Abstract
Introduction
The plasma concentration of beta-amyloid (Aβ) has been considered another biomarker of Alzheimer's disease and was reportedly associated with cortical Aβ accumulation.
Methods
We analyzed 28 subjects with apolipoprotein E4 (ApoE4; E4 group) and 89 subjects without ApoE4 (non-E4 group) to determine the association between cortical Aβ accumulation by standard uptake value ratio with [18F]florbetapir positron emission tomography and plasma Aβ1–40 and Aβ1–42.
Results
Aβ1–42/Aβ1–40 correlated significantly with mean regional [18F]florbetapir standard uptake value ratio in the non-E4 group (R2 = 0.06, P = .02) but not in the E4 group, and receiver operating characteristic curve analysis for Aβ1–42/Aβ1–40 in the non-E4 group showed sensitivity (92.9%) and specificity (45.9%) with a cutoff value of 0.150 for Aβ positivity.
Discussion
We verified that the correlation between Aβ1–42/Aβ1–40 and Aβ accumulation differed according to ApoE phenotype. The high sensitivity of plasma Aβ1–42/Aβ1–40 for Aβ positivity in non-E4 subjects indicated a possible role of plasma Aβ1–42/Aβ1–40 as a screening biomarker before amyloid positron emission tomography in clinical settings.
Keywords: Alzheimer's disease, Plasma beta-amyloid1–40, Plasma beta-amyloid1–42, Beta-amyloid1–40/beta-amyloid1–42, Positron emission tomography, Phenotype of apolipoprotein
1. Introduction
For early diagnosis of Alzheimer's disease (AD), researchers have been searching for useful biomarkers in terms of accuracy. To the present, both amyloid positron emission tomography (PET) imaging and cerebrospinal fluid (CSF) have been most useful biomarkers, showing good sensitivity and specificity (amyloid PET, 82% and 95% [1]; CSF beta-amyloid (Aβ)1–42, 96.4% and 76.9% [2]). However, these methods have some disadvantages that prevent them from coming into wide use, such as high cost, difficulty of ligand synthesis, and high invasiveness. Blood testing has been considered to offer another possible biomarker of AD, and it is less costly and invasive than PET and CSF. Recently, rapid progressing protein analysis has found some proteins as possible plasma biomarker candidates of AD. Proteomic analysis reported that plasma concentrations of apolipoprotein E (ApoE) protein and related proteins (e.g., Apo-A1, Apo-A2M) were identified as preclinical biomarkers of neocortical amyloid burden [3], [4], and other studies reported that combinations of plasma proteins and other clinical information could predict neocortical amyloid burden [5], [6].
Because Aβ accumulates in cerebral cortex and is detected in both CSF and plasma, plasma Aβ is one of the hopeful biomarker candidates of AD. Some studies examined plasma Aβ as a predictor of AD [7], [8], [9], but the results were inconsistent. Recently, two large-scale multicenter studies, Australian Imaging, Biomarkers and Lifestyle (AIBL) and Alzheimer's Disease Neuroimaging Initiative (ADNI), successively reported a correlation between amyloid PET and blood test. The AIBL study reported that Aβ1–42/Aβ1–40 was negatively correlated with the neocortical Aβ burden evaluated by [11C]PiB uptake [10]. On the other hand, the ADNI study reported a positive correlation between plasma Aβ1–40/Aβ1–42 and [11C]PiB uptake, although this correlation was shown only in subjects without ApoE-ε4 [11].
In this study, we examined the correlation between plasma Aβ and the results of amyloid PET imaging with [18F]florbetapir in a Japanese population and a single-center study setting. We also tried to verify the effect of ApoE on the relationship between plasma Aβ and Aβ accumulation.
2. Methods
2.1. Subjects
We analyzed 117 Japanese participants (49 males and 68 females). Participants were excluded if they had other current clinically relevant neurologic illness, were receiving any investigational medications, or had ever received antiamyloid experimental therapy. Participants were also excluded by brain imaging (i.e., magnetic resonance imaging or computed tomography) if it showed evidence of significant brain damage (e.g., stroke, hemorrhage, or traumatic brain injury) that could explain the patients' cognitive decline or dementia.
Their cognitive function was evaluated by Mini–Mental State Examination (MMSE) [12]. The criteria for dementia were based on the Diagnostic and Statistical Manual of Mental Disorder (Fourth Edition, text revision). Participants diagnosed with dementia because of other general medical conditions were evaluated if they met the criteria for dementia with Lewy bodies [13] or frontotemporal lobe dementia [14]. The criteria for mild cognitive impairment (MCI) and healthy controls were the same as in our previous report [15].
