Abstract
Formation of neutrophil extracellular traps (NETs) can perpetuate sterile inflammation; thus, it is important to clarify their pathophysiological characteristics. Free heme, derived via hemolysis, is a major contributor to organ damage, and reportedly induces neutrophil activation as well as reactive oxygen species (ROS) production and NET formation. For this study, we examined hemin (Fe3+ -protoporphyrin IX)-induced NET formation quantitatively in vitro as well as the effects of oxidative stress.
NETs formed in vitro from cultured neutrophils were quantitatively detected by using nuclease treatment and Sytox Green, a nucleic acid stain. Hemin-induced NET production was found to be in a dose-dependent manner, NADPH oxidase-dependent and toll-like receptor (TLR)-4 independent. Additionally, the iron molecule in the porphyrin ring was considered essential for the formation of NETs. In the presence of low concentrations of hydrogen peroxide, low concentrations of hemin-induced NETs were enhanced, unlike those of phorbol myristate acetate (PMA)-induced NETs.
Quantitative analysis of NET formation may prove to be a useful tool for investigating NET physiology, and hemin could function as a possible therapeutic target for hemolysis-related events.
Abbreviations: NET, neutrophil extracellular traps; MPO, myeloperoxidase; ROS, reactive oxygen species; ELISA, Enzyme-Linked Immuno-Sorbent Assay; PMA, phorbol myristate acetate; DPI, diphenyleneiodonium; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; TLR, toll-like receptor; PAD4, peptidylarginine deiminases 4; LPS, lipopolysaccharide; HO-1, heme oxygenase-1
Chemical compounds studied in this article: hemin (PubChem CID: 121225420), phorbol myristate acetate (PubChem CID: 22833501), protoporphyrin IX (PubChem CID: 4971), hydrogen peroxide (PubChem CID: 784), sytox green (PubChem CID: 46863923), TAK-242 (PubChem CID: 11703255), diphenylene iodonium (PubChem CID: 3101), polymyxin B (PubChem CID: 4868)
Keywords: Hemin, Neutrophil, Extracellular trap, Quantitative detection, Hydrogen peroxide
Highlights
-
•
Hemin induces NET formation dose-dependently.
-
•
Hemin-induced NETs are ROS-dependent and TLR-4-independent.
-
•
Hydrogen peroxide enhances hemin-induced NET formation.
1. Introduction
Neutrophil extracellular traps (NETs) are actively discharged from activated neutrophils, and are composed of decondensed chromatin fibers coated with antimicrobial granular and cytoplasmic proteins, such as myeloperoxidase (MPO), neutrophil elastase, and alpha-defensin [1], [2]. Although NETs form to prevent dissemination of pathogens [1], excessive release of DNA and DNA-associated proteins can also perpetuate sterile inflammation as well as lung injury, thrombosis, sepsis, autoimmune diseases, and metastasis of cancers [2], [3], [4], [5], [6].
In addition to damage-associated molecular patterns (DAMPS), free heme is a major contributor to organ damage induced by sepsis [7] or hemolysis [8]. Several studies have postulated that free heme activates human neutrophils and induces reactive oxygen species (ROS) production [9], [10], [11] and NET formation [12], [13].
The in vitro method to detect NETs is mainly based on morphological observation, but quantitative detection is also important. Released NETs are quantitatively measurable using nucleic acid staining agents such as Sytox Green, fluorescent nucleic acid stain [14] or double strand DNA quantification kit [15], or by means of ELISA detecting myeloperoxidase and DNA complex [16]. In addition, plasma cell-free DNA (cfDNA) is reportedly as useful for several pathological conditions [17], [18], while the application of flow cytometric techniques is also being developed [19]. We employed a nucleic acid quantification method using Sytox green which has been used for microscopic observation and identification of NETs [20].
For this study, we evaluated hemin (Fe3+ (ferri)-protoporphyrin IX)-induced NET formation quantitatively and confirmed its concentration-dependency. Moreover, hemin-induced NET formation was found to increase in the presence of oxidative stress.
2. Methods
2.1. Preparation of neutrophils
Heparinized peripheral blood was collected from healthy volunteers after obtaining their written informed consent. Neutrophil separation (>90% purity) was performed at room temperature (RT) using the density gradient method with the Polymorphprep separation medium (Alere Technologies AS, Oslo, Norway). This study was approved by the Ethics Committee of Himeji Dokkyo University (12-01) and by Sysmex Corporation (2014-50).
