Abstract
Background
Recent studies have showed that nonsteroidal anti-inflammatory drugs (NSAIDs) could reduce the risk of several types of cancer. However, epidemiological evidence of the association between NSAIDs intake and the risk of hepatocellular carcinoma (HCC) remains controversial.
Methods
To assess the preventive benefit of NSAIDs in HCC, we simultaneously searched the databases of PubMed, EmBase, Web of Science, and Scopus and screened eligible publications.
Results
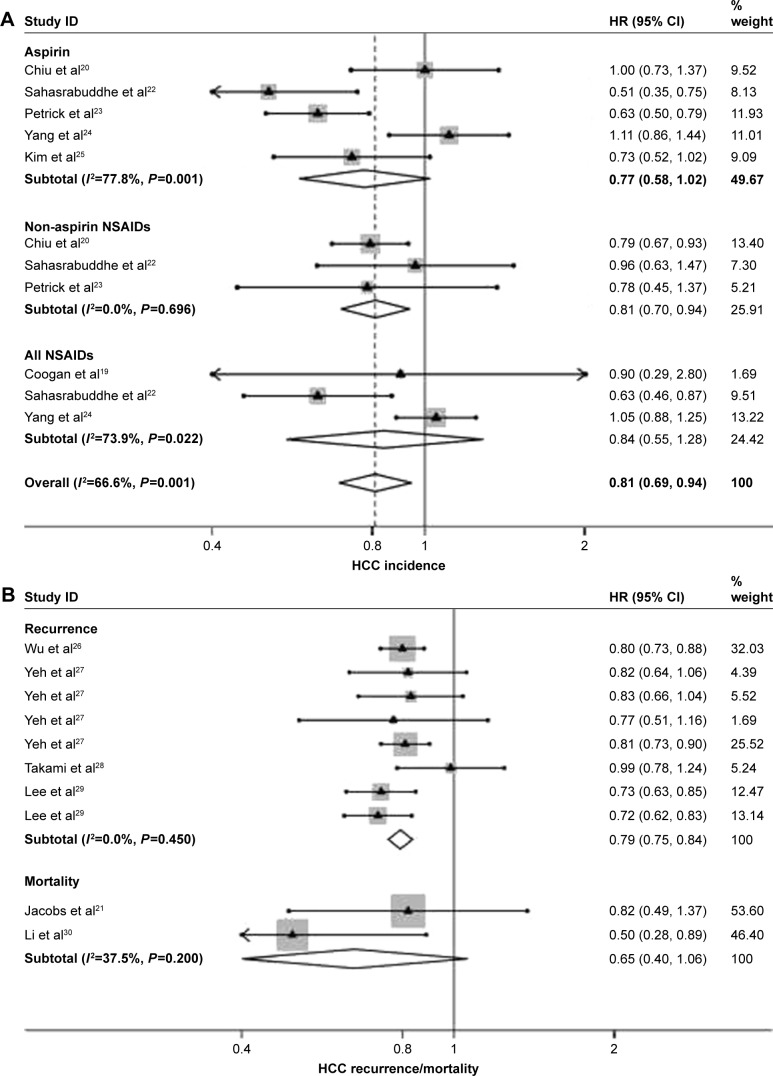
A total of twelve articles (published from 2000 to 2017) from five countries were identified by retrieval. We observed a significantly lower risk of HCC incidence among users of NSAIDs than among those who did not use NSAIDs (pooled hazard ratio [HR] value =0.81, 95% confidence interval [CI]: 0.69–0.94). No evidence of publication bias was observed (Begg’s test, P=0.755; Egger’s test, P=0.564). However, when stratified according to the categories of NSAIDs, users of non-aspirin NSAIDs (HR =0.81, 95% CI: 0.70–0.94), but not aspirin (HR =0.77, 95% CI: 0.58–1.02), showed a statistically significant reduced HCC incidence. We also found that NSAIDs use significantly reduced the recurrent risk of HCC, with a HR value of 0.79 (95% CI: 0.75–0.84), whereas there was no statistically significant association between NSAIDs use and HCC mortality, with a HR value 0.65 (95% CI: 0.40–1.06).
Conclusion
Taken together, our meta-analysis demonstrates that NSAIDs significantly reduce the incident and recurrent risk of HCC.
Keywords: NSAIDs, aspirin, hepatocellular carcinoma, incidence, recurrence
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide, especially in less developed countries. According to the Global Cancer Statistics of 2012,1 the yearly burden of HCC is estimated to be 782,500 new cases, resulting in 745,500 deaths. To lower the incidence and mortality of HCC, preventative drugs for HCC need to be explored.
