Abstract
Metabolic profile of urine from piglets administered with single boluses contaminated with mycotoxin mixture (deoxynivalenol, aflatoxin B1, fumonisin B1, zearalenone, and ochratoxin A) were studied by 1H NMR spectroscopy and chemometrics (PCA, PLS-DA, and OPLS-DA). The mycotoxin levels were close to the established maximum and guidance levels for animal feed (2003/100/EC and 2006/576/EC). Urine samples were obtained from four groups of four piglets before (control, C) or within 24 h (treated, T) after receiving a contaminated boluses with increasing doses of mycotoxins (boluses 1–4). For the two highest dose groups, the urines were collected also after one week of wash out (W). For the two lowest doses groups no significant differences between the C and T samples were observed. By contrast, for the two highest doses groups the T urines separated from the controls for a higher relative content of creatinine, p-cresol glucuronide and phenyl acetyl glycine and lower concentration of betaine and TMAO. Interestingly, a similar profile was found for both W and T urines suggesting, at least for the highest doses used, serious alteration after a single bolus of mycotoxin mixture.
Keywords: Mycotoxin, Urine, 1H NMR spectroscopy, Chemometrics, Metabolomics, Biomarker
Highlights
-
•
A single dose of mycotoxins mixture (DON, AFB1, FB1, ZEN, and OTA ) could produce serious impairments of gut microbiota of piglets.
-
•
The explorative metabolomic analysis on urine piglets, fed with boluses contaminated with mycotoxins mixtures, was performed.
-
•
NMR-based MVA resulted in a higher urinary concentrations of creatinine, p-cresol glucuronide and phenyl acetyl glycine and lower concentrations of betaine and TMAO after treatment with respect to own controls.
1. Introduction
Mycotoxins can be frequently found in the food chain because of fungal infection of crops to be either consumed directly by Humans or used as livestock feed. Consumption of contaminated foodstuffs can produce teratogenic, carcinogenic, neurotoxic, estrogenic, and immunosuppressive effects (both acute and chronic) in Humans and animals [1], [2]. It is worth noting that the most frequently contaminated food crops are cereals and dried fruits. The production and accumulation of mycotoxins can take place at different levels in the food chain (pre-harvest, harvest and storage) and inadequate agricultural, harvesting, storage, packaging and transport practices could increase their content. The EU and several countries worldwide have in force specific regulations to restrain Human and animal exposure to the principal mycotoxins but also to avoid an impact on commerce worldwide.
The toxicokinetic of some mycotoxins such as ochratoxin A (OTA) and aflatoxin B1 (AFB1) could result in accumulation in different organs or tissues and excretion in milk. The mycotoxins can therefore enter the food chain also through meat, milk, or eggs obtained from livestock fed with contaminated feed, with a significant health risk for consumers. Recently, Streit and coworkers analyzed for contamination with several mycotoxins 17316 samples of feed and feed raw materials from all over the world during an 8-year period, reporting that overall, 72% of the samples tested positive for at least one mycotoxin and 38% were found to be co-contaminated [3].
The importance of the mycotoxin contamination of feed for farm animals has been recognized by the European Food Safety Authority [4], [5], [6], [7], [8]. Based on these EFSA opinions the Commission of the European Communities (CEC) established a maximum level for AFB1 (20 µg/kg) in animal feed (Commission Directive 2003/100/EC) and the so-called guidance levels for deoxynivalenol (DON, 900 µg/kg), zearalenone (ZEN, 100–250 µg/kg) [9], OTA (50 µg/kg) and fumonisin B1+B2 (FB1+FB2, 5000 µg/kg) (Commission Recommendation 2006/576/EC). The purpose of these guidance levels is to protect farm animals from possible deleterious effects of contaminated feed and to ensure awareness of all involved economic parties and supervising authorities if the critical concentrations are exceeded [1]. A large number of fungal genera, belonging to various toxigenic fungi such as Apergillus, Penicillium, Alternaria and Fusarium spp. are able to produce several mycotoxins as secondary metabolites. The most relevant mycotoxins found in food and feed are produced by three fungal genera: aflatoxins, produced by Aspergillus species; OTA by both Aspergillus and Penicillium; trichothecenes (e.g. DON), ZEN and FB1 produced mainly by Fusarium species [1]. Moreover, also specific environmental conditions, such as temperature, water activity, oxygen level, physical damage, insects, amount of fungal inoculum, are important factors that affect fungal growth and mycotoxin formation and accumulation both in the field and during storage of food crops [10]. DON, AFB1, FB1, ZEN and OTA are the main toxicologically relevant mycotoxins frequently occurring in cereals and cereal-based feed. These mycotoxins and their metabolites have been recently quantified in vivo in piglet urines in dose dependent manner [11].
In the present work the metabolic profile of 39 urine samples has been studied by proton nuclear magnetic resonance (1H NMR) spectroscopy and multivariate analysis (MVA). These samples were already analyzed for their mycotoxin content [11]. This approach aims to assess potential differences in the overall metabolic response of piglets fed with single boluses contaminated, in most cases, within the guidance values for mycotoxins in feed. Metabolomic is a well-established methodology, based on 1H NMR spectroscopy or HPLC-MS techniques assisted with multivariate statistical analysis [12], which provides an effective method for evaluating the metabolic responses of living organisms to physiological and pathological stresses. Due to its efficient and non-invasive characteristics, NMR spectroscopy, used in combination with multivariate data analyses, has been indicated as an effective analytical tool that can provide comprehensive information on the metabolic profiles in different fields. These include, among the others, drug toxicity evaluations [13], [14], nutritional studies [15], [16] and mammalian–parasite interactions [17], [18].
The pig is an important model of human disease and nutrition. Moreover, urine is potentially the most useful metabolic diagnostic matrix in the pig to examine the relationship between metabolites and a specific pathophysiological status [19], [20]. However, only few studies on animal or human biological system response to mycotoxin supplementation have been reported [21], [22], [23]. To the best of our knowledge, this work is the first in vivo study on an animal model, the pig, to a mycotoxin mixture administration considering the EC guidelines concentration limits.
2. Materials and methods
The urine samples of piglets analyzed in this study were previously obtained with an in vivo experiment aimed to validate urinary biomarkers of DON, AFB1, FB1, ZEN and OTA [11]. The design of the in vivo experiment used in the study [11] is reported below whereas the preparation of contaminated boluses and mycotoxin analysis of commercial diet are reported elsewhere [11].
2.1. Design of the in vivo experiments with piglets
Sixteen 4-week-old weaned piglets (Pietrain/Duroc/Large-white) and weighing 10.56±1.88 kg at the beginning of the experiment were procured locally. Animals were acclimatized for 1 week in the animal facility of the INRA ToxAlim Unit (Toulouse, France) prior to being used in experimental protocols. During the acclimation and experimental periods, animals were given free access to water. Except during the urine collection periods, animals were fed a commercial diet ad libitum. Four groups of piglets (four piglets per group) were administered boluses contaminated with mixtures of DON, AFB1, FB1, ZEN and OTA at different levels (Bolus 1–4, Table 1). The feed was removed the evening before the experiment thus the animal had no access to feed overnight. Depending of the animal, the bolus was eaten within 1–2 h. After consumption of the bolus each piglet was housed in a metabolic cage to collect 24 h urine. Urine samples from each piglet were collected 3 times at regular intervals within 24 h (sample T) and their volumes were measured then the urines were pooled. Control urine samples (sample C) were collected from the same piglet the day before giving the contaminated bolus. Wash out urines were collected for 24 h for two groups (Bolus 3 and 4) after one week from the administration of contaminated bolus (sample W). Urine samples were frozen and stored at −20 °C until the NMR spectroscopy analysis. More details on the preparation of contaminated boluses, administration of boluses to piglets and urine collection are reported elsewhere [11].
