Abstract
Background
Toxoplasmosis constitutes a large global burden that is further exacerbated by the shortcomings of available therapeutic options, thus underscoring the urgent need for better anti-Toxoplasma gondii therapy or strategies. Recently, we showed that the anti-parasitic action of inorganic nanoparticles (NPs) could, in part, be due to changes in redox status as well as in the parasite mitochondrial membrane potential.
Methods
In the present study, we explored the in vitro mode of action of the anti-T. gondii effect of NPs by evaluating the contributions of host cellular processes, including the tryptophan pathway and hypoxia-inducing factor activity. NPs, at concentrations ranging from 0.01 to 200 µg/ml were screened for anti-parasitic activity. Sulfadiazine and/or pyrimethamine served as positive controls.
Results
We found that interplay among multiple host cellular processes, including HIF-1α activity, indoleamine 2,3-dioxygenase activity, and to a larger extent the tryptophan pathway, contribute to the anti-parasitic action of NPs.
Conclusion
To our knowledge, this is the first study to demonstrate an effect of NPs on the tryptophan and/or kynurenine pathway.
General significance
Our findings deepen our understanding of the mechanism of action of NPs and suggest that modulation of the host nutrient pool may represent a viable approach to the development of new and effective anti-parasitic agents.
Keywords: Hypoxia; Indoleamine 2,3-dioxygenase; Mechanism of action; Nanomedicine; Toxoplasmosis
Graphical abstract
Highlights
-
•
L-tryptophan relieved parasite growth restriction by nanoparticles.
-
•
Nanoparticles modulate host HIF-1α and IDO activity while mildly activating kynurenine pathway.
1. Introduction
Toxoplasma gondii is the causative agent of toxoplasmosis, a parasitic disease that constitutes a serious public health challenge worldwide [1], [2]. T. gondii has low specificity and infects a range of hosts; accordingly, the parasitic disease it causes is common and widespread, affecting more than 60% of the world population [3], [4]. The T. gondii infection is usually asymptomatic in healthy individuals, but can be fatal in pregnant or immunocompromised individuals [5]. In healthy individuals, the T. gondii infection is controlled by the immune system and appropriate medication, but cysts remain in all infected tissues including the brain and these may serve as a source for exacerbations particularly in immunocompromised individuals. Available treatment options for toxoplasmosis patients are limited, but include the use of anti-malarial drugs or antibiotics, which often cause serious side effects including bone marrow suppression and rashes [5]. Consequently, toxoplasmosis remains a large global burden that is further enhanced by the shortcomings of current therapeutic options. These factors drive the search for better anti-T. gondii drugs and/or new approaches to the treatment of toxoplasmosis.
Recently, we showed that inorganic nanoparticles (NPs) including Au, Ag, and Pt nanoparticles caused T. gondii death partially via changes in redox status and parasite mitochondria membrane potential [6]. However, since nanomedicine is still in its infancy, the modes of action of many NPs that appear to be bioactive remain poorly understood [7]. To further our understanding of the mode of action of NPs as it relates to their anti-T. gondii activity [6], we examined the host contribution to the anti-parasitic action of nanoparticles. In our earlier report [6], we determined that oxidative stress plays a part in the anti-parasitic action of NPs, but evidence [6] suggests that modulation of host cellular processes also contributes to the NP-induced anti-parasitic effect. Interestingly, NPs have the potential to affect several cellular signaling processes, including the activity of hypoxia-inducible factor 1 (HIF-1) [8], [9], [10]. HIF-1 is a heterodimer consisting of α and β subunits. It plays a remarkable role in T. gondii survival in the host by regulating pro-parasite genes including glycolytic metabolic genes, transferrin receptor, and vascular endothelial growth factors [11], [12], [13]. Moreover, increased levels of HIF-1 protein and activity are not restricted to hypoxic stress as many pathogens including T. gondii activate HIF-1 [14], and loss of the HIF-1α subunit has been shown to cause a significant reduction in parasite growth at physiological oxygen levels [15].
Furthermore, given that T. gondii is an obligate intracellular parasite, it must satisfy its nutritional needs by scavenging essential nutrients such as tryptophan from its host [16]. Therefore, this may represent an opportunity for the host to naturally restrict parasite growth by modulating nutrient pools. For example, in human cells, the inducible enzyme indoleamine 2,3-dioxygenase (IDO) reduces local tryptophan levels and is therefore able to mediate broad spectrum effector functions including restricting the growth of various clinically relevant pathogens [17]. IDO belongs to the family of heme enzymes that catalyze the oxidative degradation of tryptophan, which the parasite cannot synthesize de novo [18]. Previous studies have shown that the parasite grows unhindered if IDO function is impaired [17] and the suppressive effect of IDO on parasite growth can be reversed by the addition of excess tryptophan to the growth medium [18]. Taken together, these studies suggest that tryptophan starvation may represent a critical anti-parasitic pathway. Moreover, hypoxia with a concomitant increase in HIF-1α level has been linked to reduced IDO expression [17] leading to a sparing effect on the local tryptophan pool that consequently may support parasite growth. Therefore, we asked whether NP treatment affects host cellular processes in a way that helps to restrict parasite growth and sought to determine likely host cellular processes involved in mediating the anti-parasitic action of NPs. The present study provides evidence that modulation of HIF-1α levels, IDO activity, and the tryptophan pathway in host cells partially mediates the anti-parasitic action of NPs.
2. Materials and methods
2.1. Materials
Nanoparticles (NPs), including gold (AuNP, 5 nm), silver (AgNP, 10 nm), and platinum (PtNP, 3 nm), were purchased from Sigma-Aldrich (St. Louis, MO, USA). The NPs were used as supplied after evaluation to confirm the supplier's specifications. The NPs were reconstituted in fresh culture medium prior to each use. L-tryptophan, L-kynurenine, cobalt (II) chloride (CoCl2), 4-(dimethylamino) benzaldehyde, 1-Methyl-D-tryptophan (DMT), and 3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1) were obtained from Sigma-Aldrich. Dexamethasone sodium phosphate and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox) were obtained from Wako Pure Chemicals (Osaka, Japan); (±)3,4-dihydro-3-hydroxy-2,2-dimethyl-4-[(phenylmethyl)amino]-2H-naphtho[2,3-b]pyran-5,10-dione (a naphthoquinone derivative – NQ) was obtained from Cayman Chemicals (Ann Arbor, MI, USA). All reagents were of analytical grade and used as supplied unless otherwise stated.
