Abstract
Objective
Approximately 200,000 women are diagnosed with breast cancer in the U.S. every year. These patients commonly suffer from oral complications of their cancer therapy. The purpose of this study was to assess dental hygienists’ knowledge and professional practice related to providing care for breast cancer patients.
Methods
A pre-tested 43-item survey was mailed to a random sample of 10% of all licensed dental hygienists in the State of Michigan (N=962). The survey assessed the respondents’ knowledge of potential oral complications of breast cancer treatments as well as their professional practices when treating patients with breast cancer. After two mailings, the response rate was 37% (N=331). Descriptive and inferential analyses were conducted using SAS.
Results
Many dental hygienists were unaware of the recommended clinical guidelines for treating breast cancer patients and lacked specific knowledge pertaining to the commonly prescribed anti-estrogen medications for pre-and postmenopausal breast cancer patients. Over 70% of the respondents indicated they were unfamiliar with the AI class of medications. Only 13% of dental hygienists correctly identified the mechanism of action of anti-estrogen therapy. Dental hygienists reported increased gingival inflammation, gingival bleeding, periodontal pocketing, xerostomia and burning tissues in patients receiving anti-estrogen therapies. Less than 10% believed that their knowledge of breast cancer treatments and the oral side effects is up to date.
Conclusions
Results indicate a need for more education about the potential oral effects of breast cancer therapies and about providing the best possible care for patients undergoing breast cancer treatment.
Keywords: Breast cancer, Anti-estrogen therapy, Dental hygienist, Oral health, Knowledge, Professional behavior, Chemotherapy, Education
INTRODUCTION
Over 200,000 women are diagnosed with breast cancer in the United States annually.1 Breast cancer occurs more frequently in postmenopausal women and the median age at diagnosis is 61.2 The etiology of most breast cancers is unknown. However, risk factors for the disease have been established, including gender, increasing age, family history of breast cancer, early menarche, late menopause, ethnicity, alcohol use, and genetic risk factors.3 The majority of women diagnosed with breast cancer can expect an excellent outcome, with a 5-year survival rate above 80%.2 Therefore long-term survivorship issues, including those related to oral health, are important components of breast cancer care and follow-up.
Range of breast cancer treatments
The rationale and selection of breast cancer treatments are complex and based on many prognostic and predictive factors4 including tumor histology and grade, the clinical and pathologic stage, lymph node involvement, tumor hormone receptor content, tumor HER2 status, comorbid conditions, age and patient preference.5 Table 1 highlights how menopausal status and hormone receptor status influence care. The National Comprehensive Cancer Network provides comprehensive descriptions of currently accepted approaches for breast cancer treatment.4
Table 1.
Broad treatment options for early stage breast cancer patients4
| Menopausal Status | Estrogen Receptor Status* | Surgical Treatment# | Chemotherapy† | Radiation Therapy‡ | Endocrine Therapy§ |
|---|---|---|---|---|---|
| Premenopausal | ER + | Mastectomy or Breast conserving | Chemotherapy† | Radiation‡ | Tamoxifen Ovarian suppression with or without an aromatase inhibitor |
| Postmenopausal | ER + | Mastectomy or Breast conserving | Chemotherapy† | Radiation‡ | Tamoxifen or aromatase inhibitor |
| Premenopausal Or Postmenopausal | ER − | Mastectomy or Breast conserving | Chemotherapy† | Radiation‡ |
Estrogen-receptor (ER) status (ER positive (ER+) or ER negative (ER−);
Surgical Treatment: Considered based upon tumor size;
Chemotherapy: May occur either before (neoadjuvant) or after surgical treatment depending upon a variety of clinical, pathologic, and genetic factors;
Radiation Therapy: Considered based upon surgical procedures and stage of disease;
Endocrine Therapy: Considered when the tumor expresses either the estrogen or progesterone receptor
Surgery for breast cancer addresses local control and provides tissue for analysis of staging and biomarkers. Depending upon the cancer stage, the histologic and molecular profile of the tumor, systemic adjuvant therapy may be recommended to decrease the risk of developing distant metastases.6 Systemic therapies may include chemotherapy, trastuzumab, or antiestrogen therapy.7,8 These therapies may be considered either before or after surgery based on the individual patient’s needs and goals. Radiation therapy (radiotherapy) to the breast, chest wall, and/or local lymph node regions may be provided as another means of obtaining local control but does not replace surgery which is the foundation of the management of early stage breast cancer.
Approximately 75% of breast cancers express the estrogen and/or progesterone receptors (ER, PR).9, 10 Breast cancer can depend on ER/PR signaling for tumor growth and survival.11 Targeting ER/PR with anti-estrogen therapies has been shown to decrease the risk of breast cancer recurrence.7 In premenopausal women, therapy may ablate ovarian estrogen production by surgery, radiation or chemical means with luteinizing-hormone releasing-hormone inhibitors (goserelin or leuprolide). More commonly, oral adjuvant systemic anti-estrogens such as Tamoxifen are used. Postmenopausal women may be prescribed either Tamoxifen or a aromatase inhibitor (AI) (FDA approved drugs: anastrozole, exemestane or letrozole).12 While breast cancer occurs in only 1% of males, nearly 90% of their tumors are ER +. Male breast cancer patients are typically treated similarly to women with surgery, followed by systemic therapy (chemotherapy and/or anti-estrogen therapy) plus or minus radiation based on the tumor stage and biomarkers.13
Risks of Breast cancer therapy
Acute side effects and long term complications of breast cancer therapies have a marked impact on the patients’ oral health, oral health-related quality of life14–16 and on therapy compliance. Cancer patients undergoing chemotherapy often suffer from oral complications including oral/pharyngeal mucositis, pain, xerostomia and dental caries, and are at an increased risk for opportunistic bacterial, fungal, and viral infections as a result of chemotherapy-induced immune suppression.17–19 Patients are also at risk for osteonecrosis20 and periodontal tissue changes including gingivitis, gingival bleeding, and periodontal infection.21–24 Patients undergoing radiotherapy may complain of transient xerostomia. Table 2 displays common oral side effects of breast cancer treatments.
Table 2.
