Abstract
Introduction
Aromatase inhibitors (AIs) are a well-established component of adjuvant therapy in postmenopausal women with hormone receptor (HR)+ early stage breast cancer (BCa). We explored in an 18-month cohort study whether subjective oral health (OH), subjective periodontal health (PH), and oral health-related quality of life (OHRQoL) of postmenopausal BCa survivors on AIs differ from those of women without cancer diagnoses, and whether saliva flow, OH, PH and OHRQoL are related.
Methods
Data were collected from 29 postmenopausal BCa survivors on AIs and 29 postmenopausal women without cancer diagnoses. Socio-demographic information, OH, PH, and OHRQoL were collected at baseline and 6, 12 and 18 months later. Unstimulated whole saliva volume per 15 minutes was determined by drooling.
Results
The two groups did not differ in background characteristics at baseline. Women on AIs had poorer OH (p=.043), PH (p=.04), and OHRQoL (p=.017), and lower saliva flow rate (p<.001) than control respondents. BCa survivors had the poorest PH at the 18 months visit. Xerostomia was correlated with OH at baseline and with OH and PH at 18 months. However, objective saliva flow rate was not correlated with OH or OHRQoL at this visit.
Conclusions
This study is the first to investigate the effects of AIs on patients’ subjective OH, subjective PH, and OHRQoL. The data suggest that women treated with AIs have worse OH, PH, and OHRQoL than women without cancer diagnoses. Interprofessional care is recommended so that women on AIs receive optimal supportive oral care to assure long-term OH and positive OHRQoL.
Keywords: Oral Health, Periodontitis, Quality of Life, Aromatase Inhibitors, Breast neoplasms, Saliva
INTRODUCTION
Oral health is a critical component of an individual’s overall health [1–3] and this connection is even found in patients undergoing cancer treatments [4, 5]. Common oral complications that these patients experience include oral/pharyngeal mucositis[6], xerostomia, and dental caries[7]. They are also at an increased risk for opportunistic bacterial, fungal, and viral infections as a result of chemotherapy-induced immune suppression[8]. They might even experience osteonecrosis of the jaw [9] and periodontal tissue changes including gingivitis, gingival bleeding, and periodontal infections[10, 11 ]. Periodontal diseases are characterized by the infection of both the soft and hard tissues and can be so severe that they result in tooth loss[12]. Poor periodontal health has been shown to be related to systemic health in patients with diabetes[2] and cardiovascular disease[3]. While research has examined the effects of chemotherapy and radiation treatment on oral health, these investigations have focused mainly on patients with stem cell transplants [13] or undergoing radiotherapy, especially for head and neck cancer[14–16]. So far, no research investigated the impact of aromatase inhibitors (AI) on patients’ oral health and oral health-related quality of life.
Breast cancer (BCa) is the most common type of cancer in women and the incidence increases with age[17]. The median age at which BCa is diagnosed in women is over 60 years of age. Standard endocrine therapy for postmenopausal estrogen receptor positive (ER+) early stage BCa includes the adjuvant use of an aromatase inhibitors (AI) to interfere with estrogen production for between 5 and 10 years. [18] The systemic effects of inhibiting estrogen with AIs include accelerated bone loss and osteoporotic fractures[19] Prior research focused on understanding how survivors’ health-related quality of life (HRQOL) is affected by BCa[20], and how endocrine or hormonal therapy impact HRQOL of BCa patients and survivors[21, 22]. The “Arimidex, tamoxifen, alone or in combination” (ATAC) trial analyzed the HRQOL effects of AIs and Tamoxifen as primary adjuvant therapy for postmenopausal women with localized BCa. The results showed that AIs and Tamoxifen had similar impacts on HRQOL and that endocrine-related HRQOL gradually improved from baseline over the next 24 months [23].
So far, no research has explored the effects of AI therapy on BCa survivors’ oral health or oral health-related quality of life (OHRQoL). OHRQoL, the degree to which oral health affects functioning, causes pain/discomfort, and intrudes on psychological and social well-being, [24] is an integral part of a women’s overall quality of life. One recent study examined the impact of Zoledronic Acid on OHRQoL in BCa patients and found that the OHRQOL of BCa patients using Zoledronic Acid vs. not using bisphosphonate therapy did not differ[25]. However, this study only assessed OHRQoL at the end of 5 years after BCa diagnosis and did not analyze oral health and OHRQOL changes over time.
Concerning the effects of AIs on objectively assessed oral and specifically periodontal health, Taichman et al. showed that AIs have an effect on alveolar bone height, periodontal health and salivary biomarkers in BCa survivors which may be related to the rapid and severe AI induced suppression of estrogen levels[26]. However, one central question is whether BCa survivors on AIs are actually aware of their periodontal health status and if it affects their OHRQOL. This question is quite significant, because research showed that patients’ subjective oral and periodontal health are not only significantly related with chewing and eating habits,[27] but that they are better predictors of food selection than objective oral health assessments determined in direct clinical examinations[28–30]. Avoiding food that is difficult to chew, such as raw vegetables, fruits and other nutritious items, can affect an individual’s nutritional status and overall quality of health[31]. In addition, suffering from xerostomia as a possible consequence of AI use might further affect these patients’ OHRQOL and its impact on food selection and mastication [32].
The objectives of this study therefore are to explore in an 18 month long cohort study (a) whether postmenopausal BCa survivors on AIs have a poorer subjective oral health (OH), subjective periodontal health and OHRQoL than postmenopausal women in a control group who never had a cancer diagnosis, and (b) whether subjective OH, subjective periodontal health, and OHRQOL are related and also have a relationship with xerostomia and objectively assessed saliva secretion in AI users.
METHODS AND MATERIALS
This prospective, longitudinal study performed serial examinations of the oral cavity concurrent with collection of saliva and survey data. Study time points were baseline, 6, 12, and 18 months. Study participants were seen in the dental research facility of the University of Michigan. The baseline data on objective periodontal health measures and saliva biomarkers have been published elsewhere[26]. For the purpose of this report, only the longitudinal saliva and subjective oral health and oral health-related quality of life data are presented here.
This study was reviewed and approved by the Institutional Board for the Medical Sciences at the University of Michigan, Ann Arbor, MI, USA prior to enrolling patients and was registered through National Institutes of Health ClinicalTrials.gov (Identifier no. NCT01272570). The authors followed the STROBE guidelines for carrying out the study and for writing the paper[33]. All study participants provided written and signed informed consent.