2.2. Parasite strain
A luciferase-expressing parasite strain, T. gondii RH-2F [19], was used for this study. The parasite was maintained by repeated passages in monolayers of human foreskin fibroblast cells (HFF; ATCC®, Manassas, VA, USA) cultured in Dulbecco's Modified Eagle Medium (DMEM; Nissui, Tokyo, Japan) and supplemented with GlutaMAX™-I (Gibco, Invitrogen, Waltham, MA, USA), 10% (v/v) fetal calf serum (FCS; Gibco, Invitrogen, Waltham, MA, USA), and penicillin and streptomycin (10,000 U/ml; Leicestershire, UK). The number of T. gondii tachyzoites was determined through a luminescence-based assay of β-galactosidase (β-gal) activity expressed by the parasite strain RH-2F. To obtain a purified parasite suspension for the assays, infected cells were passed through a 27-gauge needle to lyse them and the lysates were filtered to remove cell debris. The parasite suspension free of host cell debris was then washed with fresh culture medium. Parasite density was measured with a hemocytometer and adjusted for in vitro experimental infection analysis.
2.2.1. The anti-T. gondii potential of NPs in vitro
NP doses were selected on the basis of our previous findings [6], and in vitro growth inhibition assays were performed as previously described [6]. Briefly, purified parasite suspension plus the NPs (reconstituted in culture medium prior to use) was added to growing HFF monolayers and incubated for 48 h. The untreated but infected cells served as controls, whereas the culture medium only well was used to correct for the background signal. Sulfadiazine (Sigma, St Louis, MO, USA) and/or pyrimethamine (Wako Pure Chemical, Osaka, Japan) were included as positive controls. After the 48-h incubation at 37 °C in a 5% CO2 atmosphere, the viability of the RH-2F parasite strain was determined by assaying for galactosidase activity by using a Beta-Glo luminescent assay kit (Promega, Madison, WI, USA). The assay was performed in triplicate and repeated three times independently. All experiments were performed in 96-well solid white plates (Nunc; Fisher Scientific, Pittsburgh, PA, USA) unless otherwise stated.
2.3. Determination of indoleamine 2,3-dioxygenase (IDO EC 1.13.11.52) activity and kynurenine levels
Briefly, growing HFF monolayers were treated with NPs in the presence or absence of RH-2F infection. After a 24- or 48-h incubation at 37 °C, cells were scrapped and washed three times with cold PBS at 2500×g for 10 min (Cold centrifuge; Hitachi, Japan). The cells were re-suspended in M-PER lysis buffer (Thermo-Fisher, Waltham, MA, USA). The mixture was gently shaken for 10 min and cell debris removed by centrifugation at 14,000×g for 15 min. The supernatant was transferred to a new tube for immediate biochemical analysis. For IDO activity determination, a Sandwich human ELISA assay kit (Cloud-Clone, Houston, TX, USA) was used. The assay was performed according to the manufacturer's instructions.
To determine the concentration of kynurenine in cell supernatant, we used the protocol described by Braun et al. [20] with slight modification. Briefly, 100 μL of 30% trichloroacetic acid (TCA) was added to 100 μL of culture supernatant and incubated for 30 min at 50 °C to hydrolyze N-formylkynurenine to kynurenine. This was then vortexed, and centrifuged at 8500×g for 5 min. An aliquot (100 μL) of the supernatant was then mixed with an equal volume of freshly prepared Ehrlich reagent (2%; 100 mg P-dimethylbenzaldehyde in 5 ml of glacial acetic acid) in a micro-titer plate well (96-well format). After a 10-min incubation at room temperature, the optical density was measured at 492 nm by using a microplate reader (MTP 500; Corona Electric, Hitachinaka, Japan). The level of kynurenine in the culture supernatant was extrapolated from a calibration curve of defined kynurenine concentrations (0–250 μM).
2.4. Chemical induction of hypoxia
Chemical hypoxia was induced in HFF cells by following the procedure described by Wu and Yotnda (2011). Briefly, growing HFF cells were treated with CoCl2 (0.1 µM final concentration) and incubated for 24 h at 37 °C. Successful hypoxia induction was confirmed by measuring the HIF-1α level and comparing it with that of the untreated control.
2.4.1. Determination of hypoxia-inducing factor 1-alpha (HIF-1α) levels
HIF-1α was detected by using a cell-based human ELISA Kit (Cell Biolabs, Inc., San Diego, CA, USA) developed for rapid detection of HIF-1α in fixed cells. The assay was performed according to the manufacturer's instructions. Briefly, growing HFF monolayers in solid white microplate wells (96-well format) were treated with NPs in the presence or absence of RH-2F infection. After a 24-h incubation at 37 °C, cells were fixed, permeabilized, and then neutralized in the well. HIF-1α was then detected with an anti-HIF-1α antibody followed by a horseradish peroxidase-conjugated secondary antibody by luminescence using a microplate reader (GloMax-Multi Detection System, Promega, Madison, WI, USA). To validate the detection assay, 3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1) a known inhibitor of HIF-1α activation [21], [22] and the chemical hypoxia inducer CoCl2 [23] were included in the assay.