Oral sequelae of common cancer treatments
| Cancer Treatment | Oral complication |
|---|---|
| Chemotherapy | Mucositis Xerostomia Fungal Infection (Candida) Viral infection (HSV) Gingival Bleeding Periodontal Infection |
| Radiotherapy | Transient xerostomia |
| Intravenous Bisphosphonates* | Osteonecrosis |
A rare condition which has generally been related to dento-alveolar surgery
Breast cancer therapies can impact skeletal bone mass. Chemotherapy is associated with premature ovarian failure25 and results in accelerated loss of bone mineral density (BMD).26, 27 In addition, anti-estrogen therapies are associated with stimulating bone loss. Changes in BMD depend on menopausal status as well as on the class of drug used.28, 29 Premenopausal breast cancer patients taking the estrogen receptor antagonist Tamoxifen are at an increased risk for reduced skeletal BMD.30 In postmenopausal women, Tamoxifen has been shown to maintain or slightly increase BMD.31 In contrast to the bone-preserving effect of Tamoxifen in post-menopausal bone, AI use is associated with significant loss of BMD.32 To mitigate the bone loss effect of cancer therapies, bisphosphonates may be prescribed.33 Importantly, an association has been established between estrogen deficiency, decreases in skeletal BMD, and oral health. Estrogen deficiency among postmenopausal women may increase risk for periodontal diseases, tooth loss, decreased salivary flow, oral dysesthesia, alterations in taste and burning mouth syndrome.34, 35 As estrogen plays a key role in maintaining bone and soft tissues of the oral cavity, drugs that affect the production and/or binding of estrogen to its receptor may also affect bone and/or soft tissue of the oral cavity.36
Provision of oral care to breast cancer patients
Dental hygienists often serve as primary oral health care providers for women undergoing breast cancer therapy.37 As prevention specialists, dental hygienists are in a strategic position to provide information and care to women and men undergoing therapy for breast cancer.37 Oral assessment prior to and during active treatment (chemotherapy and radiotherapy), and following therapy is a critical aspect of oral health care for cancer patients.38,39, 40 The National Institute of Dental and Craniofacial Research (NIDCR) indicates that an oral evaluation is necessary prior to cancer therapy for the identification of any outstanding dental needs that could increase the risk or severity of oral complications during breast cancer treatments. For patients undergoing chemotherapy, communication between the oncology and dental teams is essential for the safety of the patient41 It is important to determine the patient’s hematologic status prior to treatment.41 In addition, there are some cases where antibiotic prophylaxis may be recommended prior to dental procedures for patients with Port-A-Caths or indwelling central venous catheters to limit secondary infections associated with the immuno-suppression produced by cancer therapies.42, 43 As there appears to be a void in clinically validated premedication guidelines specific to these devices, inter-professional communication and collaborative practice is needed.
Obtaining blood pressure measurement is another important aspect of dental care for the breast cancer patient. Breast cancer patients who receive axillary surgery and/or radiation are at risk for lymphedema. Clinical recommendations include the avoidance of blood pressure measurements on the affected arm(s) of patients who have undergone lymph node removal to mitigate the risk of lymphedema associated with squeezing the lymph channels by a blood pressure cuff.44–46
While oral health guidelines for cancer patients have been in place for over 20 years, research is scarce concerning dental hygienists’ provision of dental care for breast cancer patients.47, 48 Currently, no information is available specific to dental hygienists’ knowledge of the potential oral complications related to anti-estrogen breast cancer therapies. The aim of this study was to determine dental hygienists’ knowledge and professional practice concerning care of patients undergoing treatments for breast cancer. In addition, this study also explored which demographic factors are associated with dental hygienists’ knowledge of cancer therapies.
METHODS AND MATERIALS
Study design
This study was a cross-sectional survey of a random sample of licensed dental hygienists in the State of Michigan. The State of Michigan was chosen due to the large numbers of registered dental hygienists residing here. This research was submitted and determined to be exempt from full board review by the Institutional Review Board for the Health and Behavioral Sciences at the University of Michigan.
Sample selection
A list of the 10,126 dental hygienists licensed in the State of Michigan was obtained from the Michigan State Board of Dentistry in March of 2011. Dental hygienists with out-of-state mailing addresses were excluded from the sample (n = 502) as they did not fit the inclusion criteria. A 10 % random sample was selected for this study (n = 962) from the remaining licensed dental hygienists.
Instrument
The survey instrument was developed based on information from a literature search and the advice of several faculty members at the University of Michigan, School of Dentistry. Content experts in breast oncology, oral medicine and public health assessed the validity of the survey. The survey was pre-tested with 10 dental hygienists who worked in private dental practices in Michigan. The survey’s test-retest reliability was evaluated by twice administering the survey 2 weeks apart. Pearson’s correlation coefficient was applied to the intra-class correlation (ICC) coefficient at the individual level. Reproducibility was strong, with ICC values as follows: anti-estrogen therapies: 0.76, provision of care: 0.83, breast cancer risk factors: 0.71,: clinical recommendations 0.81, overall: 0.88.
The survey consisted of 43 questions concerning the respondents’ demographic background, practice characteristics, care recommendations for breast cancer patients, and a series of items assessing their knowledge concerning risk factors for breast cancer, knowledge of anti-estrogen cancer therapies and possible oral complications related to anti-estrogen cancer therapies, and the use of bisphosphonates as related to breast cancer therapy. Radiation therapy, other than for patients with head and neck cancer, has not shown a significant impact on oral health.49 Therefore, no questions concerning potential oral complications or care recommendations were included. The survey contained both closed and open ended questions. Specific open-ended questions were asked concerning oral complications related to cancer therapy.
Data collection
Data were collected using a self-administered questionnaire mailed with a cover letter and a return stamped, addressed envelope to a random sample of registered dental hygienists in the State of Michigan in May of 2011. Alternatively, participants had the option to respond to a web-based survey. Respondents were asked to return the questionnaire within nine days of receipt. By returning the questionnaire, the dental hygienists implicitly provided their consent to participate in this research. Confidentiality for hygienists responding to the web-based survey was assured by using a SSL encrypted data network. Before being mailed, the surveys were coded with a unique number so that one-follow up mailing could be sent to the non-respondents. This second mailing, containing a different cover letter, a second copy of the questionnaire, and a self-addressed stamped return envelope, was sent approximately 4 weeks after the first mailing to all non-respondents.