Our participants included 35 patients with dementia (10 males and 25 females, age 76.2 ± 7.4 [mean ± standard deviation] years, MMSE score 19.7 ± 6.5, 22 AD, 11 dementia with Lewy bodies, one frontotemporal lobe dementia, one dementia unspecified), 58 patients with MCI (27 males and 31 females, age 68.1 ± 14.8 years, MMSE score 25.1 ± 4.1), and 24 healthy controls (12 males and 12 females, age 73.6 ± 5.3 years, MMSE score 28.8 ± 1.4).
2.2. PET analysis
A PET scanner system, Eminence SET-3000GCT/X (Shimadzu Corp, Kyoto, Japan), was used to measure regional brain radioactivity. For quantitative analysis of florbetapir-PET images, we used the same method as described in previous studies [15], [16]. Mean cortical (six regions: medial orbital frontal, temporal, anterior and posterior cingulate, parietal lobe, and precuneus) and whole-cerebellar region of interest (ROI) templates were applied to all PET scans to calculate mean regional cerebral-to-cerebellar standard uptake value ratio (SUVR) [16]. A threshold of SUVR greater than 1.099 was used to signify positive Aβ [15].
2.3. Blood test
Blood samples were drawn before the injection of [18F]florbetapir. Blood collected in a vacuum collection tube with ethylenediaminetetraacetic acid (EDTA) 2K was centrifuged (5000g) at 4°C for 15 minutes to separate plasma. Plasma samples were stored at −80°C until use. The thawed samples were analyzed and discarded after analysis every month. Then ApoE phenotype and plasma concentrations of Aβ1–40 and Aβ1–42 were examined by the methods given subsequently.
2.3.1. Determination of ApoE phenotype
We pretreated plasma by incubating 10 μL with 100 μL of 5 mmol/L dithiothreitol containing 2.5 mL/L Tween 20 for 15 minutes at 4°C. The ApoE phenotypes were determined by isoelectric focusing using the method of Kataoka et al. [17] with a slight modification. The number of each phenotype of ApoE was as follows: E2/E3, N = 16 (13.7%); E3/E3, N = 71 (60.7%); E3/E4, N = 26 (22.2%); E3/E5, N = 2 (1.7%); and E4/E4, N = 2 (1.7%). We divided the subjects according to ApoE4, with 28 subjects having ApoE4 (E4 group) and 89 subjects being without ApoE4 (non-E4 group).
2.3.2. Measurement of plasma Aβ1–40 and Aβ1–42
For measurement, the plasma sample was diluted fourfold by standard diluent to avoid the effect of interfering substances in plasma. A sandwich Aβ enzyme-linked immunosorbent assay kit was used (Wako, Osaka, Japan).
The plasma samples were analyzed as follows. We added 100 μL of standard diluent to samples, and they were incubated with a plate seal in a refrigerator overnight. After washing samples five times, 100 μL horseradish peroxidase–conjugated antibody solution was added and incubated in the refrigerator for 1 hour. After washing samples five times, 100 μL 3,3′,5,5′-tetramethylbenzidine solution was added and incubation under a plate seal was done at room temperature in the dark for 30 minutes. Finally, 100 μL of stop solution was added, and absorbance of each well was read at 450 nm with a microplate reader. Human Aβ1–40 and Aβ1–42 concentrations for the samples and controls were read from the standard curve.
Sensitivity was 0.019 pmol/L (dynamic range, 1.0–100 pmol/L) for Aβ1–40 and 0.06 pmol/L (dynamic range, 0.1–20 pmol/L) for Aβ1–42. Average intra-assay and interassay coefficients of variation were 2.18% and 6.94%, respectively.