A growing body of convincing evidence demonstrates that host systemic and local inflammation promotes tumorigenesis and tumor progression.2–5 More than 70%–90% cases of HCC gradually unfold on the background of chronically inflamed hepatic parenchyma and thus HCC represents one of the pro-inflammatory stimuli-related malignancies.6 In addition to the elevated risk of HCC incidence, there is increasing evidence supporting the adverse role of systemic inflammation in the prognosis of HCC.7,8
The close association between inflammation and tumorigenesis forms the basis of the hypothesis that anti-inflammatory drugs may have antitumor effects. Based on this, recently considerable evidence from experimental and clinical studies indicates that nonsteroidal anti-inflammatory drugs (NSAIDs) could reduce the risk of several types of cancer, including gastric cancer,9 prostate cancer,10 colorectal cancer,11 esophageal cancer,12 breast cancer,13 bladder cancer,14 and head and neck cancers.15 The key mechanism of the protective action of NSAIDs is the inhibition of the cyclooxygenase (COX) enzymes, which could catalyze the synthesis of prostaglandins (PGs) in inflammatory processes. Taking into account that HCC is a chronic inflammation-related disease and is characterized by high expression of COX,16 it is tempting to speculate that NSAIDs may reduce HCC risk. Herein, we used the approach of meta-analysis to summarize all eligible epidemiological evidence for an association between aspirin or non-aspirin NSAIDs use and the incident or recurrent risk of HCC.
Materials and methods
Search strategy and selection criteria
A systematic search through databases of PubMed, EmBase, Web of Science, and Scopus databases until April 2017 was performed by two independent investigators (PQ and JH). Our core search consisted of terms (“nonsteroidal anti-inflammatory drugs” or NSAIDs or aspirin) AND (“hepatocellular carcinoma” or “liver cancer”). Additionally, we retrieved the reference lists of the included publications and related reviews manually. Endnote X7 software (Thomson Corporation, Stam-ford, CT, USA) was used to analyze and manage references.
Studies that met the following predetermined selection criteria were included: 1) published as an original article in English; 2) designed as an observational study; 3) exposure was use of aspirin or non-aspirin NSAIDs; 4) outcome was the incidence, recurrence, or mortality of HCC; 5) reported hazard ratio (HR), relative risk, or odd ratio value and 95% confidence interval (CI),17,18 or provided sufficient data to assess them. We excluded the following studies: 1) those that assessed the effect of NSAIDs on the survival of patients with HCC; 2) those that just provided P-value or other conditions wherein effect size and 95% CI could not be estimated; 3) animal studies, reviews, conference abstracts, commentaries, editorials, and duplicate studies.
Data abstraction
Based on the above inclusive and exclusive criteria, two authors (PQ and JH) independently evaluated the retrieved publications for inclusion and discrepancy was resolved by discussion. Kappa concordance coefficient was then calculated to assess the agreement between the two screeners. We extracted the following data with a standardized data collection protocol: tumor location, first author, publication year, country, study period, duration of follow-up, information source, types of NSAIDs, median age of study population, number of participants, number of cases, HR value (adjusted HR value in preference) and 95% CI, and controlled confounders. The research quality of each included study was assessed by MZ by using the modified Newcastle–Ottawa Scale (NOS) scores. The score ranged from zero to nine and ≥7 points was defined as high quality. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for reporting the current meta-analysis. All data were double checked by the first author (PQ).
Statistical analysis
We estimated the incident risk of HCC in users of any NSAIDs, aspirin, and non-aspirin NSAIDs compared with nonusers, respectively. With the eligible studies, we uniformly used a random-effects model to calculate the pooled HR value and 95% CI. Heterogeneity across studies was estimated by Q value and I2 statistic. I2 values of 25%, 50%, and 75% correspond to cutoff points of low, moderate, and high degrees of heterogeneity, respectively. A study was considered to demonstrate statistically substantial heterogeneity provided P<0.1 in Q statistic or I2>50%; otherwise, there was no significant heterogeneity. Based on the factors that might bring in potential heterogeneity, we subsequently performed subgroup analyses and meta-regression to seek the potential source of heterogeneity.
We also did influence analyses to evaluate whether any single study could markedly affect the pooled results. We compared the pooled HR values calculated by using a fixed-effects model with that calculated by using a random-effects model. Then, publication bias was determined by Begg’s funnel plot and Egger’s test. Finally, the Galbraith plot was studied to detect potential outliers, which might bias the results. We considered a statistically significant difference to be present if a bilateral P-value was <0.05.
Results
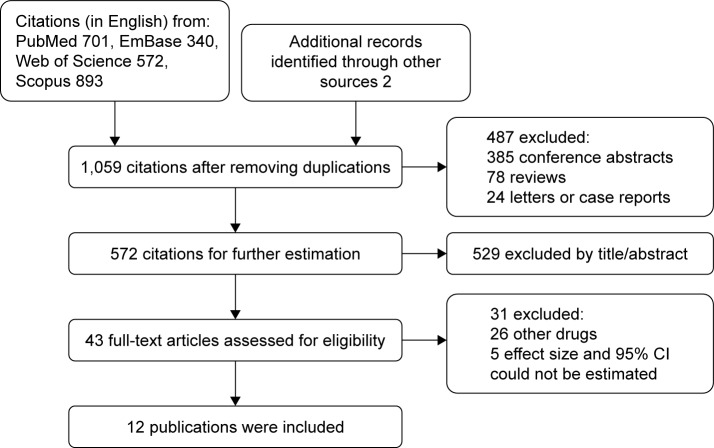
The flow chart of the literature retrieval and screening process is shown in Figure 1. Of the total 1,059 citations, 43 full text articles were assessed and 31 were further excluded. Thus, we finally included 12 publications in this meta-analysis. Agreement between the two investigators on which studies were to be included was good (kappa =0.917).