Respondents
The cohort of women included in this study consisted of 29 postmenopausal BCa survivors on adjuvant AIs and 29 women without a BCa diagnosis with at least 15 teeth. The BCa survivors were enrolled at least 3 months after the end of their radiation/chemotherapy treatment in order to avoid including women in the acute recovery period.
Procedure
Enrollment criteria have been described in full by Taichman et al. [26] Participants were consecutively recruited between April 2009 and September 2010 and followed for 18 months. Menopausal status was determined using the National Comprehensive Cancer Network (NCCN) criteria. Postmenopausal women with a histologic confirmed diagnosis of early stage (I-IIIA) BCa newly on adjuvant AI therapy (within 2–11 months of start) were recruited from the Breast Cancer Clinic at the University of Michigan Comprehensive Cancer Center (UMBCC). AIs prescribed included anastrozole, exemestane or letrozole. Subjects may have had a history of Tamoxifen use, chemotherapy and/or radiation therapy. Women were excluded if they had received a diagnosis of metastatic BCa. The participants in the control group were recruited from the University of Michigan Breast Imaging clinic where they had been seen for routine mammograms. Exclusion criteria included any cancer (basal cell skin cancer), uncontrolled diabetes (A1c >7.2) or Sjögren Syndrome, and the use of medications that affect periodontal status or were highly xerogenic (i.e. medications for depression or asthma). Oral bisphosphonate use dosed for low bone mass was allowed. No participant received bisphosphonates for cancer treatment.
Survey
Participants completed a self-administered questionnaire concurrently with the dental examinations at baseline and after 6, 12, and 18 months. Part 1 of this survey collected demographic information such as age, ethnicity/race, education, and income. Part 2 included oral health-related questions such as the subjective oral health question from the National Health and Nutrition Examination Survey (NHANES)[34] which asks the respondents to rate the health of their teeth and gums on a 5-point scale from 1 = “Poor” to 5 = “Excellent”. Additional questions asked how important their oral health was, how much saliva they had, and how dry their mouth felt. Oral health-related behavior questions assessed the frequency of dental visits (1 = “Never” to 5 =”Over 13 months”) and of brushing and flossing (Answer scale: 1 = “Never” to 5 = “More than once a day”). Part 3 consisted of the short version of the Oral Health Impact Profile (OHIP-14) which assesses oral health-related quality of life[35]. The OHIP-14 consists of 14 questions concerning the frequency with which respondents had experienced oral health-related symptoms during the last month. Answers were given on a 5-point scale with 1 = “Never”, 2 = “Hardly ever”, 3 = “Occasionally”, 4 = “Fairly often”, and 5 = “Very often”. The final section contained eleven Yes/No answer format questions concerning the respondents’ periodontal health such as whether they had swelling of their gums, bleeding during tooth brushing, or had noticed any other symptoms related to gum disease.
Saliva collection
Unstimulated whole saliva was collected via passive drooling into a sterile plastic tube from all study participants only at baseline, 12 months and 18 months [36]. Participants were asked to refrain from eating, drinking (with the exception of water), smoking and use of alcohol and oral hygiene measures for at least 1 hour prior to the study visit. In addition, no beverages other than water were available to the participants during the approximately 1 hour when other data (consent, vitals, patient survey) were collected before the collection of the saliva sample. Saliva collection was stopped once a total of 2 ml was collected or 15 minutes had elapsed whichever occurred first. Saliva flow rate was determined by saliva volume divided by time. The sample was immediately placed on ice, aliquoted, supplemented with proteinase inhibitors Aproptonin and Phenylmethane-sulfonyl fluoride (PMFS) and stored at −80 C [37]. Patients were scheduled for saliva collection at the same time of day for follow-up visits.
Statistical Analysis
Data were analyzed using SPSS (Statistics for Windows, Version 22.0; IBM Corp. 2013. Armonk, NY). Repeated measurement multivariate analyses of variance were computed to compare the responses of BCa survivors on AIs at baseline, 6, 12 and 18 months following their initiation of AIs versus women without a cancer diagnosis. Chi-square analyses were used to determine whether the responses to the “yes”/“no” answer format questions were significantly different at each of the given time points. In addition, Pearson Correlation coefficients were computed to determine whether there were significant associations between subjective oral and periodontal health, importance of oral health, saliva-related responses and OHRQoL at baseline and the 18 month follow-up visits. A significance level of 0.05 was assumed for statistical analysis.
RESULTS
A total of 142 potential participants were assessed for eligibility in this study. Of these patients, 34 patients were excluded due to systemic conditions or the use of xerogenic medications. Another 45 patients declined participation in the study due to being “overwhelmed with cancer treatments” or the distance from the cancer center. Five additional patients withdrew before the baseline assessment.
Based on the a prior design, 58 participants met study eligibility criteria and were enrolled; 29 control participants, and 29 AI treatment participants. At the six month follow up visit, one BCa survivor dropped out, and at the 12 months follow up visit three BCa survivors left this study because they had to discontinue AI therapy due to AI toxicity. This resulted in 25 AI study participants and 29 controls at the 18 month study visit. The two groups did not differ in their background characteristics at baseline as previously reported[26]. Their mean age was close to 62 years (AI use: 61.7 years; No AI Use: 61.6 years; p=.929). Twenty-six of the 29 women in each group were white and the majority of women in both groups had more than a high school level of education (AI use: 69%; No use: 64%; p=.804). Eight of the women in the AI group had incomes below $40,000 and 21 women had higher incomes compared to 13 women in the no AI group with lower incomes and 16 with higher incomes (p=.137). Twenty-one women in the AI group and 13 in the no AI use group were married (p=.228). Only one person in each group currently smoked. Sixteen of the AI users and 13 of the no AI users reported alcohol use (p=.296). More of women in the AI group reported the concurrent use of a bisphosphonate (AI use: 38%; No AI 17%; p=.071).
The women in the two groups did not differ in their oral health-related behavior. All respondents brushed at least once a day. A total of 48% of AI users and 42% of non AI users flossed daily (p=.209). While 27.6% of the AI users and 20.7% of the respondents in the control group had no dental insurance (p=.768), all but one of the AI users reported having a dental visit during the 12 months prior to the baseline appointment[26]. A high pattern of dental utilization was evident at the 6, 12 and 18 month visits with 97% of all patients indicating a dental visit within the last 6 months.