2.4. Statistical analysis
Student t test and chi-square test were used for group comparisons (Fisher's exact test if the smallest expected value of cells of the divided figure was less than 5). The influence of ApoE4 status on the relationship between plasma Aβ and SUVR was assessed using the method of Swaminathan et al. [11]: (1) SUVR = Aβ1–40 + ApoE status + (Aβ1–40 × ApoE status); (2) SUVR = Aβ1–42 + ApoE status + (Aβ1–42 × ApoE status); and (3) SUVR = Aβ1–42/Aβ1–40 + ApoE status + (Aβ1–42/Aβ1–40 × ApoE status). Linear correlation coefficients for pairs of variables were calculated for the correlations between SUVR and plasma Aβ1–40, Aβ1–42, and Aβ1–42/Aβ1–40. Receiver operating characteristic (ROC) curve analysis allowed us to examine the diagnostic power of Aβ1–42/Aβ1–40 against Aβ positivity, and the area under the curve (AUC) was calculated. We calculated the point that maximized the Youden Index (i.e., sensitivity − (1 − specificity)) as the cutoff value of ROC curve analysis. In all tests, a P value <.05 was considered statistically significant. Statistical analysis of data was carried out using JMP 11.0.0 (SAS Inc, Cary, NC) on Windows 7.
3. Results
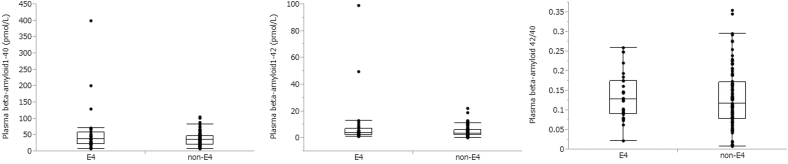
As shown in Table 1, group comparison revealed no differences between the E4 and non-E4 groups in gender distribution (chi-square = 0.58, df = 1, P = .45), mean age (t = 0.03, df = 115, P = .98), mean MMSE score (t = 1.29, df = 115, P = .26), distribution of diagnosis (chi-square = 4.84, df = 2, P = .09), and Aβ1–42/Aβ1–40 (t = −0.26, df = 115, P = .79). However, compared with the non-E4 group, the E4 group had significantly higher SUVR (t = −2.27, df = 115, P = .03), rate of Aβ positivity (chi-square = 7.54, df = 1, P = .006), Aβ1–40 (t = −2.30, df = 115, P = .02), and Aβ1–42 (t = −2.28, df = 115, P = .02).
Table 1.
Group comparison of subjects with ApoE4 (E4 group) and without ApoE4 (non-E4 group)
| E4 group | Non-E4 group | P value | |
|---|---|---|---|
| N (male/female) | 28 (10/18) | 89 (39/50) | NS |
| Age, mean ± SD | 71.6 ± 11.2 | 71.7 ± 12.2 | NS |
| MMSE, mean ± SD | 23.2 ± 6.6 | 24.6 ± 5.3 | NS |
| Diagnosis | NS | ||
| HC, N (%) | 4 (14.3) | 20 (22.5) | |
| MCI, N (%) | 11 (39.3) | 47 (52.8) | |
| Dementia, N (%) | 13 (46.4) | 22 (24.7) | |
| [18F]florbetapir PET | |||
| SUVR, mean ± SD | 1.18 ± 0.18 | 1.09 ± 0.17 | .03 |
| Beta-amyloid positivity, N (%) | 17 (60.7) | 28 (38.5) | .006 |
| Plasma (pmol/L), mean ± SD | |||
| Aβ1–40 | 57.76 ± 77.67 | 36.99 ± 20.41 | .02 |
| Aβ1–42 | 9.56 ± 19.8 | 4.54 ± 3.83 | .02 |
| Aβ1–42/Aβ1–40 | 0.14 ± 0.06 | 0.13 ± 0.08 | NS |
Abbreviations: Aβ, beta-amyloid; HC, healthy control; MCI, mild cognitive impairment; MMSE, Mini–Mental State Examination; NS, not significant; PET, positron emission tomography; SD, standard deviation; SUVR, standard uptake value ratio.
Multiple linear regression analysis showed that the multiple regression coefficient was not significant.
No significant interactions between plasma Aβ1–40 and ApoE4 status (t = −0.52, P = .61), Aβ1–42 and ApoE4 status (t = 1.28, P = .20), and Aβ1–42/Aβ1–40 and ApoE4 status (t = 1.90, P = .06) were observed on SUVR.