Statistical analysis
The data were entered into Excel spreadsheets twice to allow for validation of correct data entry. The data were then imported into SAS for Windows, Release 11 (SAS). Frequency and percentile distributions as well as means were calculated for all responses. Chi square values and probabilities were calculated for appropriate questions to determine the independence of variables from each other. To measure dental hygienists’ knowledge, Likert type items were used with a five-point answer scale ranging from “strongly agree,” “agree,” “neutral,” “disagree,” to “strongly disagree.” A “don’t know” answer category was provided for these questions. For purposes of this study, the “strongly agree” and “agree” responses were added to identify the degree of agreement with the statements and the “disagree” and “strongly disagree” responses were added to identify any disagreement with a statement. Statistical significance was judged at the level of p<0.05.
RESULTS
Respondent characteristics
Fifty-seven of the 962 surveys mailed to randomly selected dental hygienists on the State of Michigan license list were returned due to invalid addresses. The total number of valid surveys returned was 331 (15 submitted by a secure web site and 316 hard copy surveys), which represented a final response rate of 37% (331/905). The demographic characteristics of the sample are summarized in Table 3. The majority of the respondents were over 25 years of age, had a certificate/associate’s level degree (69%), worked full time (72%) in a general dental practice (83%) and had graduated before 1998. Five percent of the dental hygienists reported a diagnosis of breast cancer.
Table 3.
Socio-demographic and breast cancer knowledge characteristics of Michigan Dental Hygienists.
| Background Characteristic | Number* (N=330) |
Percentages ** |
|---|---|---|
| Age | ||
| 20–25 | 11 | 3% |
| 26–35 | 66 | 21% |
| 36–45 | 67 | 21% |
| 46–50 | 49 | 15% |
| 51–55 | 65 | 20% |
| > 55 | 68 | 21% |
| Level of Education | ||
| Diploma/Certificate/Associates | 222 | 69 % |
| Bachelors | 94 | 26% |
| Masters/Doctorate | 15 | 5% |
| Year of Graduation | ||
| Graduated before 1985 | 106 | 34% |
| Graduated between 1985–1998 | 101 | 33% |
| Graduated after 1998 | 104 | 33% |
| Currently Employed | ||
| Yes – Full Time | 238 | 72% |
| – Part Time | 73 | 22% |
| No | 19 | 6% |
| Type of Practice | ||
| General Practice | 270 | 83% |
| Periodontal Practice | 17 | 5% |
| Dental/Dental Hygiene School | 12 | 4% |
| Community Health Agency | 10 | 3% |
| Public School | 5 | 2% |
| Hospital/Nursing Home | 2 | 1% |
| Treated Patient with Breast Cancer | ||
| Yes | 314 | 95% |
| No | 17 | 5% |
| Personal Diagnosis of Breast Cancer | ||
| Yes | 18 | 5% |
| No | 309 | 95% |
| Breast Cancer Knowledge and Assessment | ||
| Breast cancer is most common cancer | ||
| Yes | 160 | 51% |
| No | 39 | 12% |
| Unsure | 118 | 37% |
| Attended CE Course with Breast Cancer Component | ||
| Yes | 21 | 7% |
| No | 298 | 93% |
| Assess Family History of Cancer | ||
| Yes | 65 | 21% |
| No | 251 | 79% |
| Assess patient history of cancer | ||
| Yes | 288 | 90% |
| No | 31 | 10% |
Note:
Frequencies for a characteristic may not add to N=330 due to missing data.
Percentages for the characteristics may not add to 100% due to rounding.
In addition, Table 1 presents knowledge regarding breast cancer prevalence and patient history assessment patterns of dental hygienists. Only 51% of the respondents knew that breast cancer is the most common cancer among women in the United States. The majority of respondents assess their patient’s history of cancer. Overall, dental hygienists were knowledgeable about the risk factors for breast cancer and were aware that smoking, alcohol use, and obesity were modifiable risk factors for breast cancer. Only 6% of the respondents indicated distributing prevention literature related to breast cancer in their dental practice (data not tabulated).
Knowledge of patient care and current breast cancer therapies
Ten items assessed the respondents’ knowledge concerning the care for breast cancer patients (Table 4). These items had a Likert-style format and were formulated in such a way that an agreement with the statement indicated a correct answer. While 56% of the dental hygienists knew that a consultation with an oncologist concerning a patient’s cell count should be done prior to dental appointments, and 55% knew that breast cancer patients should not have blood pressure measurements taken on the side where lymph nodes were removed, only 25% were aware that breast cancer patients may develop breast-cancer-related metastases as radiolucent areas in the mandible or maxilla. Only 20% of dental hygienists were aware that breast cancer patients may need to be pre-medicated prior to dental treatment while having a port for chemotherapy.
Table 4.
Dental hygienists’ responses concerning their knowledge of breast cancer patient care and anti-estrogen cancer treatments
| Patient Care | Strongly Agree/Agree n (%) |
Neutral n (%) |
Strongly Disagree/Disagree n (%) |
Don’t Know n(%) |
|---|---|---|---|---|
| Consultation with an oncologist concerning a breast cancer patient’s white blood (neutropenia) cell count should be done prior to dental appointments to avoid potential dental infections. | 180 (56%) |
27 (8%) |
33 (10%) |
83 (26%) |
| Breast cancer patients should avoid having blood pressure measurements taken on side where lymph nodes were removed. | 177 (55%) |
16 (5%) |
36 (11%) |
93 (29%) |
| Breast cancer patients may develop breast cancer related metastases as radiolucent areas in the mandible or maxilla. | 80 (25%) |
27 (8%) |
15 (5%) |
198 (62%) |
| Breast cancer patients need to be pre-medicated prior to dental treatment while having a port for chemotherapy. | 66 (20%) |
14 (4%) |
129 (40%) |
113 (36%) |
| Anti-estrogen Therapy | ||||
| The current anti-estrogen therapy for premenopausal women with estrogen receptor + breast cancer is Tamoxifen. | 69 (21%) |
28 (9%) |
19 (6%) |
207 (64%) |
| The current anti-estrogen therapy for postmenopausal women with estrogen receptor + breast cancer is Tamoxifen and/or aromatase inhibitors. | 66 (21%) |
22 (7%) |
10 (3%) |
224 (70%) |
| Breast cancer patients may report increased musculoskeletal pain including decreased grip strength while on aromatase inhibitor drugs. | 59 (18%) |
24 (8%) |
3 (1%) |
235 (73%) |
| Aromatase inhibitors given to breast cancer patients act by severely decreasing anti-estrogen activity. | 42 (13%) |
13 (4%) |
9 (3%) |
257 (80%) |
| Bisphosphonate Use | ||||
| Bisphosphonates (Fosamax, Boniva, Actonel) are commonly prescribed for prevention and treatment of osteoporosis. | 251 (81%) |
13 (4%) |
37 (12) |
22 (7%) |
| Bisphosphonates are commonly prescribed to women prior/while using aromatase inhibitors. | 45 (14%) |
21 (7%) |
6 (2%) |
249 (77%) |
Concerning the respondents’ knowledge of current anti-estrogen for breast cancer patients, only 21% knew that current guidelines indicate the use of Tamoxifen for pre-menopausal women with ER + cancer, and that AIs and/or Tamoxifen are the current standards of care for postmenopausal breast cancer patients. The majority of the respondents did not know that potential side effects of AIs include increased musculoskeletal problems (83%), increased need for bisphosphonate use (77%), or that AIs act by severely decreasing anti-estrogen activity (87%).
