Summary
Background
Sleep difficulties might be a contributory causal factor in the occurrence of mental health problems. If this is true, improving sleep should benefit psychological health. We aimed to determine whether treating insomnia leads to a reduction in paranoia and hallucinations.
Methods
We did this single-blind, randomised controlled trial (OASIS) at 26 UK universities. University students with insomnia were randomly assigned (1:1) with simple randomisation to receive digital cognitive behavioural therapy (CBT) for insomnia or usual care, and the research team were masked to the treatment. Online assessments took place at weeks 0, 3, 10 (end of therapy), and 22. The primary outcome measures were for insomnia, paranoia, and hallucinatory experiences. We did intention-to-treat analyses. The trial is registered with the ISRCTN registry, number ISRCTN61272251.
Findings
Between March 5, 2015, and Feb 17, 2016, we randomly assigned 3755 participants to receive digital CBT for insomnia (n=1891) or usual practice (n=1864). Compared with usual practice, the sleep intervention at 10 weeks reduced insomnia (adjusted difference 4·78, 95% CI 4·29 to 5·26, Cohen's d=1·11; p<0·0001), paranoia (−2·22, −2·98 to −1·45, Cohen's d=0·19; p<0·0001), and hallucinations (−1·58, −1·98 to −1·18, Cohen's d=0·24; p<0·0001). Insomnia was a mediator of change in paranoia and hallucinations. No adverse events were reported.
Interpretation
To our knowledge, this is the largest randomised controlled trial of a psychological intervention for a mental health problem. It provides strong evidence that insomnia is a causal factor in the occurrence of psychotic experiences and other mental health problems. Whether the results generalise beyond a student population requires testing. The treatment of disrupted sleep might require a higher priority in mental health provision.
Funding
Wellcome Trust.
Introduction
Sleep problems are a common occurrence in patients with mental health disorders. The traditional view is that disrupted sleep is a symptom, consequence, or non-specific epiphenomenon of the disorders; the clinical result is that the treatment of sleep problems is given a low priority. An alternative perspective is that disturbed sleep is a contributory causal factor in the occurrence of many mental health disorders.1 An escalating cycle then emerges between the distress of the mental health symptoms, effect on daytime functioning, and struggles in gaining restorative sleep. From this alternative perspective, the treatment of sleep problems attains a higher clinical importance. We are particularly interested in the putative causal association between disturbed sleep and psychotic experiences.2, 3 The interventionist–causal model approach to establishment of a causal association is to manipulate the hypothesised mechanistic variable and observe the effect on the outcome of interest;4 if a causal association exists then the outcome variable should alter. The effects of the manipulation can then be substantiated further by use of mediation analysis.5, 6 In the present study, we aimed to improve sleep in individuals with insomnia to determine the effect on psychotic experiences. This approach therefore informs both theoretical understanding and clinical practice.
The most common form of sleep disruption is insomnia, comprising sustained difficulties in initiating or staying asleep, or both, which cause problems during the day. The association of insomnia with psychotic experiences in the general population has been established.3 There are multiple, independent, psychotic experiences. Each psychotic experience exists on a spectrum of severity in the general population with differing heritability and differing strength of association with insomnia.7 Paranoia and hallucinations have the strongest links with insomnia.2, 7, 8 However, the effect of altering the amount of sleep disruption—eg, by targeted sleep treatment—on these psychotic experiences remains to be established. Clinical guidelines recommend the use of cognitive behavioural therapy (CBT) as the first-line treatment for insomnia.9 Digital forms of CBT for insomnia that require no therapist to be present have been shown to be efficacious as well.10, 11, 12 In patients with current delusions and hallucinations, results of our pilot randomised controlled trial13 have shown that insomnia can be substantially reduced with CBT, but the trial was underpowered to establish with sufficient precision the consequences for psychotic experiences. Therefore, we undertook a clinical trial that was large enough to definitively test the causal association between insomnia and self-reported psychotic experiences.