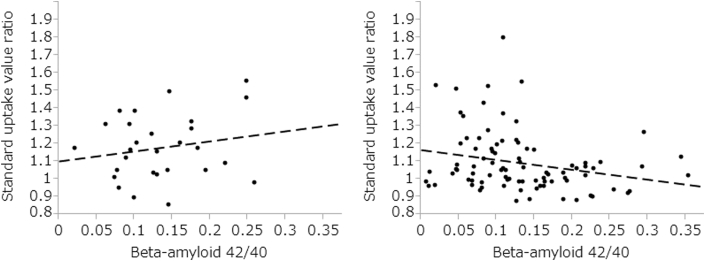
There was no significant correlation between plasma Aβ1–42/Aβ1–40 and SUVR (R2 = 0.02, t = −1.59, df = 116, P = .11) in all 117 subjects. The correlation results between the value of SUVR and plasma Aβ1–42/Aβ1–40 by ApoE status are shown in Fig. 1. Aβ1–42/Aβ1–40 was significantly correlated with the value of SUVR in the non-E4 group (slope = 0.57, R2 = 0.06, t = −2.37, df = 88, P = .02) but not in the E4 group (slope = −0.56, R2 = 0.04, t = 0.98, df = 27, P = .36) (Fig. 2). Partial correlation analysis including covariate age and gender did not change the results (E4 group: r = 0.17, df = 24, P = .41; non-E4 group: r = −0.25, df = 85, P = .02).
Fig. 1.
Distribution of plasma beta-amyloid (Aβ)1–40, Aβ1–42, and plasma Aβ1–42/Aβ1–40.
Fig. 2.
Correlation results between the value of standard uptake value ratio and plasma beta-amyloid (Aβ)1–42/Aβ1–40 by ApoE status. Left side: E4 group, y = 0.57x+1.10, R2 = 0.04, t = 0.98, df = 27, P = .34. Right side: Non-E4 group, y = −0.56x+1.17, R2 = 0.06, t = −2.37, df = 88, P = .02.
The ApoE4 status and plasma Aβ1–42/Aβ1–40 terms by themselves explained 4% and 1% variations in SUVR, respectively. The two terms together explained 5% variation in SUVR, and inclusion of the (plasma Aβ1–42/Aβ1–40 × ApoE status) interaction term along with the two terms in the model explained 7% variation in SUVR.
In the E4 group, ROC curve analysis for Aβ1–42/Aβ1–40 showed best sensitivity (63.6%) and specificity (52.9%) with a cutoff value of 0.123 for Aβ positivity (AUC, 0.519; 95% confidence interval, 0.285–0.752). There was no significant difference between AUC of E4 group and a random classifier (P = .81). In non-E4 group, ROC curve analysis for the Aβ1–42/Aβ1–40 ratio showed best sensitivity (92.9%) and specificity (45.9%) with a cutoff value of 0.150 for Aβ positivity (AUC, 0.648; 95% confidence interval, 0.528–0.767). There was a significant difference between AUC of non-E4 group and a random classifier (P = .01). Positive predictive value (PPV) was 44.1% and negative predictive value (NPV) was 93.3%.
4. Discussion
Our results from a Japanese population could not reconfirm the finding that plasma Aβ1–42/Aβ1–40 was correlated with the degree of Aβ accumulation as reported by the AIBL study [10], but they did support the hypothesis that this correlation differed according to the ApoE phenotype as indicated by the ADNI study [11]. Compared with multicenter studies, a single-center study has reported larger intervention effects [18], whereas in multicenter studies, standardization of the methods to maintain the quality of the examinations is required, and sometimes this is difficult because of disparities among the various facilities. Both our study (single-center study, Japanese participants) and the ADNI study (multicenter study, most participants were Caucasian) demonstrated the same association of plasma Aβ, the degree of Aβ accumulation varied according to ApoE phenotype, and therefore our study was important for allowing us to generalize the results that Aβ1–42/Aβ1–40 may be correlated with the accumulation of Aβ among non-E4 subjects.
The extracellular concentration of Aβ might be a result of the balance between synthesis and clearance rates of Aβ. A previous study reported that human ApoE allele might differentially regulate the clearance of Aβ from the brain and that the clearance of soluble Aβ from brain interstitial fluid depends on the isoform of the human ApoE expressed (ApoE4 < ApoE3 < ApoE2) [19]. Permeability of blood-brain barrier (BBB) might be another factor to account for the correlation between plasma Aβ and cortical Aβ accumulation. We suppose that Aβ excretion mechanisms through BBB might account for the small contribution of plasma Aβ and ApoE4 toward the variance in SUVR, because the correlation between Aβ and cortical Aβ accumulation in CSF samples [20] was stronger than in plasma samples.