Figure 1.
Flow chart of search strategy and study selection.
Abbreviation: CI, confidence interval.
Article characteristics
The main characteristics of the twelve articles, seven of which were published after 2013, are summarized in Table 1. Five of the publications were performed in western countries and others were from Asia. Seven, four, and one were cohort, case-control, and nested case-control studies, respectively. The mean (or median) follow-up time (reported) ranged from 2.1 to 17.0 years. Nine publications controlled potential confounders and reported adjusted HR values. The assessment of study quality scores from the NOS scale demonstrated that the median score was 8 with a range from 6 to 9, and 91.6% of the studies were high quality.
Table 1.
Baseline characteristics for studies included in meta-analysis
| Study | Area | Design | Study period | Follow-up (years) | Information source | Drug type | Definition of user | Median age | Total size | Cases size | Adjusted variables | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC incidence | ||||||||||||
| Coogan et al19 | America | CC | 1977–1998 | – | Questionnaire | NSAIDs | Regular use ≥1 y | 50–70 | 5,833 | 51 | Age, sex, interview year, center, race, religion, smoking, family education, drinking | 7 |
| Chiu et al20 | Taiwan | CC | 1996–2008 | NR | Database | Aspirin, NSAIDs | ≥1 prescriptiona | 66.08/65.92 | 1,166 | 1,166 | None | 6 |
| Jacobs et al21 | America | Cohort | 1992–2008 | Up to 16 | Questionnaire | Aspirin | Regular use | 60 | 58,848/41,291 | 70/48 | Age, sex, race, education, smoking, BMI, physical activity, heart disease, stroke | 8 |
| Sahasrabuddhe et al22 | America | Cohort | 1995–2008 | 17 | Questionnaire | Aspirin, non-aspirin NSAIDs | Any use | 62.8 | 260,555/39,949 | 199/51 | Age, sex, race, BMI, smoking, drinking, diabetes | 8 |
| Petrick et al23 | America | Cohort | 1985–2010 | 11.9 | Questionnaire | Aspirin, non-aspirin NSAIDs | Any use | ≥40 | 477,470/606,663 | 315/364 | Age, sex, race, cohort, BMI, smoking, drinking, diabetes | 8 |
| Yang et al24 | UK | CC | 1988–2011 | NR | Database | Aspirin, non-aspirin NSAIDs | ≥2 prescriptions | 67.2/67.0 | 4,640 | 1,195 | BMI, smoking, alcohol disorders, HBV, HCV, diabetes, other NSAIDs, antidiabetic medications, statins | 7 |
| Kim et al25 | Korea | Nested CC | 2002–2013 | NR | Database | Aspirin | Any use | ≥40 | 1,374 | 229 | None | 7 |
| HCC recurrence | ||||||||||||
| Wu et al26 | Taiwan | Cohort | 2003–2010 | 2.23 | Database | NSAIDs | Any use | 54.5 | 2,452/2,117 | 939/932 | Age, sex, resection extent, liver cirrhosis, diabetes, use of statins, metformin | 9 |
| Yeh et al27 | Taiwan | Cohort | 1997–2010 | 2.9 | Database | Aspirin, non-aspirin NSAIDs | Any use | 58.3 | 9,811/5,763 | 7,229 | Age, sex, HBV, HCV, cirrhosis, diabetes, hypertension, stains, metformin | 8 |
| Takami et al28 | Japan | CC | 2008–2011 | NR | Database | Non-aspirin NSAIDs | 15 mg daily | 69.5/70.7 | 111/113 | 62/64 | None | 7 |
| Lee et al29 | Taiwan | Cohort | 1997–2011 | 3.5 4.3 |
Database | Aspirin, non-aspirin NSAIDs | Any use | 62.3/62.2 | 442/1,786 728/1,482 |
215/906 404/717 |
Age, sex, liver cirrhosis, diabetes, coronary artery disease, cerebral vascular accidents, peptic ulcers, and use of nucleoside analogs, statins, metformin | 9 |
| HCC mortality | ||||||||||||
| Jacobs et al21 | America | Cohort | 1992–2008 | Up to 16 | Questionnaire | Aspirin | Regular use | 60 | 58,848/41,291 | 70/48 | Age, sex, race, education, smoking, BMI, physical activity, heart disease, stroke | 8 |
| Li et al30 | China | Cohort | 2008–2013 | 2.1 | Database | Aspirin | Full dose use | 67.2/66.0 | 92 | 46 | Age, gender, total bilirubin and GGT levels, PLT, cirrhosis, tumor size and number, tumor vascular invasion, treatment | 8 |
Note:
One or more NSAIDs prescription(s) recorded from the database or questionnaire.
Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs; HCC, hepatocellular carcinoma; CC, case-control; RCT, randomized clinical trial; NR, not reported; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; NOS, Newcastle–Ottawa Scale; GGT, gamma-glutamyl transferase; PLT, platelet count.
