Abstract
Background
Topoisomerase poisons are important drugs for the management of human malignancies. Nitric oxide (•NO), a physiological signaling molecule, induces nitrosylation (or nitrosation) of many cellular proteins containing cysteine thiol groups, altering their cellular functions. Topoisomerases contain several thiol groups which are important for their activity and are also targets for nitrosation by nitric oxide.
Methods
Here, we have evaluated the roles of •NO/•NO-derived species in the stability and activity of topo II (α and β) both in vitro and in human MCF-7 breast tumor cells. Furthermore, we have examined the effects of •NO on the ATPase activity of topo II.
Results
Treatment of purified topo IIα and β with propylamine propylamine nonoate (PPNO), an NO donor, resulted in inhibition of the catalytic activity of topo II. Furthermore, PPNO significantly inhibited topo II-dependent ATP hydrolysis. •NO-induced inhibition of these topo II (α and β) functions resulted in a decrease in cleavable complex formation in MCF-7 cells in the presence of m-AMSA and XK469 and induced significant resistance to both drugs in MCF-7 cells.
Conclusion
PPNO treatment resulted in the nitrosation of the topo II protein in MCF-7 cancer cells and inhibited both catalytic-, and ATPase activities of topo II. Furthermore, PPNO significantly affected the DNA damage and cytotoxicity of m-AMSA and XK469 in MCF-7 tumor cells.
General significance
As tumors express nitric oxide synthase and generate •NO, inhibition of topo II functions by •NO/•NO-derived species could render tumors resistant to certain topo II-poisons in the clinic.
Keywords: Topoisomerase, Nitric oxide, ATPase inhibition, m-AMSA, XK469, Resistance
Highlights
-
•
Nitric oxide (•NO) induces nitrosylation of many proteins, including topoisomerases.
-
•
Nitrosation of topo II inhibited catalytic-, and ATPase activities of topo II.
-
•
Inhibition of topo II activity resulted in resistance to topoisomerase II poisons.
1. Introduction
Nitric Oxide (•NO) is a small gaseous free radical molecule which easily diffuses in cells and tissues. Extensive research has now shown that •NO acts as a cellular signaling molecule and is involved in many biological processes, including cell survival, cell death, cancer progression, and the innate immune response [1], [2], [3], [4], [5]. It has been shown that the effects of •NO are biphasic: At high concentrations it induces DNA damage, apoptosis, and cell death while at low concentrations it induces cell survival and tumor progression [6], [7]. •NO and/or its reactive metabolites (NO+, N2O3, -OONO) are known to induce nitrosation (nitrosylation) of many proteins by reacting with free thiol groups, resulting in altered protein functions [8], [9]. Furthermore, it is believed that nitrosation plays a significant role in the signaling functions of •NO. In vivo, •NO is formed from l-arginine by nitric oxide synthase (NOS). Three forms of NOS have been identified, including neuronal (nNOS), endothelial (eNOS), and a Ca2+-independent inducible isoform (iNOS). High expression of iNOS and increased production of •NO have been described in many human tumors, including breast, prostate and colorectal cancers [10], [11], [12], [13].
Recent studies from our laboratory have shown that •NO and/or its reactive metabolites, delivered via an NO donor (PPNO), induce significant resistance to both topoisomerase I and II poisons [14], [15]. Topoisomerases (topo) are nuclear enzymes responsible for maintaining the topology of DNA and many of DNA's functions in cells [16], [17], [18], [19]. Inhibition of topoisomerase enzyme functions results in the inhibition of cellular synthesis and ultimately cellular death. It has been shown that the resistance to the clinically active topo poisons results from mutation of the topo gene, and/or decreases in activity of the proteins [18], [20], [21], [22]. We have found that •NO nitrosylates both topo I and II in human breast MCF-7 tumor and colon tumor HT-29 cells when treated with an NO-donor [14], [15]. Interestingly, this nitrosylation of the topo I had no significant effects upon the activity of the protein, nor did it decrease topo I-dependent DNA damage in tumor cells. We have also found that •NO reacts directly with etoposide, a topo II poison, in the presence of molecular O2, and the products of the reaction are significantly less toxic to tumor cells than the parent drug [23]. Furthermore, we have recently shown that •NO also causes nitrosylation of topo II-SH functional groups in cells [15]. Unlike topo I, nitrosylation of topo IIα leads to a decrease in its catalytic and relaxation activities both in vitro and in tumor cells [15]. An •NO-induced decrease in topo IIα activity leads to a decrease in DNA damage and induction of significant resistance to etoposide in MCF-7 breast tumor cells. While •NO-derived species nitrosylated free thiol groups of both topo I and II similarly, only the activity of topo IIα was significantly compromised, resulting in decreased DNA damage.
In the present study, we have further characterized the interactions of •NO (via PPNO, an NO donor) with topo II (α and β) both in vitro and in human MCF-7 breast cancer cells. We show here that •NO significantly modulates the cytotoxicity of XK469, a topo IIβ selective drug [24], and m-AMSA, used clinically for the treatment of lymphoma and adult acute leukemia [25]. In this study we found that •NO significantly inhibits the ATPase activity of topo II, resulting in decreased topo II catalytic activity, and suppresses the DNA damage induced by both m-AMSA and XK469 in vitro and in MCF-7 cells. Furthermore, PPNO pretreatment modulates cytotoxicities of both XK469 and m-AMSA in MCF-7 breast tumor cells.
2. Materials
The nitric oxide donor, propylamine propylamine nonoate (PPNO), and XK469 were obtained from Cayman Chemicals (Ann Arbor, MI). m-AMSA (Amsacrine, N-[4-(acridin-9-ylamono)-3-methoxyphenyl] methanesulfonamide), and the nitric oxide donor, S-nitrosoglutathione (GSNO) were obtained from Santa Cruz Biotechnology, Inc (Dallas, TX). Purified topo IIα, kDNA, and SDS/KCl precipitation assay kits were obtained from Topogen (Port Orange, FL). Purified topo IIβ was obtained from Inspiralis Limited (Norwich, UK). Primary antibody for detection of topo IIβ was obtained from BD Biosciences (Catalog number 611493, San Jose, CA). Primary antibody, anti-gamma H2AX, for the detection of DNA double strand breaks was obtained from Abcam, (Cambridge, MA). Stock solutions of PPNO or GSNO were prepared in 0.2 N NaOH and were stored at −80 °C. Stock solutions of XK469 (in DMSO), and m-AMSA (in double-distilled water) were prepared and stored at −80 °C. Fresh drug solutions prepared from stock solutions were used in all experiments.