2.2. Quantitative method for detection of NETs
Quantitative analysis was performed using Palmer's method [14] with partial modification. A 96-well plate was coated with 1% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO) and incubated over night at 4 °C. After addition of 0.2 mL of purified neutrophils in RPMI 1640 to each well at a concentration of 0.5×109 cells /L, the cells were stimulated with hemin, protoporphyrin IX (PP IX, lacking iron from hemin), phorbol myristate acetate (PMA) or Escherichia coli 0111:B4 lipopolysaccharide (LPS), all from Sigma-Aldrich, for 3 h at 37 °C in humidified air with 5% CO2. Hydrogen peroxide (Nacalai Tesque, Kyoto, Japan) was added simultaneously to the samples. After incubation, nuclease from Staphylococcus aureus (Sigma-Aldrich) treatment at a final concentration of 1 U/mL was performed for 10 min at 37 °C, followed by treatment with 2 mM ethylene glycol tetraacetic acid (EGTA, Sigma-Aldrich) at 4 °C. After being transferred to Eppendorf tubes, the samples were centrifuged at 1800g for 10 min at 4 °C. Sytox Green (Molecular Probes, Eugene, OR) was added to 0.17 mL of the supernatants at a final concentration of 2.9 nM, and fluorescence was detected at excitation and emission wavelengths of 488 nm and 520 nm, respectively, using ARVO MX (Perkin Elmer, Waltham, MA, USA). Relative fluorescence was calculated against control.
For the experiments with various inhibitors, 0.01 mM diphenylene iodonium (DPI), a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor, 0.01 mM Mito TEMPO (a mitochondria-restricted anti-oxidant), and 1 mg/L polymyxin B (Poly B, an LPS inhibitor), all from Sigma-Aldrich, were added to the samples 10 min before the addition of stimulators.
Following the protocol prescribed by Hussey [21], TAK-242, resatorvid, a small molecular inhibitor of Toll-like receptor (TLR)-4 signaling (Chem Scene, Monmouth Junction, NJ, USA) was added at 0.01 mM or 0.03 mM and incubated for 1 h at 37 °C in humidified air with 5% CO2.
2.3. Morphological observations
Purified neutrophils (1 × 109 cells/L) were suspended in Hanks' balanced salt solution (HBSS) with 2% heat-inactivated autologous serum at a concentration of 1×109/L. The cell suspension (250 µL) was added to 35-mm glass-bottom dishes coated with Poly-l-Lysine (Sigma-Aldrich), and incubated under the same conditions as for quantitative analysis. Next, Sytox Green was added at a concentration of 4 nM, and stained neutrophils were observed under a fluorescence microscope (TH4-100 Cell Sens; Olympus, Tokyo, Japan).
Immunostaining of NETs with antibodies was performed as described previously [15]. Treated neutrophils were fixed with 1% paraformaldehyde (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 1 h at RT. Fixed samples were incubated with anti-MPO mouse monoclonal antibody (ab25989; Abcam plc, Bristol, UK) and anti-citrullinated histone H3 rabbit polyclonal antibody (ab5103; Abcam) at a concentration of 20 mg/L in 1% BSA/PBS for 16 h at 4 °C. Normal rabbit IgG (sc-2027; Santa Cruz Biotechnology, Santa Crus, TX, USA) and mouse IgG (x0931; Agilent Technologies, Palo Alto, CA, USA) antibodies were used as negative controls. Following incubation, samples were washed with 1% BSA in PBS, incubated with Alexa Fluor 633 conjugated anti-mouse IgG (Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor 488 conjugated anti-rabbit IgG (Thermo Fisher Scientific) antibodies, each at a concentration of 2 mg/L in 1% BSA in PBS for 2 h at 4 °C, and observed with a fluorescence microscope system (TCS SP8; Leica Microsystems, Wetzlar, Germany).
2.4. Flow cytometry (FCM)
For flow cytometric detection of CD11b expression after LPS stimulation monoclonal antibodies to CD11b-conjugated with phycoerythrin (anti-CD11b-PE; BD Biosciences, San Jose, CA) were used as previously described [22]. Briefly, 0.1 mL of heparinized whole blood was stimulated with 10 ng/mL LPS for 15 min, followed by the addition of 5 µL of anti-CD11b-PE and incubation for 15 min in the dark at RT. The samples were then fixed and hemolyzed using 1 mL of FACS Lysing Solution (BD Biosciences) for 10 min, after which the washed samples were analyzed by means of FACS Calibur (BD Biosciences) to determine the mean fluorescence intensity (MFI) of the monocytes.
2.5. Statistical analyses
EZR (Easy R) was used for all statistical analyses [23]. A paired t-test was employed to compare data from two groups.
3. Results
3.1. Quantitative detection of hemin-induced NETs
Feasibility of quantitative analysis was checked by using lambda DNA in RPMI 1640, and linearity between 0.0625 ng/mL and 5.0 ng/mL was confirmed (r2 = 0.994) (Supplemental Fig. 1).