Effects of NSAIDs on HCC incidence
Seven articles with eleven studies estimated the risk of HCC incidence in individuals with NSAIDs intake compared with unexposed persons. Among five, three studies were available to estimate the associations between use of aspirin, non-aspirin NSAIDs, and risk of HCC incidence, respectively. The remaining three studies failed to distinguish aspirin from non-aspirin NSAIDs. A random-effects model was used to pool the included studies which were stratified according to the categories of NSAIDs. As shown in Figure 2A, on the whole, NSAIDs use significantly reduced the incident risk of HCC, with a HR value 0.81 (95% CI: 0.69–0.94) and a moderate degree of between-study heterogeneity (I2=66.6%, P<0.001). The stratified analysis showed that non-aspirin NSAIDs (HR =0.81, 95% CI: 0.70–0.94), but not aspirin (HR =0.77, 95% CI: 0.58–1.02), significantly reduced HCC incidence and was identified as potential antitumor drugs.
Figure 2.
Forest plots of meta-analysis on the use of NSAIDs and risk of HCC incidence (A) and recurrence (B), stratified according to the categories of NSAIDs.
Note: Weights are from random-effects analysis.
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs; HR, hazard ratio; CI, confidence interval.
Effects of NSAIDs on HCC recurrence and mortality
Accumulating evidence suggests that postoperative recurrence of HCC may arise from de novo tumor in the remnant liver. By summarizing eight relevant studies with a random-effects model, we subsequently analyzed the effects of NSAIDs on HCC recurrence. We demonstrated that NSAIDs use significantly reduced the recurrent risk of HCC, with a HR value 0.79 (95% CI: 0.75–0.84) and a low degree of between-study heterogeneity (Figure 2B; I2=0%, P=0.460). In addition, two studies reported the association between NSAIDs and HCC mortality, and the pooled HR value was 0.65 (95% CI: 0.40–1.06).
Exploration of heterogeneity
The meta-analysis on the association between NSAIDs and HCC incidence included eleven studies (more than ten) and produced substantial heterogeneity (P<0.10). To explore the source of the heterogeneity, we subsequently performed subgroup and meta-regression analyses (Table 2). We analyzed the factors that might cause potential heterogeneity and there were no less than two studies in each subgroup. The covariates included NSAIDs categories (aspirin, non-aspirin, mixed NSAIDs), information source (database and questionnaire), publication year (before and after 2011), region (Asia and America/Europe), design (cohort and others), and whether the potential confounders were adjusted (yes or no). Among these factors, information source and study design were found to be the potential sources of heterogeneity by subgrouped analysis and univariate meta-regression (P<0.05). However, no independent factor was identified when multivariable meta-regression was performed.
Table 2.
Subgroup analysis and meta-regression analysis for studies that assessed the preventive effect of NSAIDs use in HCC incident risk
| Covariates | Subgroup | No of studies | HR (95% CI)
|
Heterogeneity
|
Subgrouped
|
Meta-regression
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Random-effects model | Fixed-effects model | P-value | I2 | Q value | P-value | Crude P-value | Adjusted P-value | |||
| Overall | 11 | 0.81 (0.69–0.94) | 0.83 (0.77–0.90) | 0.001 | 66.6 | |||||
| NSAIDs categories | Aspirin | 5 | 0.77 (0.58–1.02) | 0.78 (0.69–0.89) | 0.001 | 77.8 | 3.52 | 0.172 | 0.647 | – |
| Non-aspirin | 3 | 0.81 (0.70–0.94) | 0.81 (0.70–0.94) | 0.696 | 0 | |||||
| All NSAIDs | 3 | 0.84 (0.56–1.28) | 0.94 (0.81–1.09) | 0.022 | 73.9 | |||||
| Information source | Database | 5 | 0.93 (0.79–1.09) | 0.92 (0.84–1.02) | 0.046 | 58.8 | 14.57 | <0.001 | 0.025 | 1.000 |
| Questionnaire | 6 | 0.66 (0.56–0.78) | 0.66 (0.57–0.76) | 0.343 | 11.3 | |||||
| Publication year | Before 2013 | 6 | 0.76 (0.63–0.93) | 0.77 (0.68–0.87) | 0.081 | 49.0 | 2.89 | 0.089 | 0.505 | – |
| After 2013 | 5 | 0.85 (0.66–1.10) | 0.89 (0.80–0.99) | 0.002 | 76.8 | |||||
| Region | Asia | 3 | 0.82 (0.71–0.94) | 0.81 (0.71–0.93) | 0.344 | 6.30 | 0.17 | 0.685 | 0.829 | – |
| America/Europe | 8 | 0.79 (0.63–1.00) | 0.84 (0.76–0.93) | <0.001 | 74.6 | |||||
| Design | Cohort | 5 | 0.66 (0.55–0.79) | 0.65 (0.56–0.76) | 0.255 | 24.9 | 14.87 | <0.001 | 0.023 | 0.731 |
| Others | 6 | 0.93 (0.80–1.08) | 0.92 (0.84–1.02) | 0.084 | 48.5 | |||||
| Adjusted confounders | No | 3 | 0.82 (0.71–0.94) | 0.81 (0.71–0.93) | 0.344 | 6.30 | 0.17 | 0.685 | 0.829 | – |
| Yes | 8 | 0.79 (0.63–1.00) | 0.84 (0.76–0.93) | <0.001 | 74.6 | |||||
Note: Bold values indicate statistical significance (P<0.05).
