Abstract
Nanotechnology is the formation, running and use of operation at the nanomaterial size scale (1–100 nm). Nanoscale materials can also be obtained by biological synthesis materials via eco-friendly green chemistry based technique. Current development and numerous strategies involved in the green synthesis of nanoparticles were focussed. This review mainly focused on plants which include scientific name, family name, common name, plant parts, its characterization, size and shape of the nanoparticles. Plant extract which was done experimentally gives its various characterization which leads to the identification of compounds of different nano size and shape. Biosynthesis of gold nanoparticles is in different shapes like spherical, rod, cubic, triangle and also in different sizes. Various application and importance of gold nanoparticles in numerous fields were discussed. The mark of the review is to provide an overview of recent learning in biosynthesized nanoparticles, its characterization and their potential applications.
Keywords: Plant, Gold nanoparticles, Characterization, Synthesis
Highlights
-
•
To synthesis the gold nanoparticles using different plant sources.
-
•
Using of different parts of plants in gold nanoparticles green synthesis.
-
•
Characterization of biosynthesized gold nanoparticles by morphologically and spectroscopically.
1. Introduction
Nanotechnology has been the subject of great research and enthusiasm for researcher's in recent years [1]. Nanomaterial having nanoscale dimension possess unique properties as compared to their bulk equivalent. The Recent approach focuses on economically alternative methods for the creation, manipulation of material at nanometre scale level [2]. Nanotechnology is one of the most exigent and fastest growing branches in the field of science and engineering. 16th century people already used noble gold nanoparticles for the medical and staining purpose. They have proved to be very useful for stomatology, pharmacy and implantology tissue engineering [3].
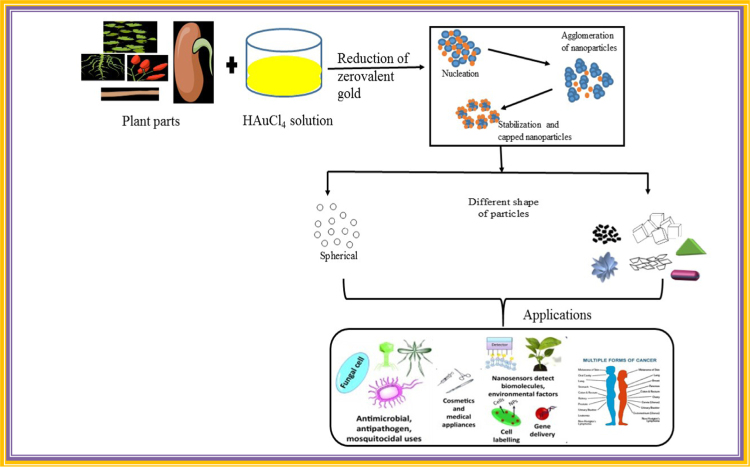
Nanoparticle synthesis is conducted by physical, chemical and biological or green method. A physical and chemical technique of synthesizing nanoparticles has proved to be quite expensive and potentially hazardous to the environment. Toxic and perilous chemicals involved in the synthesis of nanoparticles in chemical synthesis technique possess various biological risks and are responsible for several health diseases. Gold nanoparticles have been extensively used for biomedical applications (Fig. 1) [4], in separation sciences and disease diagnosis [5].
Fig. 1.
Reactions involved in green synthesis of nanoparticles and its application.
Use of plant materials for the green synthesis of nanoparticles has evolved in the last decade. The ability of plant extract to reduce metal ions has been known since the early 1900s but it was used only in last 30 years for the reduction of metal salt. Plants contain certain bioactive compounds like flavonoids, phenols, citric acid, ascorbic acid, polyphenolic, terpenes, alkaloids and reductase which act as reducing agents. Plant-mediated synthesis of nanoparticles is a very promising area of nanotechnology because the plant itself acts as both reducing and capping agent. Plant system can synthesize nanoparticles both intracellularly and extracellularly [6]. Intracellular methods for synthesizing of nanoparticles includes growing the plant in metal-rich organic media [7], metal-rich soil [8], metal-rich hydroponic solution [9]. At the same time, extracellular methods include nanoparticles synthesis by using leaf extract prepared by boiling and crushing of leaves [10].
Gold nanoparticles have bactericidal effect against animal pathogens, food pathogens and it has other pharmacological activities. Applications include tumor destruction via heat separation, pharmacokinetic studies [11], separation science [12], health care, environmental, drug delivery, gene delivery, optics, food industry, space industry [13]. The aim of this review is to focus on different plants extract mediated synthesis of gold nanoparticles, particularly where plant compounds react with metal ions and salt and lead to its reduction. These nanoparticles are further characterized by different size, shapes and morphology.
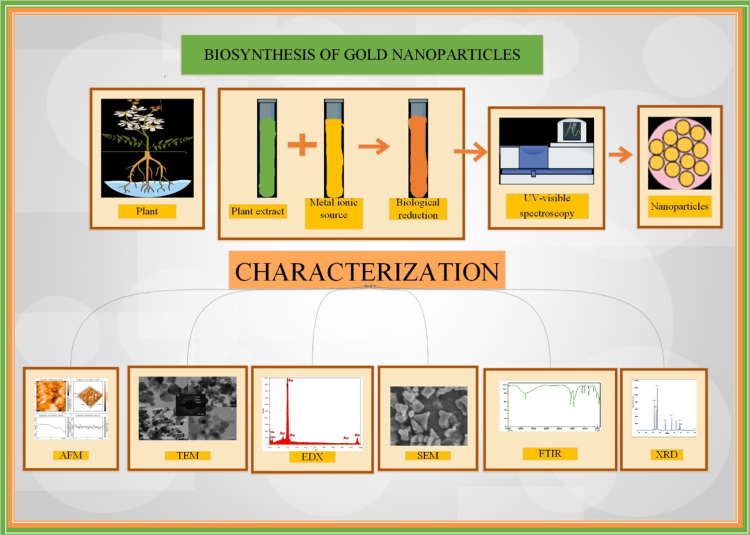
2. Characterization techniques
Technically two broad approaches are used for the synthesis of nanoparticle: top down approach and bottom up approach. The top-down approach deals with material size reduction of particles via the physical and chemical process to produce nanoparticles. The size, shape, overall physiochemical properties and surface structure are processed throughout the process [14]. Bottom-up approach deals with engineering at atomic, molecular level [15]. Nanoparticles of different size, shape, surface area are characterized by various techniques like UV-visible spectroscopy (UV-vis), powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Gas chromatography- mass spectrometry (GC-MS), High performance liquid chromatography (HPLC), energy dispersive spectroscopy (EDS), dynamic light scattering (DLS), Zeta potential, scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM) (Fig. 2) [16], [17].
Fig. 2.
Synthesis and different characterization.
Composition, size, structure, crystal phase is also determined from spectroscopy technique like UV-vis, XRD, FT-IR, DLS, EDS and Raman. UV spectra wavelengths range between 300 and 800 nm depicts the presence of various metallic nanoparticles of size range 2 nm to 100 nm. Generally, gold nanoparticles are detected by peak range between 500 and 580 nm [18]. DLS analysis estimates the size distribution and quantifies the surface charges of nanoparticles. Element composition is the determination by EDAX analysis [19]. XRD identifies the crystallite size. FTIR Spectroscopy identifies the surface residues and functional groups like flavonoid, phenol, hydroxyls which attach to the surface of nanoparticles during the course of their synthesis for their efficient reduction and stabilization.
3. Different parts of plant
The large number of different plants are reported to further gold nanoparticles synthesis are mentioned (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6) and are discussed briefly in the present review.
Table 1.
Leaf mediated synthesis of gold nanoparticles.