Table 1 provides an overview of the cancer-related characteristics of the women with BCa at baseline. At the time of the BCa diagnosis, the women ranged in age between 42 and 73 years (mean age=59.3 years). Fifteen women had been diagnosed with Stage I (51.7%), 9 with Stage II (31%), and 5 with Stage III (17%) estrogen receptor positive BCa. The time between the BCa diagnosis and the baseline study assessment ranged from 8 to 19 months (mean=15 months) and the time that the women had been treated with AIs ranged from 2 to 11 months (mean=5.7 months). Adjuvant cancer treatments included chemotherapy (37.9%), radiation therapy (89.6%), and tamoxifen therapy (17.2%) prior to the treatment with AIs. Twenty women used anastrozole, 7 used letrozole and 2 exemestane. Table 1 shows the coexisting systemic diseases and daily medication intake at baseline.
Table 1.
General characteristics of women with BCa with Aromatase Inhibitor (AI) Use and controls at baseline
| Cancer-related responses | Frequencies Mean ± SD |
Percentage Range |
|---|---|---|
|
| ||
| Age at breast cancer diagnosis in years | 59.3 ± 7.1 | 42 – 73 |
|
| ||
| Time since breast cancer diagnosis in months | 15.1 ± 6.1 | 8 – 19 |
|
| ||
| AI duration in months | 5.7 ± 3.1 | 2 – 11 |
|
| ||
| N | % | |
|
| ||
| Tumor stage at diagnosis: | ||
| - Stage I | 15 | 51.7 |
| - Stage II | 9 | 31.0 |
| - Stage III | 5 | 17.3 |
|
| ||
| Breast cancer treatment | N | % |
|
| ||
| Prior adjuvant chemotherapy: | ||
| Yes | 18 | 62.1 |
| No | 11 | 37.9 |
|
| ||
| Number of months between end of chemotherapy and baseline survey: | ||
| 4–6 months | 4 | 22.2 |
| >6 months – 9 months | 6 | 33.3 |
| >9 months – 12 months | 4 | 22.2 |
| > 12 months | 4 | 22.2 |
|
| ||
| Prior radiation: | ||
| Yes | 26 | 89.6 |
| No | 3 | 10.4 |
|
| ||
| Prior Tamoxifen use: | ||
| Yes | 5 | 17.2 |
| No | 24 | 82.8 |
|
| ||
| Distribution of AIs: | ||
| Anastrozole | 20 | 68.9 |
| Exemestane | 2 | 6.9 |
| Letrozole | 7 | 24.2 |
|
| ||
| Breast Cancer and Control group | ||
|
| ||
| Medications intake and systemic disease | AI (n=29) N (%) |
Control (n=29) N (%) |
|
| ||
| Bisphosphonate Use: | ||
| - Yes | 11(38%) | 5(17%) |
|
| ||
| Coexisting systemic diseases | ||
| Number of patients, (%) | 20(68%) | 16(55%) |
|
| ||
| Daily Medications | ||
| Number of patients, (%) | 29(100%)* | 22(76%) |
| Number of medications-Mean, SD | 2.3±0.9 | 1.7±1.4 |
| Range | (1–5) | (0–5) |
|
| ||
| Number of patients, (%) | 20(69%) | 18(62%) |
| Potentially xerogenic medications-Mean, SD | 1.02±1.0 | 0.8±0.9 |
| Range | (0–4) | (0–3) |
AI –Aromatase inhibitors
All BCa patients took an AI daily
Table 2 provides an overview of the oral health-related responses of women with versus without AI use at baseline and at the 6, 12, and 18 months follow-up visits. Overall, women with AI use reported a less positive mean overall subjective teeth-related health than women in the control group. However, the two groups did not differ in their mean responses concerning the health of their gums. When asked about the amount of saliva they had, no significant difference was found between the women in the two groups despite the fact that the women with AI use had produced less saliva when unstimulated saliva was collected compared to the women in the control group.
Table 2.
Oral health-related responses of Aromatase Inhibitor (AI) users and controls at baseline, 6, 12 and 18 months
| Oral health-related responses1 | AI use | Baseline | 6 months | 12 months | 18 months | P(Tx)/P(Time) P(Tx xTime) |
|---|---|---|---|---|---|---|
| How would you describe the health of your teeth?2 | No | 3.70 | 3.55 | 3.79 | 3.79 |
.043/.125 .884 |
| Yes | 3.15 | 3.17 | 3.35 | 3.43 | ||
| How would you describe the health of your gums?2 | No | 3.34 | 3.55 | 3.45 | 3.75 | .209/.167 .144 |
| Yes | 2.98 | 3.17 | 3.35 | 3.30 | ||
| How important is your dental health to you?3 | No | 4.97 | 4.97 | 5.00 | 5.00 | .449/.117 .056 |
| Yes | 4.73 | 4.83 | 4.83 | 4.47 | ||
| How much saliva do you have?4 | No | 4.34 | 4.21 | 4.10 | 4.21 | .062/.468 .816 |
| Yes | 3.96 | 3.52 | 3.74 | 3.52 | ||
| Ml of unstimulated saliva per 15 minutes 5,6 | No | 2.25 (0.5–3.5) |
n/a | 2.50 (0.5–6.0) |
3.00 (0.5–7.0) |
.001/.315 .049 |
| Yes | 1.75 (0.5–3.0) |
n/a | 2.00 (0.5–3.0) |
2.00 (0.0–4.0) |
||
| Did you ever have these problems?7 | AI use | % Yes | % Yes | % Yes | % Yes |
P(TX) P(Time) |
| Do you think you might have gum disease (swollen gums, receding gums, infected gums or loose teeth)? | No | 21% | 14%* | 14% | 10.%* | <.001 .712 |
| Yes | 38% | 38% | 28% | 38% | ||
| Have you noticed bleeding from your gums during tooth brushing in the last month? | No | 28% | 17% | 21% | 28% | .08 .721 |
| Yes | 10% | 17% | 12% | 21% | ||
| Have you noticed gingival swelling? Did tissues look puffy in the last month? | No | 14% | 7% | 7% | 3% | .426 .733 |
| Yes | 10% | 7% | 8% | 13% | ||
| Have you ever had any teeth become loose on their own, without an injury? | No | 3% | 3%* | 3% | 3% | <.001 .708 |
| Yes | 21% | 24% | 12% | 17% | ||
| Have you ever had treatment for gum disease, such as scaling and root planing, also called “deep” cleaning? | No | 21% | 21% | 21% | 17%* | .041 .547 |
| Yes | 17% | 28% | 36% | 46% | ||
| Can you see more of the roots of your teeth than in past? | No | 31% | 17% | 10% | 17% | .04 .693 |
| Yes | 24% | 31% | 28% | 38% | ||
| Have you ever been told by a dental professional that you lost bone around your teeth? | No | 14% | 10%** | 10%* | 7%*** | <.001 .996 |
| Yes | 36% | 41% | 40% | 46% | ||
| During the past 3 months, have you noticed a tooth that doesn’t look right? | No | 3% | 3% | 0% | 7% | .150 .079 |
| Yes | 17% | 3% | 0% | 8% | ||
| Within the last month have you had sore areas on your gums that have lasted more than a week? | No | 3% | 0% | 0% | 1% | .038 .523 |
| Yes | 7% | 7% | 1% | 3% | ||
| Within the last month have you had painful tingling in your gums or tongue? | No | 0% | 1% | 1% | 0% | .067 .966 |
| Yes | 10% | 1% | 1% | 2% | ||
| Do you have any teeth that are sensitive to hot, cold or sweets? | No | 21%* | 4%* | 17% | 10%** | <.001 .270 |
| Yes | 52% | 11% | 32% | 42% |
Legend: Asterisk/s in cell indicate/s significant differences between women using AIs vs. control at each point in time
= p < .05;
= p < .01;
= p < .001
The average responses to these questions were compared with repeated measurement analyses of variance.
