334. doi: 10.1016/8756-3282(85)90324-2. [DOI] [PubMed] [Google Scholar]
- 32.Petrtýl M., Hert J., Fiala P. Spatial organization of the haversian bone in man. J. Biomech. 1996;29:161–169. doi: 10.1016/0021-9290(94)00035-2. [DOI] [PubMed] [Google Scholar]
- 33.Marotti G. Osteocyte orientation in human lamellar bone and its relevance to the morphometry of periosteocytic lacunae. Metab. Bone Dis. Relat. Res. 1979;1:325–333. [Google Scholar]
- 34.Nagayama K., Kimura Y., Makino N., Matsumoto T. Strain waveform dependence of stress fiber reorientation in cyclically stretched osteoblastic cells: effects of viscoelastic compression of stress fibers. Am. J. Physiol. Cell Physiol. 2012;302:C1469–1478. doi: 10.1152/ajpcell.00155.2011. [DOI] [PubMed] [Google Scholar]
- 35.Teti A., Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–16. doi: 10.1016/j.bone.2008.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material