2.5. Data analysis
Data were analyzed by using one-way ANOVA (GraphPad Software Inc., San Diego, CA, USA) and are presented as the mean ± standard error of mean (SEM). Comparisons among groups were determined by using Tukey's test. P-values < 0.05 were considered to be statistically significant (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Anti-parasitic action of NPs may be linked to modulation of the host tryptophan pathway
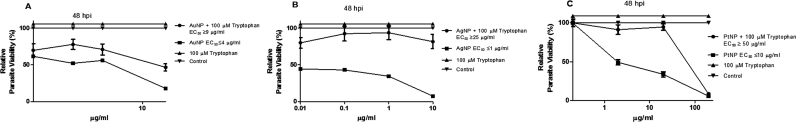
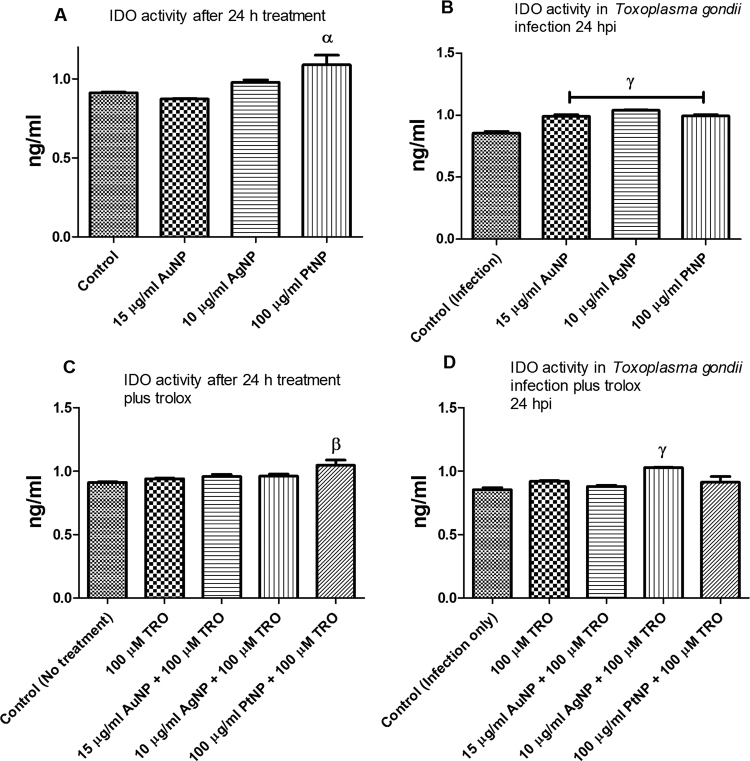
Previously, we determined that a host cell target might partly mediate the anti-parasitic action of NPs [6]. Therefore, here we sought to identify likely host cellular processes involved in mediating the anti-parasitic action of NPs. We found that addition of L-tryptophan to the culture medium relieved the NP-induced restriction on parasite growth (Fig. 1a–c). For all three types of NP (AuNP, AgNP, and PtNP), the EC50 values were significantly increased when L-tryptophan was added to the culture medium. This finding suggests that the host tryptophan pathway might contribute to the anti-parasitic action of NPs. This is consistent with the fact that T. gondii is an auxotroph for tryptophan and restricting access to this nutrient may limit its growth. To further confirm the involvement of the host tryptophan pathway in the anti-parasitic action of NPs, we examined IDO activity in the presence and absence of T. gondii infection and/or NP treatment. NP treatment increases IDO activity in the presence of T. gondii infection (Fig. 2a,b), whereas in the untreated control, IDO activity was reduced in the presence of T. gondii infection compared with when there was no infection. Furthermore, in light of our earlier finding [6] that the anti-parasitic action of NPs was linked to the production of reactive oxygen species (ROS) and that the presence of an antioxidant (trolox) reversed the anti-parasitic effect of NPs, we added trolox to the culture medium to assess its effect on IDO activity. We found that trolox attenuated the effect of NPs on the activity of IDO in the presence of T. gondii infection (Fig. 2c,d). In the absence of T. gondii infection, trolox addition failed to suppress the IDO activity particularly in the presence of PtNP.
Fig. 1.
Parasite viability. Toxoplasma gondii-infected HFF monolayers were co-treated with nanoparticles and/or L-tryptophan at the indicated concentration and parasite viability was determined after a 48-h incubation. [A] Treatment with AuNP and/or 100 µM L-tryptophan; [B] Treatment with AgNP and/or 100 µM L-tryptophan; [C] Treatment with PtNP and/or 100 µM L-tryptophan. Data are expressed as the mean ± standard error of mean (SEM). The experiment was performed in triplicate and repeated three times independently. ‘hpi’ is hours post-infection.
Fig. 2.
Indoleamine 2,3-dioxygenase (IDO) activity. IDO activity was assessed in the absence or presence of Toxoplasma gondii infection and following a 24-h treatment with nanoparticles and/or trolox. [A] IDO activity determined in the absence of T. gondii infection; [B] IDO activity determined in the presence of T. gondii infection; [C] IDO activity determined in the absence of T. gondii infection but in the presence of 100 µM trolox; [D] IDO activity determined in the presence of T. gondii plus 100 µM trolox. Data are expressed as the mean ± SEM (n = 3); α is significant at p < 0.05, β is significant at p < 0.001, γ is significant at p < 0.0001 relative to the control. ‘hpi’ is hours post-infection.