3. Methods
3.1. Effects of •NO on topoisomerase II activity
Evaluations of the catalytic activities for topo II (α and β) were carried out as described previously [26]. Briefly, topo II catalytic activity was determined by incubating 0.2 µg kDNA with topo II (2 Units) in the presence or the absence of various concentrations of PPNO (25–100 µM) in topo II buffer in the presence of ATP for 30 min at 37 °C. For the inhibition studies, topo II in the presence of DNA (without ATP) was incubated with PPNO for 2.0 min on ice and the mixture incubated for an additional 30 min following the addition of 1 mM ATP. The reaction was stopped by adding loading dye and the content was loaded onto a 1% agarose gel.
3.2. ATP hydrolysis and inhibition studies
Topo II-dependent ATP hydrolysis was determined by the one-step colorimetric assay using malachite green and molybdate as described previously by Chan et al. [27]. Inhibition studies with PPNO were carried out by including various concentrations of the NO-donor with topo II (α and β) on ice for 5 min and incubating the mixtures at 37 °C for 30 min in the presence of 1 mM ATP. Incubations (with PPNO) without ATP were used as blanks.
3.3. Cell culture and cytotoxicity studies
Human breast MCF-7 (ATCC, Rockville, MD) was grown in Phenol Red-free RPMI media supplemented with 10% fetal bovine serum and antibiotics. MCF-7 cells (doubling times of about 24 h) were routinely used for 15–20 passages, after which the cells were discarded and a new cell culture was started from fresh, frozen stock. For the treatment with PPNO (25–100 µM), cells were plated in RPMI media containing 1.0% FBS without antibiotics. After the prescribed treatment period (0–2 h), the medium was changed to a regular medium containing 10% FBS with antibiotics.
The cytotoxicity studies were carried out with a cell growth inhibition assay. For the cell count-based growth inhibition studies, 150,000–200,000 cells/well were plated in 2 ml of complete medium onto a 6-well plate (in triplicate) and allowed to attach for 18 h. The medium was removed and fresh warm medium (2 ml) containing 1% FBS without antibiotics and 200 μl of PBS (pH 7.0) was added. Cells were treated with PPNO (100 μM) for 2 h, followed by the addition of various concentrations of drugs (XK469 or m-AMSA), and were incubated for 48 h in the complete medium. DMSO and PPNO were included as the controls. Cells were trypsinized, and the numbers of surviving cells were determined by counting the cells in a cell counter (Beckman, Brea, CA). We used PPNO as our •NO donor because it has a short half-life (15 min at 37 °C, pH 7.0) and found PPNO to be the least toxic to cells compared to various other NO donors (e.g., diproylenetriamine nonoate). Even at 100 µM, only 10–15% of cells were found to be dead after 48 h of PPNO exposure.
3.4. Confocal microscopy studies in MCF 7 cells
1×105 cells were plated for 18 h at 37 °C in culture plates on glass coverslips. The medium was removed and replaced with fresh medium, and cells were treated with 100 µM PPNO and 500 µM GSNO for 18 h. To investigate the extent of nitrosylation and co-localization of s-nitrosated adducts with topo II (α and β) following PPNO or GSNO treatment, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature, washed twice for 5 min, permeablized for 5 min with 0.5% Triton X-100, and washed twice for 5 min. After blocking with 4% fish gelatin in PBS (pH 7.4) for 2 h at room temperature, the cells were incubated with rabbit anti-s-nitrosylated-cysteine igG (diluted 1:2000) and mouse anti-topo II (diluted 1:2000) for 2 h, followed by secondary anti-rabbit Alexa Fluor 488 and anti-mouse Alexa Fluor 568 antisera (both diluted 1:1000) for 1 h. Coverslips containing cells were washed four times and mounted on glass slides using Prolong Gold anti-fade reagent. Confocal images were taken with a Zeiss LSM 510-UV Meta microscope (Carl Zeiss Inc., Oberkochen, Germany) using a Plan-NeoFluar 40X/1.3 Oil DIC objective with zoom 3. The 488 nm line from an Argon laser was used for producing polarized light for fluorescence excitation of the Alexa Fluor 488 secondary antibody. All images were acquired with equal excitation power (5%) and identical detection gain (532 V).
3.5. SDS-KCl precipitation assay
The formation of covalent topo II/DNA complexes with drugs in MCF-7 cells was quantitated by the SDS-KCl precipitation assay as described by Liu et al. [28]. Briefly, the DNA of cells growing in the logarithmic phase (1–2×105 cells/ml), seeded in triplicate in six-well plates, was labeled with [methyl-3H]-thymidine (1.0 μci, 20 Ci/mmol; Perkin-Elmer, Waltham, MA) for 18–24 h. Cells were washed twice with medium, and various concentrations of drugs (XK469 in DMSO, or m-AMSA dissolved in doubly distilled water) were added. Treatment with PPNO was carried out in medium containing 1% FBS for 2 h. Drug treatment was carried out in the complete medium for 1 h. Cells were lysed with 1 ml of prewarmed lysis solution (Topogen). After lysis and shearing of DNA, DNA-drug-topo II-complexes were precipitated with KCl. The precipitate was collected by centrifugation and washed extensively (4×) with the washing solution (Topogen) per the manufacturer's instructions. The radioactivity was counted in a scintillation counter after adding 5 ml of scintillation fluid. PPNO alone had no significant effect on SDS-KCl precipitate formation.
3.6. The Western Blot
The Western Blot analyses for topo IIβ and for the detection of double-strand breaks induced by m-AMSA and XK469 were carried out with standard methods, and samples (40 μg of total protein) were electrophoresed under reducing conditions on 3–8% Tris-acetate gels (Novex, Life Technologies, Carlsbad, CA) for 50 min at 200 V. After electrophoresis, proteins were transferred onto nitrocellulose membranes and probed with anti-topo IIβ, anti-gamma H2AX, and anti-beta actin antibodies. An Odssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE) was used to acquire images.
