Abstract
Objective:
To describe clinical and radiologic outcomes of children with incidental findings on neuroimaging suggestive of CNS demyelination (termed “radiologically isolated syndrome” or RIS).
Methods:
Clinical and radiologic data were obtained from a historical cohort of children with no symptoms of demyelinating disease who had MRI scans that met the 2010 MRI criteria for dissemination in space for MS.
Results:
We identified 38 children (27 girls and 11 boys) with RIS now being prospectively followed at 16 sites in 6 countries. The mean follow-up time was 4.8 ± 5.3 years. The most common reason for initial neuroimaging was headache (20/38, 53%). A first clinical event consistent with CNS demyelination occurred in 16/38 children (42%; 95% confidence interval [CI]: 27%–60%) in a median of 2.0 years (interquartile range [IQR] 1.0–4.3 years). Radiologic evolution developed in 23/38 children (61%; 95% CI: 44%–76%) in a median of 1.1 years (IQR 0.5–1.9 years). The presence of ≥2 unique oligoclonal bands in CSF (hazard ratio [HR] 10.9, 95% CI: 1.4–86.2, p = 0.02) and spinal cord lesions on MRI (HR 7.8, 95% CI: 1.4–43.6, p = 0.02) were associated with an increased risk of a first clinical event after adjustment for age and sex.
Conclusions:
We describe the clinical characteristics and outcomes of children with incidental MRI findings highly suggestive of CNS demyelination. Children with RIS had a substantial risk of subsequent clinical symptoms and/or radiologic evolution. The presence of oligoclonal bands in CSF and spinal cord lesions on MRI were associated with an increased risk of a first clinical event.
The incidental finding of abnormalities on MRI scans of the brain and spinal cord has become more common due to the increasing use of MRI in the evaluation of a wide range of medical conditions in children.1,2 Some of these abnormalities are highly suggestive of CNS demyelination based on their size, location within the white matter, and shape. This finding has previously been described in adults and has been termed “radiologically isolated syndrome” or RIS.3–5 Criteria for RIS in adults were proposed in 2009 and require both clinical and imaging factors including the incidental detection of MRI abnormalities meeting the following criteria: (1) ovoid and well-circumscribed homogenous foci with or without involvement of the corpus callosum, (2) T2 hyperintensities ≥3 mm in diameter fulfilling at least 3 of the 4 Barkhof MRI criteria for dissemination in space (DIS), as adopted in the 2005 diagnostic criteria for MS,6 and (3) the CNS abnormalities are not consistent with a vascular pattern.3 We recently reported a teenager with such incidental white matter abnormalities detected on brain MRI.7 However, outcomes following the detection of RIS in children are not known, and there are no criteria for RIS in children.
Adults identified with RIS have a 34% risk of developing a first clinical event consistent with CNS demyelination within 5 years.8,9 Factors associated with the development of a first clinical event in adults with RIS include age <37 years, male sex, and the presence of spinal cord lesions on MRI. Radiologic evolution occurred in 59% of adult RIS subjects after a median of 2.7 years.3 The risk of developing either a first clinical event consistent with CNS demyelination or radiologic evolution in children (age <18 years) with RIS is unknown.
The objectives of this historical cohort study in children newly enrolled in a multicenter longitudinal observational cohort study of outcomes following pediatric RIS were therefore (1) to propose criteria for RIS in children, (2) to determine the clinical and radiologic outcomes of children with RIS over time, and (3) to determine whether any clinical, MRI, or laboratory marker was associated with an increased risk of either clinical or radiologic evolution.
METHODS
Study participants.
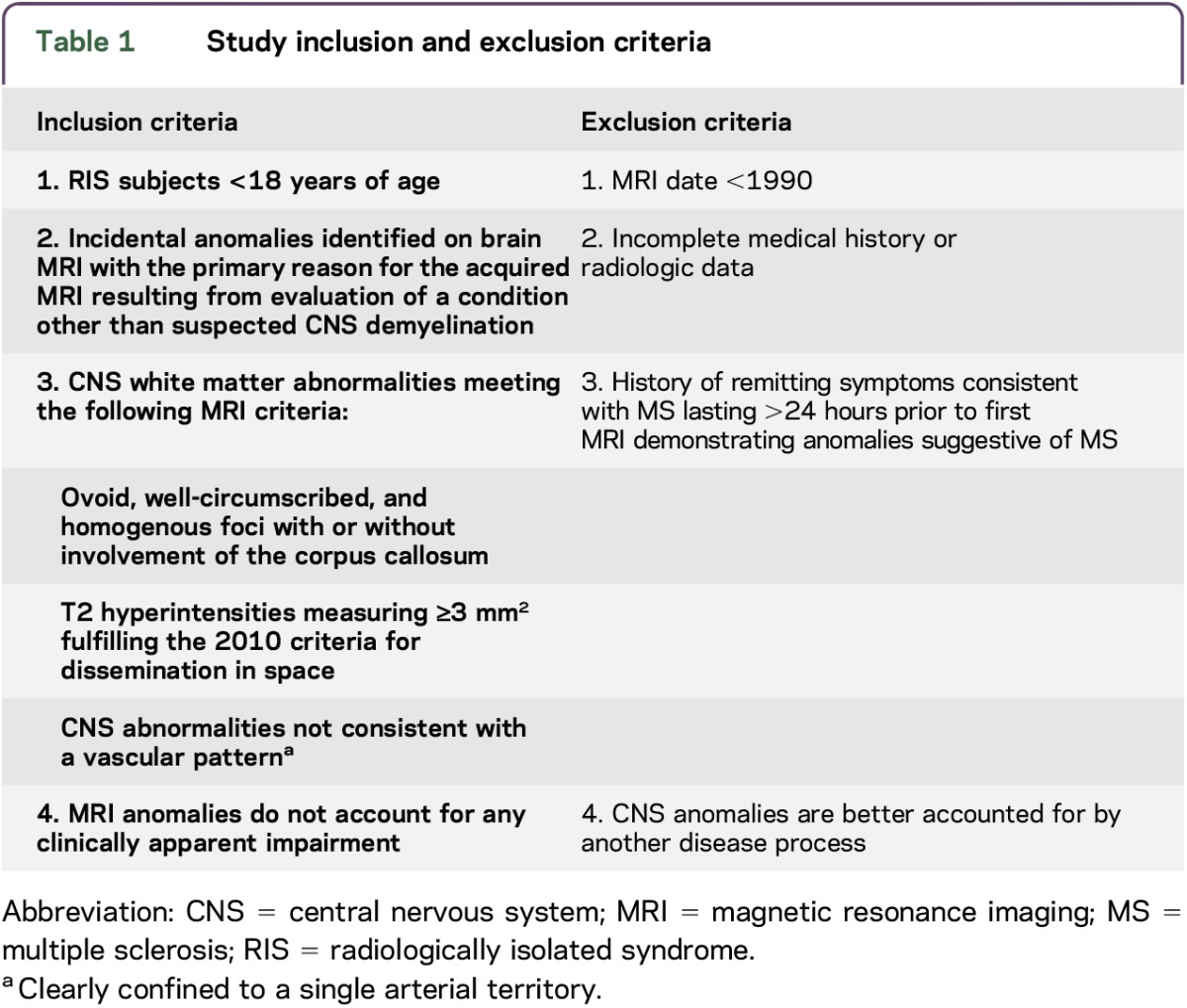