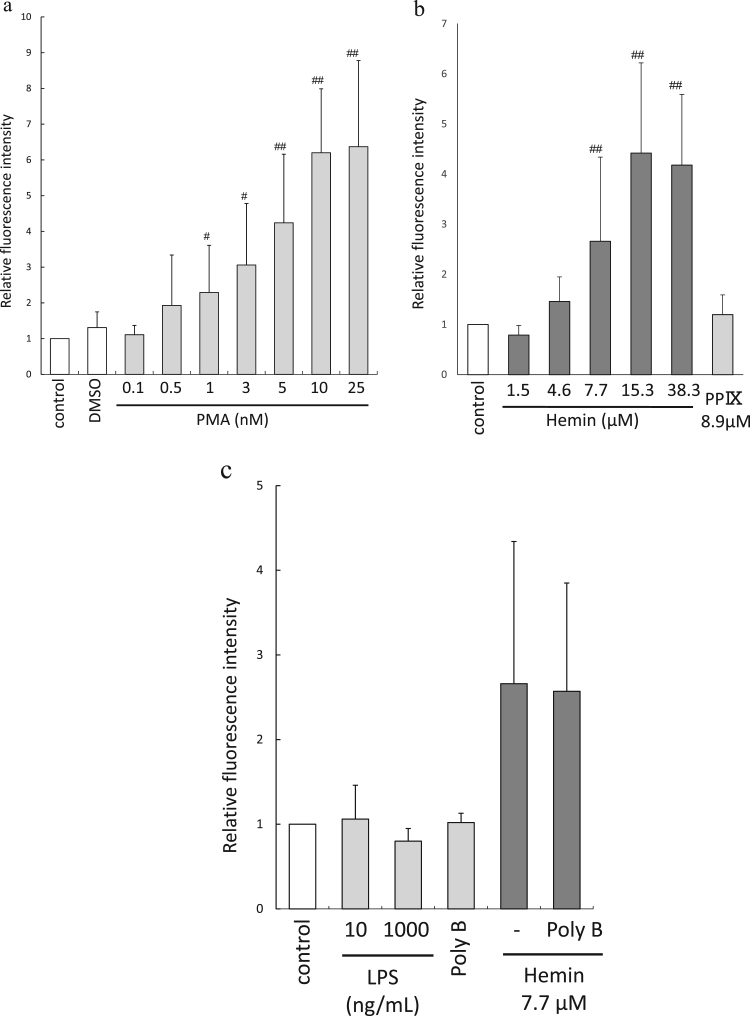
NET formation induced by PMA and hemin is shown in Fig. 1-a and b, respectively. The NET induced by PMA (0.1–10 nM) and hemin (1.5–15.3 µM) was dose-dependent. Although we previously demonstrated that PP IX had a more potent ROS-producing capability than hemin [11], PP IX did not induce NETs in this case (Fig. 1-b). The probability of LPS contamination in reagents was unlikely because LPS alone did not induce NETs under these conditions, and treatment with polymyxin B did not affect the fluorescence intensity (Fig. 1-c).
Fig. 1.
Quantitative analyses of PMA- or hemin-induced NET generation. (a) PMA-induced NET formation. NETs were formed dose-dependently (0.1 and 10 nM of PMA; n = 10–20). #, p < 0.05, ##, p < 0.01 against control. (b) Hemin-induced NET formation. NETs were generated dose-dependently (1.5 µM–38.3 µM of hemin; n = 10–20). PP IX did not induce NET production. ##, p < 0.01 against control. (c) The effects of Poly B on hemin-induced NETs. LPS or Poly B alone did not induce NETs. Hemin-induced NETs were not inhibited by the presence of Poly B (n = 4–10).
3.2. Hemin-induced NETs depend on NADPH oxidase and ROS but not on TLR-4
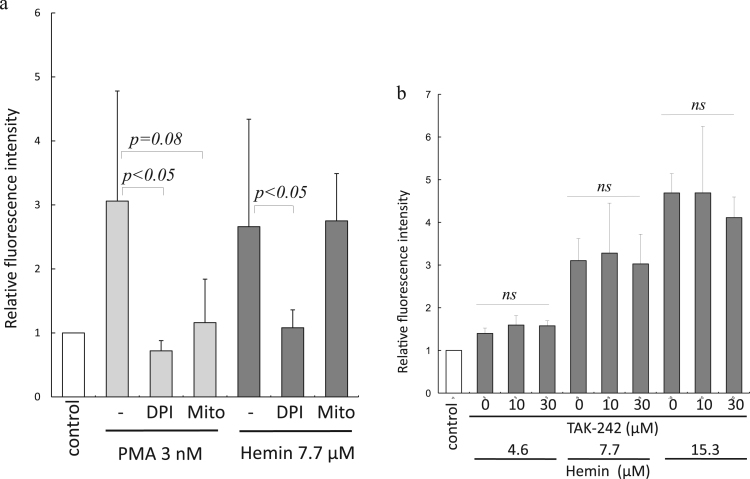
NET formation is mediated by several integrated mechanisms comprising autophagy, ROS production through NADPH oxidase, neutrophil elastase, and histone citrullination by peptidylarginine deiminases 4 (PAD4) [24]. For this study, we investigated whether hemin induced-NETs are ROS- dependent. We found that although DPI inhibited NET formation induced by PMA and hemin, Mito-TEMPO inhibited only PMA-induced NET formation (Fig. 2-a). Thus, the effect of hemin on NET formation was shown to be NADPH oxidase-derived ROS-dependent.