3.6.1. Statistical analysis
Data are presented as means±SE of at least three independent experiments and are considered significant when p≤0.05. Statistical analysis was performed using a paired Student's t-test. ** and * denote p values<0.005, and 0.05, respectively.
4. Results
4.1. Effects of •NO on topoisomerase II activity
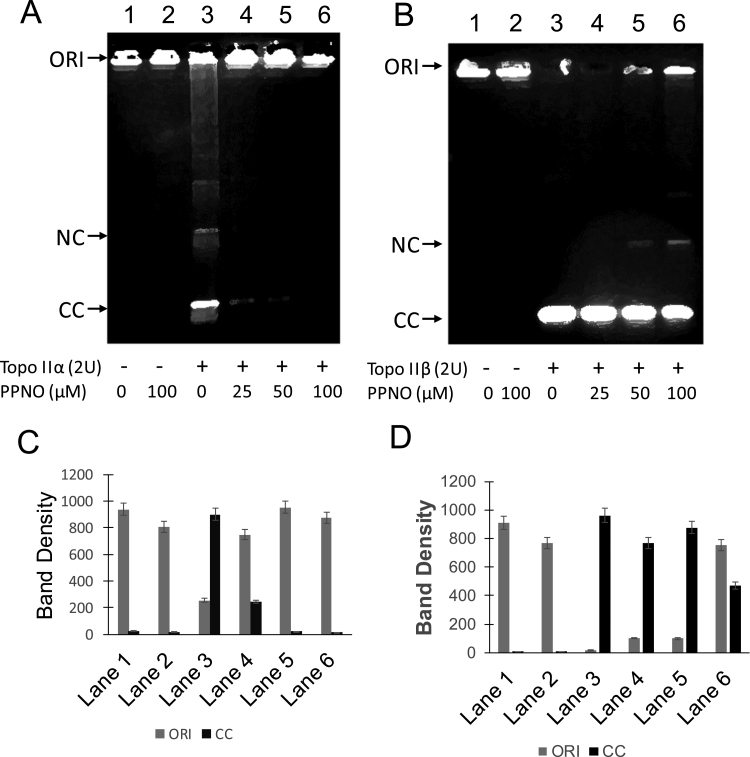
Preincubation of topo IIβ with PPNO resulted in a dose-dependent inhibition of decatenation of kDNA induced by 2 U of topo IIβ (Fig. 1B) and a 50% inhibition was observed with 100 µM PPNO (Lane 6; and Fig. 1D). We also compared PPNO-induced inhibition of this decatenation by topo IIα under identical conditions. It is interesting to note that 2 U of topo IIα was not as active as 2 U of topo IIβ in inducing decatenation of kDNA (Fig. 1A). This may represent different enzyme preparations from two different sources. However, it is important to note that the pattern of inhibitions induced by PPNO is different from that observed with topo IIβ, as PPNO was effective at lower concentrations, induced significant inhibition at 25 µM, and resulted in almost complete inhibition of the decatenation between 50 and 100 µM of PPNO (Fig. 1C). In our previous study [15], the inhibition of decatenation was carried out by preincubating topo IIα for 5 min at 37 °C using higher amounts of kDNA while in this study, we preincubated both enzymes on ice for 5.0 min to minimize denaturation/inactivation of the enzymes and used lower amounts of kDNA.
Fig. 1.
Effect of PPNO (100 µM) on topoisomerase II-induced decatenation of kDNA. The decatenation of topo II α (A) was carried out using kDNA as described in the Section 3. Lane 1, control kDNA; Lane 2, with 100 µM PPNO; Lane 3 with topo IIα 2 U; lane 4, with topo IIα 2 U and 25 µM PPNO; Lane 5, 50 µM PPNO and Lane 6, 100 µM PPNO. (B) Decatenation induced by topo IIβ Lane 1, control kDNA; lane 2, with 100 µM PPNO; Lane 3, topo IIβ 2 U; Lane 4 with topo IIα 2 and 25 µM PPNO; Lane 5, with 50 µM PPNO and Lane 6, with 100 µM PPNO. NC, nicked open circular kDNA; CC, closed circular kDNA; ORI, origin. (C) and (D) Quantifications of DNA following decatenation with purified topoisomerases IIα and β, respectively.
4.2. Effects of •NO on ATPase activity of topoisomerase II
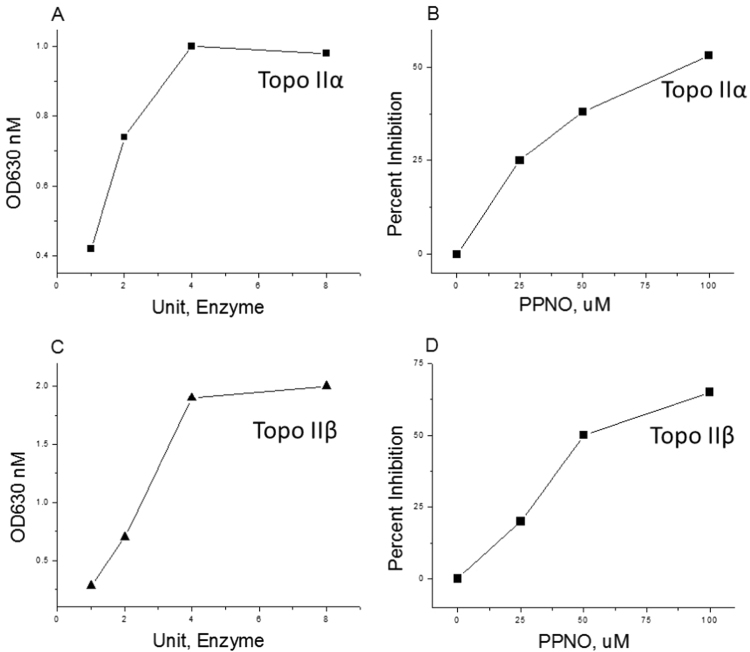
As reported previously by other investigators [29], [30], both topo IIα and β were effective in inducing the hydrolysis of ATP. Inclusion of PPNO in the reaction mixtures containing either isomer of topo II resulted in a significant inhibition of this enzyme-mediated ATP hydrolysis, and the inhibition was PPNO dose-dependent (Fig. 2).
Fig. 2.