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs; HR, hazard ratio; CI, confidence interval.
Then, Galbraith’s plots identified four outliers as potential sources of heterogeneity (Figure S1). When the meta-analysis was further performed after excluding the four outliers, the heterogeneity nearly disappeared (Figure S2; I2=0%, P=0.530).
Sensitivity analysis and test of publication bias
Afterwards, we carried out a sensitivity analysis to validate our findings. First, we compared the pooled HR values (that were calculated by using a random-effects model) with the estimated effect sizes by using a fixed-effects model (Table 2). There were no significant differences between the two models except that aspirin significantly reduced the risk of HCC when a fixed-effects model was used. Second, we carried out an influence analysis and the results suggested that no study could affect the summary of HCC incidence and recurrence risk estimate (Figure S3).
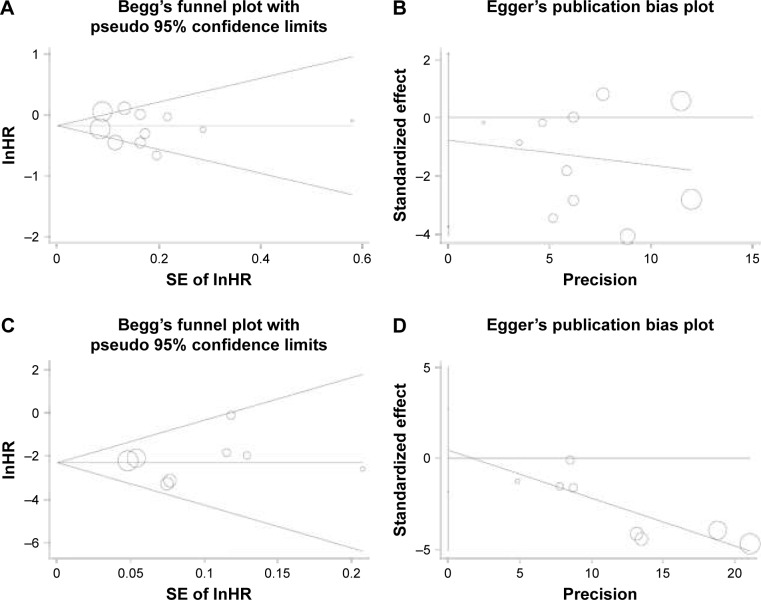
Eventually, we drew funnel plots for the meta-analysis of HCC incidence and the plots reflected basic symmetry (Figure 3A and B). Furthermore, no significant publication bias was identified by Begg’s test and Egger’s test, with a P-value of 0.755 and 0.564, respectively. In addition, there was also no publication bias in the meta-analysis of HCC recurrence (Figure 3C and D; P-value =0.902 and 0.656, respectively).
Figure 3.
Begg’s funnel plots and Egger’s funnel plots of studies that assessed the effects of NSAIDs use in risk of HCC incidence (A, B) and recurrence (C, D).
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs; HR, hazard ratio.
Discussion
In the recent decades, there has been great progress in the diagnosis and treatment of HCC. However, to date, diagnosis at early stages of this malignancy is still difficult and the prognosis remains unsatisfactory.31 We need to urgently seek several effective strategies for decreasing the risk of HCC incidence and mortality.
Accumulating evidence suggests that systemic inflammatory responses play irreplaceable roles in different stages of tumor progression, such as initiation, promotion, invasion, and metastasis.32–34 Tumor could accelerate the inflammatory process, which in turn predisposes to tumor progression via inhibiting apoptosis, promoting angiogenesis and DNA damage.35 Recently, it has been reported that inflammation increases the incident risk of several types of cancer, such as colon cancer, prostate carcinoma, and pancreatic cancer,36–38 whereas the possible mechanisms remain unclear. There is no denying that, early in tumorigenesis, inflammatory cells produce numerous cytokines, reactive oxygen species, inflammatory mediators, and eventually provide an attractive environment for tumor formation and angiogenesis.39–41 COX-1 and COX-2, which catalyze the oxidative conversion of arachidonic acid to PG, are crucial inflammatory mediators.42 By increasing PG synthesis and stimulating inflammation processes, COX-1 and COX-2 can promote apoptosis, angiogenesis, and tumorigenesis.
In view of the causal relationship between inflammation response and tumorigenesis, it is postulated that the development of cancer could be inhibited by alleviating inflammation. NSAIDs have been widely used for the prevention and treatment of various diseases by relieving the inflammation process. Recently, several epidemiological studies have indicated that frequent use of NSAIDs significantly reduces the incident risk of several solid cancers, such as prostate cancer,10 colorectal cancer,11 and breast cancer.13 Nevertheless, the antineoplastic mechanism of NSAIDs is not well understood and may be partially related to the inhibition of COX and PG synthesis.43 Aspirin is a strong, irreversible inhibitor of COX-1 and an inhibitor of COX-2 only at higher doses, whereas non-aspirin NSAIDs are nonselective and reversible inhibitors of both COX-1 and COX-2.44 It is notable that, as the expression of COX can be induced by various inflammatory cytokines and tumor promoters, the effects of NSAIDs may differ by tissues.45 To date, the antitumor effect of NSAIDs in HCC remains controversial.