Another interesting finding was that plasma Aβ1–42/Aβ1–40 in the non-E4 group showed strong sensitivity (92.9%), NPV (93.3%), and the negative likelihood ratio was 0.16 for Aβ positivity by amyloid PET. A previous study of other minimally invasive biomarkers for MCI converting to AD reported an AUC of combined cytokine, clinical measures, and ApoE genotype of 0.61 [21]. In that study, the combination of cytokine and magnetic resonance imaging showed the best AUC of 0.78, sensitivity of 0.75, specificity of 0.78, PPV of 0.75, and NPV of 0.78 [21]. It has been reported that the ApoE-ε3 allele is present in 50% to 90% of people of all populations, whereas ApoE-ε4 is present in 5% to 35% and ApoE-ε2 in 1% to 5% [22]. Our results suggested that Aβ1–42/Aβ1–40 among non-E4 subjects represented good screening for excluding subjects not needing unnecessary cost and exposure, because the prevalence of cortical Aβ positivity is known not to be so high in non-E4 subjects. However, E4 subjects and non-E4 subjects with lower plasma Aβ1–42/Aβ1–40 need to undergo further testing with high specificity for Aβ positivity, as lower PPV of plasma Aβ1–42/Aβ1–40 among the non-E4 group and ApoE4 is known to be a high-risk factor of AD.
We need to acknowledge several methodological limitations in our study. First, we studied only 117 subjects, so this result will need to be verified with a larger number of subjects in the future. Second, our subjects comprised three clinically diagnosed groups. Therefore, one might suppose that our results may have been driven primarily by the effect of diagnosis. Unfortunately, our study group, especially the E4 group, was not sufficiently large to allow analyses within each clinically diagnosed group, so we could not exclude the possibility of a diagnosis effect. Third, our non-E4 group contained ApoE2, ApoE3, and ApoE5. Although the phenotype of ApoE might influence the clearance of Aβ, the number of these subgroups was not large enough to allow analyses within them. Thus, further study will be needed to address whether the relationship of Aβ1–42/Aβ1–40 and SUVR differed only with ApoE4 or also with other ApoE phenotypes. Finally, we should conduct further study among subjects with suspected asymptomatic to preclinical stage of AD to clarify the usefulness of Aβ1–42/Aβ1–40 as an AD biomarker.
In conclusion, the relationship of Aβ1–42/Aβ1–40 and SUVR was affected by ApoE phenotype. Although this correlation was very weak for a differential diagnosis, our results suggested that plasma Aβ1–42/Aβ1–40, especially among non-E4 subjects, might be a possible screening modality for deciding whether subjects should be examined by amyloid PET or not in clinical settings.
Research in Context.
-
1.
Systematic review: The authors used PubMed and the keywords “Alzheimer’s disease”, “biomarker”, “amyloid positron emission tomography”, “plasma beta-amyloid”, “apolipoprotein”, and “ApoE4”.
-
2.
Interpretation: Both the ADNI study, a multi-center study of Caucasians, and our study, a single-center study of Japanese, revealed the same finding that plasma Aβ1–42/Aβ1–40 was correlated with the degree of Aβ accumulation and that this correlation differed by ApoE phenotype. The combination of the measurement of plasma Aβ1–42/Aβ1–40 and ApoE phenotype might be a useful screening test before amyloid PET in clinical settings.
-
3.
Future directions: Minimally-invasive screening test for AD is required. Thus, in order to confirm our findings, further study with large numbers of subjects and different cohorts, and examining whether the correlation changes by clinical stages, will be needed.
Acknowledgments
The authors thank Mr Koji Nagaya, Mr Koji Kanaya, Ms Megumi Hongo, and Mr Minoru Sakurai for their assistance in performing the PET experiments and magnetic resonance imaging scanning, and Ms Michiyo Tamura for her help as clinical research coordinator (Clinical Imaging Center for Healthcare, Nippon Medical School, Tokyo, Japan).