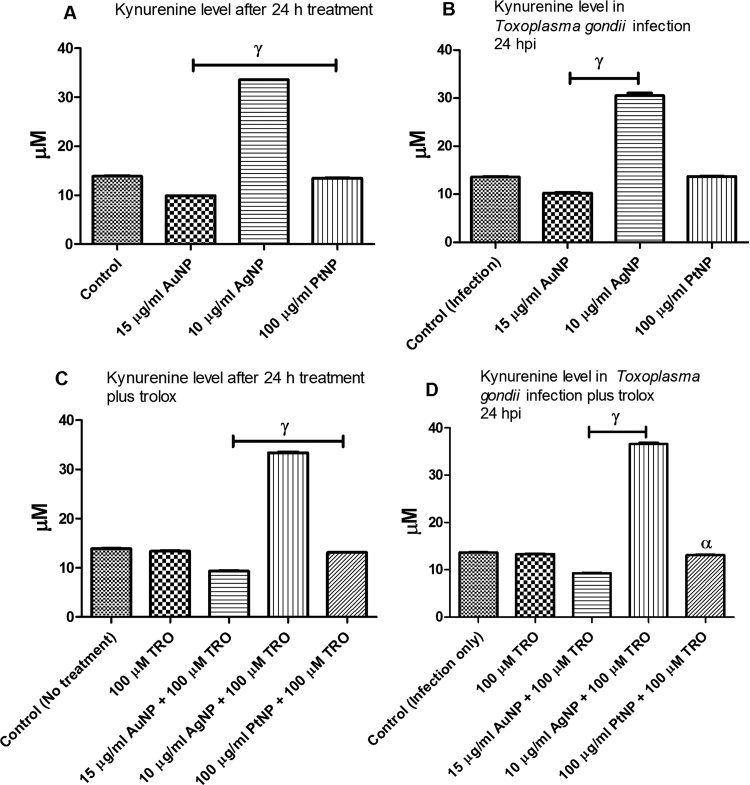
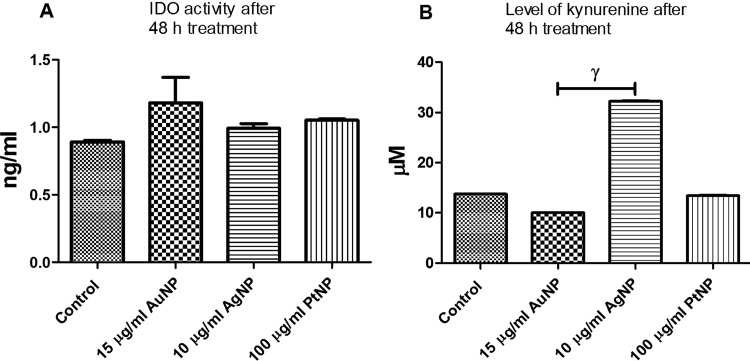
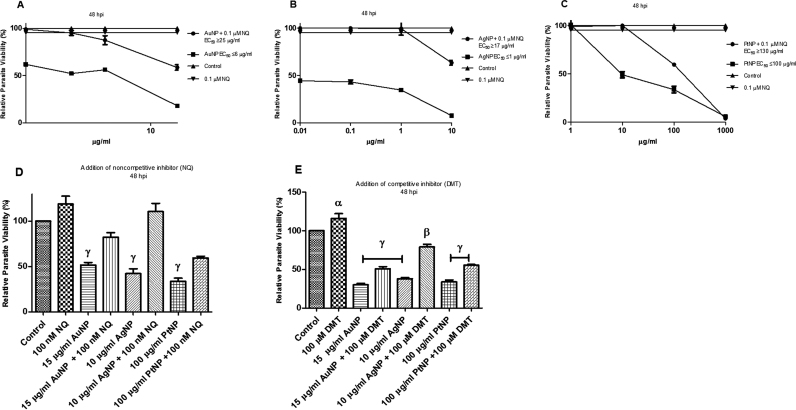
IDO catalyzes the regulatory step in the degradation of tryptophan. The increase in IDO activity by NP treatment may indicate that tryptophan was being degraded. Therefore, we determined the level of kynurenine (the degradation product of tryptophan) in the absence and presence of T. gondii infection and/or NP treatment. We found that only AgNP treatment appreciably increased the level of kynurenine in the absence as well as in the presence of T. gondii infection compared with the control (Fig. 3a,b). Moreover, for AgNP treatment, the addition of trolox had no detectable effect on the level of kynurenine in the absence or presence of T. gondii infection (Fig. 3c,d). While this is consistent with our earlier findings [6] that antioxidants reduce the anti-parasitic action of NPs, the findings herein may indicate that the interplay between cellular oxidative stress and modulation of the host tryptophan pathway might contribute to the NP anti-parasitic action. Furthermore, our data indicate that the effect of NP treatment on IDO activity and the level of kynurenine may not be time dependent because there was no significant change in IDO activity or the level of kynurenine between the 24 and 48 h NP treatments (Figs. 2a, 3a, and 4a, b). When we checked to see whether a non-competitive (naphthoquinone derivative; NQ) or a competitive (1-Methyl-D-tryptophan; DMT) inhibitor of IDO could abate the anti-parasitic action of NPs, we found that both IDO inhibitors ameliorated the NP-induced restriction of parasite growth (Fig. 5a–e). Together, the findings underscore the likely involvement of the host tryptophan pathway in the anti-parasitic effect of NPs.
Fig. 3.
Level of kynurenine. The level of kynurenine was determined in the absence or presence of Toxoplasma gondii infection and following a 24-h treatment with nanoparticles and/or trolox. [A] Level of kynurenine determined in the absence of T. gondii infection; [B] Level of kynurenine determined in the presence of T. gondii infection; [C] Level of kynurenine determined in the absence of T. gondii infection but in the presence of 100 µM trolox; [D] Level of kynurenine determined in the presence of T. gondii plus 100 µM trolox. Data are expressed as the mean ± SEM (n = 3); α is significant at p < 0.05 and γ is significant at p < 0.0001 relative to the control. ‘hpi’ is hours post-infection.
Fig. 4.
Indoleamine 2,3-dioxygenase (IDO) activity and level of kynurenine after a 48-h treatment with nanoparticles and/or trolox. IDO activity and level of kynurenine were determined in the absence of Toxoplasma gondii infection and following a 48-h incubation. [A] IDO activity determined in the absence of T. gondii infection; [B] Level of kynurenine determined in the absence of T. gondii infection. Data are expressed as the mean ± SEM (n = 3); γ is significant at p < 0.0001 relative to the control. ‘hpi’ is hours post-infection.
Fig. 5.
Parasite viability. Toxoplasma gondii-infected HFF monolayers were either singly or co-treated with nanoparticles and (±)3,4-dihydro-3-hydroxy-2,2-dimethyl-4-[(phenylmethyl)amino]−2H-naphtho[2,3-b]pyran-5,10-dione (a naphthoquinone derivative – NQ) or 1-Methyl-D-tryptophan (DMT) at the indicated concentration and parasite viability was determined after a 48-h incubation. [A] Treatment with AuNP and/or 0.1 µM NQ; [B] Treatment with AgNP and/or 0.1 µM NQ; [C] Treatment with PtNP and/or 0.1 µM NQ; [D] Single dose treatment with NPs and/or 0.1 µM NQ; [E] Single dose treatment with NPs and/or 100 µM DMT. Data are expressed as the mean ± SEM. The experiment was performed in triplicate and repeated three times independently. α is significant at p < 0.05, β at p < 0.001 and γ at p < 0.0001 versus control. ‘hpi’ is hours post-infection.