While 81% of the respondents were aware that bisphosphonates are commonly prescribed for the prevention or treatment of osteoporosis, only 14% knew that bisphosphonates are commonly prescribed to breast cancer patients using AIs.
Treatment recommendations for breast cancer patients
Several questions were asked about oral care recommendations that dental hygienist provide for breast cancer patients at different stages of cancer treatment (Table 5). For patients receiving dental care during chemotherapy, the majority of respondents reported provision of oral hygiene instruction, use of mouth rinses, palliative care for xerostomia, and use of fluoride rinses. However, only half of the respondents provided nutrition counseling for breast cancer patients during this segment of their therapy. The most frequently recommended mouthwash mentioned in the open-ended comment section was MI paste, a rinse containing the milk protein. Dental hygienists were less likely to provide treatment recommendations for breast cancer patients receiving anti-estrogen therapy. Oral hygiene instruction was provided by only 72% of the respondents and only 64% recommended mouth rinses or fluoride rinses for these patients.
Table 5.
Dental hygienists’ recommendations for breast cancer patients during chemotherapy and anti-estrogen therapy (n=330).
| Provided/recommended treatment | Which clinical dental care do you provide/recommend for patients receiving: | |||
|---|---|---|---|---|
| Chemotherapy | Anti-estrogen Therapy (Tamoxifen & Aromatase Inhibitors) | |||
| N | Percentages | N | Percentages | |
| Xerostomia alleviating strategies such saliva substitutes | 293 | 93% | 206 | 66% |
| Fluoride treatments/toothpastes/rinses | 291 | 92% | 200 | 64% |
| Oral Hygiene instruction | 287 | 91% | 224 | 72% |
| Nutrition counseling | 180 | 57% | 132 | 42% |
Knowledge of potential complications related to breast cancer therapies
Figure 1 shows that 60% of dental hygienists knew that mucosal changes are a common oral complication of chemotherapy. Nearly 80% of respondents correctly stated that xerostomia was related to chemotherapy, and 71% noted a potential increased risk for gingival tissue changes during chemotherapy. While increased risk of osteoporosis was noted as a potential long-term complication of chemotherapy by 32% of the respondents, even fewer respondents knew that osteoporosis could be related to Tamoxifen use (12%) or AI use (10%), depending on menopausal status. Few respondents knew that xerostomia or gingival changes, dental caries, or mucosal changes are potential complications of the use of Tamoxifen or AIs.
Figure 1.
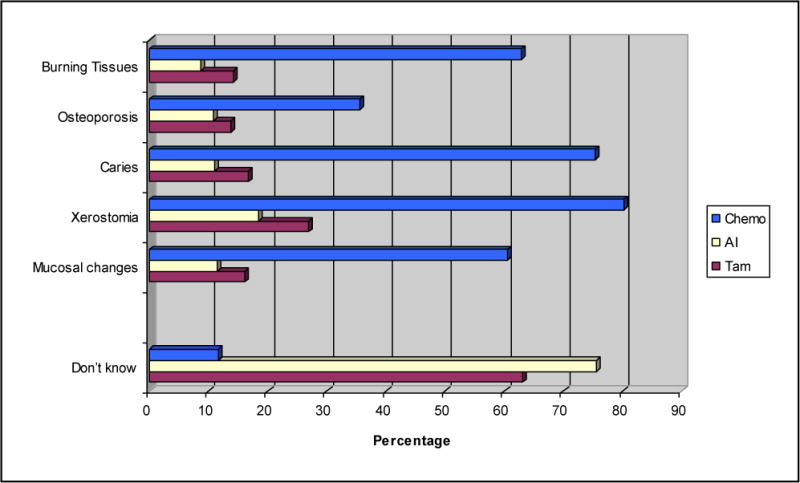
Dental hygienists’ knowledge of possible complications associated with breast cancer treatments
Specific reported conditions related to anti-estrogen cancer therapy
When respondents were asked to share specific oral/other complaints related to anti-estrogen therapy that either patients had reported or that they themselves had identified, 14% of dental hygienists reported oral side effects of Tamoxifen and 7% reported oral side effects related to the use of AIs (Table 6). Common oral health-related complaints of patients using either an AI or Tamoxifen included increases in gingival inflammation, gingival bleeding, xerostomia, and burning sensations in oral tissues. A dental hygienist reported oral side effect unique to Tamoxifen use was the report of increased dental caries. Patient-reported complaints specific to AI use included generalized joint pain and hand and wrist pain. This type of pain was related to difficulties with tooth brushing. A specific patient complaint related to Tamoxifen use was jaw pain (Table 6).
Table 6.
Responses concerning oral conditions associated with anti-estrogen therapy (n=276)
| Anti-estrogen treatment | Dental Hygienists indicating treating patients with oral side effects | Specific reported side effects* | |
|---|---|---|---|
| N | Percentages | ||
| Aromatase Inhibitors | 17 | 7% | Gingival inflammation Xerostomia Burning tissues/mouth Joint pain Pain in hands – difficulty brushing Increase in periodontal pocketing |
| Tamoxifen | 39 | 14% | Gingivitis Burning tissues/mouth Bleeding on probing Xerostomia Increased caries Pain in jaws Increase in periodontal pocketing |
Specific oral/other complaints identified by the dental hygienist or reported by a patient with breast cancer using endocrine therapy.