Answers ranged from 1=Poor, 2=Fair, 3= Good, 4=Very good, to 5=Excellent.
Answers ranged from 1 = Not at all to 5 = very important.
Answers ranged from 1 = Very little saliva” to 5 = Perfect amount of saliva.
Saliva volume was determined by collecting unstimulated saliva for up to 15 minutes at baseline, 12 and 18 months.
Saliva volume reported as median and range.
Answers were 1= “Yes” and 2 = “No”. Chi-square tests were used to compare the responses of women with BCa treated with AI vs. controls at the four assessments.
In addition to these general oral health-related questions, the respondents also reported whether they had encountered eleven specific problems related to the health of their teeth and gums. The women in the AI group were significantly more likely than the women in the control group to report that they had 7 of the 11 issues in question. For example, overall the women with AI use were more likely to think they might have gum disease, had loose teeth, had experienced deep cleaning, had been told that they had lost bone around their teeth, and had sore areas on their gums that lasted for more than a week compared to the women without AI use. When a sum score was computed by adding one point for each reported symptom, the data suggested the women with AI use had a significantly worse mean periodontal health score than the women in the control group (p=.040) (see Figure 1). In addition, the mean scores at the four points in time differed (p=.016), with the mean number of symptoms reported at the last visit (18 months) being the worst sum score.
Figure 1. Average number of subjective periodontal disease indicators of women with breast cancer (BCa) treated with aromatase inhibitors (AI) vs. women with no cancer diagnoses at the four points in time.
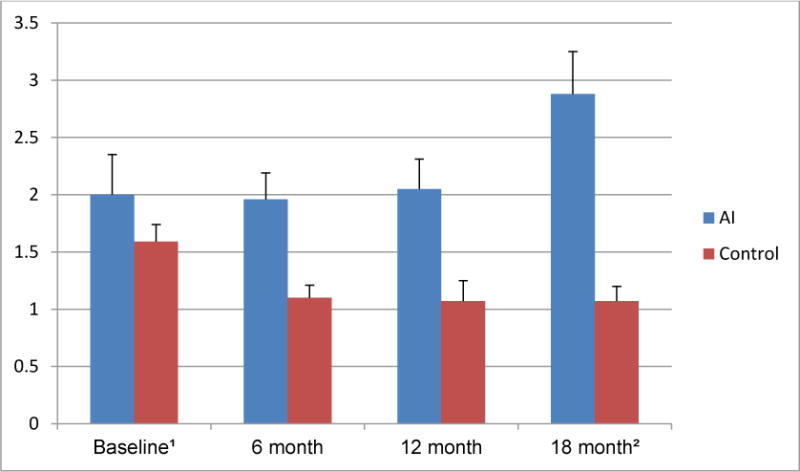
1 Note that the maximum number of subjective oral/periodontal disease indicators the women could report was 11. The wording of these eleven indicators is included in Table 2. The scores could therefore range from 0=”Best oral/periodontal health” to 11= “Worst oral/periodontal health”.
2 The results of the repeated measurement univariate analysis of variance showed that the main effect “Treatment” (AI use vs. control group) (p= 0.040) and the main effect “Time” (Baseline vs. 6 month vs. 12month vs. 18month visits) (p= 0.016) were significant, while the interaction effect “Treatment” × “Time” was not significant (p=0.059).
In addition to providing OH, periodontal health and saliva responses, the women also reported their oral health-related quality of life (OHRQoL) at each of the four study time points. The 14-item version of the Oral Health Impact Profile (OHIP-14) was used to assess their OHRQoL. Table 3 shows that overall, women with AI use reported poorer OHRQoL for 4 of the 14 items compared to the women in the control group. When an OHIP-14 index was computed by averaging the responses to the 14 items, the women with AI use had significantly poorer overall OHRQoL than the women without AI use (p=.017). Women with AI use reported to be more tense, self-conscious and irritable with other people and had more difficulty doing their usual tasks because of problems with their teeth and mouth compared to the women in the control group. When the OHRQoL scores at the four points in time were compared, a significant difference was found (p=.005). Figure 2 shows that the mean OHIP-14 scores of women with BCa on AIs was poorest at baseline, improved at 6 and 12 months and slightly worsened again at 18 months.
Table 3.
