Abstract
5-Aminolevulinic acid (ALA) is a precursor for the biosynthesis of porphyrins and heme. Although the oral administration of ALA has been widely applied in clinical settings, the dynamics of its absorption, metabolism, and excretion within enterocytes remain unknown. In this study, after enterocytic differentiation, Caco-2 cells were incubated with 200 µM ALA and/or 100 µM sodium ferrous citrate (SFC) for up to 72 h. Both ALA and the combination of ALA and SFC promoted the synthesis of heme, without affecting the expression of genes involved in intestinal iron transport, such as DMT1 and FPN. The enhanced heme synthesis in Caco-2 cells was more pronounced under the effect of the combination of ALA and SFC than under the effect of ALA alone, as reflected by the induced expression of heme oxygenase 1 (HO-1), as well as a reduced protein level of the transcriptional corepressor Bach1. Chromatin immunoprecipitation analysis confirmed Bach1 chromatin occupancy at the enhancer regions of HO-1, which were significantly decreased by the addition of ALA and SFC. Finally, Transwell culture of Caco-2 cells suggested that the administered ALA to the intestinal lumen was partially transported into vasolateral space. These findings enhance our understanding of the absorption and metabolism of ALA in enterocytes, which could aid in the development of a treatment strategy for various conditions such as anemia.
Abbreviations: ALA, 5-aminolevulinic acid; ALAS2, 5-aminolevulinic acid synthase 2; ChIP, chromatin immunoprecipitation; CSA, congenital sideroblastic anemia; DMT1, divalent metal transporter 1; FPN, ferroportin; HO-1, heme oxygenase 1; PP IX, protoporphyrin IX; RT-PCR, reverse transcription polymerase chain reaction; SFC, sodium ferrous citrate
Keywords: 5-Aminolevulinic acid, Caco-2 cell, Heme oxygenase 1, Bach1
Highlights
-
•
Combination of ALA and SFC promotes heme synthesis than ALA alone in Caco-2 cells.
-
•
Heme induces HO-1 by inhibiting transcriptional corepressor Bach1 in Caco-2 cells.
-
•
Addition of ALA to intestinal lumen was partially transported to vasolateral space.
-
•
Our data enhance the understanding of the dynamics of ALA in enterocytes.
1. Introduction
5-Aminolevulinic acid (ALA) is an important precursor of heme. This compound is synthesized from glycine and succinyl-CoA in mitochondria; this process is catalyzed by two different ALA synthases (ALAS): one expressed ubiquitously (ALAS1) and the other expressed only by erythroid precursors (ALAS2) [1]. During synthesis, ALA is converted to protoporphyrin IX, and heme is generated by the insertion of ferrous iron into protoporphyrin IX. The oral administration of ALA has recently been widely used in various clinical settings. For example, because porphyrin is known to be a strong photosensitizer, ALA has been used to diagnose and treat various cancers [2]. Furthermore, ALA was expected to have therapeutic effects on some types of anemia. It has been suggested that ALA may represent a novel therapeutic option for congenital sideroblastic anemia (CSA), attributable to the mutation of ALAS2, which converts glycine and acetyl-coenzyme A to generate ALA [3]. In addition to ALA, some studies have suggested the efficacy of orally administering the combination of ALA and iron for pre-diabetic subjects in reducing serum glucose levels [4], [5]. The increase in the heme synthesis in the body by the combination of ALA and iron, rather than ALA alone, was estimated to have a beneficial effect on the serum glucose levels [4], [5]. Whereas pharmacokinetic studies of ALA have demonstrated good oral bioavailability [6], [7], [8], other reports have suggested that the administration could cause an excessive accumulation of ALA in enterocytes [9], [10]. In addition, the detailed differences of the combination of ALA and iron versus ALA only on the enterocytes remain unknown. Thus, there is a need to clarify the dynamics of absorption, metabolism, and excretion of ALA and/or iron in enterocytes.
Herein, based on a model of enterocytic differentiation using human intestinal Caco-2 cells, we evaluated the effects of ALA on enterocytes.
2. Materials and methods
2.1. Cell culture and reagents
Caco-2 cells were obtained from American Type Culture Collection (ATCC; Manassas, VA). Cells were grown in a humidified incubator at 37 °C with 5% carbon dioxide, and maintained in a DMEM medium (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (Biowest, Miami, FL). ALA hydrochloride and sodium ferrous citrate (SFC) (SBI Pharmaceuticals Co., Ltd., Tokyo, Japan) were prepared using distilled water. Cells were seeded at a density of 1 × 104 cells/cm2 onto 60-mm plastic flasks or 6-well Transwell permeable support plates (Costar, Corning Inc., New York, USA), and cultured for 21 days. The medium was refreshed every 2–3 days. Cells were incubated with a medium containing 200 µM ALA and/or 100 µM SFC for a culture period of up to 72 h. ALA and/or SFC were added to the upper chamber to the Transwell inserts, corresponding to the intestinal lumen. The cells used for all experiments are described here in passages 24–35.