Perceptions of continuing education
Less than 10% of respondents considered their knowledge about breast cancer risk factors and treatments up to date. Only 7% of dental hygienists reported having taken a continuing education class that had included information on potential oral complication of cancer treatments within the last 5 years. The majority of dental hygienists (95%) desired further education in this area. The most popular choices for updating knowledge were continuing education lectures (80%), reading journal articles (28%) and receiving specific topic booklets with self-tests (41%) (Data not tabulated).
Socio-demographic and practice factors and knowledge of oral consequences of breast cancer treatment
To assess the impact of background characteristics on dental hygienists level of knowledge related to the effects of breast cancer treatments on their patients’ oral health, bivariate analyses were performed (Table 7). Respondents who had been diagnosed with breast cancer (p=0.004) and respondents who asked their patients about their family history with cancer (p=0.026) were more likely to indicate that their knowledge in this area was up to date than other dental hygienists.
Table 7.
Associations between demographic/professional attributes and dental hygienists’ knowledge of breast cancer and breast cancer treatments (n = 318)
| Background Characteristic | Knowledge of Breast Cancer Treatments on Oral Health Up-to-date | ||||
|---|---|---|---|---|---|
|
| |||||
| Yes (n=29) |
Yes % |
No (n=289) |
No % |
P-Value | |
|
| |||||
| Age | |||||
| 20–25 | 1 | 10% | 9 | 90% | 0.45 |
| 26–35 | 6 | 9% | 58 | 91% | |
| 36–45 | 4 | 6% | 62 | 94% | |
| 46–50 | 5 | 11% | 42 | 89% | |
| 51–55 | 8 | 13% | 56 | 88% | |
| 56+ | 5 | 8% | 60 | 92% | |
|
| |||||
| Level of Education | |||||
| Diploma/Certificate/Associates | 17 | 8% | 200 | 92% | 0.28 |
| Bachelors | 10 | 12% | 71 | 88% | |
| Masters/Doctorate | 1 | 7% | 13 | 93% | |
|
| |||||
| Year of Graduation | |||||
| Graduated before 1985 | 6 | 5% | 97 | 94% | 0.29 |
| Graduated between 1985–1998 | 12 | 12% | 87 | 88% | |
| Graduated after 1998 | 9 | 9% | 93 | 91% | |
|
| |||||
| Currently Employed | |||||
| Full Time | 14 | 7% | 196 | 93% | 0.07 |
| Part Time | 13 | 13% | 90 | 87% | |
|
| |||||
| Type of Practice | |||||
| General Practice | 25 | 7% | 237 | 93% | 0.67 |
| Other | 4 | 13% | 48 | 87% | |
|
| |||||
| Diagnosis of Breast Cancer | |||||
| Yes | 5 | 28% | 13 | 72% | 0.004 |
| No | 24 | 8% | 276 | 92% | |
|
| |||||
| Knowledge of BCa prevalence | |||||
| Yes | 13 | 8% | 143 | 92% | 0.16 |
| No | 6 | 16% | 32 | 84% | |
| Unsure | 7 | 6% | 110 | 94% | |
|
| |||||
| Assess Family Cancer History | |||||
| Yes | 10 | 16% | 53 | 84% | 0.026 |
| No | 17 | 7% | 227 | 93% | |
Note: Frequencies for a characteristic may not total N=318 due to missing data.
DISCUSSION
Over 2.5 million women in the U.S. have been diagnosed with breast cancer.50 As the survival rate is increasing,2 long-term survivorship issues including oral health status are important components of breast cancer care and follow-up. This is the first study examining dental hygienists’ knowledge of anti–estrogen therapies and professional practice related to providing care for these patients.
Knowledge of patient care and anti-estrogen therapies
While 95% of the respondents indicated that they had treated a patient with a diagnosis of breast cancer, just over half knew that breast cancer is the most common cancer among women, aside from non-melanoma skin cancer.1 In addition, quite a high percentage of respondents reported that they did not know the answers to the questions concerning patient care (26% to 62%), the consequences of using anti-estrogen therapy (64% to 80%), and bisphosphonate use (7% to 77%). A lack of knowledge concerning these issues can put patients at risk and should therefore be addressed both in dental hygiene programs as well as in continuing education courses. For example, large percentages of dental hygienists were not aware of the recommended clinical guidelines for treating breast cancer patient when taking blood pressure readings, for consultation with an oncologist for determining patient white blood cell counts before treatment, and for the need for possible premedication of breast cancer patients who have a port for chemotherapy.
Dental hygienists’ knowledge concerning anti-estrogen therapy for breast cancer patients showed significant deficiencies, with large majorities of respondents indicating that they did not know the answers to the questions about these issues.21–25 Only a small percentage (21%) were aware of the current anti-estrogen treatment standards for pre and postmenopausal women (21%), and fewer still responded correctly to the questions concerning the mechanism of action of anti-estrogen therapy (13%). These findings are of concern because the American Society of Clinical Oncology (ASCO) has developed clinical practice guidelines on adjuvant anti-estrogen therapy for postmenopausal women with hormone receptive positive (ER+ or PR+) breast cancer,51 which recommend that, for optimal adjuvant anti-estrogen therapy for postmenopausal women with ER+ disease, an AI should be used either as initial therapy or following a course of Tamoxifen.51 At present, the recommended duration of initial anti-estrogen therapy is 5 years, and extended anti-estrogen therapy for an additional 5-year period has proven beneficial for some patients.52 In consideration of this long duration of anti-estrogen therapies for breast cancer patients, treatment-related adverse effects are not only relevant, but absolutely crucial for assuring patients’ long-term oral health.
Over three quarters of dental hygienists were unaware that patients on anti-estrogen therapies may develop potential musculoskeletal issues related to the use of AIs. Musculoskeletal toxicities occur in up to 50% of patients. Symptoms include joint stiffness, myalgias and arthralgias, especially of the wrists, hands, and fingers.53 The etiology of AI-associated musculoskeletal symptoms remains unclear, but may be a result, in part, of estrogen deprivation.54 Patients with these side effects may find maintenance of oral health difficult because of pain or inability to brush and floss their teeth. Dental hygienists need to be aware of these issues to provide educational interventions and treatments to support these patients.