In our study, we first hypothesized and verified the anticancer role of NSAIDs in HCC. We showed that users of NSAIDs experienced a statistically significant lower risk of HCC incidence as well as HCC recurrence than nonusers. When the analysis was stratified by the categories of NSAIDs, non-aspirin NSAIDs were found to be superior to aspirin. This difference may be due to the different pharmacological and toxic mechanisms of NSAIDs. Further basic and clinical studies are still needed to clarify the differences of antitumor effects between aspirin and other NSAIDs. In addition, we found no significant benefit of NSAIDs in reducing the risk of HCC mortality.
Potential mechanisms linking NSAIDs and reduced HCC risk might be manifold. First, the pathogenesis of HCC is strongly related to sustained inflammation. As the last and the most redoubtable clinical consequence of liver cirrhosis, HCC is developed mainly based on a myriad of pro-inflammatory stimuli and is triggered by well-recognized noxae such as hepatotropic viruses infection, obesity, and alcohol consumption.46 Several signaling pathways have been identified to be associated with the inflammation process of HCC, including NF-κB, JAK-STAT, and MAPK pathways, etc. Second, COX-2 demonstrates high expression in HCC and can promote tumor initiation, differentiation, invasion, and metastasis.16,47,48 A meta-analysis with 11 publications further indicates that high expression of COX-2 is associated with decreased survival and worse prognosis in HCC.49 In addition, it is suggested that COX-1 plays a similar role, and COX-1 inhibitors may show potential therapeutic implications in HCC.50 Third, there is increasing evidence that platelets promote tumor growth, angiogenesis, and metastasis.51 Meanwhile, tumor patients are often accompanied by thrombocytosis and functional abnormality of platelets. Thus, the protective role of NSAIDs in HCC may be also related to the antiplatelet function.
Though widely recognized as anti-inflammatory and antitumor agents, NSAIDs still are offset by their complications of major gastrointestinal bleeding and intracranial bleeding even with low-dose usage.52,53 Moreover, bleeding risk in HCC patients treated with NSAIDs is also higher than in those not treated with NSAIDs.29 Therefore, the clinical benefit of NSAIDs in an actual clinical setting may be not satisfactory. Although NSAIDs may cause gastrointestinal bleeding and ulcer perforation, several quantitative reviews report that NSAIDs use, long-term (≥4 years) and (or) low frequency (1–4.5 times per week) in particular, reduced the risk of gastric cancer (around 26%–33%).54–56
Our analysis embraces several limitations and shortcomings. First, there was substantial heterogeneity in our meta-analysis. In fact, owing to many potential confounders such as population characteristics, year of publication, follow-up time, and so forth, between-study heterogeneity was inevitable in any meta-analysis. By meta-regression analysis, we identified the potential source of heterogeneity: information source and study design. NSAIDs showed better antitumor effects in the subgroup of information source from questionnaire and in the subgroup of cohort study, both of which provide better evidence compared with matched subgroups. To reduce potential confounders and obtain more robust evidence, a generalized worldwide guideline for conducting observational studies of NSAIDs in HCC is needed. Second, the association of NSAIDs and aspirin with HCC risk may be related to the dose, duration, and frequency of these medicines. However, few included studies reported the effects of different dose or duration of NSAIDs in HCC risk. Further study should focus on the dose–response relationship between NSAIDs and the risk of HCC. Third, when a fixed-effects model was used, aspirin significantly reduced the risk of HCC, unlike the result obtained from the random-effects model. This may due to the high heterogeneity among the included studies. The significance of aspirin in HCC risk should be further verified through larger, multicentric prospective studies or randomized clinical trials.
In conclusion, our meta-analysis showed that use of NSAIDs significantly reduced the incident and recurrent risk of HCC, but had no effect on HCC mortality. Our results provide significant clinical value in HCC prevention.
Supplementary materials
Galbraith’s plot of studies that assessed the preventive effect of NSAIDs use in risk of HCC incidence.
Notes: *Aspirin; #non-aspirin NSAIDs; &all NSAIDs.
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs.
Forest plots of the meta-analysis on the association between NSAIDs and risk of HCC incidence after removal of the outliers of the heterogeneity.
Note: Weights are from random-effects analysis.
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs; HR, hazard ratio; CI, confidence interval.
Influence analysis of studies that assessed the effects of NSAIDs use in the risk of HCC incidence (A) and recurrence (B).
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs; CI, confidence interval.