Effects of PPNO on topoisomerase-induced ATP hydrolysis. ATP hydrolysis was assayed calorimetrically using malachite green as described in the Section 3 (A) effects of topo IIα concentrations on ATP hydrolysis; (B) effects of various concentrations of PPNO on topo IIα-induced ATP hydrolysis; (C) effects of topo IIβ concentrations of ATP hydrolysis; (D) effects of various concentrations of PPNO on topo IIβ-induced ATP hydrolysis.
4.3. Effects of •NO on DNA double strand breaks induced by m-AMSA and XK469
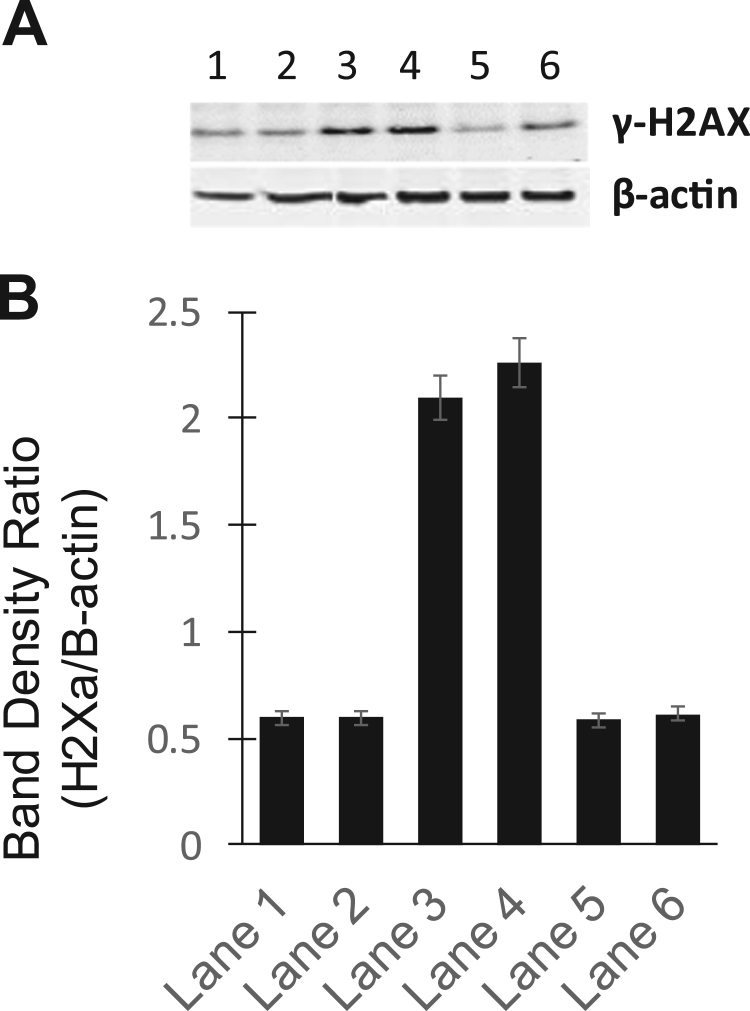
Chene et al. [31] have shown that the catalytic inhibitors ICRF-193 and QAP1 inhibit doxorubicin-induced DNA double-strand breaks as detected by decreases in ϒ-H2AX signals. We examined the effects of PPNO on m-AMSA and XK469-induced DNA damage. Results presented in Fig. 3A and B clearly indicate that preincubation of PPNO with MCF-7 cells significantly decreased DNA damage induced by both m-AMSA (75%) and XK469 (75%).
Fig. 3.
(A) Effects of PPNO on DNA damage induced by m-AMSA (2.0 µM) and XK469 (50 µM) in MCF-7 cells as detected by ϒH2AX signals. MCF-7 (400–500,000 cells/ml) were treated with drug in the presence or absence of 100 µM PPNO for 2 h. Cells were preincubated with PPNO in 1% FBS media before adding drugs and incubating mixtures in the complete media for an additional 2 h. The cells were washed with ice-cold PBS and collected, lysed and analyzed by the standard Western blots as described in the Section 3. (B) Quantifications of DNA damage (γ-H2AX band) shown in (A).
4.4. Effects of •NO on nitrosation of topoisomerase II
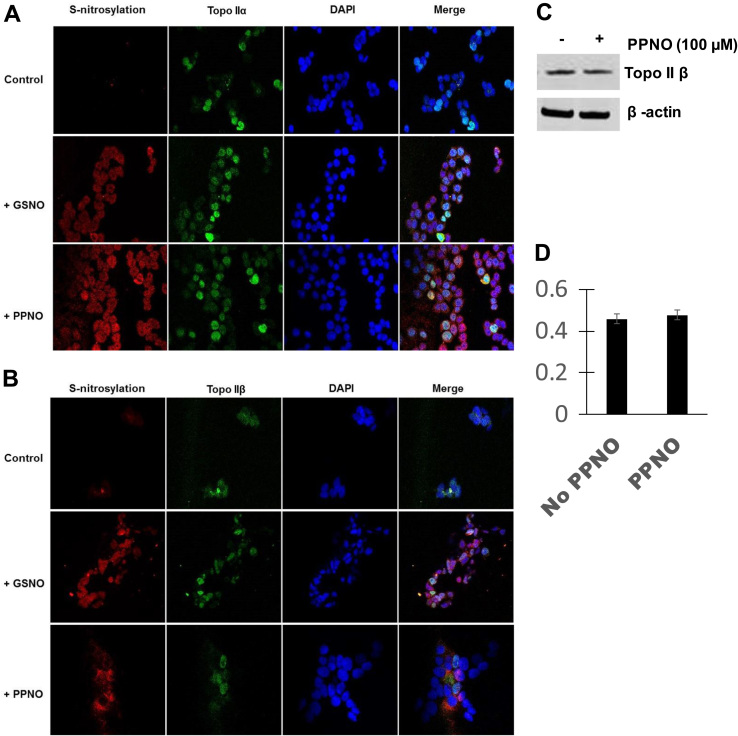
We have previously reported that both PPNO and GSNO induced significant nitrosation of topo IIα in MCF-7 cells [15]. In this study, significant nitrosation of topo IIβ was also observed in MCF-7 cells (Fig. 4). Furthermore, our results show no significant differences between the two topo II isomers in the extent of nitrosation in MCF-7 cells. We also used Western blots to test for the stability/degradation of topo IIβ protein resulting from this nitrosation of topo IIβ. Results shown in Fig. 4C and D show that PPNO had no significant effects on the topo IIβ protein levels in MCF-7 cells, as we have previously reported with topo IIα.