Fig. 2.
ROS-dependent and TLR-4 independent characteristics of hemin-induced NETs. (a) Inhibition of PMA- and hemin-induced NETs by DPI. Mito-TEMPO inhibited only PMA-induced NETs (n = 7–12). (b) Effects of TAK-242 on hemin-induced NETs. Addition of TAK-242 did not suppress hemin-induced NETs (n = 4). ns = not significant.
Neutrophils have been found to be activated through TLR-4 by high-mobility group box-1 (HMGB1) [25], and heme reportedly activates endothelial cells through TLR-4 [26]. For this study, we investigated the effects of TLR-4 signaling blockade on hemin-induced NET generation. The addition of 10 and 30 µM of TAK-242 was found to significantly inhibit the expression of LPS-induced monocytes CD11b (p < 0.01), indicating the efficacy of TAK-242 action on TLR-4 inhibition (Supplemental Fig. 2). However, since TAK-242 did not inhibit hemin-induced NET generation (Fig. 2-b), the NET-producing effects of hemin were not considered to have been generated through TLR-4 signaling.
3.3. Hydrogen peroxide enhanced hemin-induced NET production
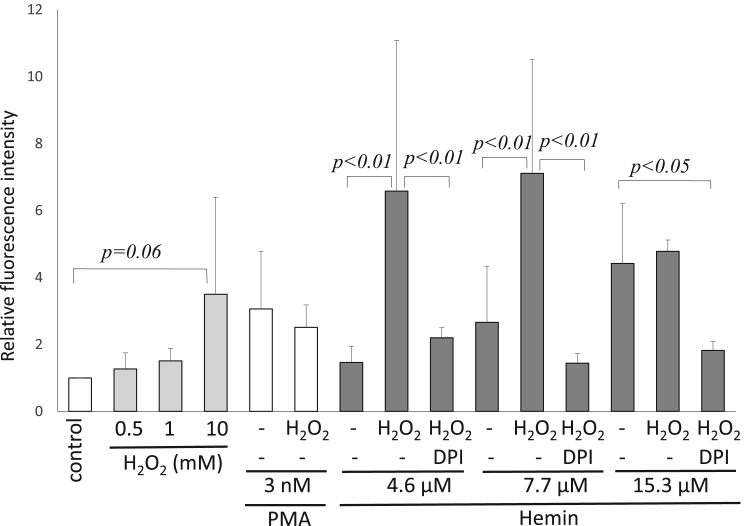
As oxidative stress is induced by several acute illnesses including sepsis, stroke, myocardial infarction, or multiple trauma [27], [28], we studied the effects of hemin under oxidative stress generated by hydrogen peroxide (H2O2). High concentrations of H2O2 (10 mM) tended to induce NETs as reported previously [29], [30], but concentrations lower than 1 mM did not induce NETs individually (Fig. 3). Changes brought about by the combination of PMA and H2O2 were insignificant alteration in comparison with those produced by PMA alone, although 0.5 mM H2O2 significantly increased NET formation induced by 4.6 or 7.7 µM hemin (Fig. 3). This augmented NET formation was inhibited by DPI (Fig. 3), similarly to the result shown in Fig. 2-a. NET formation from 15.3 µM was not enhanced by H2O2 probably due to the toxic effects of hemin [31].
Fig. 3.
Effects of hydrogen peroxide on NET formation. H2O2 (10 mM) induced NET formation, while concentrations lower than 1 mM failed to do so. Addition of H2O2 did not affect PMA-induced NETs, but, enhanced hemin-induced NET production. H2O2 -enhanced NETs were sensitive to DPI (n = 10–20).