These findings concerning dental hygienists’ knowledge about standard cancer treatments and potential adverse effects of anti-estrogen therapy should serve as a call to action for dental educators involved in dental hygiene programs as well as in continuing education courses.
Oral complications and care recommendation related to breast cancer treatments
Most dental hygienists reported that chemotherapy places patients at an increased risk for xerostomia, and mucosal and gingival changes (Figure 1). Fewer respondents were knowledgeable about the oral complications associated with anti-estrogen therapies. A similar pattern emerged regarding patient care recommendations given to breast cancer patients during different stages of cancer treatment. While the majority of dental hygienists provided or recommended xerostomia alleviating strategies, mucosal rinses, and oral hygiene education for patients undergoing chemotherapy, only about two-thirds of the respondents provided or recommended these treatments for patients undergoing anti-estrogen therapies.
Gingival inflammation, gingival bleeding, periodontal pocketing, xerostomia, and burning tissues were reported by the small number of respondents who had been told by patients or had observed themselves consequences of using Tamoxifen (n=39) and AIs (n=17). More than twice as many dental hygienists reported Tamoxifen-related oral side effects as compared to AI side effects. The low number of responses may be attributable to the fact that three quarters of the respondents indicated they were unfamiliar with AI medications, which may have limited the reporting of oral side effects related to their use.
As the majority of dental complications that occur in cancer patients are related to changes in saliva production and function,55 knowledge of potential side effects of anti-estrogen therapies is important. Sex hormone receptors have been detected in the oral mucosa and salivary glands.56,57 Estrogen deficiency among post-menopausal women has been associated with decreased salivary flow unrelated to medications.58 Decreased saliva flow can result in xerostomia, gingival bleeding, increase in dental caries, and may be responsible for an increased prevalence of oral dysesthesia and alterations in taste.59–62
Breast cancer treatments, such as chemotherapy and anti-estrogen therapies, which may promote a low estrogen status, have also been linked to an increased risk of osteoporosis,31 which is known to be a risk factor for periodontitis.63,64 Therefore cancer therapies may be risk factors for periodontitis as well as for osteoporosis. Consequently, women with a diagnosis of cancer, especially postmenopausal cancer survivors, may experience higher levels of xerostomia and dental caries as well as a possible increase in their risk for periodontal disease due to the substandard estrogen levels associated with the use of AI medications.
An important finding in this study is that less than 10% of respondents believed that their knowledge of breast cancer treatments and their oral side effects is up to date. It is not surprising that nearly all respondents indicated an interest in taking a continuing education course on this subject. Educational interventions in which dental, dental hygiene, nursing, and medical professionals learn about these issues together may be the optimal path to promoting understanding of the impact of breast cancer treatments on oral health and the treatment needs of these patients.
Overall, these findings suggest the need to increase the educational material about breast cancer survivorship issues in dental hygiene and continuing education programs. In addition, it would be helpful to conduct a study to determine the scope of information provided within the entry-level dental and dental hygiene curricula.
In this survey, dental hygienists with a diagnosis of breast cancer as well as those who assessed the patients’ family history of cancer were more confident about breast cancer treatments and their impact on oral health. These dental hygienists may have more knowledge or may have a practice philosophy of incorporating systemic health evidence into their dental hygiene practice. One limitation of this study is that only 37% of the dental hygienists who received a mailing responded to this survey. However, recent research concerning survey response rates in studies with dentists showed that this response rate is actually higher than the response rate in most surveys. This recent study compared the response rates of postal mail surveys and electronic surveys used to collect data from practicing dentists. It found that the response rates for mailed surveys were 28% and those for web-based surveys were 11%.51 The response rate in this study therefore is acceptable. Nevertheless, future research should replicate this study in other geographical locations to assure that these findings can be generalized to dental hygienists in other parts of the U.S.
CONCLUSIONS
Our results suggest that dental hygienists lack knowledge concerning the oral health-related effects of common drugs used in breast cancer treatment including AIs and Tamoxifen. Given the high number of women undergoing these treatments over the course of many years, it is important that dental care providers are aware of the issues related to breast cancer treatment and have the skills to provide the best possible care for these patients to assure their oral health in the long run. Careful monitoring of the oral health of women with breast cancer is important during all stages of cancer therapy to prevent, detect, and treat complications as soon as possible.
Most dental hygienists surveyed thought that their own knowledge concerning the management of breast cancer patients was not current and wished to learn more about this topic. Developing interdisciplinary educational interventions for dental hygiene programs as well as continuing education courses about dental care and breast cancer treatments is important. Further research is needed concerning the long-term oral health-related consequences of breast cancer treatments, as is research into the best practices that would provide optimal care for these patients.
Acknowledgments
The authors wish to thank the dental hygienists in the State of Michigan who participated in this study. The study was supported by funding from the Michigan Institute for Clinical Health Research/CTSA pilot grant UL1RR024986 and the National Institute of Dental and Craniofacial Research (NIDCR) grant 5K23DE020197. The authors appreciate the assistance of Felicia Billings and Jessica Humfleet with data collection and management.
This study supports the NDHRA priority area, Clinical Dental Hygiene Care: Investigate how dental hygienists identify patients who are at-risk for oral/systemic disease.
Biographies
L. Susan Taichman, R.D.H., M.S., M.P.H., Ph.D. is an Assistant Professor/Research Scientist, Division of Dental Hygiene, Department of Periodontics and Oral Medicine; University of Michigan School of Dentistry, Ann Arbor, Michigan;
Grace Gomez, B.D.S., M.P.H., is a doctoral student in the Dental Sciences graduate program, Indiana University School of Dentistry, Indianapolis, Indiana;
Marita Rohr Inglehart, Dr. phil. habil. is an Associate Professor, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry and an Adjunct Associate Professor of Psychology, College of Literature, Sciences & Arts, University of Michigan, Ann Arbor, Michigan.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Breast cancer statistics. 2011. [Internet] 2013 [cited 2013 January 28]. Available from: http://www.cancer.gov/cancertopics/types/breast.