Research in context.
Evidence before this study
If insomnia is a contributory cause of psychotic experiences, then the key test is whether improving sleep will lead to a reduction in psychotic experiences. We therefore searched for randomised controlled studies that set out to reduce insomnia and examine the effects on psychotic experiences. On June 23, 2017, we searched the entire archive (ie, using no date restrictions) of PubMed for: (Sleep OR Insomnia) AND (Delus* OR Hallucinat* OR Psychosis OR Psychotic OR Schizophren*) AND (CBT OR hypnotic OR medication) AND (Random* OR RCT). 130 papers were identified and only two were randomised controlled trials that tested the effects of sleep treatment on psychotic experiences, with the larger of the trials being our own with 50 patients with schizophrenia or related disorders. These trials were underpowered to determine with any precision the potential link between insomnia and psychotic experiences.
Added value of this study
We undertook what might be the largest randomised controlled trial to date of a psychological treatment. It is the first study adequately powered to determine the effects of treating sleep dysfunction on psychotic experiences. It shows very clearly that treatment of insomnia in students leads to a reduction in psychotic experiences. A mediation analysis supports this interpretation. Furthermore, the trial is consistent with a small number of other randomised controlled trials that indicate multiple other benefits for mental health when treating sleep problems.
Implications of all the available evidence
Sleep disruption might have a contributory causal role in the occurrence of psychotic experiences and a wide range of other mental health problems. Adequately powered tests in other populations would be helpful, but research indicating that the treatment of disrupted sleep requires a higher priority in mental health provision is accumulating.
To test thousands of individuals, we did an online study using digital CBT for insomnia treatment. A participant pool (university students) was selected that would be easily reachable (since we would have access to large email lists) and at an age when mental health disorders emerge. We have previously shown in a student population that sleep problems are associated with elevated levels of paranoia, hallucinations, anxiety, depression, and manic symptoms.8 Our main aim required a comparison between the effects of a reduction in insomnia (in a group receiving recommended treatment) and continued sleep disruption (in a group receiving usual care, which is likely to mean receiving little or no treatment). Clear change in sleep in one group relative to another was required to test the mechanistic hypothesis. We were not investigating the intervention elements that might lead to change. Our primary aim was to find out whether CBT for insomnia, compared with a usual practice control group, reduced insomnia and reduced paranoia and hallucinations by the end of treatment, and whether the changes in insomnia mediated the changes in psychotic experiences. We also aimed to determine the potential effects of sleep improvement on a wider range of mental health outcomes in this general population group. Our secondary aims were to investigate whether digital CBT for insomnia, compared with usual practice, reduced depression, anxiety, nightmares, and mania; improved psychological wellbeing; and led to the occurrence of fewer mental health disorders.
Methods
Study design and participants
We did this single-blind, randomised controlled trial (OASIS; Oxford Access for Students Improving Sleep) of digital CBT versus treatment as usual (usual practice). Screening, informed consent, assessments, allocation to condition, and the delivery of the intervention were carried out online using an automated system, a specially configured instance of True Colours, which is a system for the scheduled collection of outcome measures.14 Participants in the control group were given access to the sleep intervention after their final assessment. The study received overall ethical approval from the University of Oxford Medical Sciences Inter-Divisional Ethics Committee and then local approvals at the other participating universities. The OASIS trial protocol has been published.15
Participants were eligible if they were attending university; had a positive screen for insomnia, as indicated by a score of 16 or lower on the Sleep Condition Indicator (SCI);16 and were 18 years or older. We had no exclusion criteria. 26 UK universities took part (appendix 1), ensuring a range in geographical locations and academic ability. The principal method of recruitment was sending a circular email within universities that contained a link to the web-based screening. When a circular email was not possible, recruitment was via advertisment on websites and displaying posters, or both. Recruitment began on March 5, 2015, and ended on Feb 17, 2016. We collected the final data on July 28, 2016.