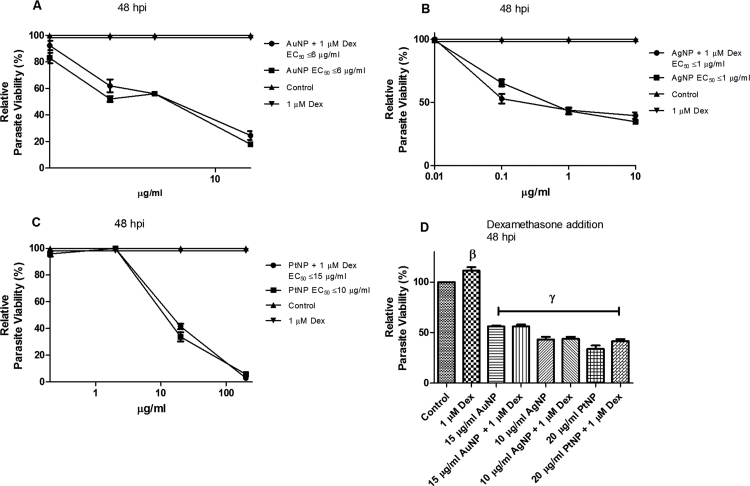
Apart from oxidative stress, activation of IFN-γ could also drive increased IDO activity. Although HFF cells are not known for IFN-γ secretion, as this is mainly secreted by natural killer cells and macrophages, HFF cells do have IFN-γ receptors. Therefore, we examined whether the NP anti-parasitic effect had any connection to IFN-γ by adding dexamethasone (1 µM final concentration) to the assay medium. Previous studies [24], [25], have shown that dexamethasone (a glucocorticoid) can inhibit IFN-γ functions. In the present study, however, addition of dexamethasone had no effect on the NP-induced restriction of parasite growth (Fig. 6a–d), thus suggesting that host IFN-γ has no role in the anti-parasitic action of the NPs.
Fig. 6.
Parasite viability. Toxoplasma gondii-infected HFF monolayers were either singly or co-treated with nanoparticles and dexamethasone (Dex) at the indicated concentration and parasite viability was determined after a 48-h incubation. [A] Treatment with AuNP and/or 1 µM Dex; [B] Treatment with AgNP and/or 1 µM Dex; [C] Treatment with PtNP and/or 1 µM Dex; [D] Single dose treatment with NPs and/or 1 µM Dex. Data are expressed as the mean ± SEM. The experiment was performed in triplicate and repeated three times independently. β is significant at p < 0.001 and γ at p < 0.0001 versus control. ‘hpi’ is hours post-infection.
3.2. NPs modulate HIF-1α levels
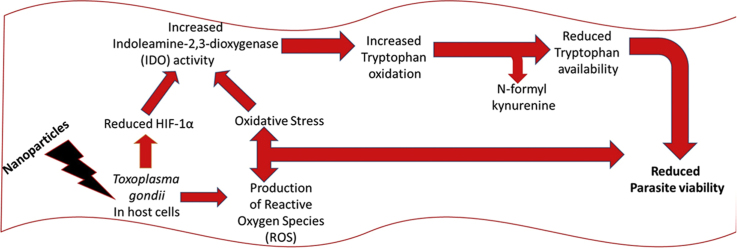
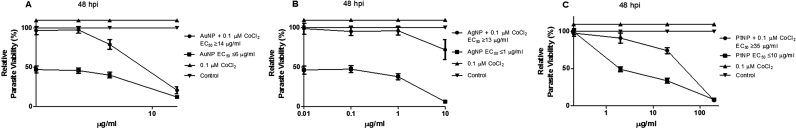
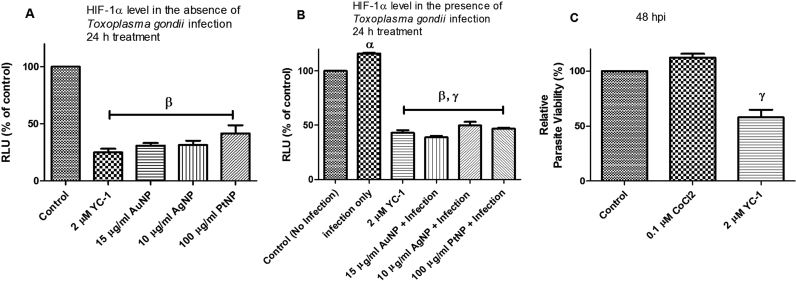
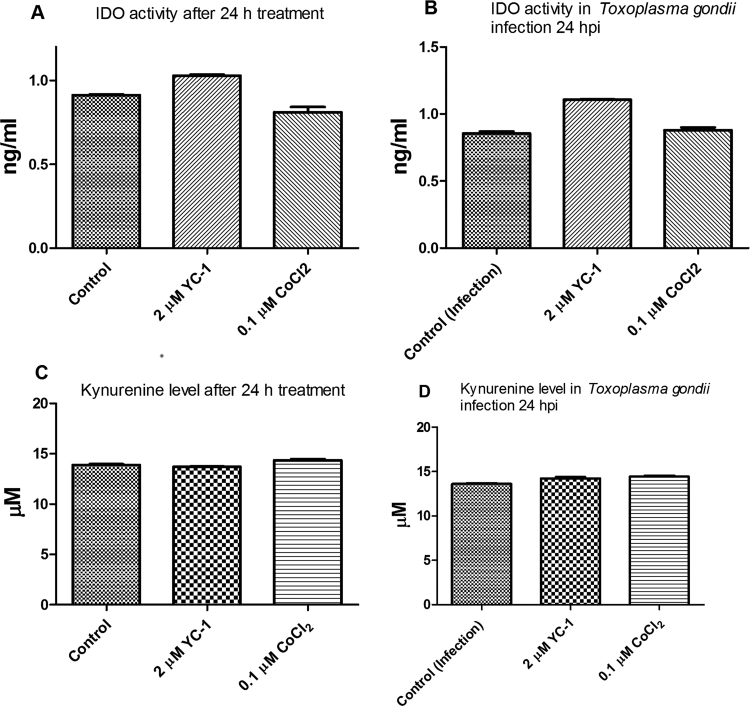
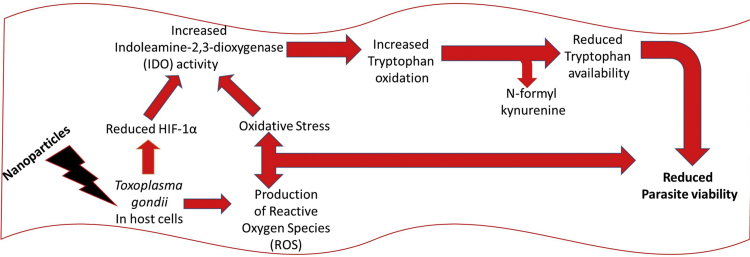
T. gondii infection causes host cell hypoxia and activates host HIF-1α signaling as part of the T. gondii growth and survival strategy [15]. Yet, modulation of HIF-1α activity affects the tryptophan pathway through IDO activity [17]. Therefore, we examined whether HIF-1α activity was involved in mediating the NP anti-parasitic action. First, we added CoCl2 (0.1 µM final concentration) to the culture medium to mimic chemical hypoxia. Addition of CoCl2 mitigated the anti-parasitic action of the NPs and raised the EC50 values (Fig. 7a–c). While these findings suggest involvement of cellular hypoxia, they are not definitive. Therefore, we determined the HIF-1α level in the presence and absence of T. gondii infection and/or NP treatments. The data showed that NPs caused a reduction in the level of HIF-1α both in the absence as well as in the presence of T. gondii infection (Fig. 8a,b). In contrast, the level of HIF-1α was elevated by T. gondii infection as well as by CoCl2 treatment. YC-1 (2 µM final concentration), which was included as positive control, reduced the HIF-1α level, thus validating the detection assay. Moreover, YC-1 restricted T. gondii infection but CoCl2 treatment allowed unhindered parasite growth (Fig. 8c). Further, YC-1 increased IDO activity in the absence as well as in the presence of T. gondii infection (Fig. 9a,b). However, in cells treated with CoCl2, IDO activity decreased both in the absence as well as in the presence of T. gondii infection. The level of kynurenine was not linked to the IDO activity as modulated by either YC-1 or CoCl2 treatment (Fig. 9c,d). Taken together, these findings suggest that the anti-parasitic action of NPs might be due in part to the interplay between multiple host processes (Fig. 10).
Fig. 7.
Parasite viability. Toxoplasma gondii-infected HFF monolayers were either singly or co-treated with nanoparticles and CoCl2 at the indicated concentration and parasite viability was determined after a 48-h incubation. [A] Treatment with AuNP and/or 0.1 µM CoCl2; [B] Treatment with AgNP and/or 0.1 µM CoCl2; [C] Treatment with PtNP and/or 0.1 µM CoCl2. Data are expressed as the mean ± SEM. The experiment was performed in triplicate and repeated three times independently. ‘hpi’ is hours post-infection.