Fig. 4.
S-Nitrosation of -SH groups of topoisomerase IIα (A) and topoisomerase IIβ (B) in MCF-7 cells by PPNO (100 µM) and GSNO (500 µM). Cells were treated with NO-donors for 18 h as described in the Section 3 and processed for confocal microscopy studies. (C) Effects of •NO on topo IIβ protein levels in MCF-7 cells. Cells were treated with 100 µM PPNO for 24 h as described in the Section 3. Cells were collected, lysed and analyzed by Western blots for topo IIβ. (D) Quantifications of topo IIβ protein shown in (C).
4.5. Cytotoxicity and DNA damage studies with m-AMSA
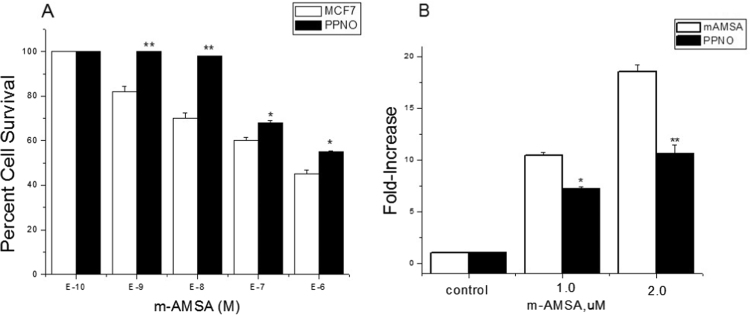
We evaluated the cytotoxicity of m-AMSA in the presence and absence of PPNO in MCF-7 cells. We found that m-AMSA induced significant cell killing in MCF-7 cells (IC50=4.0±1.0×10−7 M, Fig. 5A). When cells were treated with 100 µM PPNO, there was a significant decrease in m-AMSA-mediated cell killing (IC50=7.5±0.5×10−6 M), which represented a 4–5-fold increase in resistance to m-AMSA (Fig. 5A). The most interesting finding was that PPNO completely abolished the effects of m-AMSA at lower drug concentrations (Fig. 5A; compare the effects of PPNO at 10−9 M and 10−8 M). This decrease in PPNO-dependent cytotoxicity of m-AMSA resulted from decreased DNA damage, as there was a significant decrease in SDS-KCl precipitate formation by m-AMSA in the presence of PPNO (Fig. 4B).
Fig. 5.
Cytotoxicity of m-AMSA in MCF-7 cells. (A) Cells were seeded in 6-well plates in triplicate and allowed to attach for 18 h. PPNO (100 µM) treatment was carried out in a medium containing 1% FBS without antibiotics for 2 h. m-AMSA alone (□); m-AMSA in the presence of PPNO (■). Formation of cleavage complexes (B) in MCF-7 cells in the presence of m-AMSA (□) and m-AMSA in the presence of PPNO (■). Cells were treated with 100 µM PPNO for 2 h in a medium containing 1% FBS without antibiotics before treating with m-AMSA for 1 h in the complete medium and carrying out SDS-KCl precipitation assays as described in the Section 3. Data represent at least three independent experiments. ** and * p values≤0.005 and ≤0.05 compared with concentration-matched samples.
4.6. Cytotoxicity and DNA damage studies with XK469
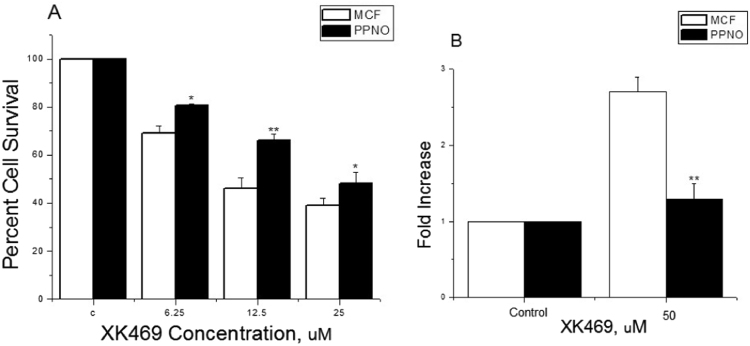
Because recent studies suggest that topo IIβ may be an important anticancer drug target [24], [32], we examined the effects of •NO/•NO-related species on its activity by using XK469, a selective topo IIβ poison in the MCF-7 cell line. Our results, presented in Fig. 6A and B, show that XK469 is moderately active in killing MCF-7 cells (IC50=12.0±1.5 µM), and PPNO pretreatment significantly decreased (2-fold) XK469 cytotoxicity in MCF-7 cells (IC50=25.0±2.0 µM), suggesting that•NO/•NO-related species inhibited topo IIβ activity in these cells. To further confirm these findings, we also examined topo IIβ-mediated DNA damage induced by XK469 in MCF-7 tumor cells. Data presented in Fig. 6B show that XK469 induced a 2–3-fold increase in SDS-KCl precipitate formation only at a high concentration of XK460 (50 µM) in these cells. PPNO treatment abolished this increase, suggesting that the induction of DNA damage was mediated by topo IIβ.
Fig. 6.
Cytotoxicity of XK469 in MCF-7 cells. (A) Cells were seeded in 6-well plates in triplicates and allowed to attach for 18 h. PPNO (100 µM) treatment was carried out in a medium containing 1% FBS without antibiotics for 2 h as described in the Section 3. XK469 alone (□); XK469 in the presence of PPNO (■). Formation of cleavage complexes (B) in MCF-7 cells by XK469 (□) alone and XK469 in the presence of PPNO (■). Cells were treated with 100 µM PPNO for 2 h in a medium containing 1% FBS without antibiotics before treating with XK469 for 1 h in the complete medium and carrying out SDS-KCl precipitation assays as described in the Methods section. Data represent at least three independent experiments. ** and * p values≤0.005 and ≤0.05 compared with concentration-matched samples.