Randomisation and masking
As recommended for large clinical trials,17 we used simple randomisation (1:1) with an automated online system, ensuring that the research team was unable to affect randomisation. Participants completed all the assessments independently online and therefore their responses could not be affected by the trial team.
Procedures
Assessments took place at weeks 0 (baseline), 3, 10 (end of therapy), and 22. The week 3 assessment comprised only the primary outcome measures. The week 3 assessment was carried out to assess in the mediation analyses the temporal order of changes. Participants received an email prompt to complete the assessments online. The order of the assessments was consistent across timepoints. If participants did not complete the assessment, then they received up to two email reminders 2 days apart.
The CBT for insomnia intervention is called Sleepio.11, 18 It is provided in six sessions, lasting an average of 20 min each. Sessions are unlocked weekly, although participants can move at a slower pace. The full programme is accessible via any web browser and all participants start the programme online. Certain tools (eg, sleep diaries and relaxation audios) could also be accessed using the web browser of a smartphone. All of the sessions, sleep diaries, relaxation audios, and the scheduling tool could be accessed with an iPhone. Completion of an initial assessment drives the algorithms that personalise the programme. For example, the assessment leads to a tailored choice of treatment goal, with progress then reviewed at each subsequent session. The treatment includes behavioural, cognitive, and educational components. The behavioural techniques include sleep restriction (ie, reducing the sleep window to enhance sleep consolidation), stimulus control (eg, getting out of bed after 15–20 min of wakefulness), and relaxation (eg, tensing and relaxing muscles when in bed). The cognitive techniques include paradoxical intention (eg, trying to stay awake), belief restructuring (eg, targeting unrealistic expectations about sleep), mindfulness (eg, acknowledging thoughts and feelings without dwelling on them), imagery (eg, generating positive mental images), and putting the day to rest (eg, setting time aside to reflect on the day). The educational component covers information about the processes of sleep and sleep hygiene. The programme is interactive, and content is presented by an animated therapist. Participants make a time for the session and are prompted via email or text message via a short message service if they do not attend. Participants complete daily sleep diaries throughout the intervention, which are used by the programme to tailor the advice. Sleep restriction is introduced in the third session of the course. The animated therapist proposes a new sleep window, which is calculated from the sleep diary data, and engages with the participant to help them select the timing of the window (eg, earlier versus later in the night). A more lenient sleep window is used for those individuals reporting substantial physical problems, other mental health problems, or moderate-to-severe sleepiness. The sleep window is regularly reviewed at each session after it has been introduced. If the sleep diary data indicate a sleep efficiency of 90% or higher, the animated therapist advises that 15 min is added to the sleep window. Throughout the course of therapy, participants had access to a moderated online community and an online library of information about sleep. Participants could also view their online case file, which included four sections: a progress review, a reminder of strategies, an agreed sleep schedule, and a list of further reading. Usual practice (treatment as usual) referred to the current care that the participants were receiving. The amount of treatment input was likely to be minimal, with prescription of medication for a small proportion. We did not attempt to alter the current care that participants received.
Outcomes
The primary outcomes were insomnia, paranoia, and hallucinatory experiences. The primary measure for insomnia was the SCI-8.16 This score is an 8-item measure, validated against DSM-5 criteria, assessing sleep and its impact on daytime functioning over the past week. Scores can range from 0 to 32 with higher scores indicating better sleep. A clinical cutoff of less than 17 correctly identifies 89% of individuals with probable insomnia disorder. We used a version of the SCI that included one additional question, as a secondary outcome, regarding early morning waking. The internal consistency (Cronbach's α at baseline) of the scale in the present study was 0·63.
Paranoia was assessed with the Green et al Paranoid Thought Scales (GPTS), part B.19 This scale assesses persecutory ideation, and the timeframe used was the past fortnight. The scale comprises 16 items, each rated on a 1 (not at all) to 5 (totally) scale. High scores indicate higher levels of paranoia. The internal consistency of the scale in the present study was 0·94.