Table 1.
Mycotoxin intakes in four groups of piglets that received a bolus containing a mixture of deoxynivalenol (DON), aflatoxin B1 (AFB1), fumonisin B1 (FB1), zearalenone (ZEN) and ochratoxin A (OTA). This table was already published in Gambacorta et al. [11].
| Mycotoxin |
Bolus 1 (n=4)a |
Bolus 2 (n=4)b |
Bolus 3 (n=4)c |
Bolus 4 (n=4)d |
||||
|---|---|---|---|---|---|---|---|---|
| μg/kg bw | μg/animal | μg/kg bw | μg/animal | μg/kg bw | μg/animal | μg/kg bw | μg/animal | |
| DON | 7.16 | 63.61 | 20.44 | 191.12 | 24.14 | 315.05 | 57.38 | 630.03 |
| AFB1 | 0.16 | 1.40 | 0.45 | 4.20 | 0.54 | 7.03 | 1.28 | 14.01 |
| FB1 | 3.71 | 32.96 | 10.60 | 99.14 | 63.19 | 824.60 | 150.19 | 1649.12 |
| ZEN | 0.68 | 6.05 | 1.94 | 18.15 | 2.38 | 31.08 | 5.66 | 62.15 |
| OTA | 0.16 | 1.45 | 0.46 | 4.35 | 0.56 | 7.25 | 1.32 | 14.50 |
Mean body weight (bw) of 4 piglets ± standard deviation (SD): 8.88±1.26 kg;
Mean bw of 4 piglets ± SD: 9.35±0.47 kg;
Mean bw of 4 piglets ± SD: 13.05±0.58 kg;
Mean bw of 4 piglets ± SD: 10.98±1.14 kg
2.2. NMR sample preparation
Thirty-nine samples of urine from sixteen piglets before and after administration of boluses contaminated with a mixture of 5 mycotoxins were obtained as described above and analyzed by 1H NMR spectroscopy. Frozen samples were thawed at room temperature and shaken before use. Aliquots of each urine sample (630 μL) were added to 70 μL of potassium phosphate buffer (1.5 M K2HPO4 in D2O, pH 7.4), to minimize variations in metabolite NMR chemical shifts arising from differences in urinary pH, also containing 0.1% sodium 3-(trimethylsilyl)-[2,2,3,3-d4]propionate (TSP) and 2 mM sodium azide. Samples were centrifuged at 14,000g for 5 min at 4 °C to remove any solid debris. 600 μL of the supernatant were placed in a 5 mm outer diameter NMR tube.
2.3. NMR measurement
All measurements were performed on a Bruker Avance III 600 Ascend NMR spectrometer (Bruker, Karlsruhe, Germany) operating at 600.13 MHz for 1H observation, equipped with a z axis gradient coil and automatic tuning-matching (ATM). A time delay of 5 min was set between sample injection and preacquisition calibrations to ensure complete temperature equilibration (300 K). For each sample a one-dimensional NOESY experiment (referred to as 1D 1H-NOESY), including solvent signal saturation during relaxation and mixing time and a spoil gradient, was acquired using 64 free induction decays (FIDs) a spectral width of 12,019 Hz, an acquisition time of 2.7 s, a relaxation delay of 4 s, and a mixing time of 10 ms. The FIDs were multiplied by an exponential weighting function corresponding to a line broadening of 0.3 Hz before Fourier transformation phasing, and baseline correction. All spectra were referenced to the TSP signal (δ = 0.00 ppm). NMR data were processed using TopSpin 2.1 (Bruker). The metabolites were assigned on the basis of 2D NMR spectra analysis (2D 1H Jres, 1H COSY, 1H–13C HSQC and HMBC) and comparison with published data [14], [19], [24], [25].
2.4. Data analysis
NMR spectra were processed using Topspin 2.1 and visually inspected using Amix 3.9.13 (Bruker, Biospin, Italy). 1H NMR spectra were segmented in rectangular buckets of fixed 0.04 ppm width and integrated. The spectral region between 6.1 and 4.5 ppm was discarded to avoid the effects of variable water suppression and to prevent cross-relaxation effects to the urea signal via proton exchange between urea and water. According to published procedures [26], the remaining 204 buckets in the range 10.00–0.30 ppm were then normalized to total area to minimize small differences due to total metabolites concentration and/or acquisition conditions among samples and subsequently mean‐centered. The resulting data set was made of the 1H NMR spectra bucket values (columns) measured for the above urine samples (rows). The description of statistical analyses refers to Pareto scaling data. The data table generated with all the spectra was submitted to multivariate data analysis.
2.5. Multivariate statistical analyses
1D 1H-NOESY spectra were analyzed by multivariate statistical procedures. Specifically, an exploratory data analysis was performed using Principal component analysis (PCA), while Partial Least Squares Discriminant Analysis (PLS‐DA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS‐DA) methods were used to maximize the separation between samples. Multivariate statistical analysis and graphics were obtained using Simca‐P version 14 (Umetrics, Sweden). PCA is a way of identifying patterns in data, expressing them in order to highlight their similarities and/or differences and was used to get an overview of the multivariate profiles. The PCA works by decomposing the X‐matrix (buckets linked with the NMR signals) as the product of two smaller matrices (loading and score matrices) and was used to get an overview of the multivariate data. Values falling outside the Hotelling T2 95% confidence limit in score plots are considered outliers [27]. The PLS-DA is the regression extension of PCA, which gives the maximum covariance between the measured data (the X‐matrix) and the response variable (Y variable, class membership). The confidence level for membership probability was considered to be 95% (observations at <5% were considered outliers) [28]. In this work, the PLS-DA method was also performed in order to justify the number of t latent variables used in OPLS-DA model. The OPLS-DA analysis is a modification of the usual PLS‐DA method which filters out variation that is not directly related to the response and produces models of clearer interpretation, as shown in several recent studies of metabolomics [29], [30]. So, the further improvements made by the OPLS‐DA resides in the ability to separate the portion of the variance useful for predictive purposes from the not predictive variance (which is made orthogonal) overcoming the problems of multicollinearity and autocorrelation of the variables. The quality of the models was described by R2, Q2 values and p[CV-ANOVA]. The R2 value is a cross validation parameter defined as the proportion of variance in the data explained by the models and indicates goodness of the fit. The Q2 parameter is defined as the proportion of variance in the data predictable by the model and indicates predictability. The models were validated using internal cross-validation default method (7-fold) and further evaluated with permutation test (400 permutations) of SIMCA 14 software, Umetrics, Umea, Sweden [28], [31]. For each PLS-DA and OPLS-DA model built, a combination of the loading scores, variable influence on projection (VIP) parameters and p(corr) were examined to identify which metabolites most contributed to the data clustering. Loading scores describe the correlations between the original variables and the new component variables. VIP parameters are essentially a measure of the extent to which a particular variable explains the Y variance (class membership) and p(corr) represents the loadings scaled as a correlation coefficient (ranging from −1.0 to 1.0) between the model and original data [32].
3. Results
Urine metabolic profile of piglet fed with boluses contaminated with mixtures of DON, AFB1, FB1, ZEN and OTA at four increasing doses were studied by 1H NMR spectroscopy and MVA. The values of mycotoxin intake in the four groups of piglets used in this study are reported in Table 1. These concentrations were chosen considering the maximum allowed and guidance levels reported in the Directive 2003/100/EC (EC, 2003) and the Recommendation 2006/576/EC (EC, 2006), respectively.
The piglets used in this work consumed about 350–400 g feed/day, therefore the values of mycotoxin intake were below the limits for all tested doses of OTA and FB1. About AFB1, DON, and ZEN, the levels were below the limits for three out of the four doses and about twice the limits for the highest dose. The urine samples were collected from the same piglets the day before administration (controls, C), within the 24 h post dose (treated, T) and after 1 week of wash out (wash out, W). All urines were analyzed by 1H NMR spectroscopy and MVA.