We identified a historical cohort of children aged <18 years with incidental MRI abnormalities consistent with CNS demyelination that met the 2010 criteria for DIS for MS on MRI.10 All children are now being prospectively followed. Inclusion and exclusion criteria are shown in table 1. Children were identified and followed according to routine clinical practice at 16 collaborating MS centers in 6 countries between December 1, 1995, and March 15, 2016 (table e-1 at Neurology.org/nn). A detailed clinical history and neurologic examination were performed on all children. Tests to exclude other infectious, inflammatory, rheumatologic, and metabolic diseases (e.g., erythrocyte sedimentation rate, C-reactive protein level, antinuclear antibody screen, rheumatoid factor level, double-stranded DNA testing, vitamin B12 level, angiotensin-converting enzyme level, anticardiolipin antibodies, and Lyme disease serology) were performed based on local practice. CSF analysis and determination of serum 25-hydroxyvitamin D levels were obtained at the discretion of the treating neurologist at nonstandardized time points and tested using local methods.
Table 1.
Study inclusion and exclusion criteria
Neuroimaging.
All children underwent MRI on either 1.5T or 3T MRI scanners. All brain and spinal cord MRI studies included T1- and T2-weighted spin-echo sequences in multiple planes of view (axial and sagittal, with coronal images for brain studies) with or without gadolinium.
MRI abnormalities were first identified by a clinical neuroradiologist and then confirmed by at least 1 MS specialist at each site to ensure that the 2010 DIS criteria were met on initial scans. The presence or absence of radiologic evolution, defined as any of ≥1 new T2 lesion, ≥1 newly enlarging T2 lesion, or ≥1 newly enhancing lesion in either the brain or spinal cord, was similarly determined.8,9
Standard protocol approvals and patient consents.
Institutional ethical approval was obtained from all sites. Written informed consent was obtained from parents/guardians, and children provided assent.
Statistical analysis.
Clinical and MRI data were collected using a standard template. We report mean values (±SD) and/or medians (with interquartile ranges, IQRs) for continuous variables and frequency (percentage) for categorical variables. We created Kaplan-Meier survival curves to illustrate time to either a first clinical event consistent with CNS demyelination (defined as a new neurologic symptom and sign lasting ≥24 hours) or radiologic evolution where zero time was the date of the initial scan that met the 2010 criteria for DIS. We used Mann-Whitney U tests (continuous variables) and Fisher's exact tests (categorical variables) to examine the statistical significance of unadjusted associations between the outcomes of either a first clinical event or radiologic evolution, and demographic variables (e.g., age, sex, and race), MRI variables (e.g., number of brain lesions, presence of enhancing lesions, presence of periventricular, infratentorial, or spinal cord lesions), and laboratory-based variables (e.g., presence of ≥2 unique oligoclonal bands in CSF). We created multivariable Cox proportional hazards models for the time to either a first clinical event or radiologic evolution. Multivariable models included predictors found to have significant associations with our outcomes in unadjusted analyses as well as age (modeled continuously in years) and sex, which we felt were clinically relevant variables. The proportional hazards assumption was assessed using graphical methods. We report hazard ratios (HRs) with 95% confidence intervals (CIs). We considered 2-sided p values <0.05 as statistically significant. We used SAS v9.4 (Carey, NC) for all statistical analyses.
RESULTS
Characteristics of children with RIS.
We screened 39 children, of whom 1 was excluded due to baseline MRI not being available for review (only neuroradiologist's report). We therefore included 38 children at 16 sites from 6 countries who met our criteria in the current study (table 1). Seventy-one percent (27/38) of children were girls. The median age at index MRI was 15.4 years (IQR 13.5–16.5 years). The mean time from index MRI to most recent clinical assessment was 4.8 ± 5.3 years (median 2.5 years, IQR 1.2–7.0 years) and was longer in children who developed a first clinical event than in those who did not (mean 7.2 ± 5.7 years vs 3.0 ± 4.1 years, p = 0.01). The clinical and demographic characteristics of the cohort are summarized in table 2.