2.2. Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was purified using TRIzol (Invitrogen) and 1 μg of the purified total RNA was used to synthesize complementary DNA (cDNA) with ReverTra Ace qPCR RT Master Mix (Toyobo). Reaction mixtures (20 μL) for real-time quantitative RT-PCR comprised 2 μL of cDNA, 10 μL of Quantitect SYBR Green PCR Master Mix (Qiagen), and 8 μL of the appropriate primers. Product accumulation was monitored by measuring SYBR Green fluorescence and normalized relative to GAPDH messenger RNA (mRNA). We used the following primers: DMT1, AGCAGGCCTTTAGAGATGCTTA and ATTATATGTGGTGGCTGCTGTG; FPN, CCTGTTAACAAGCACCTCAGC and TTGCAGAGGTCAGGTAGTCG; ALAS1, GGCAGCACAGATGAATCAGA and CCTCCATCGGTTTTCACACT; heme oxygenase 1 (HO-1), ATGAACTCCCTGGAGATGACTC and CCTTGGTGTCATGGGTCAG; GAPDH, GAAGGTCGGAGTCAACGGATTT and GAATTTGCCATGGGTGGAAT; solute carrier family 36 (proton/amino acid symporter), member (SLC36A1), GACTACCACGACTACAGCTCCA and CCTTTTAACAGGTGGATCAAGG; solute carrier family 15 (oligopeptide transporter), member 1 (SLC15A1), ATACGTTTGTGGCTCTGTGCTA and TTACTGAGGTGACTGCTTGTCC. To evaluate absolute expression levels of human ALA transporters (SLC36A1 and SLC15A1), an amplified cDNA fragment of each gene was cloned into the pGEM™-T Easy Vector (Promega, Madison, WI), and was used as an internal standard in quantitative RT-PCR. The plasmid copy number was calculated as follows: copy number (copy/μL) = 6.02 × 1023 × [plasmid DNA concentration (μg/μL)] × 10−6/[total plasmid size (base pair)] × 660, as described previously [3].
2.3. Intracellular heme and protoporphyrin IX content
Intracellular heme content was determined fluorometrically, as described previously [11]. In brief, cell pellets were suspended in 2 M oxalic acid and boiled (100 °C) for 30 min to dissociate protoporphyrin IX and iron from heme. The fluorescence for protoporphyrin IX was then measured at 400 nm (excitation) and 662 nm (emission). To exclude endogenous levels of protoporphyrin IX, the fluorescence of unboiled samples was subtracted.
2.4. Measurement of ALA, total porphyrin, and heme
Heme content in medium was determined using a chemiluminescence assay as described previously [12]. In brief, the same amounts of medium and chemiluminescence detection reagents (Pierce western blotting substrate plus; ThermoFisher, USA) were mixed and incubated at room temperature for 5 min. Chemiluminescence intensities were then measured using a luminometer (GloMax 20/20; Luminometer, Promega, USA).
ALA levels in medium were analyzed by synthesizing a fluorescent ALA derivative and measuring its concentration with a fluorometric HPLC system, as described previously [13]. Porphyrin levels in medium were also measured with the HPLC system, as described previously [14].
2.5. Western blotting
Western blotting was conducted as described previously [15], [16]. Antibodies for ferritin (ab75973) and HO-1 (ab 13248) were purchased from Abcam (Cambridge, UK). The antibody for Bach1 was a generous gift from Prof. Kazuhiko Igarashi (Tohoku University, Japan). A densitrometric analysis of Western blot was conducted with ImageJ software (http://rsbweb.nih.gov/ij/). For calculating relative intensity, control samples were set to 1.
2.6. Quantitative ChIP analysis
Real-time PCR-based quantitative chromatin immunoprecipitation (ChIP) analysis was conducted as described previously [17]. We used the following primers: HO-1 E1, CATTTCTGCTGCGTCATGTT and GAGGCTTCTGCCGTTTTCTA; and HO-1 E2, CCCTGCTGAGTAATCCTTTCC and GGCGGTGACTTAGCGAAAAT. Primer sequences for the RPII215 and NECDIN promoters were as previously reported [16].