Fig. 8.
Level of hypoxia inducing factor – 1 alpha (HIF-1α) and parasite viability. [A] Level of HIF-1α determined in the absence of Toxoplasma gondii infection after a 24-h treatment; [B] Level of HIF-1α determined in the presence of Toxoplasma gondii infection after a 24-h treatment; [C] Toxoplasma gondii viability determined after a 48-h treatment with 3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1) and CoCl2. Data are expressed as the mean ± SEM. The experiment was performed in triplicate and repeated three times independently. α is significant at p < 0.05 versus control (no infection), β at p < 0.001 versus control (no infection), γ at p < 0.0001 versus infection only. ‘hpi’ is hours post-infection.
Fig. 9.
Effect of 3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1) and CoCl2 on indoleamine 2,3-dioxygenase (IDO) activity after 24 h of treatment with or without trolox. [A] IDO activity determined in the absence of Toxoplasma gondii infection; [B] IDO activity determined in the presence of Toxoplasma gondii infection; [C] level of kynurenine determined in the absence of Toxoplasma gondii infection; [D] level of kynurenine determined in the presence of Toxoplasma gondii infection. Data are expressed as the mean ± SEM (n = 3). ‘hpi’ is hours post-infection.
Fig. 10.
Proposed mechanism of the anti-parasitic action of nanoparticles.
4. Discussion
Although studies have shown that ROS generation by NPs, including AgNP and AuNP, could in part be responsible for the anti-parasitic action of NPs [26], [27], the anti-microbial and/or anti-parasitic mode of action of these NPs remains largely unknown. We recently showed that production of intracellular ROS and by the extension of cellular oxidative stress contributes in part to the anti-T. gondii action of NPs [6]. Herein, we provide evidence that suggests the interplay among multiple host cellular processes partly mediates the anti-parasitic action of NPs. Firstly, addition of tryptophan attenuated the anti-parasitic action of NPs, thus indicating the likely involvement of the host tryptophan pathway in the anti-parasitic action of NPs. NP treatment probably caused depletion of the local tryptophan concentration in the host cells thereby starving the T. gondii of this required nutrient. This is consistent with previous reports of the host tryptophan pathway as a critical anti-parasitic strategy [11], [17], [18].
Our data further showed that NP treatment elevated IDO activity in the presence but not in the absence of T. gondii infection. The reason for this disparity is not known but may be connected to infection-induced alterations in host cell physiology, which suggests that NPs may act differently depending on whether a physiological stressor or stimulator like infection is present. However, the fact that IDO activity was elevated by NP treatment in the presence of T. gondii supports the notion that modulation of the host tryptophan pathway might be involved in the anti-parasitic action of NPs. Although, in the present study, the level of kynurenine did not increase concomitantly with the elevated IDO activity, except for in response to AgNP treatment (which resulted in an appreciable increase in the level of kynurenine), NP-induced elevation of IDO activity suggests involvement of the host tryptophan pathway in the anti-parasitic action of NPs. Moreover, non-competitive and competitive inhibitors of IDO reduced the anti-parasitic effect of NPs, thus providing additional evidence that host tryptophan pathway is probably involved in mediating the anti-parasitic action of NPs. In addition, the changes in IDO activity and kynurenine levels as presented herein suggest that activation of the host kynurenine pathway was subtle and possibly a minor and/or secondary effect.