3.4. Morphological confirmation of enhancement of NET production by hemin with hydrogen peroxide
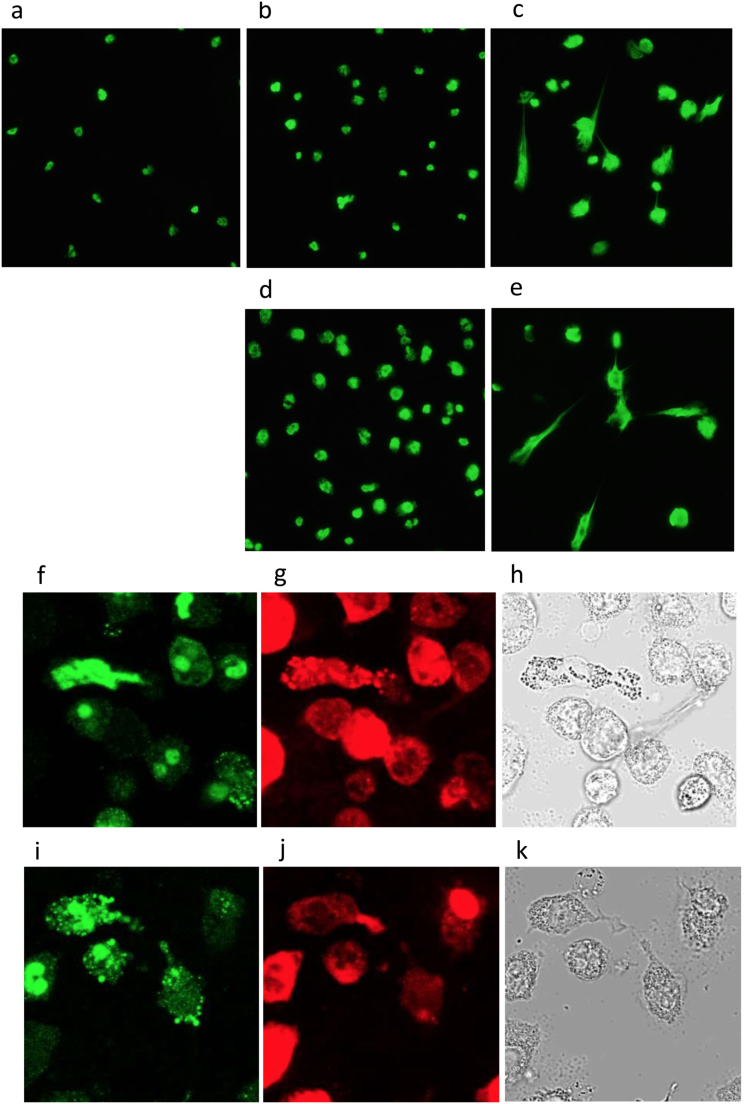
Morphological observation under a fluorescence microscope was carried out after staining with Sytox Green. Hemin alone, at a concentration of 7.7 µM or 15.3 µM, induced nuclear swelling with little NET-like structure formation (Fig. 4-b or d, respectively) in comparison with control (Fig. 4-a). However, co-stimulation with 0.5 mM H2O2 induced accelerated formation of NET-like structures. Representative results of stimulation with 7.7 µM hemin with H2O2 are shown in Fig. 4-c, and those of 15.3 µM hemin with H2O2 are shown in Fig. 4-e. Immunostaining studies revealed that both PMNs stimulated with 15.3 µM hemin alone (Fig. 4-f, g, h) and those stimulated with hemin combined with 0.5 mM H2O2 (Fig. 4-i, j, k) displayed positive staining with citrullinated histone H3 (Fig. 4-f, i, green) as well as with MPO (Fig. 4-g, j, red), indicating that both hemin alone and hemin in conjunction with H2O2 induced NET formation.
Fig. 4.
Morphological observation of hemin-induced NETs. Representative fluorescence microscopy images after Sytox Green staining (a~e), and immunostaining with anti-citrullinated histone H3 (f, i) and MPO (g, j) are shown as phase contrast microscopy images (h, k). (a) control, (b) 7.7 µM hemin, (c) 7.7 µM hemin with 0.5 mM H2O2, (d) 15.3 µM hemin, (e) 15.3 µM hemin with 0.5 mM H2O2, (f,g,h) 15.3 µM hemin, (i,j,k) 15.3 µM hemin with 0.5 mM H2O2.
4. Discussion
NETs are though to exert protective effects because of their anti-microbial activity. However, NETs also contribute to destructive events, such as thrombus formation and organ damage. Although morphological observation using fluorescent microscopy or confocal laser scanning microscopy is most frequently used in in vitro studies of NETs, a quantitative analysis of morphological changes is neither simple nor easy. For the study reported here, a quantitative method described by Palmer [14] was used to detect NET formation by using Sytox Green, which is frequently employed for morphological observation. As linearity between 0.0625 and 5.0 ng/mL lambda DNA had been confirmed, quantitative analysis was feasible and carried out.
It confirmed the capability of hemin to induce NET production. As PP IX did not induce NET formation, hemin-induced NETs were considered to be iron dependent, a finding consistent with that from a previous report [13].