2.7. Statistics
Statistical significance was assessed by one-way ANOVA followed by Tukey's post hoc test. In all analyses, differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Enterocytic differentiation of human intestinal Caco-2 cells
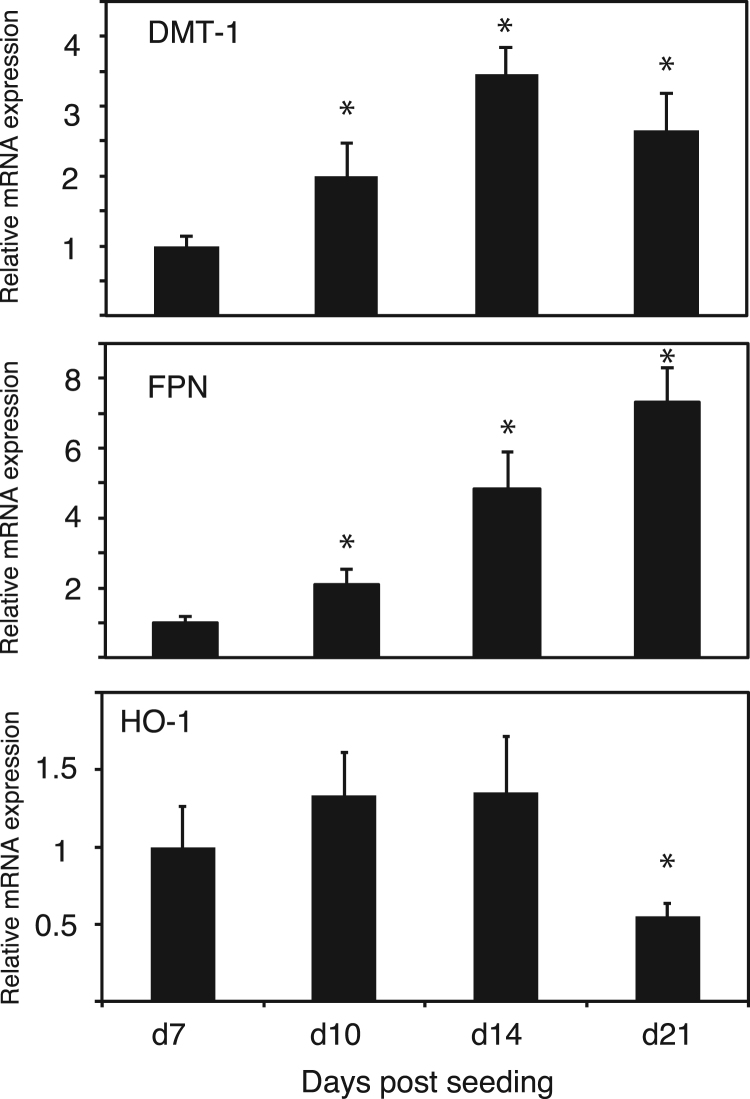
To confirm the enterocytic differentiation of Caco-2 cells, we examined the expressions for DMT1 and FPN, which are known to be an intestinal iron importer and exporter, respectively [18], [19]. As shown in Fig. 1, the levels of DMT1 mRNA gradually increased to reach a maximum at day 14, whereas those of FPN mRNA steadily increased until day 21, which were in line with a previous study demonstrating the enterocytic differentiation of Caco-2 cells [20]. On the other hand, the levels of HO-1 mRNA remained the same until day 14 and then decreased until day 21 during Caco-2 cell differentiation. The decrease of HO-1 expression during Caco-2 cell differentiation was similar to that found in a previous study, demonstrating that the HO-1 protein is undetectable after its confluency [21]. Taken together, Caco-2 cells that are subjected to our culture conditions are the representatives of intestinal epithelial cells.
Fig. 1.
Differentiation of human intestinal Caco-2 cells. Quantitative RT-PCR analysis of DMT1, FPN, and HO-1 in Caco-2 cells during days 7–21 after seeding. Data are expressed as mean ± standard error (SE). n = 3; *, p < 0.05. Asterisks indicate levels that are statistically significantly different from those on day 7.