The measure for hallucinations was the Specific Psychotic Experiences Questionnaire—Hallucinations subscale.20 The scale comprises nine items rated on a 0 (not at all) to 5 (more than once per day) scale. The timeframe was the past fortnight. Higher scores indicate greater occurrences of hallucinatory experiences. The internal consistency of the scale in the present study was 0·93.
The secondary outcome measures for sleep were the Insomnia Severity Index (ISI;21 Cronbach's α in the present study=0·67) and the Disturbing Dreams and Nightmare Severity Index22 (Cronbach's α in the present study=0·91). The secondary outcome measure for psychotic experiences was the 16-item version of the Prodromal Questionnaire23 (Cronbach's α in the present study=0·79). A score of 6 or more has 87% specificity and 87% sensitivity to correctly classify ultra-high risk for psychosis mental states in a help-seeking sample.
The measures to assess affective symptoms were the Patient Health Questionnaire 9-item version24 (PHQ-9; Cronbach's α in the present study=0·85), the Generalised Anxiety Disorder 7-item version25 (GAD-7; Cronbach's α in the present study=0·89), and the Altman Mania Scale26 (Cronbach's α in the present study=0·64). Psychological wellbeing was assessed with the Warwick–Edinburgh Mental Wellbeing Scale (WEMWBS;27 Cronbach's α in the present study=0·89), and the Work and Social Adjustment Scale (WSAS;28 Cronbach's α in the present study=0·83).
To assess the development of mental health disorders, we used established cutoffs on the Prodromal Questionnaire,23 Altman Mania Scale,26 PHQ,24 and GAD-7.25 Participants were also asked at each assessment timepoint whether they were in contact with mental health services, had received a mental health diagnosis, took medication for a mental health problem, or were currently receiving any other psychological therapy.
If the trial team were informed of the occurrence of a serious adverse event for a trial participant, then this was recorded. Serious adverse events were defined as deaths, suicide attempts, serious violent incidents, admissions to secure units, and formal complaints about the online intervention.
Statistical analysis
We calculated the sample size on the basis of change in paranoia (one of the two psychotic symptoms studied), since following this intervention change would be expected to be lower in psychotic experiences than in insomnia. Based on the SDs observed from a previous study29 for the GPTS (SD 10·4), a total sample size of 2614 participants (ie, 1307 per group) would provide 90% power to detect a small effect size in paranoia, with a standardised mean difference of 0·15, while accounting for a high amount of expected attrition (40%). In a study amendment, the sample size was increased because of a higher than initially expected dropout rate.
An outline of the analysis strategy was provided in the published trial protocol15 and a full statistical analysis plan was agreed before the trial analysis (appendix 2). All the analyses were validated by a second statistician. Analyses were by intention to treat and were carried out at the end of the last follow-up assessment (with no interim analyses). We analysed each continuous outcome with a linear mixed effects regression model to account for the repeated measures over time, and we analysed binary outcomes with a logistic mixed effects model. Mixed effects models are the recommended statistical technique for analysing clinical trials when outcomes are collected at repeated timepoints,30 and in this trial included outcome data at weeks 3, 10, and 22 available for all participants who had been randomly assigned. The method has the advantage of implicitly accounting for data missing at random. The estimated (adjusted) treatment differences from these analyses are therefore reported. The linear mixed effects models included the outcome as the response variable, timepoint, randomised group, and baseline score as fixed effects and random effects were estimated for students nested within universities. A student is located within one university, and so to estimate the random intercepts, we accounted for random variation between universities and between students within the same university. We modelled an interaction between time and randomised group as a fixed effect to allow estimation of treatment effect at all three timepoints. Sex and course level were included as covariates in the model. We used an unstructured variance–covariance matrix to model the within-subject error correlation structure. Results are presented as mean adjusted differences in scores between the randomised groups, with 95% CIs and associated two-sided p values. We confirmed the normality assumption of the residuals for each outcome. No deviations from normality were apparent and therefore maximum likelihood estimates were reported. We did sensitivity analyses (pattern mixture models, inclusion of baseline covariates predictive of missing data, and imputation) for the three main outcomes, examining the robustness of the results to different assumptions regarding missing data. We calculated standardised effect sizes with Cohen's d, dividing the treatment effect by the shared SD at baseline. We used similar logistic mixed effects models for the secondary binary outcomes.