Table 2.
Clinical, demographic, imaging, and laboratory data from the entire pediatric RIS cohort (n = 38)
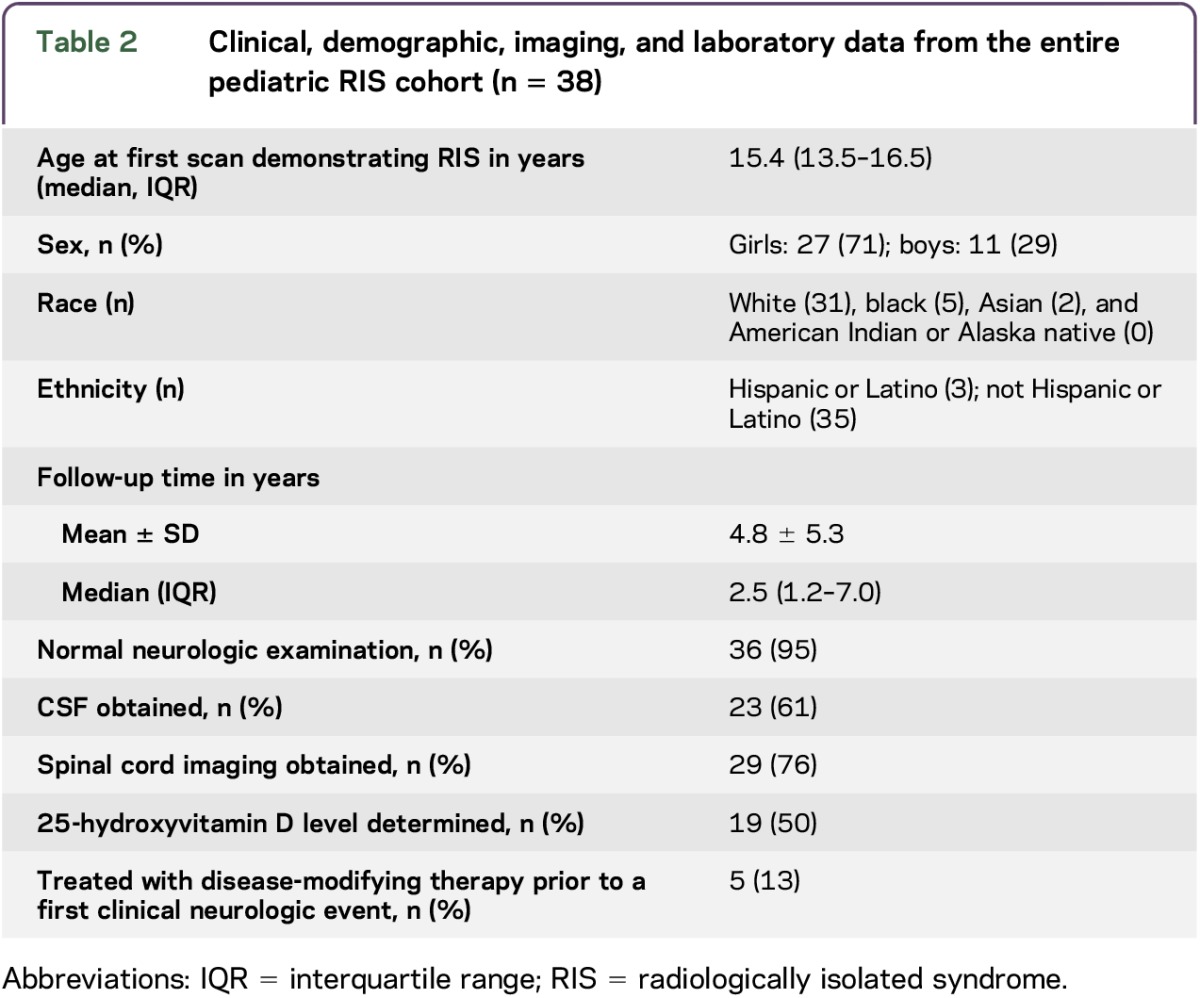
The most common reason for obtaining initial neuroimaging was headache (20/38, 53%). Other reasons were depression (2), seizure/epilepsy (4), concussion (2), attention-deficit disorder (1), Adie tonic pupil (1), endocrinopathy (1), leukemia (1), syncope/loss of consciousness (2), known Chiari I (1), neck mass (1), ear pain (1), and healthy control in a research study (1).
Initial neurologic examinations were normal in 36/38 children (95%). One child had symmetrically brisk reflexes and 1 had an uncorrected bilateral visual acuity of 20/25 without optic disc pallor. At least 2 unique oligoclonal bands were present in CSF, but not serum, in 57% (13/23) of children in whom CSF was obtained. Serum 25-hydroxyvitamin D levels were determined in 19/38 (50%) children.
All children had initial MRI scans that met the 2010 criteria for DIS (representative images in figure 1). The most common lesion type was periventricular; ≥1 periventricular lesion was detected in all 38 children (100%). Nineteen of 38 children (50%) also met the definition of RIS in adults (i.e., initial MRI scans met ≥3 of 4 Barkhof criteria/2005 DIS criteria for MS). Two of 24 children (8%) in whom gadolinium was administered on initial brain MRI scans had enhancing lesions. Spinal cord imaging was obtained in 29/38 children (76%), among whom 5 (17%) had spinal lesions and 2 of these 5 children had enhancing lesions. All 5 children had cervical cord lesions; 1 child also had lesions in the thoracic cord.