3.2. The combination of ALA and SFC enhanced heme and protoporphyrin IX synthesis in Caco-2 cells
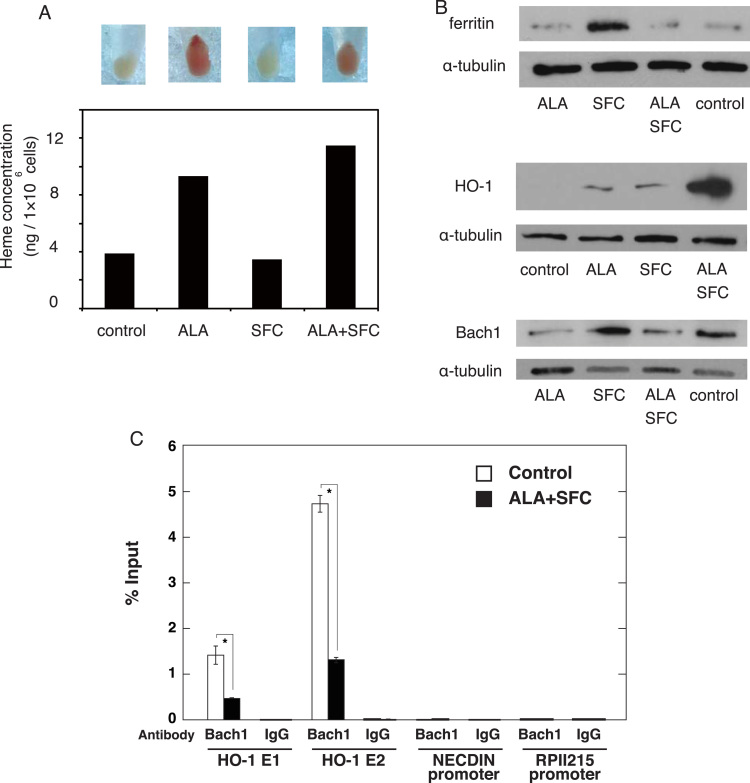
As shown in Fig. 2A, we found that cell pellets treated with ALA were reddish, implying that the ALA-treated cells may contain excessive porphyrin as observed in a patient with porphyria in which reddish urine was exhibited [22]. Notably, whereas the ALA treatment induced heme synthesis, the combination of ALA and SFC promoted heme synthesis (Fig. 2A). We next conducted a western blot analysis to confirm the changes in intracellular heme/iron status. Whereas SFC increased the level of ferritin protein, the combined use of ALA and SFC did not increase the level of ferritin protein, suggesting that iron was efficiently utilized to generate heme (Fig. 2B). It has been reported that heme induced the expressions of globins and HO-1, by repressing the activity of Bach1, which is known as a transcriptional repressor that binds the maf-recognition element located at the regulatory region of globin and HO-1 [23]. Induced heme accumulation and HO-1 expression in Caco-2 cells have recently been demonstrated [24]. Noticeably, we demonstrated that the combination of ALA and SFC strongly increased the level of HO-1 protein (Fig. 2B). We further confirmed that ALA reduced the level of Bach1 protein (Fig. 2B), which was confirmed by densitrometric analysis (Relative Bach1/tubulin intensity for ALA, SFC, ALA/SFC, and control were 0.48, 1.09, 0.61 and 1, respectively). Furthermore, we conducted quantitative ChIP analysis to assess whether Bach1 directly binds to the regulatory element of HO-1 in Caco-2 cells. The analysis revealed Bach1 occupancy at the HO-1 enhancer region, which was significantly decreased by the addition of ALA/SFC (Fig. 2C). As a reference, we also examined Bach1 occupancy at the NECDIN and RPII215 promoter regions, which are considered to be negative control sites. Taken together, the enhanced heme synthesis in Caco-2 cells was more pronounced under the effect of the combination of ALA and SFC than under ALA alone.
Fig. 2.
ALA treatment enhances heme biosynthesis and changes intracellular iron status. A: Cellular pellets (top) and heme levels (bottom) of Caco-2 cells treated with ALA (200 μM) and/or SFC (100 μM) for 72 h. A representative data was shown among 3 independent experiments. B: Western blot analysis of ferritin (left), HO-1 (center), and Bach1 (right). α-tubulin was used as a loading control. C: Quantitative ChIP analysis to detect endogenous Bach1 occupancy at HO-1 enhancers (i.e., the distal E2 and proximal E1 enhancers), based on control and ALA/SFC-treated Caco-2 cells. The RPII215 promoter and the NECDIN promoter were used as negative controls. Data are expressed as mean ± standard error (SE). n = 3; *, p < 0.05. Asterisks indicate levels that are statistically significantly different from those of the control.
Heme synthesis mostly occurs in developing erythroblasts located in the bone marrow to produce hemoglobin; however, approximately 15% of daily synthesis occurs in the liver to form heme-containing enzymes such as cytochrome P450 [25]. Previous study showed that the induction of HO-1 expression by ALA and SFC occurred in a macrophage cell line (RAW264 cells). The authors demonstrated that the exposure of RAW264 cells to the combination of ALA and SFC increases the HO-1 expression via MAPK activation with the negative regulation of Bach1 [26]. Additional studies, based on a human hepatoma cell line [27] and mouse kidney cells [28], reported ALA-mediated upregulation of HO-1 mRNA levels. Heme has been reported to inhibit the transcriptional repressor activity of Bach1, resulting in the derepression of its target genes such as globin in erythroid cells and HO-1 in diverse cell types [23]. These results are in line with our findings that the combination of ALA and SFC efficiently induces heme biosynthesis in enterocytes.