To test the mediation hypotheses, we determined the extent of mediation of the week 3 and week 10 insomnia scores on the week 10 paranoia and hallucination outcomes. The approach used was similar to the method of Baron and Kenny,5, 31 but made use of linear mixed effects models at each step. The approach involved four steps and three separate model fits. In two separate linear mixed effects models, the intervention was shown to be correlated with the outcome and then with the mediator. We then fitted the data to a third model with the outcome as the response and both the intervention and mediator as covariates. The parameters were extracted as per Baron and Kenny31 to obtain the total, direct, and indirect effects, and finally the percentage mediation was determined. In all models, we included baseline amounts of both the outcome and mediator as covariates. This method is similar to the mediation analysis in the study by Freeman and colleagues,32 but made use of linear mixed effects models to account for repeated measurements, rather than through structural equation modelling. We used STATA version 14.1 for the statistical analysis.
The trial is registered with the ISRCTN registry, number ISRCTN61272251.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
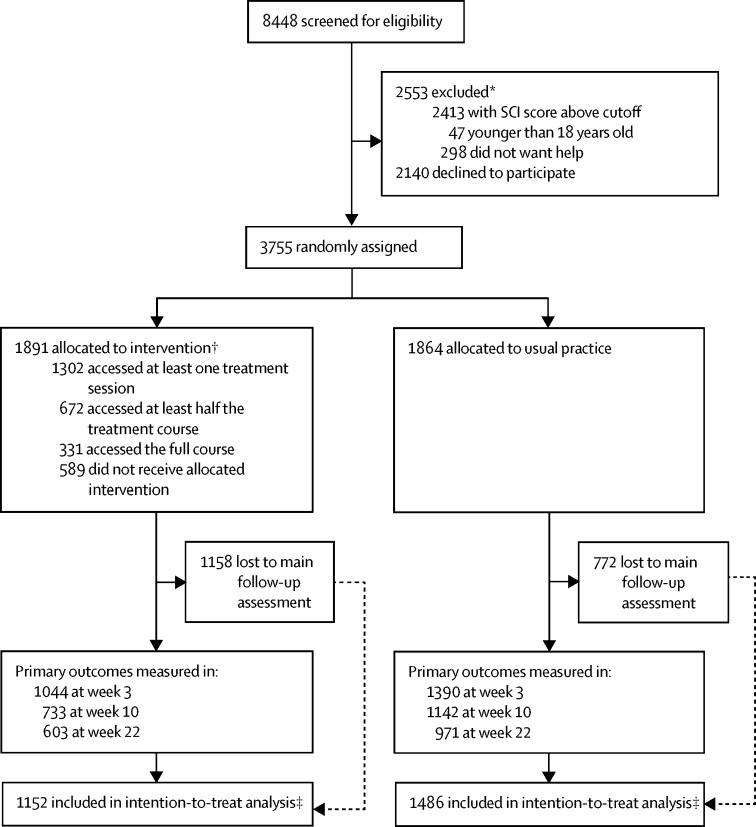
Between March 5, 2015, and Feb 17, 2016, we randomly assigned 3755 participants to receive digital CBT for insomnia (n=1891) or usual practice (n=1864; figure). The sample was predominately female, studying for their first university degree, and two-thirds were of white British ethnicity (table 1). Around a fifth of the participants were in contact with mental health services (table 1). The two randomised groups were well matched at baseline (table 1).