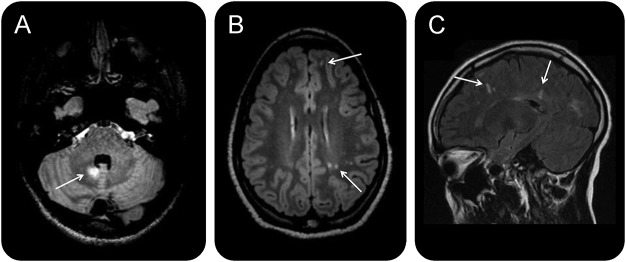
Figure 1. Representative MRIs from select children with RIS.
Axial FLAIR images demonstrate (A) an infratentorial hyperintensity within the cerebellar white matter in a child with RIS at baseline (other lesions not shown) and (B) juxtacortical and ovoid hyperintensities (arrows) in a different child. (C) Sagittal FLAIR image from the child shown in B demonstrates hyperintensities extending over the long axis of the lateral ventricles and oriented perpendicularly to the corpus callosum (arrows, other lesions not shown). To date, neither child has developed a first clinical event consistent with CNS demyelination. RIS = radiologically isolated syndrome; FLAIR = fluid-attenuated inversion recovery.
Clinical and radiologic outcomes.
A first clinical event consistent with CNS demyelination occurred in 16/38 children (42%; 95% CI: 27%–60%) with a median of 2.0 years (IQR 1.0–4.3 years) and median clinical event-free survival time (which takes into account variable follow-up times in the cohort) of 5.0 years (95% CI: 2–7 years). Among the 19 children who met the definition of RIS for adults, 10 (53%; 95% CI: 30%–75%) developed a first clinical event. The phenotypes of a first clinical event included optic neuritis (4), monofocal brainstem syndromes (4), myelitis (3), other monofocal signs (3), and polyfocal signs without encephalopathy (2). Eight of 20 children (40%) who presented with headache and 2 of 4 children (50%) with a seizure developed a first clinical event. No child developed either clinical acute disseminated encephalomyelitis or a primary progressive course.
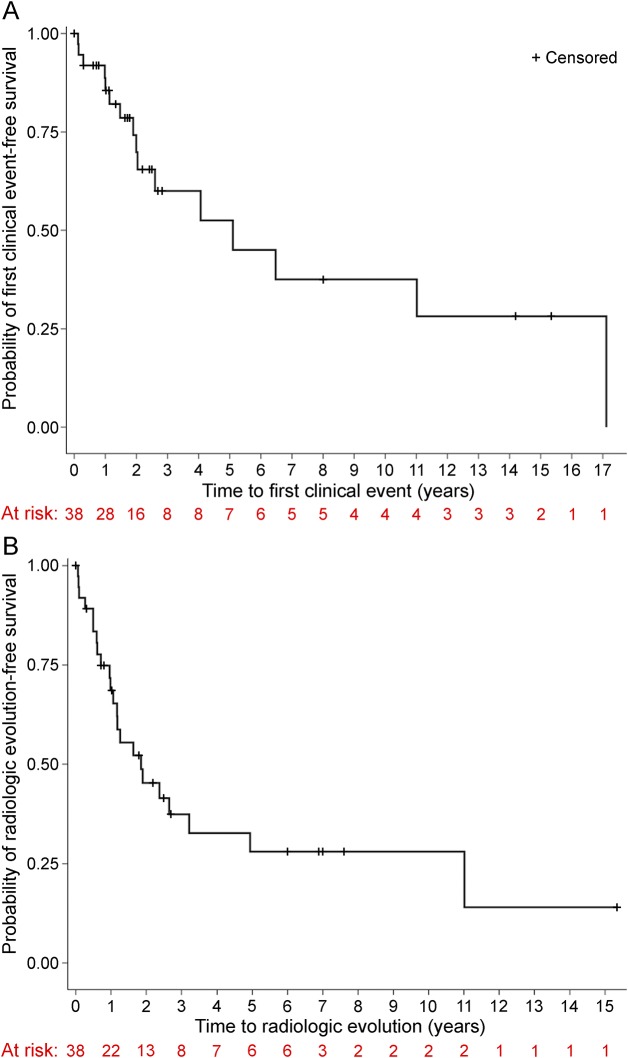
Of the 16 children who developed a first clinical event consistent with CNS demyelination, 14 (88%) had ≥1 routine follow-up surveillance of brain MRI prior to the onset of clinical symptoms (6 children had 2 scans and 3 children had ≥3 scans). The median interval between the index MRI and the first follow-up scan was 380 (range 22–1,803) days. Two of the 16 children developed a first clinical event in close proximity to the index MRI scans (after 1 and 3 months, respectively) and had follow-up MRIs performed at the time of clinical symptoms. Of the 22 children who did not develop a first clinical event, all but 1 child (who refused additional MRIs) had ≥1 follow-up MRI scan of the brain performed (median = 2 scans/child). The median interval between the index MRI and the first follow-up scan in children who did not develop a first clinical event was 258 (range 13–1,422) days. Radiologic evolution developed in 23/38 children (61%; 95% CI: 27%–60%) with a median of 1.1 years (IQR 0.5–1.9 years) and a median radiologic event-free survival time of 1.8 years (95% CI: 1.1–4.9 years). Kaplan-Meier curves for clinical and radiologic endpoints are shown in figure 2.
Figure 2. Kaplan-Meier survival curves.