Average OHIP-143 responses of survivors of breast cancer treated with aromatase inhibitor (AI) vs. women without cancer diagnoses at the 4 points in time
| How often in the last month, have you | AI use | Base Line1 | 6 months | 12 months | 18 months | P(Tx)/P(Time) P(int)2 |
|---|---|---|---|---|---|---|
| - had trouble pronouncing any words because of problems with your teeth or mouth? | No | 1.07 | 1.03 | 1.03 | 1.07 | .195/.629 .629 |
| AI | 1.26 | 1.22 | 1.22 | 1.17 | ||
| - felt that your sense of taste has worsened because of problems with your teeth or mouth? | No | 1.03 | 1.00 | 1.00 | 1.00 | .052/.002 .019 |
| AI | 1.45 | 1.13 | 1.17 | 1.04 | ||
| - had painful aching in your mouth? | No | 1.03 | 1.17 | 1.17 | 1.17 | .145/.515 .687 |
| AI | 1.41 | 1.48 | 1.35 | 1.22 | ||
| - felt tense because of problems with your teeth or mouth? | No | 1.07 | 1.21 | 1.18 | 1.04 | .050/.055 .027 |
| AI | 1.62 | 1.30 | 1.17 | 1.12 | ||
| - found it uncomfortable to eat any foods because of problems with your teeth or mouth? | No | 1.10 | 1.14 | 1.07 | 1.07 | .093/.076 .840 |
| AI | 1.65 | 1.48 | 1.30 | 1.26 | ||
| Has your diet been unsatisfactory because of problems with your teeth or mouth? | No | 1.00 | 1.03 | 1.00 | 1.00 | .063/.090 .060 |
| AI | 1.17 | 1.13 | 1.09 | 1.13 | ||
| - been self-conscious because of your teeth or mouth? | No | 1.07 | 1.14 | 1.14 | 1.11 | .017/.936 .017 |
| AI | 1.48 | 1.52 | 1.48 | 1.43 | ||
| - found it difficult to relax because of problems with your teeth or mouth? | No | 1.10 | 1.10 | 1.03 | 1.14 | .615/.203 .025 |
| AI | 1.22 | 1.13 | 1.04 | 1.17 | ||
| - had to interrupt meals because of problems with your teeth or mouth? | No | 1.07 | 1.03 | 1.00 | 1.10 | .092/.123 .141 |
| AI | 1.39 | 1.26 | 1.09 | 1.22 | ||
| - been a bit irritable with other people because of problems with your teeth or mouth? | No | 1.00 | 1.00 | 1.00 | 1.03 | .035/.017 .231 |
| AI | 1.27 | 1.00 | 1.05 | 1.09 | ||
| - been embarrassed because of problems with your teeth or mouth? | No | 1.00 | 1.14 | 1.10 | 1.10 | .061/.444 .016 |
| AI | 1.26 | 1.37 | 1.39 | 1.40 | ||
| - had difficulty doing your usual tasks because of problems with teeth and mouth? | No | 1.00 | 1.00 | 1.00 | 1.00 | .011/.288 .097 |
| AI | 1.14 | 1.04 | 1.05 | 1.10 | ||
| - felt that life in general was less satisfying because of problems with your teeth and mouth? | No | 1.03 | 1.00 | 1.00 | 1.00 | .072/.263 .061 |
| AI | 1.17 | 1.22 | 1.02 | 1.13 | ||
| - been totally unable to function because of problems with your teeth and mouth? | No | 1.00 | 1.00 | 1.03 | 1.0 | .350/.662 .020 |
| AI | 1.14 | 1.03 | 1.10 | 1.10 |
Answers ranged from 1 = “Never”, 2 = “Hardly ever”, 3 = “On occasion”, 4 = “Fairly often”, to 5 = “Very often”.
The average responses to these questions were compared with repeated measurement univariate analyses of variance. P(Tx) is the p-value for “Treatment” AI use vs. control group; P(Time) is the p-value for the main effect “Time” Baseline vs. 6 month vs. 12month vs. 18month visits; P(int) is the p value of interaction effect between “Treatment” × “Time”.
OHIP-14- Oral Health Oral Health Impact Profile 14- item short form
Figure 2. Average OHIP-14 scores of women with BCa treated with AI vs. women without cancer diagnoses at the four points in time.
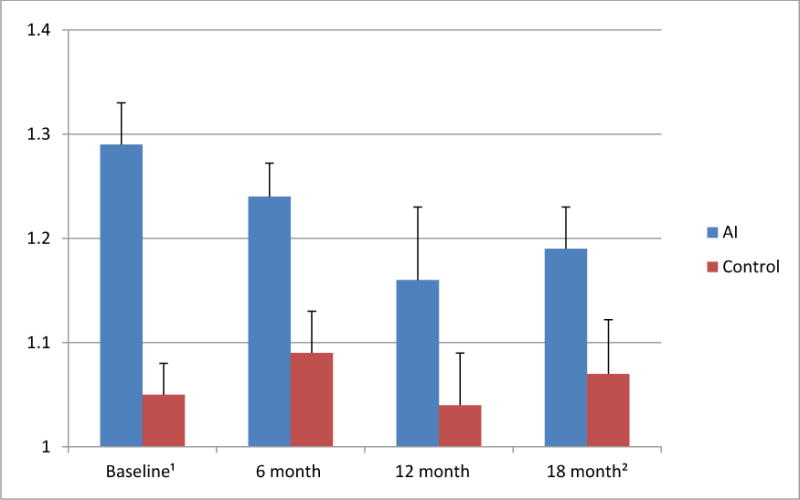
1 Note that the average OHIP-14 score can range from 1 = best oral health-related quality of life (OHRQoL) to 5 = worst OHRQoL. The wording of the 14 OHIP items is included in Table 3.
2 The results of the repeated measurement univariate analysis of variance showed that the main effect “Treatment” AI use vs. control group (p= 0.017) and the main effect “Time” Baseline vs. 6 month vs. 12month vs. 18month visits (p= 0.005) were significant, while the interaction effect “Treatment” × “Time” was not significant (p=0.061).
Note that the Cronbach alpha inter-item consistency coefficients for the mean OHIP-14 scores at each point in time showed sufficient reliability (alpha at Time 1= 0.856; alpha at Time 2=0. 829; alpha at Time 3=0.829; alpha at Time 4=0.898).