References
- 1.Yang B, Petrick JL, Chen J, et al. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink. Cancer Epidemiol. 2016;43:105–111. doi: 10.1016/j.canep.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coogan PF, Rosenberg L, Palmer JR, et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev. 2000;9(1):119–123. [PubMed] [Google Scholar]
- 4.Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol. 2011;106(5):894–898. doi: 10.1038/ajg.2010.475. [DOI] [PubMed] [Google Scholar]
- 5.Kim G, Jang SY, Han E, et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: a nationwide nested case-control study. Int J Cancer. 2017;140(4):798–806. doi: 10.1002/ijc.30506. [DOI] [PubMed] [Google Scholar]
- 6.Petrick JL, Sahasrabuddhe VV, Chan AT, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the liver cancer pooling project. Cancer Prev Res (Phila) 2015;8(12):1156–1162. doi: 10.1158/1940-6207.CAPR-15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 8.Takami Y, Eguchi S, Tateishi M, et al. A randomised controlled trial of meloxicam, a Cox-2 inhibitor, to prevent hepatocellular carcinoma recurrence after initial curative treatment. Hepatol Int. 2016;10(5):799–806. doi: 10.1007/s12072-016-9704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh CC, Lin JT, Jeng LB, et al. Nonsteroidal anti-inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recur rence after curative liver resection: a nationwide cohort study. Ann Surg. 2015;261(3):521–526. doi: 10.1097/SLA.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 10.Lee PC, Yeh CM, Hu YW, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol. 2016;23(Suppl 5):874–883. doi: 10.1245/s10434-016-5520-9. [DOI] [PubMed] [Google Scholar]
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no 81600452).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Demaria S, Pikarsky E, Karin M, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33(4):335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambhir S, Vyas D, Hollis M, Aekka A, Vyas A. Nuclear factor kappa B role in inflammation associated gastrointestinal malignancies. World J Gastroenterol. 2015;21(11):3174–3183. doi: 10.3748/wjg.v21.i11.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma – epidemiological trends and risk factors. Dig Dis. 2009;27(2):80–92. doi: 10.1159/000218339. [DOI] [PubMed] [Google Scholar]
- 7.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 8.Pinato DJ, Stebbing J, Ishizuka M, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI) J Hepatol. 2012;57(5):1013–1020. doi: 10.1016/j.jhep.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Tian W, Zhao Y, Liu S, Li X. Meta-analysis on the relationship between nonsteroidal anti-inflammatory drug use and gastric cancer. Eur J Cancer Prev. 2010;19(4):288–298. doi: 10.1097/CEJ.0b013e328339648c. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Lin YW, Wu J, et al. Meta-analysis of nonsteroidal anti-inflammatory drug intake and prostate cancer risk. World J Surg Oncol. 2014;12:304. doi: 10.1186/1477-7819-12-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen V, Vogel U. Systematic review: interactions between aspirin, and other nonsteroidal anti-inflammatory drugs, and polymorphisms in relation to colorectal cancer. Aliment Pharmacol Ther. 2014;40(2):147–159. doi: 10.1111/apt.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124(1):47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 13.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100(20):1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Jiang D, Li X. Use of nonsteroidal anti-inflammatory drugs and bladder cancer risk: a meta-analysis of epidemiologic studies. PLoS One. 2013;8(7):e70008. doi: 10.1371/journal.pone.0070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang L, Hu H, Liu H, Jian C, Wang H, Huang J. Association of non-steroidal anti-inflammatory drugs and aspirin use and the risk of head and neck cancers: a meta-analysis of observational studies. Oncotarget. 2016;7(40):65196–65207. doi: 10.18632/oncotarget.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Z, Jiang JH, Zhang J, et al. COX-2 promotes migration and invasion by the side population of cancer stem cell-like hepatocellular carcinoma cells. Medicine (Baltimore) 2015;94(44):e1806. doi: 10.1097/MD.0000000000001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Yan L, Wang Z, et al. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Oncotarget. 2017;8(9):16017–16026. doi: 10.18632/oncotarget.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coogan PF, Rosenberg L, Palmer JR, et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev. 2000;9(1):119–123. [PubMed] [Google Scholar]
- 20.Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol. 2011;106(5):894–898. doi: 10.1038/ajg.2010.475. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs EJ, Newton CC, Gapstur SM, Thun MJ. Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst. 2012;104(16):1208–1217. doi: 10.1093/jnci/djs318. [DOI] [PubMed] [Google Scholar]
- 22.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrick JL, Sahasrabuddhe VV, Chan AT, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the liver cancer pooling project. Cancer Prev Res (Phila) 2015;8(12):1156–1162. doi: 10.1158/1940-6207.CAPR-15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang B, Petrick JL, Chen J, et al. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink. Cancer Epidemiol. 2016;43:105–111. doi: 10.1016/j.canep.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim G, Jang SY, Han E, et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: a nationwide nested case-control study. Int J Cancer. 2017;140(4):798–806. doi: 10.1002/ijc.30506. [DOI] [PubMed] [Google Scholar]