Addition of trolox abated the NP-induced elevation in IDO activity, suggesting that the anti-parasitic action of NPs might be partially due to interplay among multiple host processes, including but not limited to oxidative stress and modulation of the host tryptophan pathway. The fact that NP treatment caused host tryptophan depletion may be connected to its potential to cause ROS production. Recently, we showed that ROS production and to a larger extent cellular oxidative stress was in part responsible for the anti-parasitic action of NPs [6]. Therefore, it is conceivable that NP-induced oxidative stress may promote modulation of the host tryptophan pathway in the way that affects parasite growth. This line of thought is supported by reports that associate oxidative stress with activation of the kynurenine pathway [28], [29]. In addition, the finding that the anti-parasitic action of NPs was not attenuated in the presence of dexamethasone, a potent inhibitor of IFN-γ, seems to indicate that the increased IDO activity was not a result of IFN-γ–driven functions. This concept is supported by the knowledge that NPs have the capacity to activate IFN-γ expression [30]. We did not determine IFN-γ levels in the present study because HFF cells may be deficient for IFN-γ secretion as this cytokine is mainly secreted by natural killer cells and macrophages. However, it would be logical that if IFN-γ secretion was involved in the NP anti-parasitic action, then the addition of dexamethasone would ameliorate the parasite growth restriction caused by NPs. However, this was not the case, thus suggesting that IFN-γ functions do not contribute to the anti-parasitic action of NPs. Our finding provides additional evidence that mediation of the NP anti-parasitic action through increased IDO activity might be linked to NP-induced oxidative stress. Taken together, our data suggest that subtle modulation of the host tryptophan pathway contributes at least in part to the anti-parasitic action of NPs. This may not be unexpected or surprising if we consider that previous studies [17], [18] have pointed to tryptophan starvation as a viable anti-T. gondii strategy.
HIF-1 is a major regulator of energy homeostasis and cellular adaptation to low oxygen stress. Increases in HIF-1 protein levels and activity are not restricted to hypoxic stress since many pathogens including T. gondii activate host HIF-1 as part of their growth strategies [14], [17]. The finding that NP treatment decreased the level of HIF-1α in the presence and absence of T. gondii infection lends additional support to our belief that the anti-parasitic action of NPs is linked to multiple host cellular processes including but not limited to oxidative stress [6] as well as the modulation of HIF-1α levels and the local pool of host tryptophan. Perhaps NP treatment causes oxidative stress and reduces the level of HIF-1α as part of its primary anti-parasitic action. In this scenario, the elevated IDO activity would be a secondary effect. The NP-induced increase in IDO activity was mild and subtle without a definite concomitant increase in the level of kynurenine except for in response to AgNP treatment. Meanwhile, studies have shown that cellular oxidative stress [28], [29] as well as HIF-1α [17] can modulate IDO activity and to a greater extent activate the kynurenine pathway. Our contention that NP-induced oxidative stress and the reduced level of HIF-1α may together trigger an increase in IDO activity is consistent with previous reports [15], [17], [31] that have linked increased IDO activity and depletion of local tryptophan levels to reduced cellular levels of HIF-1α. Moreover, in the present study, YC-1 (a potent HIF-1α inhibitor) increased IDO activity in the presence as well as in the absence of T. gondii infection. Therefore, it is plausible that the NP-induced reduction in HIF-1α levels contributes to the elevated IDO activity. Additional support that interplay between host HIF-1α and IDO activity contributes to the anti-parasitic action comes from the fact that CoCl2 treatment decreased IDO activity in the present study. This was likely due to the ability of CoCl2 to induce chemical hypoxia [23] and thus increase the expression of HIF-1α, as was the case herein. Interestingly, while YC-1 restricted T. gondii growth, CoCl2 showed no detectable anti-parasitic effect in the present study (at least at the dose that induced chemical hypoxia). Together, our findings implicate modulation of host HIF-1α, IDO activity, and the tryptophan pathway in the anti-parasitic action of NPs. To this end, a proposed mechanism and/or connection might be that NP treatment primarily causes oxidative stress and modulates the level of HIF-1α, which subsequently leads to an increase in IDO activity thus pushing the tryptophan pathway towards kynurenine production. The probable outcome of activating the kynurenine pathway would be a decrease in the local tryptophan pool, which would starve the parasite of an essential nutrient and thus restrict its growth.
5. Conclusion
Our data suggest that interplay among multiple host processes, including modulation of HIF-1α activity, IDO activity, and the tryptophan pathway, contributes to the anti-parasitic action of NPs. To our knowledge, this is the first study to demonstrate an effect of NPs on the tryptophan and/or kynurenine pathway. Further, our findings suggest that NP treatment might produce different outcomes depending on whether a physiological stressor or stimulator like infection is present or not. Taken together, these findings not only deepen our understanding of the mechanism of action of NPs but also demonstrate that modulation of the host nutrient pool is a viable approach to the development of new and effective anti-parasitic agents. Future investigations should include evaluating the anti-parasitic potential of NPs in a mouse or other animal model.
Conflicts of interest
The authors have no competing interests.
Acknowledgements
The research was funded through a JSPS Fellowship to Dr. Adeyemi. This study was supported by grants-in-aid for Scientific Research, Scientific Research on Innovative Areas (3308 and 3407) from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan; by the "Nanotechnology Platform Japan" program, the Program to Disseminate Tenure Tracking System and the Adaptable & Seamless Technology Transfer Program through Target-driven R&D (A-STEP) from the Japan Science and Technology Agency (JST); and by the Ito Foundation.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.07.004.