| S. No | Scientific name | Family | Common name | Characterization | Size (nm) | Shape | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Bacopa monnieri (BLE) | Scrophulariaceae | Valaarai | UV–vis spectro- photometer, TEM, FTIR, TGA, XRD | 3–45 | Spherical | [16] |
| 2. | Terminalia catappa | Combretaceae | Country-almond | UV-Vis spectroscopy, XRD, FTIR,TEM | 10–35 | Spherical | [10] |
| 3. | Dracocephalum kotschyi | Lamiaceae | – | TEM-SEAD, SEM-EDAX, XRD, Zeta potential, DLS, and FT-IR | 11 | Spherical | [20] |
| 4. | Olea europaea | Oleaceae | Sweet oil | UV–Vis spec, photoluminescence, TEM, XRD, FTIR | 50 to 100 | Triangular, hexagonal and spherical | [17] |
| 5. | Mangifera indica | Anacardiaceae | Mango | UV–vis, TEM, XRD. | ∼ 20 | Spherical | [21] |
| 6. | Cacumen Platycladi | Cupressaceae | Biota | AGE, TEM, DGC | 2–70 | Spherical and triangular | [22] |
| 7. | Abutilon indicum | Malvaceae | Thuthi | UV-visible Spectroscopy, TEM, GC-MS and FTIR, ZETA | 1–20 | Spherical | [23] |
| 8. | Butea monosperma | Fabaceae | Parasu | UV visible spectroscopy, XRD, TEM, DLS, FTIR | 20–80 | Large spherical | [24] |
| 9. | Suaeda monoica | Amaranthaceae | Seepweeds | SEM, EDAX, TEM, XRD, FT-IR, DLS | 14.5 | Spherical and rarely triangular | [25] |
| 10. | Ipomoea carnea | Convolvulaceae | Pink morning glory | UV–Vis spectra, SEM, TEM, EDAX, XRD, FT-IR, | 25–100 | Triangular, hexagonal, pentagonal, rod and truncated | [27] |
| 11. | Geranium sp., | Geraniaceae | Cranesbills | UV–Visible, TEM, STEM, HAADF, EDS, FTIR | 12 ± 3 | – | [28] |
| 12. | Phoenix dactylifera | Arecaceae | Date palm | UV–vis spectra, TEM FTIR | 32 and 45 | Spherical | [29] |
| 13. | Sesbania grandiflora | Fabaceae | Agati | SPR, TEM, AFM, SEM, EDX. FTIR | 34.11 | Spherical | [30] |
| 14. | Salix alba | Salicaceae | Willow | UV–Vis spectrophotometer, AFM, SEM, FTIR | 50–80 | Non-spherical | [31] |
| 15. | Magnolia kobus and Diopyros kaki | Magnoliaceaeand Ebenaceae | Kobus Magnolia and Ebony | ICP, EDS, SEM, TEM, AFM, XPS, FTIR | 5–300 | Spherical structures | [32] |
| 16. | Cacumen Platycladi | Cupressaceae | Chinese arborvitae | UV–Vis spectroscopy, TEM, EDX, SAED, FTIR, | 2.2 to 42.8 | Spherical | [15] |
| 17. | Cinnamomum zeylanicum | Cinnamomum verum | Cinnamon | UV–vis, TEM, XRD, FTIR, | 25 | Spherical | [33] |
| 18. | Coriander | Apiaceae | Coriander | UV-vis, XRD,EDAX, FTIR, TEM. | 6.75 to 57.91 | Spherical,Triangle, truncated triangles and decahedral | [34] |
| 19. | Nerium oleander | Apocynaceae | Oleander | HRTEM, SEM, XRD, FT-IR | 2–10 | Spherical | [35] |
| 20. | Euphorbia hirta | Euphorbiaceae | Pill-bearing spurge | TEM, XRD, EDAX, AFM | 50 | Spherical | [36] |
| 21. | Terminalia arjuna | Combretaceae | Arjuna | UV–visible, FTIR, XRD, TEM, AFM | 20 to 50 | Cubic, Spherical | [37] |
| 22. | Hibiscus sabdariffa | Malvaceae | Hibiscus | UV–vis spectroscopy, XRD, FTIR, and XPS, TEM | 10–60 | Spherical | [38] |
| 23. | Mimosa pudica | Fabaceae | Sensitive plant | UV–vis, FT-IR, XRD, HR-TEM, | 12.5 | Spherical | [39] |
| 24. | Argemone mexicana | Papaveraceae | Mexican poppy | UV- Vis, XRD and SEM. | 22–26 | Spherical | [40] |
| 25. | Ocimum sanctum | Lamiaceae | Vernacular | UV−vis-NIR, TEM, GC−MS, HRTEM and SAED | 10–300 | Disk,coral shape | [41] |
| 26. | Azadirachta indica L | Meliaceae | Neem | TEM, DLS, SEM, FTIR, XRD, UV-vis near-infrared spectra | 15–18 | Isomorphic spherical | [42] |
| 27. | Silybum marianum | Asteraceae | Milk thistle | – | – | – | [43] |
| 28. | Ficus benghalensis | Moraceae | Banyan | UV-Spec, FTIR, TEM, XRD, SPR, | 2 nm to 100 | Spherical | [44] |
| 29. | Diospyros ferrea | Ebenaceae | Diospyros | UV-VIS spectroscopy, FT-IR, SEM | 70 – 90 | – | [45] |
| 30. | Bauhinia tomentosa | Fabaceae | Yellow bauhinia | UV-Visible Spectrophotometer, FTIR, FESEM, EDAX, HR-TEM, XRD | 31.32 | Crystalline | [46] |
| 31. | Bougainvillea glabra | Nyctaginaceae | Paper flower | UV-visible spectral, FTIR, | – | – | [47] |
| 32. | Costus igneus | Costaceae | Spiral flag | UV-Vis Spectroscopy, SEM | 54–62 | Spherical | [48] |
| 33. | Aloe perfoliata L | Asphodelaceae | Barbados aloe | UV-vis-NIR spectroscopy, TEM, and FTIR, AFM, EDAX | 50–350 | Spherical | [49] |
| 34. | Magnolia kobus | Magnoliaceae | Kobus magnolia | UV-visible absorption, EIS, Cyclic voltammograms, SEM | 100 to 300 | Plate and spherical | [50] |
| 35. | Hibiscus rosa-sinensis | Malvaceae | China rose | UV–vis spectroscopy, TEM, FTIR | 16–30 | Spherical | [51] |
| 36. | Nepenthes khasiana | Nepenthaceae | Ksete-phare | TEM, SEM, FT-IR, UV-VIS and XRD | 50 nm to 80 | Triangular and spherical | [52] |
| 37. | Hygrophila spinose | Acanthaceae | Lasia spinosa | XRD, SEM-EDAX, DLS, FT-IR and UV-spectroscopy | 50 nm to 80 | Triangular and spherical shape | |
| 38. | Amaranthus spinosus | Amaranthaceae | Spiny pigweed | UV-Vis, TEM, XRD, FT-IR, EDX, | 10.74 | Spherical, few triangular | [53] |
| 39. | Vitis vinifera | Vitaceae | Grape | UV–visible spectra, TEM, XRD, FTIR | 10–17 | Spherical | [54] |
| 40. | Azadirachta indica, Eucalyptus camaldulensis | Meliaceae,Geraniaceae | Neem, murray red gum, | EDS spectra, UV/Vis spectrum, TEM | 1.25 – 17.5 and 2.5 – 27.5 with an average size of 5.5 and 7.5 | – | [55] |
| 41. | Mentha piperita, Melissa officinalis, Salvia officinalis | Lamiaceae | Peppermint, lemon balm, garden sage | UV–Vis absorption, DLS, SEM, TEM | – | – | [56] |
| 42. | Cicer arietinum L. | Fabaceae | Chick pea | UV-Vis spectra, TEM, SEM | 30 to 80 | Spherical, pentagonal and triangle shape | [57] |
| 43. | Tamarindus indica L | Fabaceae | Tamarind | UV visible Spectroscopy, GC/MS, HPLC, SEM, EDX, FTIR | 52 | Spherical sizes | [58] |
| 44. | Erythrina Variegate | Fabaceae | Indian coral tree | UV- Vis,FTIR, XRD, EDX, SEM and TEM | 20–50 | Cubical | [59] |
| 45. | Gymnema sylvestre | Apocynaceae | Gymnema | UV-vis, SEM, EDAX, FTIR, XRD | 72.8 | Spherical | [60] |
Table 2.
Fruit mediated synthesis of gold nanoparticles.
| S. No | Scientific name | Family | Common name | Characterization | Size (nm) | Shape | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Citrus maxima | Rutaceae | Pomelo | TEM, DLS, SEM, XRD, FTIR, UV–vis spectroscopy, | 25.7±10 | Rod and spherical | [61] |
| 2. | Citrus limon, Citrus reticulata andCitrus sinensis | Rutaceae | Sweet orange | UV–visible spectra, TEM, XRD, SAED | 15–80 | Spherical | [62] |
| 3. | Genipa americana | Rubiaceae | Genipapo | ESI-MS, TEM, SAED, FTIR, XRD | 15–40 | Spherical | [63] |
| 4. | Averrhoa bilimbi | Oxalidaceae | Bilimbi | UV-Vis, FTIR, EDX,SEM, SPR | 75 to 150 | Rhomboidal | [64] |
| 5. | Lansium domesticum | Meliaceae | Duku | UV-Vis spectroscopy, FTIR, TEM | 20–40 | Triangular and hexagonal | [65] |
| 6. | Punica granatum | Pomegranate | Punicaceae, | UV, IR, TEM | 70 | – | [66] |
| 7. | Garcinia combogia | Clusiaceae | Brindall Berry | UV–Visible, HRTEM, XRD, FTIR, | 17 | Hexagonal and spherical | [67] |
| 8. | Couroupita guianensis Aubl. | Lecythidaceae | Kanuunankuulapuu | UV–Visible and FTIR, TEM and XRD, DLS and Zeta potential analysis, | 25 ± 6 | Spherical, triangular and hexagonal | [68] |
| 9. | Terminalia arjuna | Combretaceae | Arjuna | UV–visible, FT-IR, XRD, TEM, EDAX, HPLC | Ta1 (60), Ta3 (20), Ta5(14) | Pentagon | [69] |
| 10. | Nitraria schoberi | Zygophyllaceae | – | UV-VIS, FESEM | 30 and 40 | Circle | [70] |
Table 3.
Flower mediated synthesis of gold nanoparticles.
| S. No | Scientific name | Family | Common name | Characterization | Size (nm) | Shape | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Ixora coccinea | Rubiaceae | Jungle geranium | UV- visible spectra, TEM | 5–10 | Spherical | [71] |
| 2. | Cassia auriculata | Caesalpiniaceae | Avaram | UV–vis spectrum, EDAX, HRSEM, HRTEM, FTIR | 12–41 | Spherical | [72] |
| 3. | Tagetes erecta | Asteraceae | Marigold | UV-visible, HRTEM | 30 −50 | Spherical | [73] |
| 4. | Moringa oleifera | Moringaceae | Moringa | UV–vis, TEM, DLS, SEM and EDX | 3–5 | Spherical | [74] |
| 5. | Nyctanthes arbortristis | Oleaceae | Night Jasmine | UV–Vis, TEM, XRD, FTIR, XRD | 19.8 ± 5.0 | Spherical | [75] |
| 6. | Prunus serotina | Rosaceae | Wild black cherry | UV- Vis Spectroscopy, XRD, TEM, SEM, FTIR | 10 to 20 | Spherical | [76] |
| 7. | Couroupita guianensis | Lecythidaceae | Cannon ball tree | UV–Visible, FR-IR, SEM, TEM | 25–45 | Spherical | [77] |
| 8. | Bauhinia purpurea | Leguminosae | Butterfly tree | UV- Vis, XRD, FTIR EDX, SEM and TEM. | 20 – 50 | Cubic | [78] |
| 9. | Plumeria alba Linn | Apocynaceae | Rubra | UV-Visible Spectro photometer, HRTEM, | 20–30 | Spherical | [79] |
| 10. | Gnidia glauca | Thymelaeaceae | Datpadi | UV-vis spectra, XRD,TEM and HRTEM, EDS, FTIR | 5 to 20 | Spherical | [49] |
Table 4.