3.3. ALA did not affect the expression of intestinal ALA/iron transporters
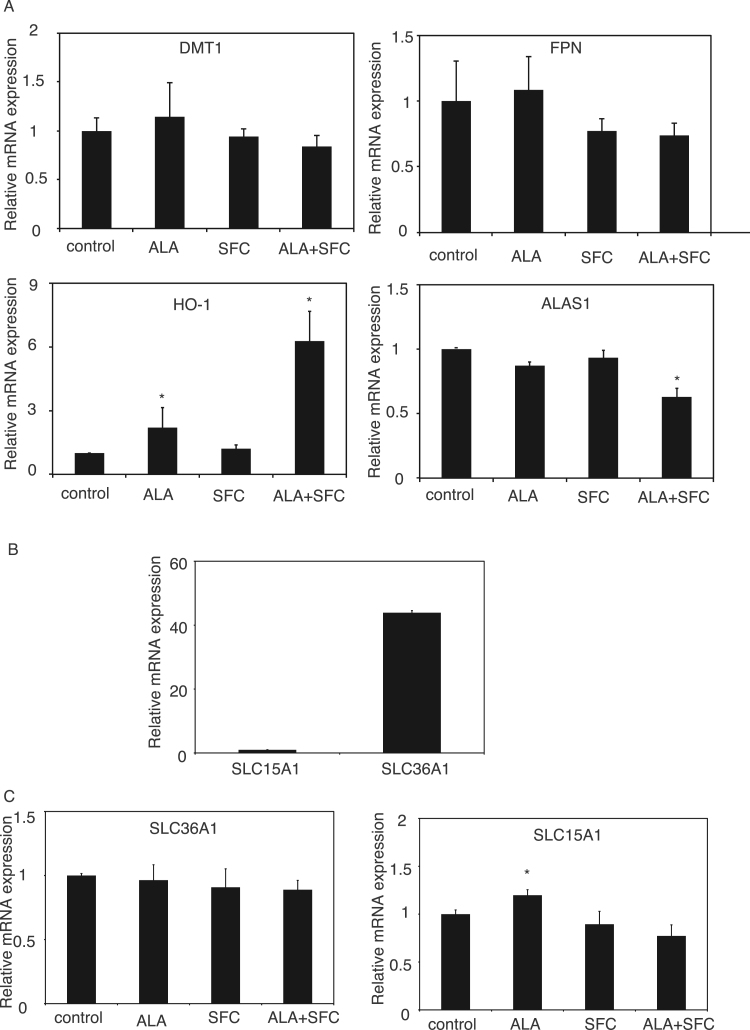
To test whether the ALA-mediated increase in heme concentration in enterocytes could be accompanied by the changes of iron absorption as well as excretion in intestinal cells, quantitative RT-PCR was conducted for DMT1 and FPN. As shown in Fig. 3A, there were no significant changes in the expression of genes involved in iron transport, including DMT1 and FPN (Fig. 3A), suggesting that the ALA-mediated increase in heme concentration may not affect iron absorption/excretion in intestinal cells. Heme has been reported to induce HO-1 expression and suppress ALAS1 expression; both contribute to a negative feedback loop in non-erythroid cells [1]. Similar to the western blot analysis, we demonstrated that HO-1 expression was significantly increased by ALA treatment and that this increase was more pronounced under the combination of ALA and SFC (Figs. 2B and 3A). Furthermore, whereas ALAS1 expression was not affected by ALA, its expression was significantly reduced by the co-administration of SFC (Fig. 3A). We also examined the expression levels of ALA transporters, SLC36A1 and SLC15A1, which were considered to be important ALA transporters in intestinal cells [29], [30]. As shown in Fig. 3B, we demonstrated that the absolute mRNA expression level of SLC15A1 was clearly low (by more than 40 times) as compared to that of SLC36A1, suggesting that in Caco-2 cells SLC36A1 may be the main ALA transporter (Fig. 3B). As shown in Fig. 3C, ALA, SFC, and the combination of ALA and SFC did not affect the expression of SLC36A1. On the other hand, whereas SFC and the combination of ALA and SFC did not affect the expression of SLC15A1, we noticed that ALA administration slightly induced its expression (Fig. 3C). According to the markedly low expression of SLC15A1 as compared to that of SLC36A1 (Fig. 3B), the contribution of the ALA-mediated increase of SLC15A1 may be negligible and thus we consider that ALA did not significantly affect the ALA import into enterocytes.
Fig. 3.
ALA did not affect the expression of intestinal iron transporters and minimally changed ALA transporters. A: Quantitative RT-PCR analysis of DMT1, FPN, HO-1, and ALAS1 in Caco-2 cells treated with ALA (200 μM) and/or SFC (100 μM) for 72 h. Data are expressed as mean ± standard error (SE). n = 3; *, p < 0.05. Asterisks indicate levels that are statistically significantly different from those of the control. B: Comparison of quantitative RT-PCR analysis of SLC36A1 and SLC15A1 on day 21. C: Quantitative RT-PCR analysis of SLC36A1 and SLC15A1 in Caco-2 cells treated with ALA (200 μM) and/or SFC (100 μM) for 72 h. Data are expressed as mean ± standard error (SE). n = 3; *, p < 0.05. Asterisks indicate levels that are statistically significantly different from those of the control.