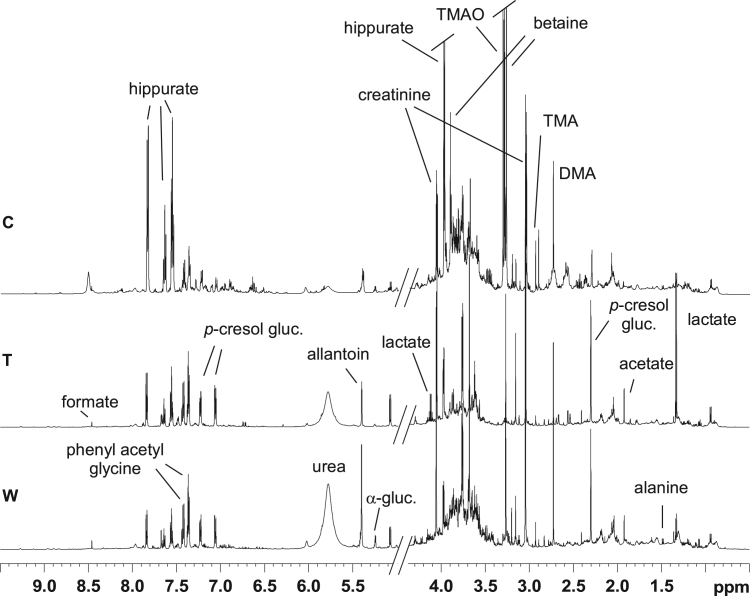
Average one-dimensional (1D) 1H NMR spectra from C, T, and W urine samples of piglets fed with Bolus 3 (characterized by the highest considered contamination values within the allowed limits) are reported in Fig. 1. Urine metabolites were identified in the 1H NMR spectra and assigned on the basis of 2D NMR spectra analysis (2D 1H Jres, 1H COSY, 1H 13C HSQC and HMBC) and by comparison with published data [14], [19], [25]. Relevant 1H NMR data are reported in Table 2. Metabolic profiles of urine characterized by 1H NMR spectroscopy were therefore studied by multivariate analyses (PCA, PLS-DA, OPLS-DA). Multivariate analysis was performed on bucket reduced 1H NMR spectra (see Materials and Methods). The original dataset (204 buckets from the spectral region 10.00–0.30 ppm) was rearranged in a new multivariate coordinate space in which the dimensions are ordered by decreasing explained variance of the considered data. The principal components were displayed as a set of scores (t) and a set of loadings (p), which highlight clustering or outliers (score plot) and influence of input variables on t (loading plot), respectively. The first PCA model was built with the C and T urine samples (Fig. 2). In this model the first two components explained the 63% of total variance (PC1 = 36%, PC2 = 27%), with a Q2 of 0.33. No relevant trends and some overlap among samples were observed in the PCA model applied on C and T urine samples of all groups, except for a marked separation of urine samples of piglets from Bolus 1 and 2 with respect to Bolus 3 and 4 before the treatments.
Fig. 1.
1D 1H NMR average spectra from C, T, and W urine samples of piglets fed with Bolus 3 (characterized by the highest considered contamination values within the allowed limits). The most representative metabolites are indicated.
Table 2.
1H peak assignments for identified metabolites.
| Compound | δH |
|---|---|
| acetate | 1.92 (s, CH3) |
| alanine | 1.49, (d, βCH3), 3.80 (q, αCH) |
| β-alanine | 3.19 (t, N-CH2), 2.57 (t, CH2COOH) |
| allantoin | 5.40 (s, C4H) |
| betaine | 3.26 (s, N-(CH3)3), 3.90 (s, CH2) |
| choline | 3.20 (s, N-(CH3)3) |
| trans-cinnamic acid | 6.50 (d, =CH), 6.67 (d, =CH), 6.70 (d, =CH), 7.33 (d, =CH), 7.49 (d, =CH) |
| creatinine | 3.04 (s, CH2-N), 4.06 (s, CH2-N) |
| p-cresol glucuronide | 7.23 (da, C3H and C5H), 7.06 (d, C2H and C6H) |
| 2.30 (s, CH3), 5.07, 3.86, 3.61 | |
| dimethylamine (DMA) | 2.72 (s, N-(CH3)2) |
| dimethylglycine | 2.93 (s, N(CH3)2), 3.73 (s, CH2) |
| formate | 8.46 (s, HCOOH) |
| fructose | 4.12, 3.89 |
| fumarate | 6.64 (s, CH=CH) |
| α-glucose | 5.25 (d, C1H), 3.53 (dd, C2H), 3.71 (dd, C3H), 3.42 (dd, C4H), 3.84 (m, C5H), 3.78 (m, C6H) |
| β-glucose | 4.65 (d, C1H), 3.89 (dd, C6H), 3.73 (dd, C5H), 3.49 (t, C3H), 3.25 (dd, C2H) |
| glycine | 3.57 (s, CH2) |
| hippurate | 7.84 (d, C2H and C6H), 7.64 (t, C4H), 7.56 (t, C3H and C5H), 3.97 (d, CH2) |
| lactate | 1.33 (d, CH3) |
| malonate | 3.15 (s, CH3) |
| methylamine (MA) | 2.62 (s, N-(CH3)) |
| 3-methylhistidine | 7.00 (s, C4H), 7.67 (s, C2H) |
| methylmalonate | 1.26 (d, CH3), 3.76 (m, CH) |
| phenyl acetyl glycine | 7.43 (m, C3H and C5H), 7.36 (m, C2H and C6H), 3.75 (d, CH2), 3.68 (s, CH2) |
| succinate | 2.41 (s, CH2) |
| taurine | 3.28 (t, N-CH2), 3.45 (s, S-CH2) |
| trigonelline | 9.10 (s, C1H), 8.82 (m, C3H and C5H), 8.07 (m, C4H), 4.43 (s, CH3) |
| trimethylamine (TMA) | 2.90 (s, N-(CH3)3) |
| trimethylamine-N-oxide (TMAO) | 3.30 (s, N-(CH3)3) |
| urea | 5.78 (broad signal, NH2) |
Letters in parentheses indicate the peak multiplicities; s, singlet; d, doublet; t, triplet; dd, doublet of doublet; m, multiplet.
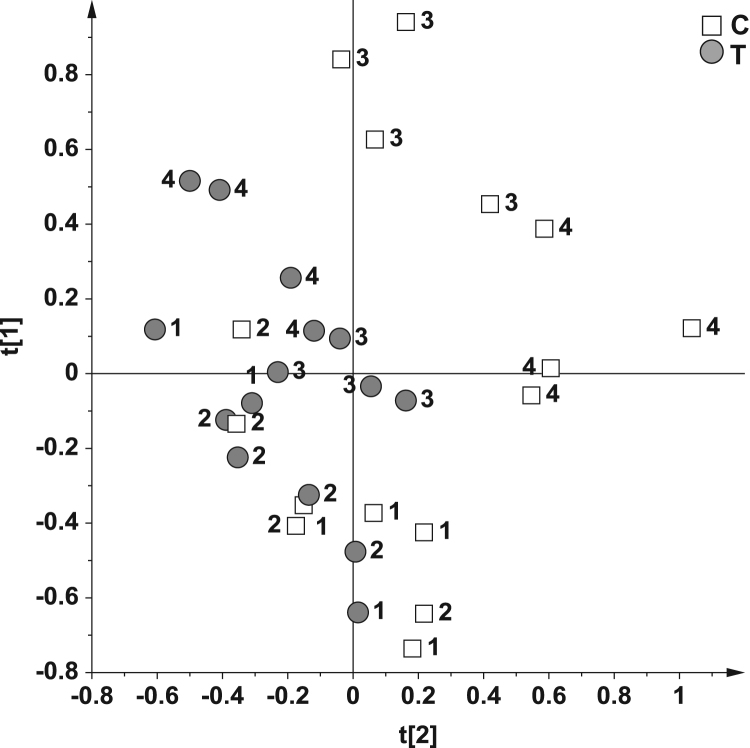
Fig. 2.