Appendix B. Supplementary material
Supplementary material
References
- 1.Beck H.P., Blake D., Darde M.L., Felger I., Pedraza-Diaz S., Regidor-Cerrillo J., Gomez-Bautista M., Ortega-Mora L.M., Putignani L., Shiels B., Tait A., Weir W. Molecular approaches to diversity of populations of apicomplexan parasites. Int. J. Parasitol. 2009;39:175–189. doi: 10.1016/j.ijpara.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Sanfelice R.A., da Silva S.S., Bosqui L.R., Miranda-Sapla M.M., Barbosa B.F., Silva R.J., Ferro E.A.V., Panagio L.A., Navarro I.T., Bordignon J., Conchon-Costa I., Pavanelli W.R., Almeida R.S., Costa I.N. Pravastatin and simvastatin inhibit the adhesion, replication and proliferation of Toxoplasma gondii (RH strain) in HeLa cells. Acta Trop. 2017;167:208–215. doi: 10.1016/j.actatropica.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 3.M.W. Black, J.C. Boothroyd, Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64 (200), pp. 607–623. [DOI] [PMC free article] [PubMed]
- 4.Hill D.E., Chirukandoth S., Dubey J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- 5.Kamau E.T., Srinivasan A.R., Brown M.J., Fair M.G., Caraher E.J., Boyle J.P. A focused small-molecule screen identifies 14 compounds with distinct effects on Toxoplasma gondii. Antimicrob. Agents Chemother. 2012;56:5581–5590. doi: 10.1128/AAC.00868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adeyemi O.S., Murata Y., Sugi T., Kato K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017;12:1647–1661. doi: 10.2147/IJN.S122178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yah C.S., Simate G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU. 2015;23:43. doi: 10.1186/s40199-015-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eom H.J., AhN J.M., Kim Y., Choi J. Hypoxia inducible factor-1 (HIF-1)-flavin containing monooxygenase-2 (FMO-2) signaling acts in silver nanoparticles and silver ion toxicity in the nematode, Caenorhabditis elegans. Toxicol. Appl. Pharmacol. 2013;270:106–113. doi: 10.1016/j.taap.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Leite P.E., Pereira M.R., do Nascimento S.C.A., Campos A.P., Esteves T.M., Granjeiro J.M. Gold nanoparticles do not induce myotube cytotoxicity but increase the susceptibility to cell death. Toxicol. In Vitro. 2015;29:819–827. doi: 10.1016/j.tiv.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Yang T., Cao F., Liu Q., Wang X. Silver nanoparticles inhibit hypoxia inducible factor function and cancer cell growth. Nanomed.: Nanotechnol. Biol. Med. 2016;12:554. [Google Scholar]
- 11.Blader I.J., Saeji J.P. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion and virulence. APMIS. 2009;111:458–476. doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE. 2007;407 doi: 10.1126/stke.4072007cm8. (cm8) [DOI] [PubMed] [Google Scholar]
- 13.Zinkernagel A.S., Johnson R.S., Nizet V. Hypoxia-inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- 14.Mandi W.M., Sweeney K.R., Chan D.A., Brown K.M., McMurtrey C., Howard E.W., Giaccia A.J., Blader I.J. Toxoplasma gondii activates hypoxia-inducible factor (HIF) by Stabilizing the HIF-1alpha subunit via type I activin-like receptor kinase receptor signaling. J. Biol. Chem. 2010;285:26852–26860. doi: 10.1074/jbc.M110.147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spear W., Chan D., Coppens I., Johnson R.S., Giaccia A., Blader I.J. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–352. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 16.Blader I.J., Koshy A.A. Toxoplasma gondii development of its replicative niche: in its host cell and beyond. Eukaryot. Cell. 2014;13:965–976. doi: 10.1128/EC.00081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt S.K., Ebel S., Keil E., Woite C., Ernst J.F., Benzin A.E. Regulation of IDO activity by oxygen supply: inhibitory effects on antimicrobial and immunoregulatory functions. PLoS One. 2013;8:e63301. doi: 10.1371/journal.pone.0063301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfefferkorn E.R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishiwa A., Kobayashi K., Takemae H. Effects of dextran sulfates on the acute infection and growth stages of Toxoplasma gondii. Par. Res. 2013;112:4169–4176. doi: 10.1007/s00436-013-3608-8. [DOI] [PubMed] [Google Scholar]
- 20.Braun D., Longman R.S., Albert M.L. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Y., Zhu H., Ling T., Hao B., Zhang G., Shi R. Effects of YC-1 targeting hypoxia-inducible factor 1 alpha in oesophageal squamous carcinoma cell line Eca109 cells. Cell Biol. Int. 2010;35:491–497. doi: 10.1042/CBI20090419. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Wu D., Yotnda P. Induction and testing of hypoxia in cell culture. J. Vis. Exp. 2011;54:2899. doi: 10.3791/2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawliczak R., Logun C., Madara P., Barb J., Suffredini A.F., Munson P.J., Danner R.L., Shelhamer J.H. Influence of IFN-γon gene expression in normal human bronchial epithelial cells: modulation of IFN-γ effects by dexamethasone. Physiol. Genom. 2005;23:28–45. doi: 10.1152/physiolgenomics.00011.2005. [DOI] [PubMed] [Google Scholar]
- 25.Hu X., Li W.P., Meng C., Ivashkiv L.B. Inhibition of IFN-gamma signaling by glucocorticoids. J. Immunol. 2003;170:4833–4839. doi: 10.4049/jimmunol.170.9.4833. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad A., Syed F., Shah A. Silver and gold nanoparticles from Sargentodoxa cuneata: synthesis, characterization and antileishmanial activity. RSC Adv. 2015;5:73793–73806. [Google Scholar]
- 27.Saini P., Saha S.K., Roy P. Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Exp. Parasitol. 2016;I60:39–48. doi: 10.1016/j.exppara.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Anisman A. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J. Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 29.Stoy N., Mackay G.M., Forrest C.M., Christofides J., Egerton M., Stone T.W., Darlington L.G. Tryptophan metabolism and oxidative stress in patients with Huntington's disease. J. Neurochem. 2005;93:611–623. doi: 10.1111/j.1471-4159.2005.03070.x. [DOI] [PubMed] [Google Scholar]
- 30.Luo Y., Chang L.W., Lin P. Metal-based nanoparticles and the immune system: activation, inflammation, and potential applications. BioMed. Res. Int. 2015;2015 doi: 10.1155/2015/143720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blader I.J., Manger I.D., Boothroyd J.C. Microarray analysis reveals previously unknown changes in Toxoplasma gondii–infected human cells. J. Biol. Chem. 2001;276:24223–24231. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material