3.4. Subsets of ALA and porphyrin in Caco-2 cells were transported into vasolateral space
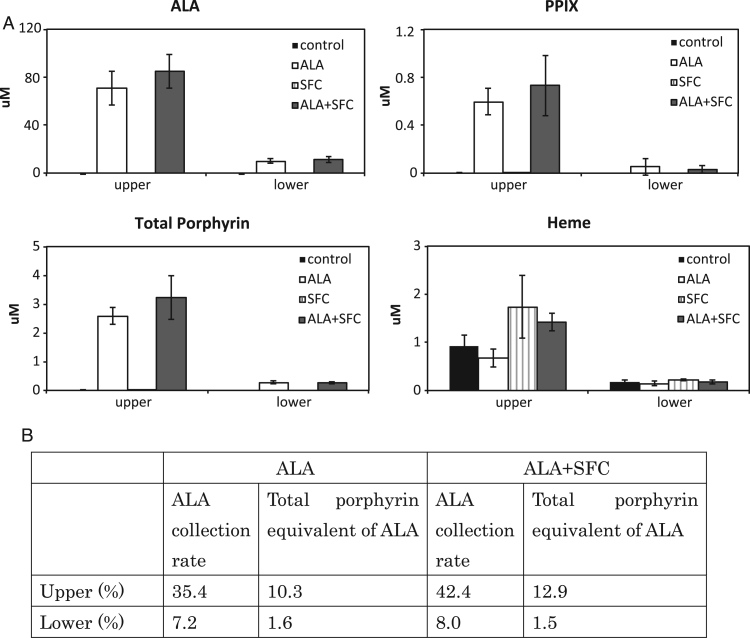
Finally, to demonstrate the dynamics of absorption, metabolism, and excretion of ALA as well as SFC within enterocytes, we measured the concentrations of ALA and porphyrin based on the Transwell culture of Caco-2 cells. The Transwell has two compartments that are separated by one microporous membrane insert. The Transwell insert system can represent the human intestinal environment, i.e., the upper and lower compartments represent the intestinal lumen and vasolateral space, respectively. We demonstrated that a majority of ALA existed in the upper compartment, and the co-administration of SFC did not affect their concentrations (Fig. 4A and B). Among the administered ALA, 35.4% remained in the intestinal lumen and 7.2% of the ALA moved to the vasolateral space in the ALA only group, whereas 42.4% and 8.0% existed in the intestinal and vasolateral lumen, respectively, in ALA and SFC group. In addition, based on the evidence that eight molecules of ALA are used to form one porphyrin ring [22], we calculated the percentage of ALA converted into porphyrins. We demonstrated that 10.3% and 1.6% of administered ALA was converted into porphyrins and existed in the intestinal and vasolateral lumen, respectively (Fig. 4B). Similar to the ALA, the addition of SFC did not affect the distribution of porphyrins. These results suggested that a subset of the administered ALA was transported to vasolateral space across the Caco-2 cell layer. Also, synthesized porphyrins in the Caco-2 cells were mainly excreted into intestinal lumen, whereas a subset of the porphyrins was moved to vasolateral lumen.
Fig. 4.
Iron administration did not affect heme, ALA, and porphyrin concentrations in medium. A: Chemiluminescent assay to detect heme concentration of medium. HPLC to detect ALA and porphyrin concentrations in medium. N = 3. B: The calculated distribution of ALA in medium after administration. Assuming that the amount of administered ALA is 100%, ALA collection rate is calculated as the percentage of ALA in each compartment 3 days after administration. Total porphyrin equivalent of ALA is the amount of total porphyrin converted to ALA.
The effect of ALA on heme synthesis has been examined in human erythroid cells such as K562 cells and human-induced pluripotent stem cell-derived erythroid progenitor cells [3]. The study demonstrated that ALA resulted in a significant dose-dependent accumulation of heme in the K562 cell line, implying that ALA may represent a novel therapeutic option for the treatment of CSA, particularly for cases involving ALAS2 mutations. Based on the finding that subsets of ALA and porphyrin were transported into vasolateral space, it may be reasonable to develop the ALA treatment for CSA. However, since Caco-2 cells are derived from colorectal adenocarcinoma, which could exhibit reduced mitochondrial respiratory function as a result of Warburg effect, it is possible that heme biosynthesis is compromised in Caco-2 cells [31]. Thus, further studies in vivo should be required to address the potential effect of iron co-administration in the absorption, metabolism, and excretion of ALA and porphyrin.