5. Discussion
The mechanism of •NO-induced drug resistance in tumor cells is currently not known. Several studies have shown that •NO can stabilize bcl2 and HIF-1α proteins by nitrosylating reactive sulfhydryl functions [33], [34], resulting in resistance to cis-platin in tumor cells [33], [34]. We have previously shown that •NO effectively modulates cancer drug resistance in human MCF-7 breast and HT-29 colon cancer cell lines by interfering with topo I and II functions [14], [15]. This modulation of the enzyme function was dependent upon the nitrosation reactions of •NO or its reactive metabolites (+NO, N2O3, •OONO) with the reactive –SH groups of topo proteins, resulting in the down-regulation of topo I protein [14] or inhibition of both catalytic and relaxation activities of topo IIα [15]. Furthermore, we have shown that •NO, generated either intracellularly or delivered via PPNO, directly reacted with VP-16 or VP-16 radical to form inactive degradation products that resulted in VP-16 resistance in both HL60 and melanoma A375 cells [23], [35].
Mammalian cells express two isoforms of topo II (topo IIα and topo IIβ) [36], [37]. Topo IIβ is reported to be involved in the induction of childhood leukemia [38], [39] following VP-16-based chemotherapy. As topo IIβ is also implicated in many cellular functions and may be a target for certain topo-poisons, we have carried out further studies to evaluate the effects of •NO on the cytotoxicity of both m-AMSA and XK469 in human breast MCF-7 cells. While the mechanism of cytotoxicity of XK469 involves its selective interactions with topo IIβ [24], the mechanisms of tumor cell killing by m-AMSA involve both isomers of topo II [40], [41]. Here, we show that •NO induces resistance to both m-AMSA and XK469 in human breast MCF-7 cells. Furthermore, we have evaluated the mechanism of this resistance and shown that •NO (or its related species) effectively inhibits topo enzyme function by inhibiting ATPase activity of both topo IIα and β. This inhibition of ATPase activity resulted in a significant inhibition of the catalytic activities of both isomers in vitro and induced a significant decrease in DNA damage in MCF-7 cells as measured by cleavable complex formation in the presence of the drugs. As reported previously by Chene et al. [31] for certain catalytic inhibitors, PPNO pretreatment significantly suppressed the DNA damage induced by both m-AMSA and XK469, suggesting that •NO (or its related species) acted as a catalytic inhibitor of topo II (α and β) in MCF-7 cancer cells. Furthermore, a close relationship was observed between DNA damage and the decrease in cytotoxicity of these drugs in MCF-7 tumor cells in the presence of PPNO, the source for •NO used in this study.
While we have previously reported that •NO inhibited the decatenation reaction of topo IIα, this is the first report showing that this inhibition results from the inhibition of topo II ATPase activity by •NO. This is a significant finding and its implications are very intriguing. First, it describes another mechanism for tumor cell death induced by •NO (or its metabolites) by interfering with topoisomerase ATPase activity, similar to known mechanisms of actions of other catalytic inhibitors including ICRF-187 and related compounds [31], [42], [43], [44]. Catalytic inhibitors do not induce DNA cleavage but inhibit DNA double-strand break formation and activate decatenation checkpoints [45], [46]. Nakagawa et al. [47] have reported that lung tumor cells with impairment in decatenation checkpoints are sensitive to ICFR-193. This would suggest that certain NO-donors can be designed as effective topo II catalytic inhibitors and used for treatment of appropriate tumors with defective decatenation checkpoints. Finally, because our present study implicates •NO as an ATPase inhibitor (we have also found that •NO inhibits pgp activity by inhibiting pgp-dependent ATPase activity in tumor cells; manuscript in preparation), NO-donors can be utilized to sensitize pgp/MRP-overexpressing resistant tumors in the clinic. •NO is known to reverse doxorubicin resistance in pgp/MRP-overexpressing cancer cells [48], [49].
It is clear from this and our previous studies that •NO /•NO-related species modulate toxicities of various clinically active topoisomerase II-poisons, which results in resistance to these drugs in tumor cells. In this study we have identified the mechanism of this resistance where nitric oxide generated via an NO donor induces significant inhibition of ATPase activity of both topo IIα and β. Furthermore, •NO /•NO-related species suppress double strand breaks induced by these topo-poisons. Under this scenario of nitrosative stress found in human tumors overexpressing iNOS where significant amounts of •NO are continuously generated, it is not surprising that significant resistance to chemotherapy develops following treatment with topo-active drugs. The situation becomes even more difficult to manage in the clinic when inflammatory conditions present in tumors induce resistance to other important clinical drugs, e.g., taxol, as reported by Heinecke et al. [50]. Our present results further strengthen that •NO is an important modulator of cytotoxicity and resistance to topoisomerase poisons in tumor cells. Understanding of the mechanism(s) of this •NO-induced resistance will lead to effective strategies for curing cancers in the clinic. Furthermore, better, and more specific NO donors can be designed to treat cancers with decatenation checkpoint defects.
6. Conclusions
Studies reported here show that •NO generated via an NO donor inhibits ATPase activity of topo II (α and β), leading to inhibition of the catalytic activity of the proteins. Furthermore, •NO also induces significant nitrosation of these proteins in human MCF-7 breast cancer cells. We found that pretreatment of MCF-7 cancer cells with PPNO resulted in significant resistance to both m-AMSA, a clinically important drug for the treatment of human tumors, and to XK469, a selective topo IIβ-poison. The resistance to these drugs appears to result from decreases in cleavage-complex formation and the suppression of drug-induced DNA double strand breaks by PPNO. These events, taken together, would suggest that S-nitrosation of topo II proteins by •NO, most likely involving two -SH functions of ATPase, leads to conformational changes which, in turn, induce inhibition of ATP binding to the protein, resulting in inhibition of decatenation of DNA (catalytic inhibition) and induction of resistance to topo II poisons.
Funding
This research was supported [in part] by the intramural research program of the National Institute of Environmental Health Sciences, NIH. Statements contained herein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the US Government.