Root mediated synthesis of gold nanoparticles.
| S. No | Scientific name | Family | Common name | Characterization | Size (nm) | Shape | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Coleus forskohlii | Lamiaceae | Forskolin | UV–Vis spectroscopy, HRTEM, | 5–18 | Nano rods | [80] |
| 2. | Ipomoea carnea | Convolvulaceae | pink morning glory | UV–Vis spectra, SEM, TEM, EDAX, XRD, FT-IR, | 25–100 | Triangular, hexagonal, pentagonal, rod and truncated | [27] |
| 3. | Morinda citrifolia | Rubiaceae | morinda | UV–vis spectroscopy, XRD, FTIR, FE-SEM, EDX and TEM | 12.17–38.26 | Spherical, triangle and Hexagonal | [81] |
| 4. | Panicum maximum | Poaceae | zaina | SEM, AFM, UV–Vis spectroscopy, XRD | 14.28 | Spherical Shape | [82] |
Table 5.
Seed-mediated synthesis of gold nanoparticles.
| S. No | Scientific name | Family | Common name | Characterization | Size (nm) | Shape | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Cajanus cajan | Leguminosae – Pea | Pigeon pea | UV–Vis spectra, FTIR, XRD, TEM, SEM | 9 to 41 | Spherical | [83] |
| 2. | Elettaria cardamomum | Zingiberaceae | Kardemumma | UV-Vis, SPR, XRD. | 432.3 | Spherical | [84] |
| 3. | Cucurbita pepo | Cucurbitaceae | Pumpkin | UV-vis spectroscopy, TEM, DLS | 600–800 | Triangular | [85] |
| 4. | Abelmoschus esculentus | Malvaceae | Lady's fingers | UV–visible spectroscopy, XRD, FTIR, AFM, FESEM and EDX | 45–75 | Spherical and narrow | [86] |
| 5. | Vitis vinifera | Vitaceae | Grape | UV–visible spectra, TEM, XRD, FTIR | 10–17 | Spherical | [54] |
Table 6.
Bark mediated synthesis of gold nanoparticles.
| S. No | Scientific name | Family | Common name | Characterization | Size (nm) | Shape | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Cassia fistula | Fabaceae | Golden rain tre | FTIR, UV−650 spectrophotometer, FTIR, SEM | 55.2–98.4 | – | [87] |
| 2. | Eucommia ulmoides | Eucommiaceae | Hardy rubber tree | UV–Visible spectroscopy, HRTEM, EDX, XRD, RA-FTIR | 16.4 | Spherical | [88] |
| 3. | Acacia nilotica | Fabaceae | Prickly acacia | UV–vis, FT-IR, XRD, TEM, | 10–50 | Quasi-spherical | [89] |
| 4. | Ficus religiosa | Moraceae | Peepal tree | UV-Vis, XRD, TEM and FTIR | 20–30 | Triangle,Pentagon's and hexagons | [90] |
| 5. | Pistia stratiotes L | Araceae | Arum | UV–visible spectroscopy, EDAX, SEM, TEM | 2–40 | Spherical | [26] |
| 6. | Hypericum hookerianum | Guttiferae | St. John's wort | UV-Vis, XRD, FESEM, | S1−10–70, S2−10–50 | Spherical shape | [41] |
3.1. Leaves
Characterization using TEM analysis revealed the presence of predominantly spherical shape nanoparticles of size range 3–45 nm. XRD pattern confirmed the crystalline nature of AuNPs. FTIR spectra revealed the presence of (N–H, N˭H, C–CH3 and OH) functional groups attributed to the bioactive molecules present in BLE. Phytochemicals facilitated the reduction of Au3+ ions to Au° and later capped AuNPs during the particle growth termination process. AuNPs was later tested on the human cancer cell line (HeLa, MCF-7) [16].
Gold nanoparticles were synthesized using Terminalia catappa leaf aqueous extract. An aqueous solution of gold nanoparticles exhibited a strong resonance at 524 nm. FTIR spectrum of the leaf extract reduced gold nanoparticles showed absorption bands at 1420 cm−1, 1715 cm−1, and 3145 cm−1. 1441 cm−1 resonance indicated the facilitation of the binding of O-H group of phenols with the gold nanoparticle surface [10].
Dracocephalum kotschyi leaf extract was prepared at different temperatures. UV–Vis analysis and color change from yellow to pink violet indicated the occurrence of the redox reaction and conversion of Au3+ ions excited state to Au° ground state by plant compounds. Surface Plasmon resonance (SPR) observed at 536 nm in UV–vis spectrophotometer confirmed the synthesis of Au Nanoparticles. FTIR analysis identified the biomolecules in leaf extract which were involved in nanoparticles synthesis. Bands obtained at 1261.17 and 799.92 cm−1 arose from –C–O–H bond and C–C stretching modes respectively in gold nanoparticles. leaf components acted as bio reductants and surfactants. Zeta potential provided the information of nanoparticles stability and their surface charge [20].
Biosynthesis of gold nanoparticles using Olea europaea leaf extract lead to the formation of stabilized nanoparticles. Optimization was performed using the different concentration of leaf extract for the synthesis process. The color change from pink to dark pink and its characterization of (SPR) showed shifting of absorption peak from about 530–545 nm. FTIR identified the potential biomolecules involved in nanoparticles synthesis. The Very strong band observed at 1077 cm−1 was assigned to the C-OH bond of the protein in the extract. Leaf extract indicated the presence of proteins and antioxidant molecules through free amine groups, C˭O and OH group respectively [17].
Green synthesis of spherical gold nanoparticles was performed using Mangifera indica leaf extract. The biomolecule compound identification by FTIR analysis suggested the presence of functional group such as –C˭C, –C˭O, –C–O and –C–O–C– and was assigned to water soluble compounds flavonoids, terpenoids and thiamine. These compounds acted as capping ligands of the nanoparticles. Evidence of crystalline nature of nanoparticles was obtained by the peak of XRD pattern corresponding to Bragg reflections angle. Fcc structure of crystal was determined from the (1 1 1), (2 0 0), (2 2 0), (3 1 1) and (2 2 2) lattice planes [21].
Bioorganic compound present in leaf extract leads to the stabilization of NPs. Cacumen Platycladi extract proved efficient to obtain AuNPs with narrow size distribution range. Use of two-step method, synthesis and purification of nanoparticles proved efficient for the complex system [22].
GCMS analysis is Abutilon indicum leaf extract (AILE) revealed the presence of pharmacologically important compound like flavonoids, various phenolic compounds, amino acids, terpenoids. SPR peak depicted the λ max at 535 nm. Hydrodynamic size distribution of gold nanoparticles estimated using DLS particle analyzer depicted the size range of (1–180) and (1–100) nm in water and media. FTIR analysis indicated the presence of possible bioactive compound absorbed on the nanoparticles surface [23].
Owing to Butea monosperma extract, FTIR analysis revealed O-H stretching mode seems to have the presence of polyphenol in the BM extract aiding the formation of AuNP [24]
The absorption peak of Suaeda monoica extract mediated AuNP was found to be centered at 535 nm. FTIR was used to identify the potential biomolecules playing a role in the synthesis of the gold nanoparticles. The appearance of the new peak at 1728 cm−1 corresponded to stretching and vibrational bending of C˭C compounds such as flavonoids, terpenoids and soluble proteins responsible for bio-reduction and stabilization of gold nanoparticles [25].
Different parts of plant Ipomoea carnea was used for the synthesis of gold nanoparticles. Characteristic change from yellow to pinkish red or violet color indicated the synthesis of the gold nanoparticles. Bragg's planes indicated the synthesis of FCC structured pure cubic nature of Au NP with its (111) facets. FTIR pattern revealed the presence of C–N stretch of amines and its role in the reduction of gold ions as well as coating or capping of nanoparticles [26], [27].
Gold nanoparticles were synthesized by using Geranium leaf extract through Sono catalysis process. FTIR indicates the presence of possible biomolecules that could be responsible for reduction and capping of nanoparticles. The peak shape and the presence of bands at 1708 and 1325 cm−1 are characteristic of the carbonyl stretching [28].
Gold nanoparticles were synthesized from Phoenix dactylifera L. leaf extract. The high and low volume of intensity could be due to increase number of nanoparticles formed as a result of a reduction of Au3+ ions present in the solution. The volume of extract 200–400 µL shifted the SPR band to 544 and 538 nm respectively. FTIR analysis identified the chemical compound in a sample. Leaf extract mediated reduction of gold salt into gold nanoparticles caused the 1632 cm−1 IR absorption band of extract to be split into two peaks at 1651 and 1613 cm−1 corresponding to –OH and -C˭O groups. This indicated the involvement of these particular groups in the synthesis of nanoparticles [29].
Gold nanoparticles were biogenically synthesized using Sesbania grandiflora leaf extract. Visually color change from ruby red to deep violet was observed and UV-Visible analysis and confirmed the synthesis of the nanoparticles by its well-defined peaks characterized by maxima centered at 534 nm. XRD analysis depicted the presence of five well-defined peaks corresponding to FCC crystal structure of metallic gold. FTIR spectrum of AuNPs demonstrated different peak in the range of 1093, 1357, 1658, 2941 and 3438 cm−1. AuNPs synthesized using the leaf extract were capped by abundant flavonoids and polyphenols. Synthesized gold nanoparticles were further applied for catalytic degrading of chemical dye Methylene blue (MB) [30].
Gold nanoparticles synthesized by Salix alba leaf extract using (15:1) Gold-willow solutions showed a uniform and sharp peak at 540 nm. FTIR spectrum of AuNPs stabilized with leaves extract shows a band at 1242 cm−1 which corresponded to -C-O-H groups while the bands at 1296 cm−1 and 1635 cm−1 corresponded to amides of proteins respectively [31].