In conclusion, our findings improve the understanding of absorption, metabolism, and excretion of ALA in enterocytes, which would be translated into clinical settings such as treatment as well as diagnosis.
Acknowledgements
We thank Dr. Kazuhiko Igarashi for providing the anti-Bach1 antibody. We thank Dr. Kiwamu Takahashi, and Dr. Motowo Nakajima (SBI Pharmaceuticals Co., Ltd.) for excellent technical supports. All authors declare no conflicts of interest.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.07.006.
Appendix A. Transparency document
Supplementary material
References
- 1.Furuyama K., Kaneko K., Vargas P.D. Heme as a magnificent molecule with multiple missions: heme determines its own fate and governs cellular homeostasis. Tohoku J. Exp. Med. 2007;213:1–16. doi: 10.1620/tjem.213.1. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy J.C., Pottier R.H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B. 1992;14:275–292. doi: 10.1016/1011-1344(92)85108-7. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara T., Okamoto K., Niikuni R., Takahashi K., Okitsu Y., Fukuhara N., Onishi Y., Ishizawa K., Ichinohasama R., Nakamura Y., Nakajima M., Tanaka T., Harigae H. Effect of 5-aminolevulinic acid on erythropoiesis: a preclinical in vitro characterization for the treatment of congenital sideroblastic anemia. Biochem. Biophys. Res. Commun. 2014;454:102–108. doi: 10.1016/j.bbrc.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez B.L., Curb J.D., Davis J., Shintani T., Perez M.H., Apau-Ludlum N., Johnson C., Harrigan R.C. Use of the dietary supplement 5-aminiolevulinic acid (5-ALA) and its relationship with glucose levels and hemoglobin A1C among individuals with prediabetes. Clin. Transl. Sci. 2012;5:314–320. doi: 10.1111/j.1752-8062.2012.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higashikawa F., Noda M., Awaya T., Tanaka T., Sugiyama M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition. 2013;29:1030–1036. doi: 10.1016/j.nut.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 6.van den Boogert J., van Hillegersberg R., de Rooij F.W., de Bruin R.W., Edixhoven-Bosdijk A., Houtsmuller A.B., Siersema P.D., Wilson J.H., Tilanus H.W. 5-Aminolaevulinic acid-induced protoporphyrin IX accumulation in tissues: pharmacokinetics after oral or intravenous administration. J. Photochem. Photobiol. B. 1998;44:29–38. doi: 10.1016/s1011-1344(98)00102-x. [DOI] [PubMed] [Google Scholar]
- 7.Dalton J.T., Meyer M.C., Golub A.L. Pharmacokinetics of aminolevulinic acid after oral and intravenous administration in dogs. Drug. Metab. Dispos. 1999;27:432–435. [PubMed] [Google Scholar]
- 8.Dalton J.T., Yates C.R., Yin D., Straughn A., Marcus S.L., Golub A.L., Meyer M.C. Clinical pharmacokinetics of 5-aminolevulinic acid in healthy volunteers and patients at high risk for recurrent bladder cancer. J. Pharm. Exp. Ther. 2002;301:507–512. doi: 10.1124/jpet.301.2.507. [DOI] [PubMed] [Google Scholar]
- 9.Loh C.S., MacRobert A.J., Bedwell J., Regula J., Krasner N., Bown S.G. Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br. J. Cancer. 1993;68:41–51. doi: 10.1038/bjc.1993.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regula J., MacRobert A.J., Gorchein A., Buonaccorsi G.A., Thorpe S.M., Spencer G.M., Hatfield A.R., Bown S.G. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX--a pilot study. Gut. 1995;36:67–75. doi: 10.1136/gut.36.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J. Exp. Med. 1976;143:305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi S., Masuda T. High throughput heme assay by detection of chemiluminescence of reconstituted horseradish peroxidase. Comb. Chem. High Throughput Screen. 2009;12:532–535. doi: 10.2174/138620709788489028. [DOI] [PubMed] [Google Scholar]
- 13.Miyajima K., Hirata M., Yoshida T., Kosaka H., Okayama A. Study on measurement of delta-aminolevulinic acid in plasma by high-performance liquid chromatography. J. Chromatogr. B. 1994;654:165–169. doi: 10.1016/0378-4347(94)00046-8. [DOI] [PubMed] [Google Scholar]
- 14.Kondo M. Porphyrins in urine, blood, and feces and delta-aminolevulinic acid and porphobilinogen. Nihon Rinsho. 1995;53:1364–1370. [PubMed] [Google Scholar]