Acknowledgements
We thank Dr. Ann Motten and Ms. Mary Mason for their invaluable help in editing the manuscript. We also thank Drs. Maria Kadiiska and Thomas van’t Erve for their critical evaluation of the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.04.011
Appendix A. Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1.Murad F. Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone? Recent Prog. Horm. Res. 1998;53:43–59. (discussion 59–60) [PubMed] [Google Scholar]
- 2.Murad F. Shattuck lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 3.Muntane J., la Mata M.D. Nitric oxide and cancer. World J. Hepatol. 2010;2:337–344. doi: 10.4254/wjh.v2.i9.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickok J.R., Thomas D.D. Nitric oxide and cancer therapy: the emperor has NO clothes. Curr. Pharm. Des. 2010;16:381–391. doi: 10.2174/138161210790232149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirst D., Robson T. Nitric oxide in cancer therapeutics: interaction with cytotoxic chemotherapy. Curr. Pharm. Des. 2010;16:411–420. doi: 10.2174/138161210790232185. [DOI] [PubMed] [Google Scholar]
- 6.Ridnour L.A., Thomas D.D., Donzelli S., Espey M.G., Roberts D.D., Wink D.A., Isenberg J.S. The biphasic nature of nitric oxide responses in tumor biology. Antioxid. Redox Signal. 2006;8:1329–1337. doi: 10.1089/ars.2006.8.1329. [DOI] [PubMed] [Google Scholar]
- 7.Xu W., Liu L., Smith G.C., Charles l G. Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents. Nat. Cell Biol. 2000;2:339–345. doi: 10.1038/35014028. [DOI] [PubMed] [Google Scholar]
- 8.Aranda E., Lopez-Pedrera C., Haba-Rodriguez J.R. De. La, Rodriguez-Ariza A. Nitric oxide and cancer: the emerging role of S-nitrosylation. Curr. Mol. Med. 2012;12:50–67. doi: 10.2174/156652412798376099. [DOI] [PubMed] [Google Scholar]
- 9.Stamler J.S., Lamas S., Fang F.C. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 10.Klotz T., Bloch W., Volberg C., Engelmann U., Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–1903. [PubMed] [Google Scholar]
- 11.Glynn S.A., Boersma B.J., Dorsey T.H., Yi M., Yfantis H.G., Ridnour L.A., Martin D.N., Switzer C.H., Hudson R.S., Wink D.A., Lee D.H., Stephens R.M., Ambs S. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J. Clin. Investig. 2010;120:3843–3854. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loibl S., Buck A., Strank C., von Minckwitz G., Roller M., Sinn H.P., Schini-Kerth V., Solbach C., Strebhardt K., Kaufmann M. The role of early expression of inducible nitric oxide synthase in human breast cancer. Eur. J. Cancer. 2005;41:265–271. doi: 10.1016/j.ejca.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Cianchi F., Cortesini C., Fantappie O., Messerini L., Schiavone N., Vannacci A., Nistri S., Sardi I., Baroni G., Marzocca C., Perna F., Mazzanti R., Bechi P., Masini E. Inducible nitric oxide synthase expression in human colorectal cancer: correlation with tumor angiogenesis. Am. J. Pathol. 2003;162:793–801. doi: 10.1016/S0002-9440(10)63876-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma N.K., Kumar A., Kumari A., Tokar E.J., Waalkes M.P., Bortner C.D., Williams J., Ehrenshaft M., Mason R.P., Sinha B.K. Nitric oxide down-regulates topoisomerase I and induces camptothecin resistance in human breast MCF-7 tumor cells. PLoS One. 2015;10:e0141897. doi: 10.1371/journal.pone.0141897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A., Ehrenshaft M., Tokar E.J., Mason R.P., Sinha B.K. Nitric oxide inhibits topoisomerase II activity and induces resistance to topoisomerase II-poisons in human tumor cells. Biochim. Biophys. Acta. 1860;2016:1519–1527. doi: 10.1016/j.bbagen.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J.C. DNA topoisomerases. Annu. Rev. Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- 17.Pommier Y., Tanizawa A., Kohn K.W. Mechanisms of topoisomerase I inhibition by anticancer drugs. Adv. Pharmacol. 1994;29B:73–92. doi: 10.1016/s1054-3589(08)61132-1. [DOI] [PubMed] [Google Scholar]
- 18.Froelich-Ammon S.J., Osheroff N. Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J. Biol. Chem. 1995;270:21429–21432. doi: 10.1074/jbc.270.37.21429. [DOI] [PubMed] [Google Scholar]
- 19.Nitiss J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pommier Y., Leteurtre F., Fesen M.R., Fujimori A., Bertrand R., Solary E., Kohlhagen G., Kohn K.W. Cellular determinants of sensitivity and resistance to DNA topoisomerase inhibitors. Cancer Investig. 1994;12:530–542. doi: 10.3109/07357909409021413. [DOI] [PubMed] [Google Scholar]
- 21.Sinha B.K. Topoisomerase inhibitors. A review of their therapeutic potential in cancer. Drugs. 1995;49:11–19. doi: 10.2165/00003495-199549010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Friche E., Danks M.K., Schmidt C.A., Beck W.T. Decreased DNA topoisomerase II in daunorubicin-resistant Ehrlich ascites tumor cells. Cancer Res. 1991;51:4213–4218. [PubMed] [Google Scholar]
- 23.Sinha B.K., Bhattacharjee S., Chatterjee S., Jiang J., Motten A.G., Kumar A., Espey M.G., Mason R.P. Role of nitric oxide in the chemistry and anticancer activity of etoposide (VP-16,213) Chem. Res. Toxicol. 2013;26:379–387. doi: 10.1021/tx300480q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao H., Huang K.C., Yamasaki E.F., Chan K.K., Chohan L., Snapka R.M. XK469, a selective topoisomerase IIbeta poison. Proc. Natl. Acad. Sci. USA. 1999;96:12168–12173. doi: 10.1073/pnas.96.21.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghaddar H.M., Pierce S., Kantarjian H.M., Freireich E.J., Keating M.J., Estey E.H. Amsacrine and continuous-infusion high-dose cytosine arabinoside as induction therapy for patients with newly-diagnosed acute myelogenous leukemia. Leuk. Lymphoma. 1996;22:71–76. doi: 10.3109/10428199609051730. [DOI] [PubMed] [Google Scholar]
- 26.J.L. Nitiss, E. Soans, A. Rogojina, A. Seth, M. Mishina, Topoisomerase assays, Curr. Protoc. Pharmacol., Chapter 3, Unit 3, 2012, p. 3. [DOI] [PMC free article] [PubMed]