PCA score plot on 32 samples using as responsible variable (Y) the two classes control (C, 16 samples) and treated (T, 16 samples). The numbers (1–4) indicate the Bolus group.
In order to improve the separation among samples based on maximizing covariance between the measured data (X) and the response variable (Y), PLS-DA and OPLS-DA models were applied. By these methods the identity of each group of samples is specified in the model such that the maximum variance of the groups can be attained in the hyperspace. Two performance indicators were used to assess the supervised model complexity and eventual overfit degree: the cross validation (CV) and the response permutation test (n=400). The OPLS-DA (1 predictive + 2 orthogonal components, R2Y = 0.71, Q2 = 0.47, p[CV-ANOVA] = 0.009) applied on all C and T samples (before/after treatment as responsible variable, Y) gave a model where the C samples moderately separated from T along the predictive component t[1] (Fig. 3).
Fig. 3.
A: OPLS-DA score plot on 32 samples using as responsible variable (Y) the two classes control (C, 16 samples) and treated (T, 16 samples). The numbers (1–4) indicate the Bolus group. B: p(corr)/VIP score plot. The numbers indicate the NMR spectra reduced buckets (ppm).
Interestingly, along the orthogonal component to [1] the Bolus 3 and 4 well separated from Bolus 1 and 2, with the presence of a single outlier (Control sample from Bolus 2 piglets group). This clustering could be attributed to the original intrinsic differences of 1, 2 and 3, 4 piglets groups obtained from different suppliers. It is worth noting that the overall intra-class variability of the samples (dispersion along to [1] axis) was reduced after the mycotoxin treatment. Moreover, the Bolus 3 and 4 T samples were more separated than the Bolus 1 and 2 T samples from the corresponding controls. The variables responsible for the class separation observed in Fig. 3A could be determined by analysis of the p(corr)/VIP score plot (Fig. 3B) (see Experimental Section). Such variables indicate the chemical shift of the buckets responsible for the observed class separation in the bucket reduced urine spectra. Therefore, the metabolite differences between T and C could be assessed. The highest level of TMAO (bucket at 3.3 ppm), betaine (buckets at 3.26 and 3.9 ppm), TMA (2.90 ppm), DMA (2.74) and MA (2.58) resulted for C samples; whereas the T samples showed the highest content of p-cresol glucuronide (buckets at 2.30, 7.06, 7.22 ppm) and phenyl acetyl glycine (buckets at 7.42 and 7.38 ppm).
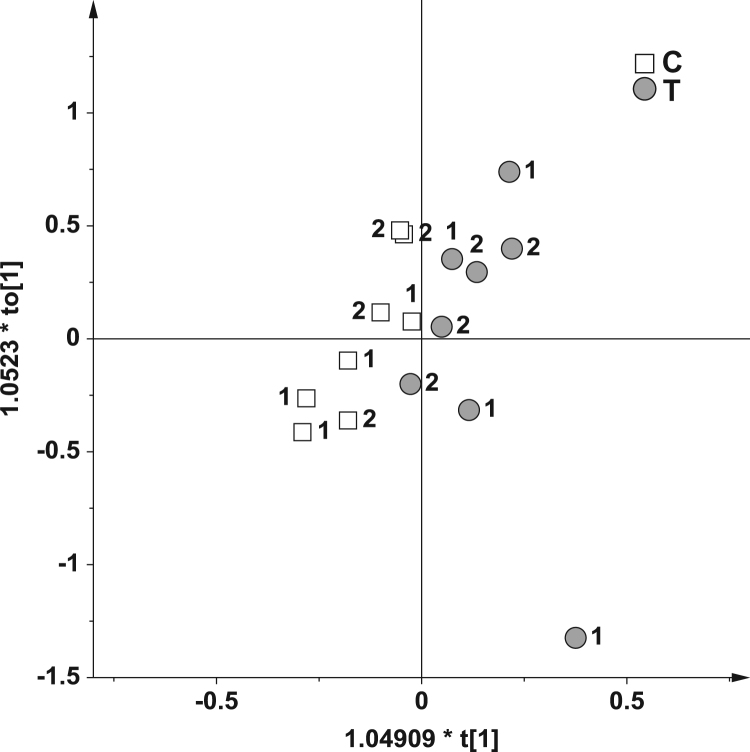
When the T samples, originated from the lower (Bolus 1 and 2) and higher doses (Bolus 3 and 4), were analyzed in comparison to the related controls (C), two models with a very different statistical significance were obtained. For Bolus 1 and 2 groups compared to the controls, unsupervised method (PCA) gave unclear results and the OPLS-DA model (Fig. 4) produced a very weak descriptive and predictive model (1 predictive and 1 orthogonal components, R2 = 0.64 and Q2 = 0.01).
Fig. 4.
OPLS-DA score plot on 16 samples from the Bolus 1 and 2 groups using as responsible variable (Y) the two classes: control (C, 8 samples) and treated (T, 8 samples).
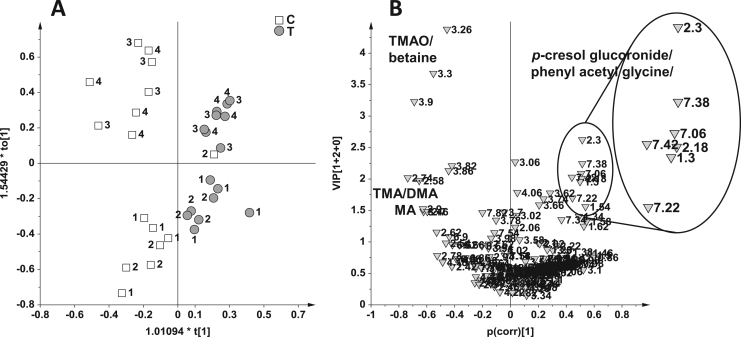
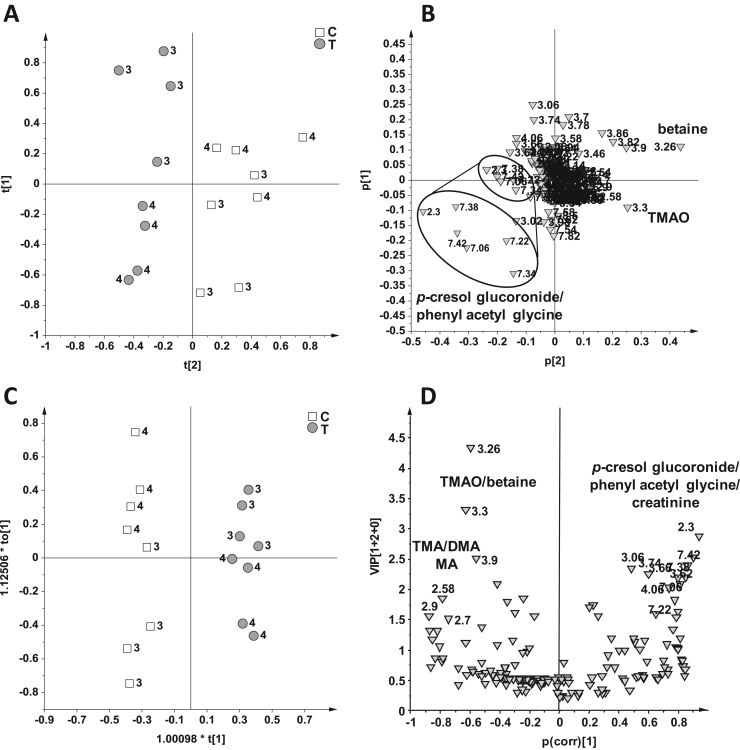
On the other hand, for the Bolus 3 and 4, a good separation was already observed with PCA method without any a priori assumptions for the sample classification (Fig. 5). In this PCA model 2 PCs explained the 70% of variance (PC1 = 45%, PC2 = 25%), with a Q2 of 0.55. In the t[2]/t[1] PCA score plot a good separation of the T from the C samples along the t[2] component was observed (Fig. 5A). By analysis of the related p[2]/p[1] loading plot (Fig. 5B) the T samples showed higher content of phenyl acetyl glycine and p-cresol glucuronide and lower level of TMAO and betaine with respect to the controls. The data were further analyzed by using OPLS-DA to maximize the separation between the classes and to further validate the model. The OPLS-DA (Fig. 5C) on the Bolus 3 and 4 produced a good descriptive and predictive model (1 predictive + 2 orthogonal components, R2Y = 0.98 and Q2 = 0.85, p[CV-ANOVA] = 0.003). By analysis of VIP/p(corr) score plot (Fig. 5D) the T samples exhibited, as well as a higher content of p-cresol glucuronide and phenyl acetyl glycine seen in the PCA model, also a higher concentration of creatinine (buckets at 3.06 and 4.06 ppm) with respect to the control. The latter showed again the highest level of TMAO, betaine, TMA, DMA and MA.