Magnolia kobus and Diopyros kaki were used for the eco-friendly extracellular synthesis of metallic gold nanoparticles. Nanoparticles exhibited ruby red color at maximum peak observed at 540 nm. The shape of nanoparticles formed at different Concentration and temperature depended on leaf broth. FTIR spectrum of leaf extract showed several bands at 1637 cm−1, 1736 cm−1 and 2916 cm−1 that could be assigned to amide I band of the proteins released by the Magnolia leaves and D. Kaki. The different band indicated the capping of synthesized gold nanoparticles by the functional group of amines, alcohols, aldehydes, ketones, proteins and metabolites [32].
Cacumen Platycladi leaf extract and its simulated solution were prepared based on the compound measurement and Fourier-transform infrared spectroscopy analysis. Characteristics SPR band for AuNPs centered around 535 nm. FTIR spectra of the extract revealed the presence of six significant bands at 3415, 2930, 1060, 639, 1384, 1610, and 3448 cm−1. The peaks were attributed to the protective biomass adhering around AuNPs which contain C˭C–H and C–O–H functional groups [15].
The experiment demonstrated synthesis and stabilization of gold nanoparticles using aqueous leaves extract of Cinnamomum zeylanicum. FTIR spectra typically showed peaks at 1037, 1367, 1455, 1538 and 1743 cm−1 and identified the possible biomolecules responsible for nanoparticles synthesis. The presence of the very intense band at 1743 cm−1 and the moderate band at 1367 cm−1 indicated the possible binding of Au nanoparticles to proteins present in the extract through the amine group [33].
Biosynthesis of gold nanoparticles was conducted by coriander leaf extract. Review of present investigation demonstrated the process of coriander leaf -mediated biosynthesis of gold nanoparticles for the first time. Gold chloride color change to pink ruby red after 12 h was used as a preliminary test for nanoparticles synthesis. UV-Visible spectra demonstrated a sharp peak at 541 nm confirming the synthesis process. FTIR analysis revealing strong bands at 1038, 1387 and 3418 cm−1 was used for plant compound identification. The band obtained at 1038 cm−1 corresponds to C–N stretching vibrations of amine. The weaker band obtained at 1632 cm−1 corresponds to amide I linkage due to carbonyl stretch in proteins [34].
The study reveals the reduction of gold ions into gold nanoparticles by Nerium oleander leaf extract using one-step green synthesis method. SPR peaks were observed at different time intervals. A strong peal observed after 70 min incubation at 560 nm which confirmed the formation of gold nanoparticles. FTIR spectra identified the reducing and stabilizing ability of leaf extract. FTIR absorption bands at 3433, 2923, 1626, 1385, 1054, and 535 cm−1 illustrated all the active groups responsible for gold nanoparticles synthesis [35].
Gold nanoparticles synthesized using Euphorbia hirta L. leaf extract was used to perform an antimicrobial activity. Formation of gold nanoparticles was confirmed by the color change from pale yellow to purplish red. Metallic nanoparticles can be synthesized by reducing metallic ions. Surface Plasmon Resonance absorption band observed at 530 nm indicated the synthesis of the gold nanoparticles. FTIR spectra of the plant extract were compared with spectra of AuNPs to identify absorbed the functional group of a plant on the nanoparticles surface. Raman spectrum analysis detected peaks at 1008, 1130, 1246, 1350, 1418, 1641 cm−1 were found to shift slightly from the reported peak values according to a characteristic feature of gold [36].
An eco-friendly approach for the synthesis of gold nanoparticles aqueous leaf extract of Terminalia arjuna has been demonstrated. Absorption spectra were recorded at multiple wavelength ranges of 350–800 nm keeping leaf extract as the blank solution. The rapid reduction process observed at different time intervals showed absorption peak at 530 nm which corresponded to the (SPR) wavelength of the AuNPs. Hydrolyzable tannins present in T. arjuna leaf extract were responsible for the observed reduction and capping during the synthesis of AuNPs. FTIR analysis identified two new strong bands at 1368 and 1229 cm−1 in the spectra of the synthesized material were assigned to NO2 stretching and C-O stretching respectively indicating the reduction of gold chloride to gold nanoparticles [37].
A facile synthesis of gold nanoparticles using Hibiscus sabdariffa leaf extract was demonstrated. The SPR band of aqueous NP solution revealed peak at 550 nm. Increase dilution rate (100 and 1000 times) produced particles with sharp SPR peak at 535 nm. Different biological molecule present in the sample observed the peak at 800 cm−1 attributed to R-CH groups. Similarly, a minor peak at 871 cm−1 may have occurred due to the leaching of HAuCl4 to Cl− ions [38].
A study on the possible route for the green synthesis of AuNPs using leaf extract of Mimosa pudica against breast cancer cell lines was demonstrated. Characterization by UV–vis spectrum revealed AuNPs absorption peak at 534 nm. The process completed within 30 min and at 55 °C. There was no change in color intensity after 30 min. FTIR peak at 1697 cm−1(C˭O stretch) and 3609 cm−1 (O-H stretch) revealed responsible functional groups for the reduction and stabilization of AuNPs [39].
The optical properties and purity of Argemone mexicana leaf extract mediated synthesized gold nanoparticles were analyzed by UV- Vis absorption peak depicting a strong peak at 545 nm. FTIR spectra analysis identified Phytoconstituents in the Argemone mexicana extract is responsible for synthesizing AuNPs. FTIR spectra depicted strong peaks at 3842 cm−1 corresponding to phosphorous compounds suggesting its role in bio-reduction gold chloride [40].
Gold Nanoparticles synthesized using Ocimum sanctum extracts were characterized by UV–Vis-NIR absorption spectra. The presence of a weak Plasmon band in the NIR region was observed alongside the sharp peak at 520 nm. Well dispersed Spherical nanoparticles ranging from 1 to 20 nm were identified to be reduced from Methyl eugenol, one of the main chemicals identified in hexane extract [41].
The Azadirachta indica L. leaf extract has many biomedical applications till date. Biosynthesis gold nanoparticles characterized UV-vis near infrared transverse plasmon resonance band at 540 nm in high wavelength and second longitudinal plasmon resonance band electromagnetic spectrum at 823 nm. FTIR absorption was observed peak at 2930 cm−1 C–H stretching vibration modes which assigned to the hydrocarbon chains. The phenol compounds are responsible for Au (III) reduction and gold nanoparticles stabilization [42].
Green synthesis of gold nanoparticles using medicinal plant Silybum marianum leaf extract was analyzed for antioxidant and phytochemical properties. Their aqueous and methanolic extracts were performed to analyze the bio- active compounds present in leaf extract. The antioxidant activity of that methanolic extract was analyzed by DPPH assay, Anti-lipid peroxidation assay and Superoxide radical scavenging activity [43].
Bioinspired gold nanoparticles synthesis from leaf extract of Ficus benghalensi, observed SPR in absorption maxima peak at 530 nm. FTIR analysis confirmed the bio stabilization of the gold nanoparticles and was due to the presence of the protein, carbohydrate and peptides. Peaks observed at O-H stretch due to carbohydrates and C-N vibrations due to a presence of protein which responsible for stabilizing the gold nanoparticles [44].
Green synthesis of gold nanoparticles synthesized by Diospyros ferrea leaf extract was tested against the pathogens. UV-vis spectra observed the peak at 541 nm, with the increase in time; the nanoparticles synthesis also increases. FTIR analysis of the extract and colloidal solution indicates the same number of functional groups and compounds [45].
Leaves of Bauhinia tomentosa were used for the synthesis of gold nanoparticles against in-vitro anticancer. UV-Visible Spectra Absorbance of SPR occurred at 563 nm. The color pale yellow immediately changed to ruby red color. This color indicates the reduction of Au3+ ions to Au°. The reason for the reduction of nanoparticles may be due to the compounds present in the extract of the leaves. FTIR peaks were found to be 3342 cm−1 (O-H stretch), 1737 cm−1 (C˭O), 1639 cm−1 (N-H bend), 1436 cm−1 (C-H bend), 1365 cm−1 (C-H rock) and 1218 cm−1 (C-O stretch) spectra has been reported for the synthesis of gold nanoparticles [46].
Bougainvillea glabra leaf extract synthesized gold nanoparticles. The bioreduction of gold nanoparticles was due to the presence of polyphenols present in the leaf extract. The visual identification of the extract was from yellow to ruby red color and the SPR absorption was at 574 nm, which depends on temperature and concentration. With the increase in a concentration of the gold chloride, there was a change in the visualization too. When 2 mM concentration was added, the color changed to violet, even though the SPR bands were observed at 574 nm. FTIR spectra absorbed peak at 3433 cm−1 [47].
Synthesis the gold nanoparticles from Costus igneus leaf extract was used in the reduction of auric chloride. The ethanolic extract of plant leaves synthesized gold nanoparticles which changed its color from yellow to ruby within 15 min and the SPR absorption peak was maximum at 536 nm. The pH played a very important role in controlling the size and shape of these nanoparticles [48].
The synthesis of gold nanoparticles from Aloe perfoliata L plant leaf extract was used in controlling the size and shape of the nanoparticles. In this case, they got nanotriangles with SPR bands observed at 560 nm. FTIR analysis found out that peaks were observed at 1120 cm−1 was assigned to compounds which were responsible for the reduction of the gold nanoparticles [49].
Magnolia kobus leaf extract was used for electrochemical characterization and green synthesis of gold nanoparticles. In this care, 5% of the leaf extract was used as reducing agent for synthesis and peaks were observed at 520 nm [50].
Stabilization and optimization of gold nanoparticles were done using Hibiscus rosa-sinensis leaf extract. The SPR band was observed at 520 nm. Hibiscentin and flavonoids play very important role in stabilizing the nanoparticles. FTIR was used for characterization by observing the metal surface for the interaction with carbonyl group/keto groups. 1611 cm−1 shifted to 1656 cm−1. The medium and sharp peak at 1059 cm−1 shifted to 1083 cm−1 and changed into small blunt peak [51].
Biosynthesis of gold nanoparticles from Nepenthes khasiana leaf extract was used for the antimicrobial assay. Synthesized nanoparticles were characterized using UV-vis spectra, absorption at different time intervals was observed the peak at 599.78 nm. The gradual increase in the intensity absorption indicates the Au3+ slowly reduced to Au°. FTIR spectra showed a strong peak at 3432.0 cm−1 indicating the presence of OH group [52].