- 27.Chan K.M., Delfert D., Junger K.D. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal. Biochem. 1986;157:375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 28.Liu L.F., Rowe T.C., Yang L., Tewey K.M., Chen G.L. Cleavage of DNA by mammalian DNA topoisomerase II. J. Biol. Chem. 1983;258:15365–15370. [PubMed] [Google Scholar]
- 29.Fortune J.M., Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIalpha by blocking DNA cleavage. J. Biol. Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- 30.West K.L., Turnbull R.M., Willmore E., Lakey J.H., Austin C.A. Characterisation of the DNA-dependent ATPase activity of human DNA topoisomerase IIbeta: mutation of Ser165 in the ATPase domain reduces the ATPase activity and abolishes the in vivo complementation ability. Nucleic Acids Res. 2002;30:5416–5424. doi: 10.1093/nar/gkf677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chene P., Rudloff J., Schoepfer J., Furet P., Meier P., Qian Z., Schlaeppi J.M., Schmitz R., Radimerski T. Catalytic inhibition of topoisomerase II by a novel rationally designed ATP-competitive purine analogue. BMC Chem. Biol. 2009;9:1. doi: 10.1186/1472-6769-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding Z., Zhou J.Y., Wei W.Z., Baker V.V., Wu G.S. Induction of apoptosis by the new anticancer drug XK469 in human ovarian cancer cell lines. Oncogene. 2002;21:4530–4538. doi: 10.1038/sj.onc.1205545. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhury R., Godoy L.C., Thiantanawat A., Trudel L.J., Deen W.M., Wogan G.N. Nitric oxide produced endogenously is responsible for hypoxia-induced HIF-1alpha stabilization in colon carcinoma cells. Chem. Res. Toxicol. 2012;25:2194–2202. doi: 10.1021/tx300274a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chanvorachote P., Nimmannit U., Stehlik C., Wang L., Jiang B.H., Ongpipatanakul B., Rojanasakul Y. Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res. 2006;66:6353–6360. doi: 10.1158/0008-5472.CAN-05-4533. [DOI] [PubMed] [Google Scholar]
- 35.Sinha B.K., Kumar A., Bhattacharjee S., Espey M.G., Mason R.P. Effect of nitric oxide on the anticancer activity of the topoisomerase-active drugs etoposide and adriamycin in human melanoma cells. J. Pharmacol. Exp. Ther. 2013;347:607–614. doi: 10.1124/jpet.113.207928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prosperi E., Sala E., Negri C., Oliani C., Supino R., Astraldi Ricotti G.B., Bottiroli G. Topoisomerase II alpha and beta in human tumor cells grown in vitro and in vivo. Anticancer Res. 1992;12:2093–2099. [PubMed] [Google Scholar]
- 37.Austin C.A., Marsh K.L. Eukaryotic DNA topoisomerase II beta. Bioessays. 1998;20:215–226. doi: 10.1002/(SICI)1521-1878(199803)20:3<215::AID-BIES5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 38.Ezoe S. Secondary leukemia associated with the anti-cancer agent, etoposide, a topoisomerase II inhibitor. Int. J. Environ. Res. Public Health. 2012;9:2444–2453. doi: 10.3390/ijerph9072444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pendleton M., Lindsey R.H., Jr., Felix C.A., Grimwade D., Osheroff N. Topoisomerase II and leukemia. Ann. N. Y. Acad. Sci. 2014;1310:98–110. doi: 10.1111/nyas.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snapka R.M., Gao H., Grabowski D.R., Brill D., Chan K.K., Li L., Li G.C., Ganapathi R. Cytotoxic mechanism of XK469: resistance of topoisomerase IIbeta knockout cells and inhibition of topoisomerase I. Biochem. Biophys. Res. Commun. 2001;280:1155–1160. doi: 10.1006/bbrc.2001.4249. [DOI] [PubMed] [Google Scholar]
- 41.Errington F., Willmore E., Leontiou C., Tilby M.J., Austin C.A. Differences in the longevity of topo IIalpha and topo IIbeta drug-stabilized cleavable complexes and the relationship to drug sensitivity. Cancer Chemother. Pharmacol. 2004;53:155–162. doi: 10.1007/s00280-003-0701-1. [DOI] [PubMed] [Google Scholar]
- 42.Hasinoff B.B., Abram M.E., Chee G.L., Huebner E., Byard E.H., Barnabe N., Ferrans V.J., Yu Z.X., Yalowich J.C. The catalytic DNA topoisomerase II inhibitor dexrazoxane (ICRF-187) induces endopolyploidy in Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 2000;295:474–483. [PubMed] [Google Scholar]
- 43.Nitiss J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen A.K., Escargueil A.E., Skladanowski A. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol. Ther. 2003;99:167–181. doi: 10.1016/s0163-7258(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 45.Deming P.B., Cistulli C.A., Zhao H., Graves P.R., Piwnica-Worms H., Paules R.S., Downes C.S., Kaufmann W.K. The human decatenation checkpoint. Proc. Natl. Acad. Sci. USA. 2001;98:12044–12049. doi: 10.1073/pnas.221430898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damelin M., Bestor T.H. The decatenation checkpoint. Br. J. Cancer. 2007;96:201–205. doi: 10.1038/sj.bjc.6603537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa T., Hayashita Y., Maeno K., Masuda A., Sugito N., Osada H., Yanagisawa K., Ebi H., Shimokata K., Takahashi T. Identification of decatenation G2 checkpoint impairment independently of DNA damage G2 checkpoint in human lung cancer cell lines. Cancer Res. 2004;64:4826–4832. doi: 10.1158/0008-5472.CAN-04-0871. [DOI] [PubMed] [Google Scholar]
- 48.Riganti C., Miraglia E., Viarisio D., Costamagna C., Pescarmona G., Ghigo D., Bosia A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005;65:516–525. [PubMed] [Google Scholar]
- 49.De Boo S., Kopecka J., Brusa D., Gazzano E., Matera L., Ghigo D., Bosia A., Riganti C. iNOS activity is necessary for the cytotoxic and immunogenic effects of doxorubicin in human colon cancer cells. Mol. Cancer. 2009;8:108. doi: 10.1186/1476-4598-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heinecke J.L., Ridnour L.A., Cheng R.Y., Switzer C.H., Lizardo M.M., Khanna C., Glynn S.A., Hussain S.P., Young H.A., Ambs S., Wink D.A. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc. Natl. Acad. Sci. USA. 2014;111:6323–6328. doi: 10.1073/pnas.1401799111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material