Although NADPH oxidase-derived ROS is required to generate NETs, another ROS-independent pathway has also been reported [32], [33], [34]. As shown in our study, hemin- and PMA-induced NETs were NADPH oxidase dependent, but the contribution of mitochondria-derived ROS might be different with these two inducers. Formation of NETs by hemin combined with tumor necrosis factor (TNF) was also reported to be dependent on ROS [13].
Heme binds to TLR-4 on endothelial cells at sites other than the LPS- binding site, and produces TNF and von Willebrand factor [26], [35]. However, our experiments with TAK-242 showed that the binding sites of hemin on neutrophils were different from TLR-4. Although hemin can penetrate the cellular membrane because of its hydrophobic character [36], a protoporphyrin ring transporter, heme carrier protein 1 (HCP1), is reportedly present and functions in duodenal cells [37], hematopoietic stem cells [38], and astrocytes [39]. Therefore, studies of the function of HCP1 in neutrophils are also necessary.
It is important for biological responses to occur that low concentrations of hemin-induced NETs are enhanced by low concentrations of hydrogen peroxide. Hemin and hydrogen peroxide have also been reported by Larsen et al. to have this additive effect on the cell death of hepatocytes in vitro, who emphasized that hemin might be a major factor in the pathophysiology of sepsis [7]. The results reported here seem to suggest that oxidative stress generated by oxidizing agents such as hydrogen peroxide [29], [30], superoxide [16] or hypochlorous acid [40] amplifies the NET producing activity induced by hemin. This means that hemin can be expected to become a therapeutic target for hemolysis-related events.
As hemin can be easily released from oxidized hemoglobin (methemoglobin) under conditions of oxidative stress [36], the pathological implications of hemin-induced NETs should be considered. Hemin combined with TNF induced NETs in vivo, and these NETs were life-threatening to mice [13]. Moreover, hemin can also induce expression of HO-1, an anti-inflammatory protein [41], [42], pointing to the need for in vivo studies under various conditions.
In summary, in the quantitative assay system, hemin alone induced NETs in a dose-dependent manner, which in turn was iron- and NADPH oxidase derived ROS-dependent. In addition, NET-forming capacity was enhanced in the presence of low concentrations of hydrogen peroxide. These results indicate that even a small amount of hemin produced by hemolysis might have pathophysiological implications that warrant further investigation.
Conflict of interest disclosure
The authors declare no conflict of interest.
Acknowledgement
This work was supported by JSPS Grant-in-Aid 15K08660 and Hyogo Medical Association Grant MRF-H-07-14 to K.S.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.07.009.
Contributor Information
Ayako Ohbuchi, Email: aohbuchi@gm.himeji-du.ac.jp.
Mari Kono, Email: Kono.Mari@sysmex.co.jp.
Kaihei Kitagawa, Email: mh53c@yahoo.co.jp.
Mariko Takenokuchi, Email: tmariko@himeji-du.ac.jp.
Shion Imoto, Email: s-imoto@kobe-tokiwa.ac.jp.
Katsuyasu Saigo, Email: ksaigo@himeji-du.ac.jp, saigok2@yahoo.co.jp.
Appendix A. Transparency document
Supplementary material
Supplementary material Supplemental Fig. 1 Quantitative detection of lambda DNA. Linearity between 0.0625 ng/mL and 5.0 ng/mL was confirmed (r2 = 0.994). Supplemental Fig. 2 Expression of LPS-induced monocytes CD11b (n = 4). FCM detection of activated monocytes demonstrated that TAK-242 successfully inhibited TLR-4 signaling.