Hygrophila spinosa aqueous leaf extract was used for the synthesis of gold nanoparticles which acted as an anticancer and antimicrobial agent. UV-vis spectra analysis showed a decrease in the intensity of a peak at 500 nm. A constant absorbance at 560 nm for an hour was observed when kept for incubation. FTIR spectra absorption peak was found at 3400.0 cm−1 indicates the presence of OH groups which was found to be responsible for interacting with the gold nanoparticles with the plant extract [91].
Biosynthesis of gold nanoparticles using Amaranthus spinosus leaf extract showed a change of color from yellow to ruby red within hours and the SPR peak was absorbed in 565 nm. FTIR analysis showed peaks for hydroxyl (3388 cm−1), aroma- tic and aliphatic amines (1391 & 1018 cm−1 respectively), carbonyl (1685 cm−1), C˭C of benzene (1607 cm−1) and C-H (2842 cm−1) functional groups [53].
In this experiment, the Vitis vinifera leaf extract was reacted to synthesize gold nanoparticles at different concentrations of both extract and gold chloride solution. There was a color change from yellow to ruby red color whose absorbance was measured at 545 nm after an hour of reaction. The IR spectra showed a strong and wide band at 3421 cm−1 which fall for hydrogen donating substituents (hydroxyl groups) which were assumed to be attached to the flavonoids compounds of the aromatic rings in the leaves. These rings reacted and a shift was observed at peak of 3282 cm−1 [54].
The methanol extract of the medicinal plant was used for the synthesis of gold nanoparticles. Screening and their reducing potential activity of two plant leaf extract were considered. The control of neem leaf extract analysis by UV- vis spectra showed absorption peak at 530 and 542 nm. Aqueous chloroauric acid solutions with E. camaldulensis and P. roseum extracts as a function of time showed the peak at 433 nm [55].
Gold nanoparticles preparation and characterization of three aqueous leaf extracts of Mentha piperita, Melissa officinalis, and Salvia officinalis was carried out and the extracts of all three studied plants successfully reduced the Au(III) ions to Au°. Visual absorption of solution color was dark yellow which changed into ruby red and then bluish. SPR absorption band was in the range within 520–580 nm. FTIR analysis for these three plants aqueous leaf extracts bands was associated with the hydroxyl groups of the phenolic compounds, secondary amines, and either nitriles, aliphatic amines [56].
The application of green chemistry via synthesis of gold nanoparticles using Cicer arietinum L. leaf extract showed the formation of gold nanoparticles which showed a color change within 45 min observed at a peak of 550 nm [57].
Biosynthesis gold nanoparticles using Tamarindus indica L. leaf extracts synthesized nanoparticles with pink color and the UV-vis analysis confirmed the wavelength at 576 nm. The functional group identification was determined using FTIR. Biomolecules commonly present in the leaf extract were –OH and –COOH groups and phenolic compounds as bio reducing agents for nanoparticles [58].
Different characterization and biosynthesis of gold nanoparticles from leaf extract showed UV-visible spectra wavelength at 537 nm. Erythrina Variegate leave extract helped in the reduction of the Au+ ions and capping of the reduced Gold nanoparticles. The two strong bands were recorded at 763 and 439 cm−1 The C-H bending peak is due to the reduction of HAuCl4 to Au NPs [59].
Bio-functionalization of gold nanoparticles were synthesized from fresh leaves of G. sylvestre plants. The UV-vis spectra function of time and reaction of SPR band was at 540 nm. The reactive site of the protein(s) is claimed to be involved in the reduction of gold nanoparticles. FTIR spectrum identification of the protein extract exhibited intense and distinct absorption bands at 1720 and 3317 cm−1, the amide bands of the polypeptides and proteins in the aqueous plant extract [60].
3.2. Fruit
Low cost and facile one-step green synthesis of gold nanoparticles were done in Citrus maxima fruit extracts. The extract was characterized by a peak at 535 nm in the UV–vis spectrum. FTIR spectra of gold nanoparticles synthesized and the biomolecules were at a peak of 1658 cm−1 and could be assigned to the vibrational modes of C˭C double bonds of biomolecules. The flavonoids, terpenes and vitamins present in C. maxima fruit are powerful reducing agents in which chloroauric acid was located at 1376 cm−1 was due to the C–N stretching vibrations [61].
Biological synthesis of gold nanoparticles using citrus fruit (Citrus limon, Citrus reticulata and Citrus sinensis) was done. The constant concentration but varying quantity of aqueous medium helped in the formation of gold nanoparticles which was identified by UV-vis spectra. The λmax obtained for the values are fairly sharp, SPR band observed for colloid to be 530 nm and is an indicative of spherical nanoparticles [62].
Photosynthetic plants was also used for the synthesis of gold nanoparticles with Genipa americana L. fruit extract. The visual appearance of extract color pink to ruby red changed and a maximum absorbance of a peak at 590 nm. FTIR spectra showed the different peak and biomolecule compounds, but secondary –OH groups interact with Au3+ via H-bonding and mediate the nanoparticles synthesis [63].
Averrhoa bilimbi Linn fruit extract synthesis of gold nanoparticles is a one step process. UV-vis spectra peak at 534–545 nm for gold nanoparticles. FTIR spectra showed extract peak values 3270, 1651, and 1023 cm−1 corresponding to alcohol, phenols and tertiary amides present in the sample along with ascorbic acid which may react and are responsible for the reduction of gold nanoparticles [64].
The biosynthesis and characterization of gold nanoparticles from Lansium domesticum fruit peel extract. The formation of gold nanoparticles changes the color and absorption peak observed at 538 nm. The fruit peel extract present in the biomolecules indicates the biosynthesis of gold nanoparticles which contains C–N group of amine, C–O group of alcohol and O–H group of carboxylic acid that takes place in the bioreduction process [65].
Ultra-fast synthesis of gold nanoparticles Punica granutum fruit peel extract. The significant of color changed to wine red, was observed within minutes. Gold nanoparticles showed SPR at 530 nm. FTIR spectrum of pomegranate extract peak at 1346 cm−1 to 1384 cm−1 also indicates that pomegranate extract with Gold nanoparticles through its adjacent phenolic hydroxyls formed quinones [66].
Shape tailored synthesis of gold nanoparticles using Garcinia Combogia fruit extract was performed. The SPR band initially occurs at 531 nm within one minute of reaction. The extract and functionalized gold nanoparticles is that the relative intensity of band is at 1631 cm−1 corresponds to C˭O stretching of carboxyl group [67].
Cannonball fruit extract mediated synthesis of gold nanoparticles were analyzed using UV–visible spectra, the synthesized gold nanoparticles displayed an intense SPR peak at 530 nm. FTIR spectroscopy identified the functional group of active bio-compounds present in the extract. The transmittance at 3447 cm−1 and 2928 cm−1 corresponding to O–H stretching vibrations of the phenol group are responsible for the reduction and capping of nanomaterials [68].
The size control nanoparticles synthesis was analyzed from Terminalia arjuna fruit pericarp aqueous extract. The different quantities of extract were added to the chloroauric acid. The yellow color to ruby red color indicated the formation of gold nanoparticles. Absorption of nano colloids with varying quantities, the UV band appeared at 563 nm for T1 colloid and T2 to T5 region peak at 527 nm. FTIR analysis proved the presence of hard ligands (–OH) to soft ligands (–C˭O) and then reduction process created a possible environment for soft metal (Au) to form carbonyl ligands. The molecular capping agent occurred on the surface of gold nanoparticles [69].
The synthesis of gold nanoparticles via green method were carried out from fresh fruits of Nitraria schoberi extract. This extract interacted with gold nanoparticles which was identified by UV-vis spectra peak at 550 nm [70].
3.3. Flower
Chetty flower (Ixora coccinea) extract was used for the synthesis of gold nanoparticles. The UV- visible spectra indicated a strong plasmon resonance peak at 550 nm [71].
Biosynthesis of gold nanoparticles using flowers of Cassia auriculata extract was done. The formation of nanoparticles was obtained in the change of color from light yellowish brown to ruby red color within three minutes. Formed nanoparticles were analysis for SPR absorption with the maximum peak at 527 nm. The reduction of Au3+ ions to Au° in the O-H peak from 3366 cm−1 to 3465 cm−1 is attributed to the involvement of hydroxyl group [72].
Mari gold plant flower extract (Tagetes erecta) for a synthesis of gold nanoparticles is used to an evaluation of antimicrobial activity. The activity of nanoparticles was noticed from in change in color yellow to deep brownish color within 30 min and was analyzed by using UV-vis spectra indicating the wavelength at 550 nm [73].
Moringa oleifera flower extract synthesis was used for stable gold nanoparticles in the biological method. The formation of gold nanoparticles changed pink color and UV–vis spectra showed absorbance at peak λmax of 540 nm. Bioreduction of Au+ ions from plant extract specifically the conversion of 1641–1635 cm−1 is assigned to the C˭O group of biomolecules to C(O)=O group which may be responsible for the reduction of Au+ to Au° [74].
Nyctanthes arbortristis flower extract was used for the biosynthesis of gold nanoparticles. Visual detection of gold nanoparticles showed a change of yellow color to red color which indicates the colloidal gold. Synthesis of nanoparticles using different volume fraction of EFE. Capping behaviour of EFE was identified by FTIR spectrum and showed bands at 3403 cm−1 (OH), 2890 cm−1 (alkane), 1611 cm−1 (C˭C of benzene), 1378 cm−1 (aromatic amines) and 1016 cm−1 (aliphatic amines) [75].
Biological synthesis of gold nanoparticles from fresh rosaceae flower extract helped in the formation of gold nanoparticles which was determined by UV-vis spectroscopy and the extract changed color from pale yellow to ruby red color. The SPR absorption peak was obtained at 545 nm. FTIR spectra indicate some phenolic compounds present on the surface of the nanoparticles, amino group and carboxylic group (–COOH) also interacts with surface of nanoparticles making highly stable [76].