Kaplan-Meier survival curves demonstrate (A) time to a first clinical event consistent with CNS demyelination and (B) time to radiologic evolution for the entire cohort.
Five children were treated with ≥1 approved disease-modifying therapy for MS prior to a first clinical event (after radiologic evolution occurred in 4 children and prior to radiologic evolution in 1 child). Interferon beta 1a was the first-line agent in all 5. Two of the treated children developed a first clinical event on treatment (after 1.1 and 17.1 years after the index MRI and after 3.4 months and 10.1 years on treatment, respectively) while the other 3 treated children remained clinically stable after 1.7–15.3 years of follow-up.
Clinical, laboratory, and MRI markers.
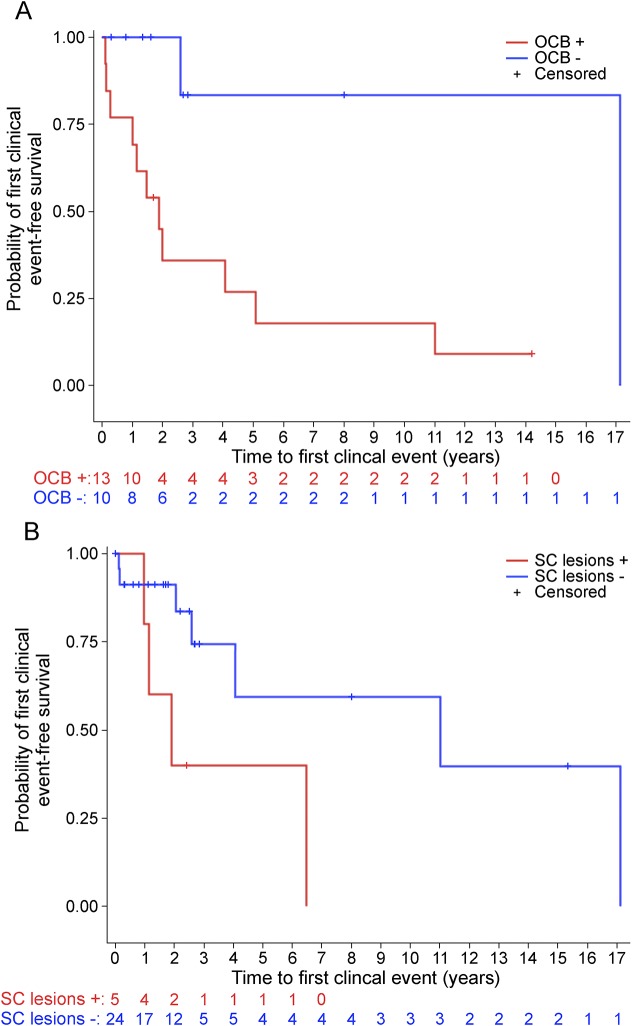
Children who had ≥2 unique oligoclonal bands detected in CSF (HR 10.9, 95% CI: 1.4–86.2, p = 0.02) or spinal cord lesions present on MRI (HR 7.8, 95% CI: 1.4–43.6, p = 0.02) were at greater risk of a first clinical event than those who did not have these factors present, after adjustment for age and sex (figure 3, A and B). Children with and without either CSF or spinal cord MRI assessments did not differ in key baseline variables, including age, sex, and race. There were no statistically significant differences in any of the following variables between children with and without either a first clinical event or radiologic evolution: age, sex, race, reason for imaging (dichotomized as headache vs not headache), first MRI scan that met the 2005 criteria for DIS, presence of gadolinium-enhancing lesions, presence of brain MRI lesions in typical locations for MS (juxtacortical, periventricular, or infratentorial), or serum 25-hydroxyvitamin D levels.
Figure 3. Risk attributable to individual risk factors.
Time to a first clinical event consistent with CNS demyelination stratified by (A) the presence of oligoclonal bands in spinal fluid (HR 10.9, 95% CI: 1.4–86.2, p = 0.02) and (B) the presence of spinal cord lesions (HR 7.8, 95% CI: 1.4–43.6, p = 0.02). All hazard ratios (HRs) are adjusted for age and sex. CI = confidence interval; OCB = oligoclonal band; SC = spinal cord.
DISCUSSION
We report findings from a longitudinal multicenter study of children with incidental MRI abnormalities consistent with CNS demyelination. We found that a substantial proportion of children that met the definition of RIS in table 1 (42%) developed a first clinical event consistent with CNS demyelination and an even higher proportion (61%) developed radiologic evolution. This is similar to the 34% and 60% of adults with RIS who have been consistently reported to develop either clinical or radiologic evolution, respectively.3,4,9 However, among the children in our study, clinical and radiologic evolution occurred faster than among adults with RIS (medians of 2.0 and 1.1 years, respectively, in children vs 5.4 years and 2.7 years, respectively, in adults).3 This is in line with prior studies that demonstrated the aggressive nature of demyelination in children with relapse rates in children with MS reported as more than twice those in adults early in the disease course.11
There are currently no formal criteria for the subsequent diagnosis of MS in either children or adults with RIS. Recently proposed modifications to existing diagnostic criteria, however, suggest that a diagnosis of MS can be made in individuals with RIS (who already demonstrate DIS on MRI) who subsequently develop a first clinical event consistent with CNS demyelination.12
All children in our study had MRI scans that met the 2010 criteria for DIS, and 50% of children also met the criteria published in 2009 for RIS in adults, which required MRI scans to meet at least 3 of the 4 Barkhof criteria for DIS (i.e., 2005 DIS criteria for MS).3 We used the newer 2010 MRI criteria for DIS in our study because there are no criteria for RIS in children and the 2010 criteria are routinely used in clinical practice for the diagnosis of MS. Because of our study design, we could not directly compare the specificity of the 2005 and 2010 MRI criteria for DIS, but it is possible that the 2010 criteria (which require fewer lesions) may have lower specificity (i.e., a higher false-positive rate). Nonetheless, given the high rate of development of a first clinical event in our cohort, we propose that our definition of RIS in children (table 1) be used in future studies.