Figure.
Trial profile
SCI=Sleep Condition Indicator. *Some participants excluded for two or more reasons. †Not all participants accessed all treatment sessions. ‡Had at least one measurement at week 3, 10, or 22.
Table 1.
Baseline characteristics
| Control group (n=1864) | Treatment group (n=1891) | |||
|---|---|---|---|---|
| Mean age (years) | 24·6 (7·6) | 24·8 (7·7) | ||
| Mean total UCAS points | 753·0 (517·3) | 720·8 (456·3) | ||
| Sex | ||||
| Male | 530 (28%) | 513 (27%) | ||
| Female | 1315 (71%) | 1361 (72%) | ||
| Other | 19 (1%) | 17 (1%) | ||
| Course level | ||||
| Undergraduate | 1352 (73%) | 1389 (73%) | ||
| Postgraduate | 480 (26%) | 473 (25%) | ||
| Other | 32 (2%) | 29 (2%) | ||
| Ethnic origin | ||||
| White | ||||
| British | 1212 (65%) | 1265 (67%) | ||
| Irish | 32 (2%) | 27 (1%) | ||
| Other | 284 (15%) | 261 (14%) | ||
| Mixed | ||||
| White and Caribbean | 13 (1%) | 11 (1%) | ||
| White and African | 9 (<1%) | 13 (1%) | ||
| White and Asian | 31 (2%) | 27 (1%) | ||
| Other | 36 (2%) | 29 (2%) | ||
| Asian | ||||
| Indian | 26 (1%) | 43 (2%) | ||
| Pakistani | 23 (1%) | 22 (1%) | ||
| Bangladeshi | 9 (<1%) | 7 (<1%) | ||
| Chinese | 95 (5%) | 73 (4%) | ||
| Other | 25 (1%) | 32 (2%) | ||
| Black | ||||
| African | 26 (1%) | 23 (1%) | ||
| Caribbean | 10 (1%) | 17 (1%) | ||
| Other | 2 (<1%) | 3 (<1%) | ||
| Arab | 12 (1%) | 14 (1%) | ||
| Other | 19 (1%) | 24 (1%) | ||
| Mean insomnia (SCI-8) score | 10·1 (4·3) | 9·9 (4·3) | ||
| Mean paranoia (GPTS) score | 24·8 (11·6) | 25·4 (11·9) | ||
| Mean hallucinations (SPEQ) score | 5·3 (6·9) | 5·3 (6·4) | ||
| Mean insomnia (SCI-9) score | 12·1 (4·9) | 11·9 (4·8) | ||
| Mean insomnia (ISI) score | 15·3 (4·0) | 15·4 (3·9) | ||
| Mean nightmares (DDNSI) score | 8·1 (8·2) | 7·7 (7·8) | ||
| Mean prodromal psychosis (PQ-16) score | 4·9 (3·4) | 4·8 (3·3) | ||
| Mean depression (PHQ-9) score | 12·7 (5·9) | 12·9 (5·8) | ||
| Mean anxiety (GAD-7) score | 9·0 (5·6) | 9·4 (5·6) | ||
| Mean mania (Altman) score | 3·5 (3·0) | 3·5 (3·0) | ||
| Mean functioning (WSAS) score | 17·7 (7·6) | 17·6 (7·6) | ||
| Mean wellbeing (WEMWBS) score | 37·9 (8·8) | 37·8 (8·5) | ||
| Ultra-high risk of psychosis (PQ-16) cutoff (≥6) | ||||
| Above | 706 (38%) | 711 (38%) | ||
| Below | 1158 (62%) | 1180 (62%) | ||
| Depressive disorder (PHQ-9) cutoff (≥10) | ||||
| Above | 1238 (66%) | 1286 (68%) | ||
| Below | 626 (34%) | 605 (32%) | ||
| Anxiety disorder (GAD-7) cutoff (≥10) | ||||
| Above | 781 (42%) | 880 (47%) | ||
| Below | 1083 (58%) | 1011 (53%) | ||
| Mania disorder (Altman) score cutoff (≥6) | ||||
| Above | 422 (23%) | 413 (22%) | ||
| Below | 1442 (77%) | 1478 (78%) | ||
| Contact with mental health services | ||||
| Yes | 328 (18%) | 346 (18%) | ||
| No | 1536 (82%) | 1545 (82%) | ||
| Any psychiatric diagnosis | ||||
| Yes | 590 (32%) | 641 (34%) | ||
| No | 1274 (68%) | 1250 (66%) | ||
| Previous diagnosis of a sleep disorder | ||||
| Yes | 93 (5%) | 96 (5%) | ||
| No | 1771 (95%) | 1795 (95%) | ||
| Any psychiatric medication | ||||
| Yes | 433 (23%) | 460 (24%) | ||
| No | 1431 (77%) | 1431 (76%) | ||
| Specific medication for a sleep disorder | ||||
| Yes | 51 (3%) | 55 (3%) | ||
| No | 1813 (97%) | 1836 (97%) | ||
| Psychological therapy | ||||
| Yes | 146 (8%) | 135 (7%) | ||
| No | 1718 (92%) | 1756 (93%) | ||