Fig. 5.
PCA score plot (A) and relative loadings plot (B), OPLS-DA (C) and relative VIP/p(corr) (D) score plot on 16 samples from the Bolus 3 and 4 using as responsible variable (Y) the two classes: control (C, 8 samples) and treated (T, 8 samples).
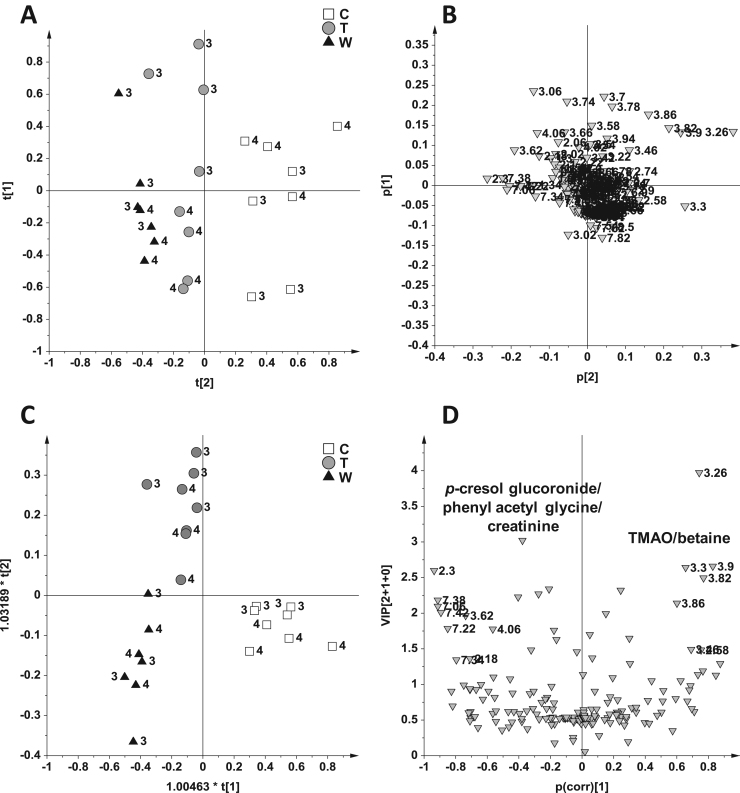
In order to investigate the prolonged effects of a single dose of high contaminated bolus, the urine samples were collected also after one week of wash out only for the animals fed with the highest doses (Bolus 3 and 4 groups). The PCA and OPLS-DA score and loadings plots on the Bolus 3 and 4 considering also the urine samples collected after one week of wash out (W) are reported in Fig. 6. In the t[2]/t[1] PCA score plot (Fig. 6A) (3 PCs, R2X = 0.70, Q2 = 0.44) the T and W urine samples separated from the control along t[2] axis (Fig. 6A). Moreover, the W samples resulted more distant from the controls. The OPLS-DA (supported by a preliminary meaningful PLS-DA analysis with 3 PLS, R2Y = 0.85 and Q2 = 0.75, p[CV-ANOVA] = 7.9*10-7) (Fig. S1), applied to maximize these differences, gave a good model (R2Y = 0.85 and Q2 = 0.69, p[CV-ANOVA] = 1.08*10-5). The OPLS-DA gave a good performance (described by 2 predictive + 1 orthogonal components, R2Y = 0.85 and Q2 = 0.69, p[CV-ANOVA] = 1.08*10-5) and it has been used as a descriptive model rather than for classification purposes, for the presence of more than two classes (control, C, treated, T, wash out, W) [29]. Interestingly, the urine samples collected after one week of wash out, when piglet had free access to diet free of mycotoxins, exhibited a marked difference from the controls along the predictive component t[1] and from treated samples along the orthogonal component t[2] (Fig. 6C). The metabolites responsible for these differences were again creatinine, p-cresol glucuronide and phenyl acetyl glycine.
Fig. 6.
PCA score plot (A) and relative loadings plot (B), OPLS-DA score plot (C) and relative loadings plot (D) on 23 samples from the Bolus 3 and 4 using as responsible variable (Y) the three classes: control (C, 8 samples), treated (T, 8 samples), wash out (W, 7 samples).
4. Discussion
In the present work the urine metabolome of piglets fed with contaminated boluses containing a mixture of mycotoxins (DON, AFB1, FB1, ZEN, and OTA) has been investigated and compared with own controls by 1H NMR spectroscopy and chemometrics (PCA, PLS-DA and OPLS-DA). To the best of our knowledge, few in vivo studies have been focused on the metabolic response to mycotoxin ingestion [21], [22], [23]. Moreover, this is the first report on explorative metabolomic analysis of piglet urine after simultaneous oral administration of several mycotoxins at different concentrations and below the allowed guideline limits for most of the tested doses. Indeed, mycotoxigenic fungi are able to produce more than one mycotoxin simultaneously and food and feed crops can be contaminated by different fungi species at the same time. Thus, Humans and animals are generally exposed to several toxins at the same time as recently confirmed for Humans resident in three different countries [33], [34], [35]. The toxicity of mycotoxins combinations cannot always be easily predicted based on their individual toxicities [36]. Interactions between concomitantly occurring mycotoxins can be antagonistic, additive or synergistic [37]. On the other hand, most of the studies concerning the toxicological effect of mycotoxins have been carried out taking into account only one mycotoxin. Therefore the information on the in vivo combined toxic effects of mycotoxin is scarce and the health risk derived from co-exposure of mycotoxins needs further studies [38].
We decided to conduct this study on piglet urines, previously collected during a mycotoxin biomarker validation study [11] for several reasons. Pigs are potentially exposed to high levels of mycotoxins due to their maize-rich diet and they are quite susceptible to mycotoxin toxicity [39], [40], [41]. On the other hand, typical lab animals, especially mice, are very resistant to most mycotoxins [41], [42]. Finally, the many biological similarities of pigs and Humans, especially for the intestinal tract, make the pig a good model for this type of study.