Table 4 shows relationships between oral and periodontal health, OHIP-14 scores and saliva indicators at baseline and at the 18 months assessments. This table shows that there are consistent correlations between the subjects’ teeth and gum related oral health responses and their periodontal sum scores over time. OH scores are also consistently correlated with oral health importance ratings. However, gum-related oral health and periodontal health scores and OHRQoL are only correlated at baseline. Perceived saliva was positively correlated with teeth and gum-related oral health at baseline, and with gum-related oral health, periodontal health and objective saliva volume at the 18-months follow-up visit.
Table 4.
Correlations between subjective oral health, OHIP-14 scores, and the saliva measures at baseline (= Time 1) and at the 18 months visit (= Time 4)
| Responses related to: | Time | Subjective oral health: Teeth1 | Subjective oral health: Gums1 | Periodontal health2 | Impor-tance3 | Average OHIP-14 Score6 | Perceived saliva4 |
|---|---|---|---|---|---|---|---|
| Subjective oral health: Teeth1 | 1 | 1 | .82*** | −.41*** | .36** | −.25 | .32* |
| 4 | 1 | .74*** | −.25 | .36** | −.26 | .22 | |
| Subjective oral health: Gums1 | 1 | .82*** | 1 | −.63*** | .35** | −.28* | .29* |
| 4 | .74*** | 1 | −.32* | .38** | −.18 | .26* | |
| Periodontal health2 | 1 | −.41*** | −.63*** | 1 | −.16 | .44*** | −.16 |
| 4 | −.25 | −.32* | 1 | −.19 | .31 | −.33* | |
| Importance3 | 1 | .36** | .35** | −.16 | 1 | −.04 | .08 |
| 4 | .36** | .38** | −.19 | 1 | .03 | −.02 | |
| Average OHIP-14 Score6 | 1 | −.25 | −.28* | .44*** | −.04 | 1 | −.18 |
| 4 | −.26 | −.18 | .31 | .03 | 1 | −.24 | |
| Perceived saliva4 | 1 | .32* | .29* | −.16 | .08 | −.18 | 1 |
| 4 | .22 | .26* | −.33* | −.02 | −.24 | 1 | |
| Saliva volume5 | 1 | .19 | .19 | −.07 | −.28* | −.03 | .17 |
| 4 | .15 | .21 | −.21 | .04 | −.08 | .43** |
Asterisk/s in cell indicate/s significant differences between women with vs. without AI use at each point in time.
= p < .05;
= p < .01;
= p < .001
Answers ranged from 1= “Poor”, 2 = “Fair”, 3 = “Good”, 4 = “Very good”, to 5 = “Excellent”.
The sum score “Periodontal health” was computed by adding 1 point for each “Yes” response to the 11 periodontal health questions asked (see Table 2 for the wording of the questions). The answers therefore ranged from 0 = “best periodontal health” to 11 = “worst periodontal health”.
Answers ranged from 1 = “Not at all” to 5 = “Very important”.
Answers ranged from 1 = “Very little saliva” to 5 = “Perfect amount of saliva”.
Saliva volume was determined by collecting unstimulated saliva for up to 15 minutes.
OHIP-14- Oral Health Oral Health Impact Profile 14- item short form
DISCUSSION
To the best of our knowledge, these findings provide the first evidence that AIs impact the subjective oral health, periodontal health, OHRQoL, and objective saliva levels in postmenopausal early stage BCa survivors. As the recommended duration of adjuvant AI therapy is 5 years and evolving data may lead to extended therapy of 15 years[38], these findings should draw attention to the potential oral health-related consequences for these BCa patients. It is therefore crucial that future research further investigates these relationships and the dental treatment these patients require over the duration of their AI therapy.
Concerning the results in this study, it is important to note that the participants in both groups were comparable at baseline in terms of their oral health and oral health behavior including the frequencies of tooth brushing, flossing and dental visits. A decrease in periodontal health over time could therefore not be attributed to differential oral hygiene practices in these two groups of women. However, one potential moderator of oral health could be the amount of saliva produced. Overall, AI users produced significantly less saliva than the control group respondents and the difference between the two groups in the amount of saliva increased significantly over time. One potential explanation might be that women on AI therapy have severely reduced estrogen levels. Research has shown that reduced estrogen levels results in poorer saliva production which in turn might affect oral health over the course of time[39]. An additional explanation could be that 18 of the BCa survivors had received chemotherapy before participating in this study. Research by Jensen and colleagues showed that in their group of BCa patients who had undergone chemotherapy, a subgroup of patients still complained about xerostomia even 6 and 12 months after the chemotherapy had ended.[40] Future research should carefully consider the role of chemotherapy on patients’ saliva rate and experiences of dry mouth and potential oral health consequences.
Women on AIs were more likely to report poorer periodontal health than women in the control group and this effect increased over time. Specifically, they were more likely to state that they had loose teeth, had experienced deep cleaning, had been told that they had lost bone around their teeth, and had sore areas on their gums that lasted for more than a week compared to control group respondents. These subjective findings complement a previously described objective periodontal health investigation of these BCa survivors[26]. Research shows that self-reported periodontal health is a valid and reliable assessment of postmenopausal women’s periodontal health status[41].
In addition to exploring subjective oral and periodontal health changes in BCa survivors, another goal of this study was to examine the oral health-related quality of life of early stage BCa survivors with AI therapy over time. The data clearly showed that women on AIs had significantly poorer OHRQoL than women in the control group. These results contradict the results obtained through analysis of the NHANES data[42] were BCa survivors did not have a lower OHRQoL compared to women with no history of cancer. This may be due to the lack of treatment data in NHANES on specific anti-estrogens used and that AI use could not be analyzed.
An additional interesting finding in this prospective, longitudinal study was that the BCa survivors’ OHRQoL was poorest at baseline. This result is not necessarily surprising because previous research showed the power of adaptation in cancer survivors and the resulting improvement in their quality of life[23 ]. It might also explain why Rathbone and colleagues found no impact on OHRQoL among their BCa survivors on bisphosphonates five years after treatment[25]. Although the impact of chemotherapy on OHRQoL was not specifically examined, we enrolled patients at least 3 months after the end of their radiation/chemotherapy treatment in order to avoid including women in the acute recovery period. Future investigations should carefully analyze change in BCa survivors OHRQoL over time as well as the impact of different anti-estrogen treatments and the overall anticancer care plan.