The synthesis of gold nanoparticles using flower extract of Couroupita guianensi was performed. A different technique was used for identification of bio reducing agents of nanoparticles. The extracted color changed to dark purple and SPR absorbance peak at 536 nm was observed within 10 min. The cyclic voltammetry analysis was used for formation and identification of electron donating methoxy and hydroxyl group contained flower extract in a suitable environment [77].
Eco-friendly synthesis of Bauhinia purpurea flower extract was done for gold nanoparticles. The visible absorption of nanoparticles peak was at 537 nm. FTIR spectrum of synthesis nanoparticles recorded strong bands at 763 and 439 cm−1. The C-H bending peak is due to the reduction of HAuCl4 to Au NPs by the flower extract [78].
Environmental benign synthesis of nanoparticles from Plumeria alba Linn flower extract was carried out. The extract with Au+ ions solution changed the brownish color within 30 min. UV-vis spectra absorption peak was formed at 550 nm [79].
Rapid synthesis of gold nanoparticles was analyzed from Gnidia glauca flower extract. The extract color changed from yellow to ruby red color and the absorption of a peak was at 540 nm in the fourth minute. FTIR spectra band at C–H stretches from 3000 to 2850 cm−1 alkanes present almost seems to attach the alkanes in synthesis GGFE to gold nanoparticles [49].
3.4. Root
The green synthesis of gold nanoparticles using Coleus forskohlii root extract was observed to have a different size and shape of the particles by zeta potential negative value of −48.6 ± 0.06 mV for gold nanoparticles. Then stability of particles was detected even after six months at room temperature [80].
The synthesis of gold nanoparticles is eco-friendly method when using Ipomoea carnea root extract, the reduction of gold ion presented in the extract medium completed within six hours. FTIR spectra of root extract had bands at 1427, 1304 and 1044 cm−1. Mostly polysaccharide and protein played a key role for bioreduction of gold nanoparticles [27].
Morinda citrifolia root extract used for the synthesis for gold nanoparticles in stabilization and reducing of biomolecules. The extract with chloroauric acid reacted and the color change to pink ruby red color was observed within 24 h and maximum peak at 540 nm. FTIR band at 1318 cm−1 and 1089 cm−1 was due to C–N stretching vibration of aromatic and aliphatic amines [81].
Synthesis and characterization of gold nanoparticles from Panicum maximum root extract was carried. After reaction, the color solution changed to pinkish red within five minutes and the reduction nanoparticles SPR occurred at 540 nm [82].
3.5. Seed
Functionalized gold nanoparticles were synthesized using seed coat of Cajanus cajan extract. The newly synthesized nanoparticles changed its color from brownish to ruby red within 10 min. SPR reaction peak was found at 535 nm. FTIR identified the active compound of peak shift from higher wavelength (carbonyl group) 1629 cm−1 to lower wavelength 1614 cm−1. The ester groups disappeared which indicated the reduction of Au+ to Au0 [83].
The green synthesis of gold nanoparticles was from Elettaria cardamomum seed pod extract, showed a color change to violet. The synthesis of nanoparticles was possible with different concentrations and pH. The solution ratio (1:1, 10:1, 5:1) was taken, the SPR absorbance peaks, and were observed at 530, 540 and 550 nm. Particle size analysis was used to find relevant articles showing the polydispersity at 0.146 and diameter around 432.3 nm [84]. One pot synthesis of gold nanoparticles using Cucurbita pepo L. seed extract, showed results for absorbance of peak at 500–600 nm by UV-VIS spectra within 30 min [85].
Abelmoschus esculentus seed aqueous extract was used for the synthesis of gold nanoparticles using different characterization. The scale of wavelength absorption of SPR peak was found at 536 nm within 10 min reaction. The identification of the bio-reduction biomolecules found in the extract was done using FTIR analysis, the absorption of peaks (1643 and 1616 cm−1) was assigned to the carbonyl stretch vibration in form of the linkage of proteins from amide I [86].
The extract of grape Vitis vinifera Seed was used for the synthesis of gold nanoparticles. The UV-VIS spectra at 565 nm were due to different concentrations of the extracts. FTIR strong absorption band at 1380 cm−1 corresponds to C–N stretching vibrations of aromatic amines which confirmed the presence of secondary alcohols as capping and reducing agents of gold nanoparticles [54].
3.6. Bark
The phytochemical synthesis of gold nanoparticles was done from Cassia fistula stem bark extract. The characteristic absorbance SPR peak was at 529 nm. The FTIR spectral data was observed at 1248.11 cm−1 and 1113.35 cm−1 which are assigned to the C–N stretching mode and the stretch at 3437 cm−1 is assigned to hydroxyl groups which help in the synthetic preparation of gold nanoparticles [87].
The biosynthesis of gold nanoparticles helped in its formation with the extract of Eucommia ulmoides (E. ulmoides) bark. The color of the gold chloride extract solution changed from pink to red. The absorption peak was observed at 534 nm from UV-vis spectra at the different concentration, temperature and pH. The constituents of phytochemicals were identified using FTIR, the absorption of various functional groups like O-H stretching of phenol and alcohol, the group of N-H, O-H, C˭O, the phytol compounds of amino acids and proteins are said to be involved in the process of nanoparticles synthesis [88].
Green synthesis of size-controlled gold nanoparticles by using Acacia nilotica twig bark extract. The UV-VIS spectra are an important technique used in the identification for its stability, size and shape of the nanoparticles. The color changes from pale yellow to violet. The different concentrations gave SPR bands in the range from 545 to 600 nm. As for the FTIR analysis, the intensity of the peak shifts from 1219 to 1236 cm−1 indicating that polyols are involved in the synthesis and the presence of C-N stretching after bioreduction shifts from 1018 to 986 cm−1 which may be due to the primary amines present during synthesis of nanoparticles [89].
The Ficus religiosa bark extract was used for the biosynthesis of gold nanoparticles. The UV-Vis spectra showed absorbance maxima at 540 nm. The FTIR spectra indicated that the peak was observed at 1745 cm−1 shows the presence of carboxylic acid in the extract of Ficus religiosa and there was a shift towards lower energy side, which clearly reflects the capping of Au nanoparticles [90].
Eco-friendly synthesis of gold nanoparticles was done using the weed pistia (Pistia stratiotes) extract. The purple-red colored solution was identified by different peaks using UV-VIS spectra, the sharp peak observed in the range of 530–570 nm and a broad peak was observed in the range of 650–800 nm. FTIR spectra weaker signals found in 1550–1350 cm−1 region can be assigned to the aromatic nitro compounds, the primary and secondary amines present in the polypeptide proteins is said to play an important role in the bioreduction and capping of gold nanoparticles [26].
The shoot culture of Hypericum hookerianum was used for the synthesis of gold nanoparticles. The different temperature was used for Au colloidal synthesis, the characterization was done using UV-vis spectra absorption peak at 540 nm and was due to the property of surface plasmon resonance (SPR) [41].
4. Conclusion
In this review, different parts of plants were used in the synthesis or the reduction of metallic nanoparticles like gold, also exploiting the various bio-compounds involved in this reaction. Plant synthesized nanoparticles are being mostly used in various applications of biomedical, agricultural, biosensor and engineering, drug delivery, tumor destruction, anticancer, antibacterial, antifungal activities and so on. These gold nanoparticles show good biocompatibility and degradability. Even though the green synthesis has many drawbacks like controlling the size and shape of these nanoparticles and formed which is not a bigger issue for other conventional methods of synthesis, this one step method of green synthesis still needs few improvements which should be further looked into. For the review, it was noticed that more researcher have to be for used on kinetics and mechanistic approaches for controlling the morphology and also the large scale production of these nanoparticles.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.06.004.
Appendix A. Transparency document
Supplementary material
References
- 1.D J.S., G S.K., Bansal Pooja. Biogenesis of nanoparticles: a review. Afr. J. Biotechnol. 2014;13:2778–2785. [Google Scholar]
- 2.Vadlapudi V., Kaladhar D.S.V.G.K. Review: green synthesis of silver and gold nanoparticles. Middle-East J. Sci. Res. 2014;19:834–842. [Google Scholar]
- 3.Sane N., Hungund B., Ayachit N. Biosynthesis and characterization of gold nanoparticles using plant extracts. Int. Conf. Adv. Nanomater. Emerg. Eng. Technol. 2013;12082:295–299. [Google Scholar]
- 4.Bhattacharya R., Mukherjee P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008;60:1289–1306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 5.M. Shah, D. Fawcett, S. Sharma, S.K. Tripathy, G.E.J. Poinern, Green synthesis of metallic nanoparticles via biological entities, 2015. http://dx.doi.org/10.3390/ma8115377. [DOI] [PMC free article] [PubMed]
- 6.Rai M., Yadav A. Plants as potential synthesiser of precious metal nanoparticles: progress and prospects. IET Nanobiotechnol. 2013;7:117–124. doi: 10.1049/iet-nbt.2012.0031. [DOI] [PubMed] [Google Scholar]
- 7.Gardea-Torresdey J.L., Parsons J.G., Gomez E., Peralta-Videa J., Troiani H.E., Santiago P., Yacaman M.J. Formation and growth of Au nanoparticles inside live Alfalfa plants. Nano Lett. 2002;2:397–401. [Google Scholar]
- 8.Haverkamp R.G., Marshall A.T., Van Agterveld D. Pick your carats: nanoparticles of gold-silver-copper alloy produced in vivo. J. Nanopart. Res. 2007;9:697–700. [Google Scholar]
- 9.Harris A.T., Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanopart. Res. 2008;10:691–695. [Google Scholar]
- 10.Ankamwar B. Biosynthesis of gold nanoparticles (Green-gold) using leaf extract of Terminalia catappa. E-J. Chem. 2010;7:1334–1339. [Google Scholar]
- 11.O. V Salata, J. Nanobiotechnol., 6, 2004, pp. 1–6. doi:10.1186/1477-3155-2-12.