References
- 1.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Porto B.N., Stein R.T. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gros A., Ollivier V., Ho-Tin-Noé B. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front. Immunol. 2015;5:678. doi: 10.3389/fimmu.2014.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashiba M., Huq A., Tomino A., Hirakawa A., Hattori T., Miyabe H., Tsuda M., Takeyama N. Neutrophil extracellular traps in patients with sepsis. J. Surg. Res. 2015;194:248–254. doi: 10.1016/j.jss.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Shimomura Y., Suga M., Kuriyama N., Nakamura T., Sakai T., Kato Y., Hara Y., Yamashita C., Nagasaki H., Kaneki M., Nishida O. Recombinant human thrombomodulin inhibits neutrophil extracellular trap formation in vitro. J. Intensive Care. 2016;4:48. doi: 10.1186/s40560-016-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remijsen Q., Kuijpers T.W., Wirawan E., Lippens S., Vandenabeele P., Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;4:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen R., Gozzelino R., Jeney V., Tokaji L., Bozza F.A., Japiassú A.M., Bonaparte D., Cavalcante M.M., Chora Â., Ferreira A., Marguti I., Cardoso S., Sepúlveda N., Smith A., Soares M.P. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 8.Belcher J.D., Chen C., Nguyen J., Milbauer L., Abdulla F., Alayash A.I., Smith A., Nath K.A., Hebbel R.P., Vercellotti G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graça-Souza A.V., Arruda M.A., de Freitas M.S., Barja-Fidalgo C., Oliveira P.L. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99:4160–4165. doi: 10.1182/blood.v99.11.4160. [DOI] [PubMed] [Google Scholar]
- 10.Porto B.N., Alves L.S., Fernández P.L., Dutra T.P., Figueiredo R.T., Graça-Souza A.V., Bozza M.T. Heme induces neutrophil migration and reactive oxygen species generation through signaling pathways characteristic of chemotactic receptors. J. Biol. Chem. 2007;282:24430–24436. doi: 10.1074/jbc.M703570200. [DOI] [PubMed] [Google Scholar]
- 11.Kono M., Saigo K., Takagi Y., Kawauchi S., Wada A., Hashimoto M., Sugimoto T., Takenokuchi M., Morikawa T., Funakoshi K. Morphological and flow-cytometric analysis of haemin-induced human neutrophil activation: implications for transfusion-related acute lung injury. Blood Transfus. 2013;11:53–60. doi: 10.2450/2012.0141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kono M., Saigo K., Takagi Y., Takahashi T., Kawauchi S., Wada A., Hashimoto M., Minami Y., Imoto S., Takenokuchi M., Morikawa T., Funakoshi K. Heme-related molecules induce rapid production of neutrophil extracellular traps. Transfusion. 2014;54:2811–2819. doi: 10.1111/trf.12700. [DOI] [PubMed] [Google Scholar]
- 13.Chen G., Zhang D., Fuchs T.A., Manwani D., Wagner D.D., Frenette P.S. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood. 2014;123:3818–3827. doi: 10.1182/blood-2013-10-529982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer L.J., Damgaard C., Holmstrup P., Nielsen C.H. Influence of complement on neutrophil extracellular trap release induced by bacteria. J. Periodontal Res. 2016;51:70–76. doi: 10.1111/jre.12284. [DOI] [PubMed] [Google Scholar]
- 15.Kono M., Saigo K., Yamamoto S., Shirai K., Iwamoto S., Uematsu T., Takahashi T., Imoto S., Hashimoto M., Minami Y., Wada A., Takenokuchi M., Kawano S. Iron chelating agent, deferasirox, inhibits neutrophil activation and extracellular trap (NET) formation. Clin. Exp. Pharmacol. Physiol. 2016;43:915–920. doi: 10.1111/1440-1681.12612. [DOI] [PubMed] [Google Scholar]
- 16.Al-Khafaji A.B., Tohme S., Yazdani H.O., Miller D., Huang H., Tsung A. Superoxide induces neutrophil extracellular trap formation in a TLR-4 and NOX-dependent mechanism. Mol. Med. 2016;22:621–631. doi: 10.2119/molmed.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arai Y., Yamashita K., Mizugishi K., Watanabe T., Sakamoto S., Kitano T., Kondo T., Kawabata H., Kadowaki N., Takaori-Kondo A. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2013;19:1683–1689. doi: 10.1016/j.bbmt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Hampson P., Dinsdale R.J., Wearn C.M., Bamford A.L., Bishop J.R., Hazeldine J., Moiemen N.S., Harrison P., Lord J.M. Neutrophil dysfunction, immature granulocytes, and cell-free DNA are early biomarkers of sepsis in burn-injured patients: a prospective observational cohort study. Ann. Surg. 2017;265:1241–1249. doi: 10.1097/SLA.0000000000001807. [DOI] [PubMed] [Google Scholar]