The most common reason for obtaining neuroimaging was headache. Brain white matter lesions, some that meet 2010 criteria for DIS on MRI, have been reported in individuals with headaches and migraines.13–15 Our finding that 40% of children with RIS who underwent imaging for evaluation of headaches developed a first clinical event (similar to the 44% in children whose indication for initial imaging was not headache) highlights the importance of following such children over time to determine which children with headaches and RIS are at greatest risk of developing clinical demyelination.
Two of 4 children who presented with a seizure subsequently developed a first clinical event. Approximately 3% of patients with established MS will have at least 1 seizure.16 Future studies to determine which children with RIS who present with a seizure are at greatest risk for the development of a first clinical event will be valuable.
Our finding that spinal cord lesions are associated with the subsequent development of a first clinical event in children with RIS is consistent with studies in adults with RIS that have found a similar association.8,9 An increased risk of a second clinical event has also been reported in adults with clinically isolated syndrome who have spinal cord lesions.17 In 1 preliminary study of children with MS, the greatest number of relapses tended to occur in children with the greatest number of spinal cord lesions.18 All children in our study who had abnormalities on spinal cord MRI scans demonstrated lesions in the cervical cord. We therefore recommend cervical cord imaging be considered in children with RIS to identify children at greatest risk of a first clinical event who may need closer follow-up.
We also found that the presence of oligoclonal bands in CSF was independently associated with a first clinical event in children with RIS. One prior study reported an increased risk of a first clinical event in adults with RIS who had abnormal CSF, but this association was only significant if there were ≥9 T2 brain lesions on brain MRI.19 In children with a first attack of symptomatic CNS demyelination, the presence of oligoclonal bands in CSF was highly predictive of a subsequent diagnosis of MS.20,21 If replicated, our findings suggest that in children with RIS, analysis of CSF for oligoclonal bands should be considered for prognostic purposes.
None of the individual brain MRI parameters we assessed were associated with either a first clinical event or radiologic evolution. A study of adults with RIS found that the presence of contrast-enhancing lesions on index MRI was associated with an increased risk of radiologic evolution, but not a first clinical event. Studies in children with acute symptomatic CNS demyelination have shown that the presence of periventricular lesions and ≥1 normalized T1-weighted hypointense lesions on brain MRI were associated with an increased likelihood of MS diagnosis.22,23 The nonstandardized clinical reading and MRI acquisition protocols used in our study did not permit the assessment of T1-weighted hypointensities, but this could be assessed in future studies.
Five children in our study were treated with immunomodulatory treatments for MS to try to prevent a first clinical event. Two multicenter clinical trials, 1 using dimethyl fumarate and 1 using teriflunomide, are currently underway to test whether immunomodulatory treatment prevents or delays clinical evolution in adults with RIS.24,25 Treatment for children with RIS should be considered, after adequate controlled trials are performed, if our finding that RIS in children is associated with a substantial risk of developing a first clinical event is confirmed.
One limitation of our study is that we analyzed historical data, and therefore, all clinical, MRI, and laboratory data were obtained in the past using nonstandardized MRI techniques, and timing and methods for the collection of clinical and laboratory data were not uniform. Prospective studies with standardized protocols are planned and will necessitate continued multinational collaborations.
Our study highlights the importance of the detection of RIS in children. As in adults, a substantial proportion of children with RIS in our cohort subsequently developed clinical symptoms, especially children with oligoclonal bands present in CSF and those with spinal cord MRI lesions. Children with RIS appear to develop clinical symptoms and radiologic evolution sooner than adults. Further research in larger cohorts is needed to confirm these findings and to identify additional risk factors for the development of clinical neurologic symptoms following the detection of RIS in children. Although this work does not address the issue of whether children with RIS should be treated with disease-modifying agents for MS, we hope that an accurate classification of the risk of clinical symptoms in children with RIS will help in the development of consensus guidelines that are urgently needed to optimize clinical care in this population.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Yale Center for Analytical Sciences for providing statistical support.
GLOSSARY
- CI
confidence interval
- DIS
dissemination in space
- HR
hazard ratio
- IQR
interquartile range
- RIS
radiologically isolated syndrome
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR AFFILIATION
From the Department of Pediatrics (N.M., E.D.S.) and Department of Neurology (N.M.), Yale University School of Medicine, New Haven, CT; Service de Neurologie (C.L.), Hopital Pasteur, Nice, France; Cerrahpasa School of Medicine (A.S., U.U.), University of Istanbul, Turkey; Centre Hospitalo Universitaire Purpan (D.B.), Toulouse; Centre Hospitalier Universitaire de Montpellier (C.C.D.); Centre Hospitalo Universitaire Strasbourg (J.d.S.), France; Yale School of Public Health (W.D., E.D.S.), New Haven, CT; Centre Hospitalo Universitaire Lyon (F.D.D.), France; Department of Neurology (O.K.), Mayo Clinic College of Medicine, Rochester, MN; Children's Hospital Los Angeles (M.L.), Keck School of Medicine of University of Southern California; Division of Neurology (S.N.), The Children's Hospital of Philadelphia, PA; APHM, CHU Timone (J.P.), Service de Neurologie, Aix Marseille University, France; Multiple Sclerosis Center of Buenos Aires (J.I.R.), Argentina; Department of Neurology (R.T.S.), University of Rochester Medical Center, NY; MS Center of Catalunya Cemcat (M.T.), Barcelona, Spain; Univ. Lille (P.V.), CHU Lille, LIRIC-Inserm U995, FHU Imminent, France; Department of Neurology (E.W.), The Birmingham Children's Hospital NHS Trust, UK; Department of Neurology and Neurotherapeutics (D.T.O.), University of Texas Southwestern Medical Center, Dallas; and Department of Neurology (D.P.), Keck School of Medicine of University of Southern California, Los Angeles.