Urine samples used in this work were previously collected from four groups of piglets (four animals per group) administered with boluses contaminated at four increasing mycotoxin levels (one level per group) [11]. The urine samples were collected from each piglet before and within the 24 h post dose administration. In order to investigate the effects of a single ingestion of mycotoxins on young animals, the urine samples were collected also after one week of wash out for the two higher doses groups (Bolus 3 and 4). At the lower dose groups (Bolus 1 and 2) no significant differences between the control and treated urine samples were detected. However, for the higher doses groups (Bolus 3 and 4) the treated samples were markedly separated from the controls due to highest content of creatinine, p-cresol glucuronide and phenyl acetyl glycine.
Creatinine is the metabolic end-product of creatine catabolism and the high concentration in 24 h post dose urines of piglets is a marker of kidney malfunction. On the other hand low creatinine concentration in urines could be an indicator of alterations in protein turnover and of metabolism in general [43]. The p-cresol glucuronide is a soluble glucuronide derivative of p-cresol. This is a uremic toxin which inhibits the proliferation of endothelial cells, prevents the repair of wounded endothelium [44], and hampers the response of endothelial cells to inflammatory cytokines [45], [46]. The increased levels of urinary p-cresol glucuronide and phenyl acetyl glycine could be the result of the disturbance of gut microbiota by single exposure to the five mycotoxins mixture. Indeed, the aromatic amino acids, phenylalanine and tyrosine, are firstly converted into phenyl acetate and p-cresol through the action of the gut microbiota [47]. Then phenyl acetate and the p-cresol conjugate with glycine and glucuronide, respectively, to form phenyl acetyl glycine and p-cresol glucuronide in the liver and the gut mucosa [48], [49], [50]. Previous studies reported that elevated levels of urinary phenyl acetyl glycine are exhibited in rat abnormally accumulating phospholipids in liver and that these levels can be used as alternative biomarker suggesting changes in gut microbiota [51]. The development and structure of the intestinal epithelium, the digestive and absorptive capabilities of the intestine, and the host immune system are considerably influenced by gut microbiota [52]. Possible disturbances of gut microbiota by mycotoxin administration can therefore affect the health status [53], [54], [55]. The two higher doses groups showed lower urinary concentration of betaine and TMAO with respect to control samples. Betaine is a well-known osmoregulatory biomolecule [56] and, therefore, its reduction in 24 h post dose urines could increase creatinine clearance. It has been reported that biomolecules with an electrophilic methyl group, e.g. as found in betaine, may represent an important control system for in vivo redox balance [57] by reducing oxidative stress in the liver. As oxidative stress has been reported to be inversely related to creatinine clearance, the higher urine creatinine excretion in treated animals could be consistent with the betaine reduction [58]. Interestingly, the three reactive methyl groups of betaine have a key role as a methyl donor in many biochemical pathways and take part in the methionine cycle [57]. A scarce dietary consumption of methyl groups rich molecules results into hypomethylation, which may correlate to several illnesses, such as coronary, cerebral, hepatic and vascular diseases [42]. Other physiological functions of betaine relate to a protective role against protein denaturation (as a ‘chemical chaperone’ [59]) and a cell volume preserving under osmotic stress [42]. Due to its physiological significance, the betaine decrease, after single contaminated bolus administration, must be considered extremely important. It has already been reported that trimethylammonium compounds such as choline and carnitine, which are ingested in the normal diet, are decomposed to TMA by several strains of gut bacteria, and further oxidized to TMAO in the liver and excreted with urine [60]. Therefore, the lower content of urinary concentration of TMAO after mycotoxin ingestion may be likely related to mycotoxin induced disruption of gut bacterial strains that degrade dietary choline to TMA in the intestinal tract. It has been reported a decline of urinary TMAO in rat treated with realgan [61]. TMAO reduction was related to realgar induced disruption of choline pathway degradation through antimicrobial activity to intestinal bacteria [61]. Our results suggest that the mixture of mycotoxins may have produced a similar anti microbial activity to intestinal bacteria with consequent disruption of choline degradation pathway. A decrease of DMA and TMAO in the urinary excretion when the gut microflora was reduced has been reported. Differently from our observation, such a decrease occurred with a concomitant increase of betaine [62].
Although our study did not address the gut microbial metabolism or activity, it has been reported that many different gut microbial species are impacted by xenobiotic toxicants, including AFB1 that induced compositional changes in gut microbial communities of male F344 rats [55]. DON and FB1 have also been demonstrated to impact the intestinal pig microbiota [53], [54]. Thus microbiological identification of specific changes in the microbiota community can be helpful in addressing the metabolic implications of mycotoxin ingestion.
The aim of this study was also to investigate the effects of a single bolus contaminated with a mixture of five mycotoxins on a young organism such as piglets after one week from the exposure. For this purpose the urine samples were collected also after one week of wash out when the piglets had free access to a diet free of mycotoxins. Interestingly, both in PCA and PLS-DA models the post wash out urine samples showed a metabolic profile similar to the treated samples exhibiting the highest level of p-cresol glucuronide, phenyl acetyl glycine and creatinine. The results obtained in this study suggest that a single dose of mycotoxin mixture, at least for the higher used doses, could produce serious alteration on urine metabolome of piglets for at least one week after exposure.
5. Conclusions
Urine metabolic profile of piglets, fed with boluses contaminated with mixtures of DON, AFB1, FB1, ZEN, and OTA at concentrations close to the maximum permitted levels in the EU showed higher urinary concentrations of creatinine, p-cresol glucuronide and phenyl acetyl glycine and lower concentrations of betaine and TMAO with respect to own controls. This suggest that the mycotoxin mixture impairs the urinary metabolome which is probably due to the alteration of the gut microbial ecosystem. Urines collected after a week of wash out showed a metabolic profile similar to that of urine collected in the 24 h post dose, exhibiting the highest level of p-cresol glucuronide, phenyl acetyl glycine and creatinine. These results also suggest that a single dose of mycotoxins could produce, serious impairments of gut microbiota of piglets. To the best of our knowledge, this work is the first in vivo metabolomic study on the response of animal, in particular pig, to a mycotoxin mixture supplementation close to the EC guidelines limits. Finally, it should be considered that in this work an explorative metabolomic analysis has been reported and a list of potential molecules which are statistically significant has been investigated. As well known, the metabolites differences observed in a metabolomic study may be statistically but not necessarily biochemically significant [63]. Some hypotheses related to the possible origin of the observed metabolic changes have been considered and are reported in this work. Nevertheless, further studies are required in order to assess the observed differences as specific biomarkers related to mycotoxins induced alterations (possibly on the basis of identified specific biochemical pathways disruption).
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the grant project, the PON (Programma Operativo Nazionale) 254/Ricerca. Potenziamento del “Centro Ricerche per la Salute dell’Uomo e dell’Ambiente” Code PONa3_00334, by MIUR (The Ministry of Education, Universities and Research).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.05.004.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.05.004.
Contributor Information
Michele Solfrizzo, Email: michele.solfrizzo@ispa.cnr.it.
Francesco Paolo Fanizzi, Email: fp.fanizzi@unisalento.it.
Appendix A. Transparency document
Supplementary material
.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Marin S., Ramos A.J., Cano-Sancho G., Sanchis V. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013;60:218–237. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Streit E., Naehrer K., Rodrigues I., Schatzmayr G. Mycotoxin occurrence in feed and feed raw materials worldwide: long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013;93:2892–2899. doi: 10.1002/jsfa.6225. [DOI] [PubMed] [Google Scholar]
- 3.Streit E., Schatzmayr G., Tassis P., Tzika E., Marin D., Taranu I., Tabuc C., Nicolau A., Aprodu I., Puel O., Oswald I.P. Current situation of mycotoxin contamination and co-occurrence in animal feed-focus on Europe. Toxins. 2012;4:788–809. doi: 10.3390/toxins4100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Aflatoxin B1 as undesirable substance in animal feed. Request N° EFSA-Q-2003-035, adopted on 3 February 2004, EFSA J. 39 (2004) pp. 1–27.