This study was partly supported by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japanese government (2011–2015), by a grant of Strategic Research Foundation Grant-aided Project for Private Universities from the MEXT, Japan (2008–2012), and by a Health and Labor Sciences Research Grant for Research on Psychiatric and Neurological Diseases and Mental Health from the Ministry of Health, Labor and Welfare, Japanese government (2010–2012). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yang L., Rieves D., Ganley C. Brain amyloid imaging—FDA approval of florbetapir F18 injection. N Engl J Med. 2012;367:885–887. doi: 10.1056/NEJMp1208061. [DOI] [PubMed] [Google Scholar]
- 2.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thambisetty M., Tripaldi R., Riddoch-Contreras J., Hye A., An Y., Campbell J. Proteome-based plasma markers of brain amyloid-beta deposition in non-demented older individuals. J Alzheimers Dis. 2010;22:1099–1109. doi: 10.3233/JAD-2010-101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westwood S., Leoni E., Hye A., Lynham S., Khondoker M.R., Ashton N.J. Blood-based biomarker candidates of cerebral amyloid using PiB PET in non-demented elderly. J Alzheimers Dis. 2016;52:561–572. doi: 10.3233/JAD-151155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton N.J., Kiddle S.J., Graf J., Ward M., Baird A.L., Hye A. Blood protein predictors of brain amyloid for enrichment in clinical trials? Alzheimers Dement (Amst) 2015;1:48–60. doi: 10.1016/j.dadm.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnham S.C., Faux N.G., Wilson W., Laws S.M., Ames D., Dedo J. A blood-based predictor for neocortical Aβ burden in Alzheimer's disease: results from the AIBL study. Mol Psychiatry. 2014;19:519–526. doi: 10.1038/mp.2013.40. [DOI] [PubMed] [Google Scholar]
- 7.Song F., Poljak A., Valenzuela M., Mayeux R., Smythe G.A., Sachdev P.S. Meta-analysis of plasma amyloid-beta levels in Alzheimer's disease. J Alzheimers Dis. 2011;26:365–375. doi: 10.3233/JAD-2011-101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyama A., Okereke O.I., Yang T., Blacker D., Selkoe D.J., Grodstein F. Plasma amyoid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69:824–831. doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lui J.K., Laws S.M., Li Q.X., Villemagne V.L., Ames D., Brown B. Plasma amyloid-beta as a biomarker in Alzheimer's disease: the AIBL study of aging. J Alzheimers Dis. 2010;20:1233–1242. doi: 10.3233/JAD-2010-090249. [DOI] [PubMed] [Google Scholar]
- 10.Rembach A., Faux N.G., Watt A.D., Pertile K.K., Rumble R.L., Trouson B.O. Changes in plasma amyloid beta in a longitudinal study of aging and Alzheimer's disease. Alzheimers Dement. 2014;10:53–61. doi: 10.1016/j.jalz.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Swaminathan S., Risacher S.L., Yoder K.K., West J.D., Shen L., Kim S. Association of plasma and cortical amyloid beta is modulated b APOE e4 status. Alzheimers Dement. 2014;10:e9–e18. doi: 10.1016/j.jalz.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.McKeith I.G., Dickson D.W., Lowe J., Emre M., O'Brien J.T., Feldman H. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 14.Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S. Frontotemporal lobar degeneration: a consensus on clinical diagnosis criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 15.Tateno A., Sakayori T., Kawashima Y., Higuchi M., Suhara T., Mizumura S. Comparison of imaging biomarkers for Alzheimer's disease: amyloid imaging with [18F]florbetapir positron emission tomography and magnetic resonance imaging voxel-based analysis for entorhinal cortex atrophy. Int J Geriatr Psychiatry. 2015;30:505–513. doi: 10.1002/gps.4173. [DOI] [PubMed] [Google Scholar]
- 16.Fleisher A.S., Chen K., Liu X., Roontiva A., Thiyyagura P., Ayutyanont N. Using positron emission tomography and florbetapir F18 to image cortical amyloid patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–1411. doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 17.Kataoka S., Paidi M., Howard B.V. Simplified isoelectric focusing/immunoblotting determination of apoprotein E phenotype. Clin Chem. 1994;40:11–13. [PubMed] [Google Scholar]
- 18.Bafeta A., Dechartres A., Trinquart L., Yavchitz A., Boutron I., Ravaud P. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study. BMJ. 2012;344:e813. doi: 10.1136/bmj.e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellano J.M., Kim J., Stewart F.R., Jiang H., DeMattos R.B., Patterson B.W. Human apoE isoforms differentially regulate brain amyloid-beta-peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimmer T., Riemenschneider M., Förstl H., Henriksen G., Klunk W.E., Mathis C.A. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furney S.J., Kronenberg D., Simmons A., Güntert A., Dobson R.J., Proitsi P. Combinatorial markers of mild cognitive impairment conversion to Alzheimer's disease—cytokines and MRI measures together predict disease progression. J Alzheimers Dis. 2011;26 Suppl 3:395–405. doi: 10.3233/JAD-2011-0044. [DOI] [PubMed] [Google Scholar]
- 22.Verghese P.B., Castellano J.M., Holtzman D.M. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]