AUTHOR CONTRIBUTIONS
N. Makhani: study concept and design, data acquisition, data analysis, data interpretation, and drafting of the manuscript. C. Lebrun, A. Siva, D. Brassat, C. Carra Dallière, and J. de Seze: data acquisition, data analysis, and critical review of the manuscript for important intellectual content. W. Du: data analysis and critical review of the manuscript for important intellectual content. F. Durand Dubief: data acquisition, data analysis, and critical review of the manuscript for important intellectual content. O. Kantarci: data analysis and critical review of the manuscript for important intellectual content. M. Langille, S. Narula, J. Pelletier, and J.I. Rojas: data acquisition, data analysis, and critical review of the manuscript for important intellectual content. E.D. Shapiro: data analysis and critical review of the manuscript for important intellectual content. R.T. Stone, M. Tintoré, U. Uygunoglu, P. Vermersch, E. Wassmer, and D.T. Okuda: data acquisition, data analysis, and critical review of the manuscript for important intellectual content. D. Pelletier: study concept and design, data analysis, critical review of the manuscript for important intellectual content, and study supervision.
STUDY FUNDING
This publication was made possible, in part by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS) at the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURE
N. Makhani received research support from Race to Erase MS Foundation. C. Lebrun served on the scientific advisory board for Biogen, Novartis, Merck, Genzyme, Teva, and Roche; received travel funding and/or speaker honoraria from Biogen, Merck, Genzyme, and Roche; served as an editorial advisory board member for Revue Neurologique (Paris) Elsevier, and Neurology and Therapy; and received research support from the French MS Society (SFSEP) EDMUS (OFSEP Center) Foundation. A. Siva received travel funding from Merck Serono, Biogen Idec/Gen Pharma of Turkey, Novartis, Genzyme, Roche, and Teva; served on the editorial board for Journal of Neurological Sciences, Journal of Headache and Pain, and Turkish Neurological Journal; consulted for Biogen Idec, Novartis, Merck Serono, Bayer, Teva, Genzyme, and Roche; served on the speakers' bureau for Excemed, Genzyme, Merck Serono, Biogen Idec/Gen Pharma of Turkey, and Teva; and received research support from The Scientific and Technological Research Council of Turkey. D. Brassat served on the scientific advisory board for Chugai Pharma; received travel funding and/or speaker honoraria from Biogen, Bayer, Novartis, Sanofi, Almirall, Merck, and Teva and received research support from FP7 and PHRC. C. Carra-Dalliere received travel funding from Genzyme, Biogen, and Novartis. J. de Seze served on the editorial board for Revue Neurologique. W. Du reports no disclosures. F. Durand-Dubief served on the scientific advisory board for Merck Serono and Roche and received travel funding and/or speaker honoraria from Biogen, Merck Serono, Sanofi-Aventis, Genzyme, Novartis Pharma, Roche, and Teva. O. Kantarci received speaker honoraria from Novartis Pharmaceuticals and Biogen; did a grant review for the National Multiple Sclerosis Society; received research support from European Regional Development Fund, National Multiple Sclerosis Society, Biogen, Multiple Sclerosis Society, Mayo Foundation, Hilton Foundation, and Mayo Clinic; received compensation for consulting activities from Teva; his spouse serves on the data safety monitoring board of Takeda Global Research and Development Center, Inc, and the data monitoring boards of Pfizer Inc and Janssen Alzheimer Immunotherapy; and is funded by the NIH and Minnesota Partnership for Biotechnology and Medical Genomics. M. Langille and S. Narula report no disclosures. J. Pelletier served on the scientific advisory board for Biogen, Novartis, and Genzyme and received research support from Arsep and Novartis. J.I. Rojas served on the speakers' bureau for Novartis Argentina, Genzyme Argentina, and Merck Serono Argentina. E.D. Shapiro received speaker honoraria from the Eastern Maine Medical Center and received research support from the NIH/NIAID/NDDK. R.T. Stone received research support from Novartis Pharmaceuticals. M. Tintoré served on the scientific advisory board from Biogen, Novartis, Genzyme, and Roche; received travel funding and/or speaker honoraria from Bayer, Teva, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis, Genzyme, Roche, and Almirall; served on the editorial board for Multiple Sclerosis Journal, Neurologia, and Revista de Neurologia; is editor of MSJ-ETC; served on the speakers' bureau from Almirall, Bayer, Teva, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis, Genzyme, and Roche; and received research support from Bayer, Teva, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis, Genzyme, Roche, Almirall, Spanish Agency for Sanitary Investigations, REEM, and Angencia de Gestio d'ajuts Universitaris I de Recerca of the Generalitat de Catalunya in Spain. U. Uygunoglu received travel funding from Merck Serono, Biogen Idec, and Novartis; served as an associate editor for Turkish Neurology Journal; and received research support from the Turkish Neurological Society. P. Vermersch served on the scientific advisory board for Sanofi, Biogen, Merck, Teva, Novartis, Roche, and