- 26.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 27.Yeh CC, Lin JT, Jeng LB, et al. Nonsteroidal anti-inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recurrence after curative liver resection: a nationwide cohort study. Ann Surg. 2015;261(3):521–526. doi: 10.1097/SLA.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 28.Takami Y, Eguchi S, Tateishi M, et al. A randomised controlled trial of meloxicam, a Cox-2 inhibitor, to prevent hepatocellular carcinoma recurrence after initial curative treatment. Hepatol Int. 2016;10(5):799–806. doi: 10.1007/s12072-016-9704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee PC, Yeh CM, Hu YW, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol. 2016;23(Suppl 5):874–883. doi: 10.1245/s10434-016-5520-9. [DOI] [PubMed] [Google Scholar]
- 30.Li JH, Wang Y, Xie XY, et al. Aspirin in combination with TACE in treatment of unresectable HCC: a matched-pairs analysis. Am J Cancer Res. 2016;6(9):2109–2116. [PMC free article] [PubMed] [Google Scholar]
- 31.Su C. Hepatobiliary cancer: all efforts for one goal. Cancer Lett. 2016;379(2):164–165. doi: 10.1016/j.canlet.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 32.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laine A, Iyengar P, Pandita TK. The role of inflammatory pathways in cancer-associated cachexia and radiation resistance. Mol Cancer Res. 2013;11(9):967–972. doi: 10.1158/1541-7786.MCR-13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 36.Allin KH, Bojesen SE, Nordestgaard BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int J Cancer. 2016;139(7):1493–1500. doi: 10.1002/ijc.30194. [DOI] [PubMed] [Google Scholar]
- 37.Aleksandrova K, Boeing H, Nothlings U, et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60(3):858–871. doi: 10.1002/hep.27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervello M, Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J Gastroenterol. 2006;12(32):5113–5121. doi: 10.3748/wjg.v12.i32.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffelt SB, de Visser KE. Cancer: inflammation lights the way to metastasis. Nature. 2014;507(7490):48–49. doi: 10.1038/nature13062. [DOI] [PubMed] [Google Scholar]
- 40.Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, Sarin SK. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer. 2013;2(3–4):367–383. doi: 10.1159/000343852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramaniam A, Shanmugam MK, Perumal E, et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta. 2013;1835(1):46–60. doi: 10.1016/j.bbcan.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271(52):33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 43.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 44.Sciulli MG, Seta F, Tacconelli S, et al. Effects of acetaminophen on constitutive and inducible prostanoid biosynthesis in human blood cells. Br J Pharmacol. 2003;138(4):634–641. doi: 10.1038/sj.bjp.0705078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagao M, Sato Y, Yamauchi A. A meta-analysis of PTGS1 and PTGS2 polymorphisms and NSAID intake on the risk of developing cancer. PLoS One. 2013;8(8):e71126. doi: 10.1371/journal.pone.0071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalasani N, Horlander JC, Sr, Said A, et al. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94(10):2988–2993. doi: 10.1111/j.1572-0241.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 47.Breinig M, Rieker R, Eiteneuer E, et al. Differential expression of E-prostanoid receptors in human hepatocellular carcinoma. Int J Cancer. 2008;122(3):547–557. doi: 10.1002/ijc.23098. [DOI] [PubMed] [Google Scholar]
- 48.Yang HJ, Jiang JH, Yang YT, et al. Cyclooxygenase-2 expression is associated with initiation of hepatocellular carcinoma, while prostaglandin receptor-1 expression predicts survival. World J Gastroenterol. 2016;22(39):8798–8805. doi: 10.3748/wjg.v22.i39.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, Li X, Yang J, et al. Prognostic significance of cyclooxygenase-2 expression in patients with hepatocellular carcinoma: a meta-analysis. Arch Med Sci. 2016;12(5):1110–1117. doi: 10.5114/aoms.2016.61916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lampiasi N, Fodera D, D’Alessandro N, et al. The selective cyclooxygenase-1 inhibitor SC-560 suppresses cell proliferation and induces apoptosis in human hepatocellular carcinoma cells. Int J Mol Med. 2006;17(2):245–252. [PubMed] [Google Scholar]
- 51.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet–cancer interactions. Semin Thromb Hemost. 2014;40(3):296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 52.Jaben EA, Mulay SB, Stubbs JR. Reversing the effects of antiplatelet agents in the setting of intracranial hemorrhage: a look at the literature. J Intensive Care Med. 2015;30(1):3–7. doi: 10.1177/0885066613487298. [DOI] [PubMed] [Google Scholar]
- 53.Cryer B. Reducing the risks of gastrointestinal bleeding with antiplatelet therapies. N Engl J Med. 2005;352(3):287–289. doi: 10.1056/NEJMe048330. [DOI] [PubMed] [Google Scholar]
- 54.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 55.Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100(3):551–557. doi: 10.1038/sj.bjc.6604880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye X, Fu J, Yang Y, Gao Y, Liu L, Chen S. Frequency-risk and duration-risk relationships between aspirin use and gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8(7):e71522. doi: 10.1371/journal.pone.0071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Galbraith’s plot of studies that assessed the preventive effect of NSAIDs use in risk of HCC incidence.
Notes: *Aspirin; #non-aspirin NSAIDs; &all NSAIDs.
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs.
Forest plots of the meta-analysis on the association between NSAIDs and risk of HCC incidence after removal of the outliers of the heterogeneity.
Note: Weights are from random-effects analysis.
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs; HR, hazard ratio; CI, confidence interval.
Influence analysis of studies that assessed the effects of NSAIDs use in the risk of HCC incidence (A) and recurrence (B).
Abbreviations: HCC, hepatocellular carcinoma; NSAIDs, nonsteroidal anti-inflammatory drugs; CI, confidence interval.