- 5.Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J. 2004;73:1–41. [Google Scholar]
- 6.Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Zearalenone as undesirable substance in animal feed Question No EFSA-Q-2003-037. EFSA J. 2004;89:1–35. [Google Scholar]
- 7.Opinion of the Scientific Panel on Contaminants in Food Chain on a request from the Commission related to ochratoxin A (OTA) as undesirable substance in animal feed. EFSA J. 2004;101:1–36. [Google Scholar]
- 8.Opinion of the Scientific Panel on Contaminants in Food Chain on a request from the Commission related to fumonisins as undesirable substances in animal feed. EFSA J. 2005;235:1–32. [Google Scholar]
- 9.Metzler M. Proposal for a uniform designation of zearalenone and its metabolites. Mycotoxin Res. 2011;27:1–3. doi: 10.1007/s12550-010-0075-2. [DOI] [PubMed] [Google Scholar]
- 10.Pitt J.I., Taniwaki M.H., Cole M.B. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control. 2013;32:205–215. [Google Scholar]
- 11.Gambacorta L., Solfrizzo M., Visconti A., Powers S., Cossalter A.M., Pinton P., Oswald I.P. Validation study on urinary biomarkers of exposure for aflatoxin B1, ochratoxin A, fumonisin B1, deoxynivalenol and zearalenone in piglets. World Mycotoxin J. 2013;6:299–308. [Google Scholar]
- 12.Nicholson J.K., Lindon J.C., Holmes E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 13.Robertson D.G. Metabonomics in toxicology: a review. Toxicol. Sci. 2005;85:809–822. doi: 10.1093/toxsci/kfi102. [DOI] [PubMed] [Google Scholar]
- 14.Waters N.J., Waterfield C.J., Farrant R.D., Holmes E., Nicholson J.K. Integrated Metabonomic Analysis of Bromobenzene-Induced Hepatotoxicity: novel Induction of 5-Oxoprolinosis. J. Proteome Res. 2006;5:1448–1459. doi: 10.1021/pr060024q. [DOI] [PubMed] [Google Scholar]
- 15.Rezzi S., Ramadan Z., Fay L.B., Kochhar S. Nutritional metabonomics: applications and perspectives. J. Proteome Res. 2007;6:513–525. doi: 10.1021/pr060522z. [DOI] [PubMed] [Google Scholar]
- 16.He Q., Tang H., Ren P., He X., Wu G., Yin Y., Wang Y. Dietary supplementation with l-arginine partially counteracts serum metabonome induced by weaning stress in piglets. J. Proteome Res. 2011;10:5214–5221. doi: 10.1021/pr200688u. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y.L., Holmes E., Nicholson J.K., Cloarec O., Chollet J.M., Tanner B., Singer H., Utzinger J. Metabonomic investigation in mice infected with Schistosoma mansoni: an approach for biomarker identification. Proc. Natl. Acad. Sci. USA. 2004;101:12676–12681. doi: 10.1073/pnas.0404878101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Utzinger J., Saric J., Li J.V., Burckhardt J., Dirnhofer S., Nicholson J.K., Singer B.H., Brun R., Holmes E. Global metabolic responses of mice to Trypanosoma brucei brucei infection. Proc. Natl. Acad. Sci. USA. 2008;105:6127–6132. doi: 10.1073/pnas.0801777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrifield C.A., Lewis M., Claus S.P., Beckonert O.P., Dumas M.E., Duncker S., Kochhar S., Rezzi S., Lindon J.C., Bailey M., Holmes E., Nicholson J.K. A metabolic system-wide characterization of the pig: a model for human physiology. Mol. BioSyst. 2011;7:2577–2588. doi: 10.1039/c1mb05023k. [DOI] [PubMed] [Google Scholar]
- 20.Bollard M.E., Stanley E.G., Lindon J.C., Nicholson J.K., Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 2005;18:143–162. doi: 10.1002/nbm.935. [DOI] [PubMed] [Google Scholar]
- 21.Liu G., Yan T., Wang J., Huang Z., Chen X., Jia G., Wu C., Zhao H., Xue B., Xiao L., Tang J. Biological system responses to zearalenone mycotoxin exposure by integrated metabolomic Studies. J. Agric. Food Chem. 2013;61:11212–11221. doi: 10.1021/jf403401v. [DOI] [PubMed] [Google Scholar]
- 22.Hopton R.P., Turner E., Burley V.J., Turner P.C., Fisher J. Urine metabolite analysis as a function of deoxynivalenol exposure: an NMR-based metabolomics investigation. Food Addit. Contam. A. 2010;27:255–261. doi: 10.1080/19440040903314015. [DOI] [PubMed] [Google Scholar]
- 23.Hopton R.P., Oswald I.P., Hardie L.J., Turner P.C., Fisher J. Nuclear magnetic resonance analysis of glucose levels in weanling piglets plasma as a function of deoxynivalenol exposure. ISRN Anal. Chem. 2012;2012:1–5. [Google Scholar]
- 24.Li J.V., Saric J., Wang Y., Keiser J., Utzinger J., Holmes E. Chemometric analysis of biofluid from mice experimentally infected with Schistosoma mansoni. Parasit. Vector. 2011;4:179–194. doi: 10.1186/1756-3305-4-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebbels T.M.D., Holmes E., Lindon J.C., Nicholson J.K. Evaluation of metabolic variation in normal rat strains from a statistical analysis of 1H NMR spectra of urine. J. Pharm. Biomed. Anal. 2004;19:823–833. doi: 10.1016/j.jpba.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Napoli C., Sperandio N., Lawlor R.T., Scarpa A., Molinari H., Assfalg M. Urine metabolic signature of pancreatic ductal adenocarcinoma by 1H nuclear magnetic resonance: identification, mapping, and evolution. J. Proteome Res. 2012;11:1274–1283. doi: 10.1021/pr200960u. [DOI] [PubMed] [Google Scholar]
- 27.Bro R., Smilde A.K. Principal component analysis. Anal. Methods. 2014;6:2812–2831. [Google Scholar]
- 28.Trygg J., Wold S. Orthogonal projections to latent structures (O-PLS) J. Chemom. 2002;16:119–128. [Google Scholar]
- 29.Consonni R., Cagliani L.R., Stocchero M., Porretta S. Triple Concentrated Tomato Paste: discrimination between Italian and Chinese Products. J. Agric. Food Chem. 2009;57:4506–4513. doi: 10.1021/jf804004z. [DOI] [PubMed] [Google Scholar]
- 30.Zotti M., De Pascali S.A., Del Coco L., Migoni D., Carrozzo L., Mancinelli G., Fanizzi F.P. 1H NMR metabolomic profiling of the blue crab (Callinectes sapidus) from the Adriatic Sea (SE Italy): a comparison with warty crab (Eriphia verrucosa), and edible crab (Cancer pagurus) Food Chem. 2016;196:601–609. doi: 10.1016/j.foodchem.2015.09.087. [DOI] [PubMed] [Google Scholar]
- 31.Holmes E., Wilson I.D., Nicholson J.K. Metabolic phenotyping in health and disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Wheelock Å.M., Wheelock C.E. Trials and tribulations of omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst. 2013;9:2589–2596. doi: 10.1039/c3mb70194h. [DOI] [PubMed] [Google Scholar]
- 33.Solfrizzo M., Gambacorta L., Visconti A. Assessment of Multi-Mycotoxin Exposure in Southern Italy by Urinary Multi-Biomarker Determination. Toxins. 2014;6:523–538. doi: 10.3390/toxins6020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shephard G.S., Burger H.M., Gambacorta L., Gong Y.Y., Krska R., Rheeder J.P., Solfrizzo M., Srey C., Sulyok M., Visconti A., Warth B., van der Westhuizen L. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem. Toxicol. 2013;62:217–225. doi: 10.1016/j.fct.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 35.Wallin S., Gambacorta L., Kotova N., Lemming E.W., Nalsen C., Solfrizzo M., Olsen M. Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi-biomarker analysis. Food Chem. Toxicol. 2015;83:133–139. doi: 10.1016/j.fct.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 36.CAST, Council for Agricultural Science and Technology, Mycotoxins, Risks in Plant, Animal, and Human System, Task Force Report 139, Ames Iowa 2003.