One clear moderating factor of patients’ oral health is a lack of saliva[43]. The fact that objectively assessed saliva production was significantly lower in BCa survivors compared to women in the control group is concerning. While this study only investigated changes over an 18-months long period, research over a longer period of time might show greater significant negative impacts of decreased saliva flow rate on patients’ oral health. The finding that there was a significant correlation at the 18-months appointment between objective saliva volume and perceived saliva should alert researchers to the need to assess the impact of lower saliva production over time not only on patients’ perceptions of saliva but also on their objective oral health and OHRQoL. Xerostomia can have significant effects on reducing patients’ quality of life of and can seriously affect functional capabilities and thus potentially patients’ nutritional status [44, 45].
Limitations
Our study is unique in that it comprehensively examines subjective oral and periodontal health, saliva flow rate and perceptions and OHRQoL of BCa survivors on AI therapy vs. women without cancer diagnoses over an 18-months timespan. However, this study had several limitations. First, this study had a relatively small number of BCa survivors. This fact limits statistical analyses because it does not allow subgroup comparisons such as detailed examinations of the effect of age or different types of AIs on the outcomes. Second, not having been able to collect data before AI therapy was initiated also limits drawing conclusions about participants’ perceptions of these issues before AI use. Third, the patients differed in the duration between their AI start and the baseline survey, Future research should assure that the data collection begins prior to AI initiation to control for potential moderating factors such as the length of AI duration. Fourth, four BCa survivors had such serious side effects to the AI use that their AI therapy was discontinued over the course of our study and they were therefore excluded from additional data collections. It is possible that these women had experienced serious oral health-related effects as well as other side effects. Future research should therefore concentrate on women who have to discontinue AI therapy and analyze the oral health-related effects of this treatment on these patients.
In conclusion, this study showed that BCa survivors treated with AIs have worse subjective oral health, periodontal health, and OHRQoL than women without cancer diagnoses. In addition, their objective saliva volume is decreased, a finding with a potential to significantly impact oral health. Future investigations are needed to focus on defining the impact of BCa therapies on oral health over a longer period of time and with larger samples of BCa survivors, including the oral health-related outcomes of BCa survivors who have to discontinue AI therapy due to serious side effects. These findings draw attention to the importance of assuring that BCa survivors on AIs receive optimal supportive oral care such as education about the management of xerostomia, the prevention of periodontal diseases at the start of AI use and more frequent dental preventive visits while being treated with AI to assure long-term oral health and positive OHRQoL.
Acknowledgments
The study was supported by a pilot grant from the Michigan Institute for Clinical and Health Research UL1RR024986 (University of Michigan, Ann Arbor, MI) and the National Institute of Dental & Craniofacial Research (NIDCR) grants 1K23DEO21779 (Bethesda, Maryland).
Footnotes
Conflict of Interest
The authors report no conflicts of interest related to this study.
References
- 1.Gift HC, Atchison KA. Oral health, health, and health-related quality of life. Med Care. 1995;33:57–77. doi: 10.1097/00005650-199511001-00008. [DOI] [PubMed] [Google Scholar]
- 2.Borgnakke WS, Ylostalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol. 2013;40:S135–152. doi: 10.1111/jcpe.12080. [DOI] [PubMed] [Google Scholar]
- 3.Brennan DS, Teusner DN. Oral health impacts on self-rated general and oral health in a cross-sectional study of working age adults. Community Dent Oral Epidemiol. 2015;43:282–288. doi: 10.1111/cdoe.12152. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JB, Murphy BA. Late effects of cancer and cancer therapy on oral health and quality of life. J Mass Dent Soc. 2010;59:22–27. [PubMed] [Google Scholar]
- 5.Ohrn K. The role of oral sequelae in health-related quality of life of cancer patients. Support Care Cancer. 2002;10:656–658. doi: 10.1007/s00520-002-0404-x. [DOI] [PubMed] [Google Scholar]
- 6.Al-Ansari S, Zecha JA, Barasch A, de Lange J, Rozema FR, Raber-Durlacher JE. Oral Mucositis Induced By Anticancer Therapies. Curr Oral Health Rep. 2015;2:202–211. doi: 10.1007/s40496-015-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonis ST, Fey EG. Oral complications of cancer therapy. Oncology (Williston Park) 2002;16:680–6. discussion 86, 91-2, 95. [PubMed] [Google Scholar]
- 8.Mosel DD, Bauer RL, Lynch DP, Hwang ST. Oral complications in the treatment of cancer patients. Oral Dis. 2011;17:550–559. doi: 10.1111/j.1601-0825.2011.01788.x. [DOI] [PubMed] [Google Scholar]
- 9.Almazrooa SA, Woo SB. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw: a review. J Am Dent Assoc. 2009;140:864–875. doi: 10.14219/jada.archive.2009.0280. [DOI] [PubMed] [Google Scholar]
- 10.Hong CH, Napenas JJ, Hodgson BD, Stokman MA, Mathers-Stauffer V, Elting LS, Spijkervet FK, Brennan MT, Dental Disease Section, Oral Care Study Group, Multi-national Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer. 2010;18:1007–1021. doi: 10.1007/s00520-010-0873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raber-Durlacher JE, Epstein JB, Raber J, van Dissel JT, van Winkelhoff AJ, Guiot HF, van der Velden U. Periodontal infection in cancer patients treated with high-dose chemotherapy. Support Care Cancer. 2002;10:466–473. doi: 10.1007/s00520-002-0346-3. [DOI] [PubMed] [Google Scholar]
- 12.AlJehani YA. Risk factors of periodontal disease: review of the literature. Int J Dent. 2014;2014:182513. doi: 10.1155/2014/182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Haverman TM, Raber-Durlacher JE, Rademacher WM, Vokurka S, Epstein JB, Huisman C, Hazenberg MD, de Soet JJ, de Lange J, Rozema FR. Oral complications in hematopoietic stem cell recipients: the role of inflammation. Mediators Inflamm. 2014;2014:378281. doi: 10.1155/2014/378281. Epub 2014: Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vozza I, Caldarazzo V, Polimeni A, Ottolenghi L. Periodontal disease and cancer patients undergoing chemotherapy. Int Dent. 2015;65:45–48. doi: 10.1111/idj.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueno AC, Ferreira RC, Barbosa FI, Ham BC, Magalhaes CS, Moreira AN. Periodontal care in patients undergoing radiotherapy for head and neck cancer. Support Care Cancer. 2013;21:969–975. doi: 10.1007/s00520-012-1614-5. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes LL, Torres SR, Garnica M, de Souza Gonçalves L, Junior AS, de Vasconcellos ÁC, Cavalcanti W, Maiolino A, de Barros Torres MC. Oral status of patients submitted to autologous hematopoietic stem cell transplantation. Support Care Cancer. 2014;22:15–21. doi: 10.1007/s00520-013-1940-2. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society. Cancer Facts & Figures. 2015 Atlanta 2015 Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf Accessed on 26-01-2016.