- 19.Masuda S., Nakazawa D., Shida H., Miyoshi A., Kusunoki Y., Tomaru U., Ishizu A. NETosis markers: quest for specific, objective, and quantitative markers. Clin. Chim. Acta. 2016;459:89–93. doi: 10.1016/j.cca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Nishinaka Y., Arai T., Adachi S., Takaori-Kondo A., Yamashita K. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochem. Biophys. Res. Commun. 2011;16:75–79. doi: 10.1016/j.bbrc.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 21.Hussey S.E., Liang H., Costford S.R., Klip A., DeFronzo R.A., Sanchez-Avila A., Ely B., Musi N. TAK-242, a small-molecule inhibitor of Toll-like receptor 4 signalling, unveils similarities and differences in lipopolysaccharide- and lipid-induced inflammation and insulin resistance in muscle cells. Biosci. Rep. 2012;33:37–47. doi: 10.1042/BSR20120098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto M., Saigo K., Jyokei Y., Kishimoto M., Takenokuchi M., Araki N., Imoto S., Taniguchi K., Kumagai S. Albumin attenuates neutrophil activation induced by stimulators including antibodies against neutrophil-specific antigens. Transfus. Apher. Sci. 2005;33:289–298. doi: 10.1016/j.transci.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remijsen Q., Kuijpers T.W., Wirawan E., Lippens S., Vandenabeele P., Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind anantimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan J., Li Y., Levy R.M., Fan J.J., Hackam D.J., Vodovotz Y., Yang H., Tracey K.J., Billiar T.R., Wilson M.A. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J. Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 26.Belcher J.D., Chen C., Nguyen J., Milbauer L., Milbauer L., Abdulla F., Alayash A.I., Smith A., Nath K.A., Hebbel R.P., Vercellotti G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bar-Or D., Bar-Or R., Rael L.T., Brody E.N. Oxidative stress in severe acute illness. Redox Biol. 2015;4:340–345. doi: 10.1016/j.redox.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prauchner C.A. Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns. 2017;43:471–485. doi: 10.1016/j.burns.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Parker H., Winterbourn C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2013;3:424. doi: 10.3389/fimmu.2012.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim M.B., Kuiper J.W., Katchky A., Goldberg H., Glogauer M. Rac2 is required for the formation of neutrophil extracellular traps. J. Leukoc. Biol. 2011;90:771–776. doi: 10.1189/jlb.1010549. [DOI] [PubMed] [Google Scholar]
- 31.Hao K., Hanawa H., Ding L., Ota Y., Yoshida K., Toba K., Ogura M., Ito H., Kodama M., Aizaw Y. Free heme is a danger signal inducing expression of proinflammatory proteins in cultured cells derived from normal rat hearts. Mol. Immunol. 2011;48:1191–1202. doi: 10.1016/j.molimm.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Tadie J.M., Bae H.B., Jiang S., Park D.W., Bell C.P., Yang H., Pittet J.F., Tracey K., Thannickal V.J., Abraham E., Zmijewski J.W. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Lung Cell Mol. Physiol. 2013;304:L342–L349. doi: 10.1152/ajplung.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilsczek F.H., Salina D., Poon K.K., Fahey C., Yipp B.G., Sibley C.D., Robbins S.M., Green F.H., Surette M.G., Sugai M., Bowden M.G., Hussain M., Zhang K., Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 34.Marcos V., Zhou Z., Yildirim A.O., Bohla A., Hector A., Vitkov L., Wiedenbauer E.M., Krautgartner W.D., Stoiber W., Belohradsky B.H., Rieber N., Kormann M., Koller B., Roscher A., Roos D., Griese M., Eickelberg O., Döring G., Mall M.A., Hartl D. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat. Med. 2010;16:1018–1023. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 35.Schaer D.J., Buehler P.W., Alayash A.I., Belcher J.D., Vercellotti G.M. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balla J., Vercellotti G.M., Jeney V., Yachie A., Varga Z., Jacob H.S., Eaton J.W., Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid. Redox Signal. 2007;9:2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 37.Shayeghi M., Latunde-Dada G.O., Oakhill J.S., Laftah A.H., Takeuchi K., Halliday N., Khan Y., Warley A., McCann F.E., Hider R.C., Frazer D.M., Anderson G.J., Vulpe C.D., Simpson R.J., McKie A.T. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Krishnamurthy P., Ross D.D., Nakanishi T., Bailey-Dell K., Zhou S., Mercer K.E., Sarkadi B., Sorrentino B.P., Schuetz J.D. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 39.Dang T.N., Bishop G.M., Dringen R., Robinson S.R. The putative heme transporter HCP1 is expressed in cultured astrocytes and contributes to the uptake of hemin. Glia. 2010;58:55–65. doi: 10.1002/glia.20901. [DOI] [PubMed] [Google Scholar]
- 40.Palmer L.J., Cooper P.R., Ling M.R., Wright H.J., Huissoon A A., Chapple I.L. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunol. 2012;(167):261–268. doi: 10.1111/j.1365-2249.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo Y.P., Jiang L., Kang K., Fei D.S., Meng X.L., Nan C.C., Pan S.H., Zhao M.R., Zhao M.Y. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int. Immunopharmacol. 2014;20:24–32. doi: 10.1016/j.intimp.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Datla S.R., Dusting G.J., Mori T.A., Taylor C.J., Croft K.D., Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50:636–642. doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material Supplemental Fig. 1 Quantitative detection of lambda DNA. Linearity between 0.0625 ng/mL and 5.0 ng/mL was confirmed (r2 = 0.994). Supplemental Fig. 2 Expression of LPS-induced monocytes CD11b (n = 4). FCM detection of activated monocytes demonstrated that TAK-242 successfully inhibited TLR-4 signaling.