- 37.Alassane-Kpembi I., Puel O., Oswald I.P. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2015;89:1337–1346. doi: 10.1007/s00204-014-1309-4. [DOI] [PubMed] [Google Scholar]
- 38.Alassane-Kpembi I., Schatzmayr G., Marin D., Taranu D., Puel O., Oswald I.P. Mycotoxins co-contamination: methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017;57:3489–3507. doi: 10.1080/10408398.2016.1140632. [DOI] [PubMed] [Google Scholar]
- 39.Halloy D.J., Gustin P.G., Bouhet S., Oswald I.P. Oral exposure to culture material extract containing fumonisins predisposes swine to the development of pneumonitis caused by Pasteurella multocida. Toxicology. 2005;15:34–44. doi: 10.1016/j.tox.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Devriendt B., Gallois M., Verdonck F., Wache Y., Bimczok D., Oswald I.P., Goddeeris B.M., Cox E. The food contaminant fumonisin B(1) reduces the maturation of porcine CD11R1(+) intestinal antigen presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet. Res. 2009;40:40–54. doi: 10.1051/vetres/2009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.(a) Pinton P., Oswald I.P. Effect of Deoxynivalenol and other Type B Trichothecenes on the Intestine: a review. Toxins. 2014;21:1615–1643. doi: 10.3390/toxins6051615. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kan M.A. Minipig: advantages and disadvantages as model in toxicity testing. J. Am. Coll. Toxicol. 1984;3:337–342. [Google Scholar]
- 42.Miller E.R., Ullrey D.E. The pig as a model for human nutrition. Annu. Rev. Nutr. 1987;7:361–382. doi: 10.1146/annurev.nu.07.070187.002045. [DOI] [PubMed] [Google Scholar]
- 43.Del Coco L., Assfalg M., D’Onofrio M., Sallustio F., Pesce F., Fanizzi F.P., Schena F.P. A proton nuclear magnetic resonance-based metabolomic approach in IgA nephropathy urinary profiles. Metabolomics. 2013;9:740–751. [Google Scholar]
- 44.Dou L., Cerini C., Brunet P., Guilianelli C., Moal V., Grau G., De Smet R., Vanholder R., Sampol J., Berland Y. p-cresol, a uremic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney Int. 2002;62:1999–2009. doi: 10.1046/j.1523-1755.2002.t01-1-00651.x. [DOI] [PubMed] [Google Scholar]
- 45.Dou L., Bertrand E., Cerini C., Faure V., Sampol J., Vanholder R., Berland Y., Brunet P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 46.Vanholder R., Glorieux G., Lameire N. New insights in uremic toxicity. In: Ronco C., Brendolan A., Levin N., editors. Vol. 149. Karger; Basel: 2005. pp. 315–324. (Cardiovascular Disorders in Hemodialysis). [Google Scholar]
- 47.Smith E.A., Macfarlane G.T. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J. Appl. Bacteriol. 1996;81:288–302. doi: 10.1111/j.1365-2672.1996.tb04331.x. [DOI] [PubMed] [Google Scholar]
- 48.Powell G.M., Miller J.J., Olavesen A.H., Curtis C.G. Liver as major organ of phenol detoxification. Nature. 1974:234–235. doi: 10.1038/252234a0. [DOI] [PubMed] [Google Scholar]
- 49.Ramakrishna B.S., Gee D., Weiss A., Pannall P., Robertsthomson I.C., Roediger W.E.W. Estimation of phenolic conjugation by colonic mucosa. J. Clin. Pathol. 1989;42:620–623. doi: 10.1136/jcp.42.6.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohus E., Coen M., Keun H.C., Ebbels T.M., Beckonert O., Lindon J.C., Holmes E., Noszál B., Nicholson J.K. Temporal metabonomic modeling of l-arginine-induced exocrine pancreatitis. J. Proteome Res. 2008;7:4435–4445. doi: 10.1021/pr800407j. [DOI] [PubMed] [Google Scholar]
- 51.Monteith D.K., Morgan R.E., Halstead B. In vitro assays and biomarkers for drug-induced phospholipidosis. Expert Opin. Drug Metab. Toxicol. 2006;2:687–696. doi: 10.1517/17425255.2.5.687. [DOI] [PubMed] [Google Scholar]
- 52.Xu J., Gordon J.I. Honor thy symbionts. Proc. Natl. Acad. Sci. USA. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burel C., Tanguy M., Guerre P., Boilletot E., Cariolet R., Queguiner M., Postollec G., Pinton P., Oswald I.P., Fravalo P. Effect of low dose of fumonisins on pig health: immune status, intestinal microbiota and sensitivity to Salmonella. Toxins. 2013;5:841–864. doi: 10.3390/toxins5040841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waché Y.J., Valat C., Postollec G., Bougeard S., Burel C., Oswald I.P., Fravalo P. Impact of deoxynivalenol on the intestinal microflora of pigs. Int. J. Mol. Sci. 2009;10:1–17. doi: 10.3390/ijms10010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Tang L., Glenn T.C., Wang J.S. Aflatoxin B1 induced compositional changes in gut microbial communities of male F344 rats. Toxicol. Sci. 2016;150:54–63. doi: 10.1093/toxsci/kfv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Craig S. Betaine in human nutrition. Am. J. Clin. Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 57.Ghyczy M., Boros M. Electrophilic methyl groups present in the diet ameliorate pathological states induced by reductive and oxidative stress: a hypothesis. Br. J. Nutr. 2001;85:409–414. doi: 10.1079/bjn2000274. [DOI] [PubMed] [Google Scholar]
- 58.Bertram H.C., Bach Knudsen K.E., Serena A., Malmendal A., Nielsen N.C., Frette X.C., Andersen H.J. NMR-based metabonomic studies reveal changes in the biochemical profile of plasma and urine from pigs fed high-fibre rye bread. Br. J. Nutr. 2006;95:955–962. [Google Scholar]
- 59.Caldas T., Demont-Caulet N., Ghazi A., Richarme G. Thermoprotection by glycine betaine and choline. Microbiology. 1999;145:2543–2548. doi: 10.1099/00221287-145-9-2543. [DOI] [PubMed] [Google Scholar]
- 60.Smith J.L., Wishnok J.S., Deen W.M. Metabolism and excretion of methylamines in rats. Toxicol. Appl. Pharmacol. 1994;125:296–308. doi: 10.1006/taap.1994.1076. [DOI] [PubMed] [Google Scholar]
- 61.Wei L., Liao P., Wu H., Li X., Pei F., Li W., Wu Y. Metabolic profiling studies on the toxicological effects of realgar in rats by 1H NMR spectroscopy. Toxicol. Appl. Pharmacol. 2009;234:314–325. doi: 10.1016/j.taap.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Zeisel S.H., Wishnok J.S., Blusztajn J.K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther. 1983;225:320–324. [PubMed] [Google Scholar]
- 63.Eriksson L., Byrne T., Johansson E., Trygg J., Wikström C. Umetrics Academy; 2013. Multi-and Megavariate Data Analysis Basic Principles and Applications. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material