- 12.Sýkora D., Kašička V., Mikšík I., Řezanka P., Záruba K., Matějka P., Král V. Application of gold nanoparticles in separation sciences. J. Sep. Sci. 2010;33:372–387. doi: 10.1002/jssc.200900677. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed S. Synthesis of gold nanoparticles using plant extract: an overview. iMedPub J. 2015;1:1–6. [Google Scholar]
- 14.Meyers M.A., Mishra A., Benson D.J. Mechanical properties of nanocrystalline materials. Progress. Mater. Sci. 2006;51:427–556. [Google Scholar]
- 15.Zhan G., Huang J., Lin L., Lin W., Emmanuel K., Li Q. Synthesis of gold nanoparticles by Cacumen Platycladi leaf extract and its simulated solution: toward the plant-mediated biosynthetic mechanism. J. Nanopart. Res. 2011;13:4957–4968. [Google Scholar]
- 16.Babu P.J., Sharma P., Saranya S., Bora U. Synthesis of gold nanoparticles using ethonolic leaf extract of Bacopa monnieri and UV irradiation. Mater. Lett. 2013;93:431–434. [Google Scholar]
- 17.Khalil M.M.H., Ismail E.H., El-Magdoub F. Biosynthesis of Au nanoparticles using olive leaf extract. 1st Nano Updates. Arab. J. Chem. 2012;5:431–437. [Google Scholar]
- 18.Mishra A., Tripathy S.K., Il Yun S. Fungus mediated synthesis of gold nanoparticles and their conjugation with genomic DNA isolated from Escherichia coli and Staphylococcus aureus. Process Biochem. 2012;47:701–711. [Google Scholar]
- 19.Jiang J., Oberdörster G., Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticles dispersions for toxicological studies. J. Nanopart. Res. 2009;11:77–89. [Google Scholar]
- 20.Dorosti N., Jamshidi F. Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: synthesis, characterization, and evaluation of anticancer and antibacterial activity. J. Econ. Financ. Adm. Sci. 2016:1–11. [Google Scholar]
- 21.Philip D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2010;77:807–810. doi: 10.1016/j.saa.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Wu W., Huang J., Wu L., Sun D., Lin L., Zhou Y., Wang H., Li Q. Two-step size- and shape-separation of biosynthesized gold nanoparticles. Sep. Purif. Technol. 2013;106:117–122. [Google Scholar]
- 23.Mata R., Nakkala J.R., Sadras S.R. Polyphenol stabilized colloidal gold nanoparticles from Abutilon indicum leaf extract induce apoptosis in HT-29 colon cancer cells. Colloids Surf. B: Biointerfaces. 2016;143:499–510. doi: 10.1016/j.colsurfb.2016.03.069. [DOI] [PubMed] [Google Scholar]
- 24.Patra S., Mukherjee S., Barui A.K., Ganguly A., Sreedhar B., Patra C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C. 2015;53:298–309. doi: 10.1016/j.msec.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 25.Rajathi F. Arockiya Aarthi, Arumugam R., Saravanan S., Anantharaman P. Phytofabrication of gold nanoparticles assisted by leaves of Suaeda monoica and its free radical scavenging property. J. Photochem. Photobiol. B: Biol. 2014;135:75–80. doi: 10.1016/j.jphotobiol.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Anuradha J., Abbasi T., Abbasi S.A. An eco-friendly method of synthesizing gold nanoparticles using an otherwise worthless weed pistia (Pistia stratiotes L.) J. Adv. Res. 2015;6:711–720. doi: 10.1016/j.jare.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbasi T., Anuradha J., Ganaie S.U., Abbasi S.A. Gainful utilization of the highly intransigent weed ipomoea in the synthesis of gold nanoparticles. J. King Saud. Univ. - Sci. 2015;27:15–22. [Google Scholar]
- 28.Franco-Romano M., Gil M.L.A., Palacios-Santander J.M., Delgado-Jaén J.J., Naranjo-Rodríguez I., De Cisneros J.L. Hidalgo-Hidalgo, Cubillana-Aguilera L.M. Sonosynthesis of gold nanoparticles from a geranium leaf extract. Ultrason. Sonochem. 2014;21:1570–1577. doi: 10.1016/j.ultsonch.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Zayed M.F., Eisa W.H. Phoenix dactylifera L. leaf extract phytosynthesized gold nanoparticles; Controlled synthesis and catalytic activity. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2014;121:238–244. doi: 10.1016/j.saa.2013.10.092. [DOI] [PubMed] [Google Scholar]
- 30.Das J., Velusamy P. Catalytic reduction of methylene blue using biogenic gold nanoparticles from Sesbania grandiflora L. J. Taiwan Inst. Chem. Eng. 2014;45:2280–2285. [Google Scholar]
- 31.Islam N.U., Jalil K., Shahid M., Rauf A., Muhammad N., Khan A., Shah M.R., Khan M.A. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab. J. Chem. 2015 [Google Scholar]
- 32.Song J.Y., Jang H.K., Kim B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009;44:1133–1138. [Google Scholar]
- 33.Smitha S.L., Philip D., Gopchandran K.G. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2009;74:735–739. doi: 10.1016/j.saa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan K.B., Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater. Lett. 2008;62:4588–4590. [Google Scholar]
- 35.Tahir K., Nazir S., Li B., Khan A.U., Khan Z.U.H., Gong P.Y., Khan S.U., Ahmad A. Nerium oleander leaves extract mediated synthesis of gold nanoparticles and its antioxidant activity. Mater. Lett. 2015;156:198–201. [Google Scholar]
- 36.Annamalai A., Christina V.L.P., Sudha D., Kalpana M., Lakshmi P.T.V. Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf. B: Biointerfaces. 2013;108:60–65. doi: 10.1016/j.colsurfb.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Gopinath K., Venkatesh K.S., Ilangovan R., Sankaranarayanan K., Arumugam A. Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Ind. Crops Prod. 2013;50:737–742. [Google Scholar]
- 38.Mishra P., Ray S., Sinha S., Das B., Khan M.I., Behera S.K., Il Yun S., Tripathy S.K., Mishra A. Facile bio-synthesis of gold nanoparticles by using extract of Hibiscus sabdariffa and evaluation of its cytotoxicity against U87 glioblastoma cells under hyperglycemic condition. Biochem. Eng. J. 2016;105:264–272. [Google Scholar]
- 39.Suganya U.S.U., Govindaraju K., Kumar G.G., Prabhu D., Arulvasu C., Dhas S.S., Karthick V., Changmai N. Anti-proliferative effect of biogenic gold nanoparticles against breast cancer cell lines (MDA-MB-231 & MCF-7) Appl. Surf. Sci. 2016;371:415–424. [Google Scholar]
- 40.S. Varun, S. Sellappa, M. Rafiqkhan, S. Vijayakumar, Research Article Green Synthesis of Gold Nanoparticles Using, 32, 2015, pp. 42–44.
- 41.Manoj L., Vishwakarma V. Green Synthesis and spectroscopic characterisations of gold nanoparticles using invitro grown hypericin rich shoot cultures of Hypericum hookerianum. Int. J. ChemTech Res. 2015;8:194–199. [Google Scholar]
- 42.Bindhani B.K., Panigrahi A.K. Green synthesis of gold nanoparticles using Neem (Azadirachta indica L.) leaf extract and its biomedical applications. Int. J. Adv. Biotechnol. Res. 2014;5:457–464. [Google Scholar]
- 43.D. Kosalai, M. Chandran, Phytochemical analysis and anti-oxidant activity of gold nanoparticles synthesizing plant - Silybum marianum, 5, 2016, pp. 469–475.
- 44.Francis G., Thombre R., Parekh F., Leksminarayan P. Bioinspired synthesis of gold nanoparticles using Ficus benghalensis (Indian Banyan) leaf extract. Chem. Sci. Trans. 2014;3:470–474. [Google Scholar]
- 45.Gayathiri K., Prabhavathi A., Tamilarasi R., Vimalavathini R., Kavimani S. Role of neprilysin in various diseases. Int. J. Pharmacol. Res. 2014;4:91–94. [Google Scholar]
- 46.Mukundan D., Mohankumar R., Vasanthakumari R. Green synthesis of silver nanoparticles using leaves extract of Bauhinia tomentosa linn and its invitro anticancer potential. Mater. Today.: Proc. 2015;2:4309–4316. [Google Scholar]
- 47.A.L. Rose, S. Vidhya, S. Ramagirija, M. Ramya, A.R. Mary, M.S. Reka, M.R. Ansilda, Kinetic study on green synthesis of gold nanoparticles using Bougainvillea Glabra leaf extract, Department of Chemistry, Holy Cross College, Tiruchirappalli 620002, India Abstract, 3, 2014, pp. 1–10.