- 3.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321(7261):624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Practice Guidelines in Oncology. Invasive breast cancer. [Internet] 2010 Jan; [cited 2012 January 28]. Available from: http://www.nccn.org/patients/patient_guidelines/breast/index.html#/2/
- 5.Carlson RW, Moench S, Hurria A, et al. NCCN Task Force Report: breast cancer in the older woman. J Natl Compr Canc Netw. 2008;6(Suppl 4):S1–S25. quiz S26–S27. [PubMed] [Google Scholar]
- 6.Bedard PL, Cardoso F. Can some patients avoid adjuvant chemotherapy for early-stage breast cancer? Nat Rev Clin Oncol. 2011;8(5):272–279. doi: 10.1038/nrclinonc.2011.19. [DOI] [PubMed] [Google Scholar]
- 7.EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 8.Piccart-Gebhart MJ. Adjuvant trastuzumab therapy for HER2-overexpressing breast cancer: what we know and what we still need to learn. Eur J Cancer. 2006;42(12):1715–1719. doi: 10.1016/j.ejca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 10.Rastelli F, Crispino S. Factors predictive of response to hormone therapy in breast cancer. Tumori. 2008;94(3):370–383. doi: 10.1177/030089160809400314. [DOI] [PubMed] [Google Scholar]
- 11.Miller WR, O’Neill J. The importance of local synthesis of estrogen within the breast. Steroids. 1987;50(4–6):537–548. doi: 10.1016/0039-128x(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 12.Burstein HJ, Griggs JJ. Adjuvant hormonal therapy for early-stage breast cancer. Surg Oncol Clin N Am. 19(3):639–647. doi: 10.1016/j.soc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114–2122. doi: 10.1200/JCO.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Oral Complications of Cancer Therapies: Diagnosis, Prevention and Treatment Consensus Statement [internet] 1989;7(7):1–11. [cited 2012 October 14] Available from: http://consensus.nih.gov/1989/1989OralComplicationsCancerTherapy073html.htm. [PubMed] [Google Scholar]
- 15.Epstein JB, Parker IR, Epstein MS, Stevenson-Moore P. Cancer-related oral health care services and resources: a survey of oral and dental care in Canadian cancer centres. J Can Dent Assoc. 2004;70(5):302–304. [PubMed] [Google Scholar]
- 16.Sheiham A, Steele JG, Marcenes W, Finch S, Walls AW. The impact of oral health on stated ability to eat certain foods; findings from the National Diet and Nutrition Survey of Older People in Great Britain. Gerodontology. 1999;16(1):11–20. doi: 10.1111/j.1741-2358.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 17.Sonis ST. Oral mucositis in cancer therapy. J Support Oncol. 2004;2(6 Suppl 3):3–8. [PubMed] [Google Scholar]
- 18.Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010 doi: 10.1016/j.oraloncology.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Sadler GR, Stoudt A, Fullerton JT, Oberle-Edwards LK, Nguyen Q, Epstein JB. Managing the oral sequelae of cancer therapy. Medsurg Nurs. 2003;12(1):28–36. [PubMed] [Google Scholar]
- 20.Almazrooa SA, Woo SB. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw: a review. J Am Dent Assoc. 2009;140(7):864–75. doi: 10.14219/jada.archive.2009.0280. [DOI] [PubMed] [Google Scholar]
- 21.Raber-Durlacher JE, Barasch A, Peterson DE, Lalla RV, Schubert MM, Fibbe WE. Oral complications and management considerations in patients treated with high-dose chemotherapy. Support Cancer Ther. 2004;1(4):219–229. doi: 10.3816/SCT.2004.n.014. [DOI] [PubMed] [Google Scholar]
- 22.Watters AL, Epstein JB, Agulnik M. Oral complications of targeted cancer therapies: a narrative literature review. Oral Oncol. 2011;47(6):441–448. doi: 10.1016/j.oraloncology.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy GM, Skillings JR. Orofacial complications of chemotherapy for breast cancer. Oral Surg Oral Med Oral Pathol. 1992;74(2):172–178. doi: 10.1016/0030-4220(92)90378-4. [DOI] [PubMed] [Google Scholar]
- 24.Ohrn KE, Wahlin YB, Sjoden PO. Oral status during radiotherapy and chemotherapy: a descriptive study of patient experiences and the occurrence of oral complications. Support Care Cancer. 2001;9(4):247–257. doi: 10.1007/s005200000214. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19(14):3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 26.Cameron DA, Douglas S, Brown JE, Anderson RA. Bone mineral density loss during adjuvant chemotherapy in pre-menopausal women with early breast cancer: is it dependent on oestrogen deficiency? Breast Cancer Res Treat. 2010;123(3):805–814. doi: 10.1007/s10549-010-0899-7. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365(1):62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 28.Hadji P, Ziller M, Maskow C, Albert U, Kalder M. The influence of chemotherapy on bone mineral density, quantitative ultrasonometry and bone turnover in pre-menopausal women with breast cancer. Eur J Cancer. 2009;45(18):3205–3212. doi: 10.1016/j.ejca.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Robinson WR, Luck M, Omar H, Blades N, Morris K, Coscia J. A pilot study of bone density loss in menopausal women treated with chemotherapy for cancer. Support Care Cancer. 2005;13(8):663–667. doi: 10.1007/s00520-005-0798-3. [DOI] [PubMed] [Google Scholar]
- 30.Vehmanen L, Saarto T, Elomaa I, Makela P, Valimaki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37(18):2373–2378. doi: 10.1016/s0959-8049(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 31.Khan MN, Khan AA. Cancer treatment-related bone loss: a review and synthesis of the literature. Curr Oncol. 2008;15(Supplement 1):S30–40. doi: 10.3747/co.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mincey BA, Duh MS, Thomas SK, et al. Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clin Breast Cancer. 2006;7(2):127–132. doi: 10.3816/CBC.2006.n.021. [DOI] [PubMed] [Google Scholar]
- 33.Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force Report: Bone Health in Cancer Care. J Natl Compr Canc Netw. 2009;7(Suppl 3):S1–S32. doi: 10.6004/jnccn.2009.0076. quiz S33–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wardrop RW, Hailes J, Burger H, Reade PC. Oral discomfort at menopause. Oral Surg Oral Med Oral Pathol. 1989;67(5):535–540. doi: 10.1016/0030-4220(89)90269-7. [DOI] [PubMed] [Google Scholar]
- 35.Forabosco A, Criscuolo M, Coukos G, Uccelli E, Weinstein R, Spinato S, Botticelli A, Volpe A. Efficacy of hormone replacement therapy in postmenopausal women with oral discomfort. Oral Surg Oral Med Oral Pathol. 1992;73(5):570–574. doi: 10.1016/0030-4220(92)90100-5. [DOI] [PubMed] [Google Scholar]
- 36.Taichman LS, Havens AM, Van Poznak CH. Potential implications of adjuvant endocrine therapy for the oral health of postmenopausal women with breast cancer. Breast Cancer Res Treat. 137(1):23–32. doi: 10.1007/s10549-012-2217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Draper C. Cancer Prevention and Treatment: The Dental Hygienist’s role. American Dental Hygienist’s Association; pp. 26–31. Access 2010(Nov) [Google Scholar]
- 38.NIH Consensus Statement Online. Oral complications of cancer Therapies: Diagnosis, Prevention, and Treatment. 1989:1–11. [PubMed] [Google Scholar]
- 39.National Institute of Dental and Craniofacial Research. Cancer Treatment and You: Three Good Reasons to See a Dentist BEFORE Cancer Treatment. [Internet] 2011 [cited 2012 December 14] Available from: http://www.nidcr.nih.gov/NR/rdonlyres/4FB39055-1788-4A9E-ADE6-FFBF2C1B6FE9/0/ThreeGoodReasons.pdf.