Data are mean (SD) or n (%). UCAS=Universities and Colleges Admissions Service. SCI-8=Sleep Condition Indicator 8-item version. GPTS=Green et al Paranoid Thought Scales. SPEQ=Specific Psychotic Experiences Questionnaire. SCI-9=SCI 9-item version. ISI=Insomnia Severity Index. DDNSI=Disturbing Dream and Nightmare Severity Index. PQ-16=Prodromal Questionnaire 16-item version. PHQ-9=Patient Health Questionnaire 9-item version. GAD-7=Generalised Anxiety Disorder 7-item version. WSAS=Work and Social Adjustment Scale. WEMWBS=Warwick–Edinburgh Mental Wellbeing Scale.
Appendix 1 has the full details of the sample, missing data patterns, sensitivity analyses, and all other analyses. The dropout from the study assessments was high (50%) during the course of the study, and was greater in the treatment group than in the control group, with this figure and pattern almost identical to the most comparable previous study.12 The baseline scores for the three primary outcomes (insomnia, paranoia, and hallucinations) were not associated with later missingness (appendix 1). Compared with participants who remained in the study, participants who dropped out from both groups were younger in age and more likely to be male (appendix 1). For the secondary measures, ISI, PHQ-9, Altman Mania Scale, and WSAS scores were slightly higher, and the WEMWBS score was slightly lower, in the missing groups than the non-missing groups (appendix 1).
Treatment uptake was relatively low. In the intervention group, 1302 participants (69%) logged on for at least one treatment session, 953 (50%) accessed at least two sessions, 672 (36%) accessed at least three sessions, 497 (26%) accessed at least four sessions, 390 (21%) accessed at least five sessions, and 331 (18%) accessed six sessions (figure).
Regarding the primary measures, the sleep treatment was associated with significant reductions, at all timepoints, in insomnia, paranoia, and hallucinations compared with the control group (all p<0·0001; table 2). The reduction in insomnia after treatment was large, while the reduction in psychotic experiences was small (table 2). After treatment, 454 (62%) of 733 individuals in the treatment group and 326 (29%) of 1142 individuals in the control group scored outside the clinical cutoff for insomnia used for trial entry. The treatment differences were robust to the three different types of sensitivity analyses for missing data (appendix 1). A conservative imputation (given the general improvement in scores in both groups) of missing data was used, whereby the last available measurement for a participant was imputed for all further missing measurements of that participant. All three primary outcome differences remained significant with last observation carried forward imputations (appendix 1). Treatment differences also remained consistent with the primary analysis when we repeated the main analyses covarying for baseline variables that predicted missingness for each outcome. We also used pattern mixture models. Treatment differences would still be significant assuming the missing individuals in the treatment group had outcome scores 2 points worse for insomnia and paranoia, and 1 point worse for hallucinations (predictably the hallucination scale scores were much lower than for the other two outcome variables; appendix 1).