- 48.Sen I.K., Maity K., Islam S.S. Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity. Carbohydr. Polym. 2013;91:518–528. doi: 10.1016/j.carbpol.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh S., Patil S., Ahire M., Kitture R., Gurav D.D., Jabgunde A.M., Kale S., Pardesi K., Shinde V., Bellare J., Dhavale D.D., Chopade B.A. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J. Nanobiotechnol. 2012;10:17. doi: 10.1186/1477-3155-10-17. (doi:10.1186/1477-3155−10−17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y., Wu T.Y., Chen S.M., Ali M.A., AlHemaid F.M.A. Green synthesis and electrochemical characterizations of gold nanoparticles using leaf extract of Magnolia kobus. Int. J. Electrochem. Sci. 2012;7:12742–12751. [Google Scholar]
- 51.Yasmin A., Ramesh K., Rajeshkumar S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg. 2014;1:12. doi: 10.1186/s40580-014-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhau B.S., Ghosh S., Puri S., Borah B., Sarmah D.K., Khan R. Green synthesis of gold nanoparticles from the leaf extract of Nepenthes khasiana and antimicrobial assay. Adv. Mater. Lett. 2015;6:55–58. [Google Scholar]
- 53.Das R.K., Gogoi N., Babu P.J., Sharma P., Mahanta C., Bora U. The synthesis of gold nanoparticles using Amaranthus spinosus leaf extract and study of their optical properties. Adv. Mater. Phys. Chem. 2012;2:275–281. [Google Scholar]
- 54.Ismail E.H., Khalil M.M.H., Al Seif F.A., El-magdoub F., Bent A.N., Rahman A., Al U.S.D. Biosynthesis of gold nanoparticles using extract of grape (Vitis vinifera) leaves and seeds. Progress. Nanotechnol. Nanomater. 2014;3:1–12. [Google Scholar]
- 55.Ramezani N., Ehsanfar Z., Shamsa F., Amin G. Screening of medicinal plant methanol extracts for the synthesis of gold nanoparticles by their reducing potential. Z. Nat. B. 2008;63:903. [Google Scholar]
- 56.Dzimitrowicz A., Jamróz P., diCenzo G.C., Sergiel I., Kozlecki T., Pohl P. Preparation and characterization of gold nanoparticles prepared with aqueous extracts of Lamiaceae plants and the effect of follow-up treatment with atmospheric pressure glow microdischarge. Arab. J. Chem. 2016 [Google Scholar]
- 57.A. Singh, M.M. Sharma, A. Batra, Synthesis of gold nanoparticles using chick pea leaf extract using green chemistry, 5, 2013, pp. 27–32.
- 58.Kaur M., Bhullar G.K. Partial characterization of Tamarind (Tamarindus indica L.) kernel starch oxidized at different levels of sodium hypochlorite. Int. J. Food Prop. 2016;19:605–617. [Google Scholar]
- 59.N. Bopana, S. Saxena, Asparagus racemosus — ethnopharmacological evaluation and conservation needs, 110, 2007, pp. 1–15. http://dx.doi.org/10.1016/j.jep.2007.01.001. [DOI] [PubMed]
- 60.Arunachalam K.D., Arun L.B., Annamalai S.K., Aarrthy M. Innovare academic sciences biofunctionalized gold nanoparticles synthesis from Gymnema sylvestre and its preliminary anticancer activity. Int. J. Pharm. Pharm. Sci. 2014;6:423–430. [Google Scholar]
- 61.Yu J., Xu D., Guan H.N., Wang C., Huang L.K., Chi D.F. Facile one-step green synthesis of gold nanoparticles using Citrus maxima aqueous extracts and its catalytic activity. Mater. Lett. 2016;166:110–112. [Google Scholar]
- 62.Sujitha M.V., Kannan S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2013;102:15–23. doi: 10.1016/j.saa.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 63.Kumar B., Smita K., Cumbal L., Camacho J., Hernández-Gallegos E., De Guadalupe Chávez-López M., Grijalva M., Andrade K. One pot phytosynthesis of gold nanoparticles using Genipa americana fruit extract and its biological applications. Mater. Sci. Eng. C. 2016;62:725–731. doi: 10.1016/j.msec.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 64.R.S.R. Isaac, G. Sakthivel, C. Murthy, Green synthesis of gold and silver nanoparticles using Averrhoa bilimbi fruit extract, 2013, 2013.
- 65.Shankar S., Jaiswal L., Aparna R.S.L., V Prasad R.G.S. Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Lett. 2014;137:75–78. [Google Scholar]
- 66.Ganeshkumar M., Sathishkumar M., Ponrasu T., Dinesh M.G., Suguna L. Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surf. B: Biointerfaces. 2013;106:208–216. doi: 10.1016/j.colsurfb.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 67.Rajan A., Meenakumari M., Philip D. Shape tailored green synthesis and catalytic properties of gold nanocrystals. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2014;118:793–799. doi: 10.1016/j.saa.2013.09.086. [DOI] [PubMed] [Google Scholar]
- 68.Sathishkumar G., Jha P.K., Vignesh V., Rajkuberan C., Jeyaraj M., Selvakumar M., Jha R., Sivaramakrishnan S. Cannonball fruit (Couroupita guianensis, Aubl.) extract mediated synthesis of gold nanoparticles and evaluation of its antioxidant activity. J. Mol. Liq. 2016;215:229–236. [Google Scholar]
- 69.Mohan Kumar K., Mandal B.K., Kiran Kumar H.A., Maddinedi S.B. Green synthesis of size controllable gold nanoparticles. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2013;116:539–545. doi: 10.1016/j.saa.2013.07.077. [DOI] [PubMed] [Google Scholar]
- 70.Rad M.S., Sharifi J., Heshmati G.A., Miri A., Jyoti Sen D. Biological Synthesis of gold and silver nanoparticles by Nitraria schoberi fruits. Am. J. Adv. Drug Deliv. 2013;1:174–179. 〈http://www.ajadd.co.uk/PA-500141-[8].pdf〉 [Google Scholar]
- 71.Nagaraj B., Malakar B., Divya T.K., Krishnamurthy N.B., Liny P., Dinesh R. Environmental benign synthesis of gold nanoparticles from the flower extracts of Plumeria alba linn. (Frangipani) and evaluation of their biological activities. Int. J. Drug Dev. Res. 2012;4:144–150. [Google Scholar]
- 72.Venkatachalam M., Govindaraju K., Mohamed Sadiq A., Tamilselvan S., Ganesh Kumar V., Singaravelu G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2013;116:331–338. doi: 10.1016/j.saa.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 73.Krishnamoorthy G., Shabi M.M., Ravindhran D., Uthrapathy S., Rajamanickam V.G., Dubey G.P. Nardostachys jatamansi: cardioprotective and hypolipidemic herb. J. Pharm. Res. 2009;2:574–578. [Google Scholar]
- 74.Anand K., Gengan R.M., Phulukdaree A., Chuturgoon A. Agroforestry waste Moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti-cancer and catalytic activity. J. Ind. Eng. Chem. 2015;21:1105–1111. [Google Scholar]
- 75.Das R.K., Gogoi N., Bora U. Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract. Bioprocess Biosyst. Eng. 2011;34:615–619. doi: 10.1007/s00449-010-0510-y. [DOI] [PubMed] [Google Scholar]
- 76.M. Reza, R. Kahkha, H.R. Kahkha, Green synthesize of gold nanoparticles using rosacea flower extract, 9, 2015, pp. 1722–1726.
- 77.I. Journal, A. Scientific, Plant mediated synthesis of gold nanoparticles, 4, 2014, pp. 891–900.
- 78.R. Radha, M. Murugalakshmi, S. Kokila, Eco friendly synthesis and characterization of Gold Nanoparticles from Bauhinia purpurea flower extract, 2016, pp. 306–310.
- 79.J. Rabadia, U. Hirani, D. Kardani, A. Kaneria, J. Rabadia, Cardioprotective effect of methanolic extract of syzygium aromaticum on isoproterenol induced myocardial infarction in rat, 2, 2014, pp. 2–7.
- 80.Naraginti S., Kumari P.L., Das R.K., Sivakumar A., Patil S.H., Andhalkar V.V. Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C. 2016;62:293–300. doi: 10.1016/j.msec.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 81.Suman T.Y., Radhika Rajasree S.R., Ramkumar R., Rajthilak C., Perumal P. The Green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim. Acta – Part A: Mol. Biomol. Spectrosc. 2014;118:11–16. doi: 10.1016/j.saa.2013.08.066. [DOI] [PubMed] [Google Scholar]
- 82.Agarwal K., Srivastava M.M. Chemistry synthesis and characterization of gold nanoparticles embedded with extract of the plant Panicum maximum with enhanced antioxidant behavior. Ijsr -Int. J. Sci. Res. 2014;63:2–4. [Google Scholar]
- 83.Ashokkumar T., Prabhu D., Geetha R., Govindaraju K., Manikandan R., Arulvasu C., Singaravelu G. Apoptosis in liver cancer (HepG2) cells induced by functionalized gold nanoparticles. Colloids Surf. B: Biointerfaces. 2014;123:549–556. doi: 10.1016/j.colsurfb.2014.09.051. [DOI] [PubMed] [Google Scholar]
- 84.Pattanayak M., Muralikrishnan T., Nayak P.L. Green synthesis of gold nanoparticles using Elettaria cardamomum (ELAICHI) aqueous extract. World J. Nano Sci. Technol. 2013;2:52–58. [Google Scholar]
- 85.Gonnelli C., Cacioppo F., Giordano C., Capozzoli L., Salvatici C., Salvatici M.C., Colzi I., Del Bubba M., Ancillotti C., Ristori S. Cucurbita pepo L. extracts as a versatile hydrotropic source for the synthesis of gold nanoparticles with different shapes. Green. Chem. Lett. Rev. 2015;8:39–47. [Google Scholar]
- 86.Jayaseelan C., Ramkumar R., Rahuman A.A., Perumal P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind. Crops Prod. 2013;45:423–429. [Google Scholar]
- 87.Daisy P., Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomed. 2012;7:1189–1202. doi: 10.2147/IJN.S26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo M., Li W., Yang F., Liu H. Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2015;142:73–79. doi: 10.1016/j.saa.2015.01.109. [DOI] [PubMed] [Google Scholar]
- 89.Emmanuel R., Karuppiah C., Chen S.M., Palanisamy S., Padmavathy S., Prakash P. Green synthesis of gold nanoparticles for trace level detection of a hazardous pollutant (nitrobenzene) causing Methemoglobinaemia. J. Hazard. Mater. 2014;279:117–124. doi: 10.1016/j.jhazmat.2014.06.066. [DOI] [PubMed] [Google Scholar]
- 90.Wani K., Choudhari A., Chikate R., Ghanekar R.K. Synthesis and characterization of gold nanoparticles using Ficus religiosa extract. Carbon – Sci. Technol. 2013;5(1):203–210. [Google Scholar]
- 91.Koperuncholan M. Bioreduction of chloroauric acid (HAuCl4) for the synthesis of gold nanoparticles (GNPs): a special empathies of pharmacological activity. Int. J. Phytopharm. 2015;5(4):72–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material