- 40.National Institute of Dental and Craniofacial Research. Oral complications of cancer treatment: What the Oncology team can do. [Internet] 2008 [cited 2012 December 28] Available from: http://www.nidcr.nih.gov/OralHealth/Topics/CancerTreatment/oralcomplicationscanceroncology.htm.
- 41.National Institute of Dental and Craniofacial Research. Oral complications of cancer treatment: What the Dental team can do. [Internet 2008] [cited 2012 28] Available from: http://www.nidcr.nih.gov/OralHealth/Topics/CancerTreatment/OralComplicationsCancerOral.htm.
- 42.Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015–2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 43.Hong CH, Allred R, Napenas JJ, Brennan MT, Baddour LM, Lockhart PB. Antibiotic prophylaxis for dental procedures to prevent indwelling venous catheter-related infections. Am J Med. 2010;123(12):1128–1133. doi: 10.1016/j.amjmed.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Hayes S, Cornish B, Newman B. Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat. 2005;89(3):221–226. doi: 10.1007/s10549-004-2045-x. [DOI] [PubMed] [Google Scholar]
- 45.Harris SR, Hugi MR, Olivotto IA, Levine M. Clinical practice guidelines for the care and treatment of breast cancer: 11. Lymphedema. CMAJ. 2001;164(2):191–199. [PMC free article] [PubMed] [Google Scholar]
- 46.McLaughlin SA. Lymphedema: separating fact from fiction. Oncology (Williston Park) 2012;26(3):242–249. [PubMed] [Google Scholar]
- 47.Epstein JB, Parker IR, Epstein MS, Gupta A, Kutis S, Witkowski DM. A survey of National Cancer Institute-designated comprehensive cancer centers’ oral health supportive care practices and resources in the USA. Support Care Cancer. 2007;15(4):357–362. doi: 10.1007/s00520-006-0160-4. [DOI] [PubMed] [Google Scholar]
- 48.McGuire DB. Barriers and strategies in implementation of oral care standards for cancer patients. Support Care Cancer. 2003;11(7):435–441. doi: 10.1007/s00520-003-0466-4. [DOI] [PubMed] [Google Scholar]
- 49.Jones JA, Avritscher EB, Cooksley CD, Michelet M, Bekele BN, Elting LS. Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer. 2006;14(6):505–515. doi: 10.1007/s00520-006-0055-4. [DOI] [PubMed] [Google Scholar]
- 50.Howlader N, Noone AM, Neyman N, et al. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; Nov, 2010. [Internet] [cited 2012 October 12] Available from: http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 51.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26(12):1965–1971. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- 53.Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park) 2008;22(12):1401–1408. [PubMed] [Google Scholar]
- 54.Henry NL, Pchejetski D, A’Hern R, Nguyen AT, Charles P, Waxman J, Li L, Storniolo AM, Hayes DF, Flockhart DA, Stearns V, Stebbing J. Inflammatory cytokines and aromatase inhibitor-associated musculoskeletal syndrome: a case-control study. Br J Cancer. 103(3):291–296. doi: 10.1038/sj.bjc.6605768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong CH, Napenas JJ, Hodgson BD, Stokman MA, et al. A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer. 2010;18(8):1007–1021. doi: 10.1007/s00520-010-0873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leimola-Virtanen R, Salo T, Toikkanen S, Pulkkinen J, Syrjanen S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas. 2000;36(2):131–137. doi: 10.1016/s0378-5122(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 57.Valimaa H, Savolainen S, Soukka T, et al. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol. 2004;180(1):55–62. doi: 10.1677/joe.0.1800055. [DOI] [PubMed] [Google Scholar]
- 58.Streckfus CF, Baur U, Brown LJ, Bacal C, Metter J, Nick T. Effects of estrogen status and aging on salivary flow rates in healthy Caucasian women. Gerontology. 1998;44(1):32–39. doi: 10.1159/000021980. [DOI] [PubMed] [Google Scholar]
- 59.Mott AE, Grushka M, Sessle BJ. Diagnosis and management of taste disorders and burning mouth syndrome. Dent Clin North Am. 1993;37(1):33–71. [PubMed] [Google Scholar]
- 60.Lopez-Jornet P, Camacho-Alonso F, Andujar-Mateos P, Sanchez-Siles M, Gomez-Garcia F. Burning mouth syndrome: an update. Med Oral Patol Oral Cir Bucal. 15(4):e562–568. doi: 10.4317/medoral.15.e562. [DOI] [PubMed] [Google Scholar]
- 61.Ferris GM. Alteration in female sex hormones: their effect on oral tissues and dental treatment. Compendium. 1993;14(12):1558–1564. 1566. quiz 1571. [PubMed] [Google Scholar]
- 62.Zakrzewska JM. Women as dental patients: are there any gender differences? Int Dent J. 1996;46(6):548–557. [PubMed] [Google Scholar]
- 63.Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol. 2001;6(1):197–208. doi: 10.1902/annals.2001.6.1.197. [DOI] [PubMed] [Google Scholar]
- 64.Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76(11 Suppl):2116–2124. doi: 10.1902/jop.2005.76.11-S.2116. [DOI] [PubMed] [Google Scholar]