Servier; received travel funding and/or speaker honoraria from Biogen, Roche, Novartis, Teva, Sanofi-Aventis, Almirall, Merck Serono, and Neurosciences et sclerose en plaques; received research support from Biogen, Bayer, Novartis, Merck, Teva, Sanofi Genzyme, and Roche; received travel funding and/or speaker honoraria from Biogen Idec, Teva, Genzyme, Shire, UCB, and Merck Serono; and received research support from Biogen Idec, Sanofi, Novartis UK MS Society, Action Medical Research, and Birmingham Children's Hospital Research Foundation. D.T. Okuda received travel funding and/or speaker honoraria from Acorda Therapeutics, Genentech, Genzyme, and Teva; consulted for Bayer, EMD Serono, Genzyme, and Novartis; served on the speakers' bureau for Acorda Therapeutics, Genzyme, and Teva Neuroscience; and received research support from Biogen. D. Pelletier consulted for Biogen, EMD Serono, Sanofi Genzyme, Novartis, Hoffmann-La Roche, and Vertex and received research support from Biogen and National Multiple Sclerosis Society. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Maher CO, Piatt JH Jr; Section on Neurologic Surgery AAoP. Incidental findings on brain and spine imaging in children. Pediatrics 2015;135:e1084–e1096. [DOI] [PubMed] [Google Scholar]
- 2.Kim BS, Illes J, Kaplan RT, Reiss A, Atlas SW. Incidental findings on pediatric MR images of the brain. AJNR Am J Neuroradiol 2002;23:1674–1677. [PMC free article] [PubMed] [Google Scholar]
- 3.Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 2009;72:800–805. [DOI] [PubMed] [Google Scholar]
- 4.Lebrun C, Bensa C, Debouverie M, et al. Unexpected multiple sclerosis: follow-up of 30 patients with magnetic resonance imaging and clinical conversion profile. J Neurol Neurosurg Psychiatry 2008;79:195–198. [DOI] [PubMed] [Google Scholar]
- 5.Siva A, Saip S, Altintas A, Jacob A, Keegan BM, Kantarci OH. Multiple sclerosis risk in radiologically uncovered asymptomatic possible inflammatory-demyelinating disease. Mult Scler 2009;15:918–927. [DOI] [PubMed] [Google Scholar]
- 6.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997;120:2059–2069. [DOI] [PubMed] [Google Scholar]
- 7.George IC, DeStefano K, Makhani N. Radiologically isolated syndrome in a pediatric patient. Pediatr Neurol 2016;56:86–87. [DOI] [PubMed] [Google Scholar]
- 8.Okuda DT, Mowry EM, Cree BA, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 2011;76:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One 2014;9:e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 2009;66:54–59. [DOI] [PubMed] [Google Scholar]
- 12.Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016;15:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Candee MS, McCandless RT, Moore KR, Arrington CB, Minich LL, Bale JF Jr. White matter lesions in children and adolescents with migraine. Pediatr Neurol 2013;49:393–396. [DOI] [PubMed] [Google Scholar]
- 14.Mar S, Kelly JE, Isbell S, Aung WY, Lenox J, Prensky A. Prevalence of white matter lesions and stroke in children with migraine. Neurology 2013;81:1387–1391. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Kullnat J, Bourdette D, et al. Prevalence of brain magnetic resonance imaging meeting Barkhof and McDonald criteria for dissemination in space among headache patients. Mult Scler 2013;19:1101–1105. [DOI] [PubMed] [Google Scholar]
- 16.Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult Scler 2015;21:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sombekke MH, Wattjes MP, Balk LJ, et al. Spinal cord lesions in patients with clinically isolated syndrome: a powerful tool in diagnosis and prognosis. Neurology 2013;80:69–75. [DOI] [PubMed] [Google Scholar]
- 18.Verhey LH, Branson HM, Makhija M, Shroff M, Banwell B. Magnetic resonance imaging features of the spinal cord in pediatric multiple sclerosis: a preliminary study. Neuroradiology 2010;52:1153–1162. [DOI] [PubMed] [Google Scholar]
- 19.Lebrun C, Bensa C, Debouverie M, et al. Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patients. Arch Neurol 2009;66:841–846. [DOI] [PubMed] [Google Scholar]
- 20.Heussinger N, Kontopantelis E, Gburek-Augustat J, et al. Oligoclonal bands predict multiple sclerosis in children with optic neuritis. Ann Neurol 2015;77:1076–1082. [DOI] [PubMed] [Google Scholar]
- 21.Banwell B, Bar-Or A, Arnold DL, et al. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol 2011;10:436–445. [DOI] [PubMed] [Google Scholar]
- 22.Verhey LH, Branson HM, Shroff MM, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet Neurol 2011;10:1065–1073. [DOI] [PubMed] [Google Scholar]
- 23.Verhey LH, van Pelt-Gravesteijn ED, Ketelslegers IA, et al. Validation of MRI predictors of multiple sclerosis diagnosis in children with acute CNS demyelination. Mult Scler Relat Disord 2013;2:193–199. [DOI] [PubMed] [Google Scholar]
- 24.Multi-center, randomized, double-blinded assessment of Tecfidera® in extending the time to a first attack in radiologically isolated syndrome (RIS) (ARISE) [online]. Available at: http://www.clinicaltrials.gov. Accessed May 21, 2016.
- 25.Multi-center, randomized, double-blinded assessment of teriflunomide in extending the time to a first clinical event in radiologically isolated syndrome (RIS) (TERIS study). Neurology 2017;88(16 suppl):P6.359. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.