- 18.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MF, Stearns V, Winer EP, Somerfield MR, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 20.Montazeri A, Gillis CR, McEwen J. Measuring quality of life in oncology: is it worthwhile? I. Meaning, purposes and controversies. Eur J Cancer Care. 1996;5:159–167. doi: 10.1111/j.1365-2354.1996.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 21.Olufade T, Gallicchio L, MacDonald R, Helzlsouer KJ. Musculoskeletal pain and health-related quality of life among breast cancer patients treated with aromatase inhibitors. Support Care Cancer. 2015;23:447–55. doi: 10.1007/s00520-014-2364-3. [DOI] [PubMed] [Google Scholar]
- 22.Helzlsouer KJ, Gallicchio L, MacDonald R, Wood B, Rushovich E. A prospective study of aromatase inhibitor therapy, vitamin D, C-reactive protein and musculoskeletal symptoms. Breast Cancer Res Treat. 2012;131:277–285. doi: 10.1007/s10549-011-1729-2. [DOI] [PubMed] [Google Scholar]
- 23.Fallowfield L, Cella D, Cuzick J, et al. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol. 2004;22:4261–4471. doi: 10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 24.Inglehart MR, Bagramian RA. Oral Health Related Quality of Life. Quintessence Publishing, Inc; Chigago: 2002. [Google Scholar]
- 25.Rathbone EJ, Brown JE, Marshall HC, et al. Osteonecrosis of the jaw and oral health-related quality of life after adjuvant zoledronic acid: an adjuvant zoledronic acid to reduce recurrence trial subprotocol (BIG01/04) J Clin Oncol. 2013;31:2685–91. doi: 10.1200/JCO.2012.46.4792. [DOI] [PubMed] [Google Scholar]
- 26.Taichman LS, Inglehart MR, Giannobile WV, Kolenic G, van Poznak C. Periodontal Health in Women With Early-Stage Postmenopausal Breast Cancer Newly on Aromatase Inhibitors: A Pilot Study. J Periodontol. 2015;86:906–916. doi: 10.1902/jop.2015.140546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheiham A. Dietary effects on dental diseases. Public Health Nutr. 2001;4(2B):569–591. doi: 10.1079/phn2001142. [DOI] [PubMed] [Google Scholar]
- 28.Lee IC, Shieh TY, Yang YH, Tsai CC, Wang KH. Individuals’ perception of oral health and its impact on the health-related quality of life. J Oral Rehabil. 2007;34:79–87. doi: 10.1111/j.1365-2842.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 29.Marino R, Schofield M, Wright C, Calache H, Minichiello V. Self-reported and clinically determined oral health status predictors for quality of life in dentate older migrant adults. Community Dent Oral Epidemiol. 2008;36:85–94. doi: 10.1111/j.1600-0528.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 30.Locker D. Oral health and quality of life. Oral Health Prev Dent. 2004;2(Suppl 1):247–53. [PubMed] [Google Scholar]
- 31.Sheiham A, Steele JG, Marcenes W, Finch S, Walls AW. The impact of oral health on stated ability to eat certain foods; findings from the National Diet and Nutrition Survey of Older People in Great Britain. Gerodontology. 1999;16:11–20. doi: 10.1111/j.1741-2358.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 32.Enoki K, Matsuda KI, Ikebe K, Murai S, Yoshida M, Maeda Y, Thomson WM. Influence of xerostomia on oral health-related quality of life in the elderly: a 5-year longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:716–721. doi: 10.1016/j.oooo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 34.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Beck DJ, Taylor GW, Borgnakke WS, Page RC, Genco RJ. Self-reported measures for surveillance of periodontitis. J Dent Res. 2013;92:1041–1047. doi: 10.1177/0022034513505621. [DOI] [PubMed] [Google Scholar]
- 35.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11:3–11. [PubMed] [Google Scholar]
- 36.Mandel ID, Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976;8:25–47. [PubMed] [Google Scholar]
- 37.Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, Rayburn LA, Tran HM, Singh AK, Giannobile WV. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, Eisen A, Fehrenbacher L, Farrar WB, Atkins JN, Pajon ER, Vogel VG, Kroener JF, Hutchins LF, Robidoux A, Hoehn JL, Ingle JN, Geyer CE, Jr, Costantino JP, Wolmark N. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–71. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- 39.Streckfus CF, Baur U, Brown LJ, et al. Effects of estrogen status and aging on salivary flow rates in healthy Caucasian women. Gerontology. 1998;44:32–39. doi: 10.1159/000021980. [DOI] [PubMed] [Google Scholar]
- 40.Jensen SB, Mouridsen HT, Reibel J, Brünner N, Nauntofte B. Adjuvant chemotherapy in breast cancer patients induces temporary salivary gland hypofunction. Oral Oncol. 2008;44:162–173. doi: 10.1016/j.oraloncology.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 41.LaMonte MJ, Hovey KM, Millen AE, Genco RJ, Wactawski-Wende J. Accuracy of self-reported periodontal disease in the Women’s Health Initiative Observational Study. J Periodontol. 2014;85:1006–1018. doi: 10.1902/jop.2013.130488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taichman LS, Griggs JJ, Inglehart MR. Periodontal health, perceived oral health, and dental care utilization of breast cancer survivors. J Public Health Dent. 2015;75:148–156. doi: 10.1111/jphd.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawes C, Pedersen AM, Villa A, et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch Oral Biol. 2015;60:863–874. doi: 10.1016/j.archoralbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Locker D. Dental status, xerostomia and the oral health-related quality of life of an elderly institutionalized population. Spec Care Dentist. 2003;23:86–93. doi: 10.1111/j.1754-4505.2003.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 45.Rhodus NL, Brown J. The association of xerostomia and inadequate intake in older adults. J Am Diet Assoc. 1990;90:1688–92. [PubMed] [Google Scholar]