- 15.Bermudez Moretti M., Correa Garcia S., Perotti C., Batlle A., Casas A. Delta-Aminolevulinic acid transport in murine mammary adenocarcinoma cells is mediated by beta transporters. Br. J. Cancer. 2002;87:471–474. doi: 10.1038/sj.bjc.6600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiwara T., Saitoh H., Inoue A., Kobayashi M., Okitsu Y., Katsuoka Y., Fukuhara N., Onishi Y., Ishizawa K., Ichinohasama R., Harigae H. 3-Deazaneplanocin A (DZNep), an inhibitor of S-adenosylmethionine-dependent methyltransferase, promotes erythroid differentiation. J. Biol. Chem. 2014;289:8121–8134. doi: 10.1074/jbc.M114.548651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiwara T., O'Geen H., Keles S., Blahnik K., Linnemann A.K., Kang Y.A., Choi K., Farnham P.J., Bresnick E.H. Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol. Cell. 2009;36:667–681. doi: 10.1016/j.molcel.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canonne-Hergaux F., Gruenheid S., Ponka P., Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. [PubMed] [Google Scholar]
- 19.Donovan A., Brownlie A., Zhou Y., Shepard J., Pratt S.J., Moynihan J., Paw B.H., Drejer A., Barut B., Zapata A., Law T.C., Brugnara C., Lux S.E., Pinkus G.S., Pinkus J.L., Kingsley P.D., Palis J., Fleming M.D., Andrews N.C., Zon L.I. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 20.Eady J.J., Wormstone Y.M., Heaton S.J., Hilhorst B., Elliott R.M. Differential effects of basolateral and apical iron supply on iron transport in Caco-2 cells. Genes Nutr. 2015;10:1–15. doi: 10.1007/s12263-015-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uc A., Britigan B.E. Does heme oxygenase-1 have a role in Caco-2 cell cycle progression? Exp. Biol. Med. (Maywood) 2003;228:590–595. doi: 10.1177/15353702-0322805-52. [DOI] [PubMed] [Google Scholar]
- 22.Sassa S. Modern diagnosis and management of the porphyrias. Br. J. Haematol. 2006;135:281–292. doi: 10.1111/j.1365-2141.2006.06289.x. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi K., Watanabe-Matsui M. Wearing red for signaling: the heme-bach axis in heme metabolism, oxidative stress response and iron immunology. Tohoku J. Exp. Med. 2014;232:229–253. doi: 10.1620/tjem.232.229. [DOI] [PubMed] [Google Scholar]
- 24.Fiorito V., Forni M., Silengo L., Altruda F., Tolosano E. Crucial role of FLVCR1a in the maintenance of intestinal heme homeostasis. Antioxid. Redox Signal. 2015;23:1410–1423. doi: 10.1089/ars.2014.6216. [DOI] [PubMed] [Google Scholar]
- 25.Ajioka R.S., Phillips J.D., Kushner J.P. Biosynthesis of heme in mammals. Biochim. Biophys. Acta. 1763;2006:723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Nishio Y., Fujino M., Zhao M., Ishii T., Ishizuka M., Ito H., Takahashi K., Abe F., Nakajima M., Tanaka T., Taketani S., Nagahara Y., Li X.K. 5-Aminolevulinic acid combined with ferrous iron enhances the expression of heme oxygenase-1. Int. Immunopharmacol. 2014;19:300–307. doi: 10.1016/j.intimp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Hagiya Y., Adachi T., Ogura S., An R., Tamura A., Nakagawa H., Okura I., Mochizuki T., Ishikawa T. Nrf2-dependent induction of human ABC transporter ABCG2 and heme oxygenase-1 in HepG2 cells by photoactivation of porphyrins: biochemical implications for cancer cell response to photodynamic therapy. J. Exp. Ther. Oncol. 2008;7:153–167. [PubMed] [Google Scholar]
- 28.Hou J., Cai S., Kitajima Y., Fujino M., Ito H., Takahashi K., Abe F., Tanaka T., Ding Q., Li X.K. 5-Aminolevulinic acid combined with ferrous iron induces carbon monoxide generation in mouse kidneys and protects from renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2013;305:F1149–F1157. doi: 10.1152/ajprenal.00275.2013. [DOI] [PubMed] [Google Scholar]
- 29.Frolund S., Marquez O.C., Larsen M., Brodin B., Nielsen C.U. Delta-aminolevulinic acid is a substrate for the amino acid transporter SLC36A1 (hPAT1) Br. J. Pharmacol. 2010;159:1339–1353. doi: 10.1111/j.1476-5381.2009.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y., Hu Y., Smith D.E. The proton-coupled oligopeptide transporter 1 plays a major role in the intestinal permeability and absorption of 5-aminolevulinic acid. Br. J. Pharmacol. 2016;173:167–176. doi: 10.1111/bph.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zong W.X., Rabinowitz J.D., White E. Mitochondria and cancer. Mol. Cell. 2016;61:667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material