Table 2.
Primary outcome results
|
Insomnia (SCI-8) |
Paranoia (GPTS) |
Hallucinations (SPEQ) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted mean |
Adjusted difference* (95% CI), d† | p value* | Unadjusted mean |
Adjusted difference* (95% CI), d† | p value* | Unadjusted mean |
Adjusted difference* (95% CI), d† | p value* | ||||
| Control | Treatment | Control | Treatment | Control | Treatment | |||||||
| Week 3 | 12·34 (5·85) | 14·96 (5·80) | 2·62 (2·19 to 3·06), 0·61 | <0·0001 | 24·63 (11·82) | 22·61 (9·89) | −1·81 (−2·49 to −1·13), 0·15 | <0·0001 | 5·06 (6·89) | 4·06 (5·84) | −0·79 (−1·15 to −0·42), 0·12 | <0·0001 |
| Week 10 | 13·31 (6·45) | 18·08 (6·66) | 4·78 (4·29 to 5·26), 1·11 | <0·0001 | 23·84 (12·16) | 21·06 (9·08) | −2·22 (−2·98 to −1·45), 0·19 | <0·0001 | 4·89 (7·24) | 3·12 (5·12) | −1·58 (−1·98 to −1·18), 0·24 | <0·0001 |
| Week 22 | 14·43 (6·71) | 19·27 (7·13) | 4·81 (4·29 to 5·33), 1·12 | <0·0001 | 23·84 (12·68) | 20·75 (9·19) | −2·78 (−3·60 to −1·96), 0·24 | <0·0001 | 4·71 (7·43) | 2·87 (5·45) | −1·56 (−1·99 to −1·14), 0·23 | <0·0001 |
Data are mean (SD). At week 3, 1398 participants were in the control group and 1044 participants were in the treatment group. At week 10, 1142 participants were in the control group and 733 participants were in the treatment group. At week 22, 971 participants were in the control group and 603 participants were in the treatment group. SCI-8=Sleep Condition Indicator 8-item version. GPTS=Green et al Paranoid Thought Scales. SPEQ=Specific Psychotic Experiences Questionnaire.
Linear mixed effects model adjusted for gender, student status, week, and interaction of week with randomisation, and including a random effect for student within university. Covariance matrix of within subject measurements was unstructured.
d is standardised effect size (Cohen's d).
For the mediation analyses, change in sleep over 3 weeks explained 30% of the intervention effect on paranoia at 10 weeks, with change in sleep over 10 weeks accounting for 58% of the treatment effect on paranoia (table 3). Change in sleep over 3 weeks explained 21% of the intervention effect on hallucinations at 10 weeks, with change in sleep over 10 weeks accounting for 39% of the intervention effect on hallucinations. Hence early changes in sleep explain approximately half of the total sleep-mediated changes in psychotic experiences by the end of treatment. In comparison, parallel analyses in the opposite direction indicated that changes in psychotic experiences explained a much smaller percentage of variation in improvements in sleep. Specifically, when paranoia and hallucination outcomes at 3 weeks were set as the mediators and the sleep outcome at 10 weeks as the main outcome, paranoia symptoms mediated just 3·8% of change in sleep and hallucinations mediated 3·4% of change in sleep. These outcomes lend further support to the causal pathway hypothesis proposed in this study.
Table 3.
Mediation analysis* results
|
Total effect |
Direct effect |
Indirect effect |
Percentage mediated | ||||
|---|---|---|---|---|---|---|---|
| Effect size (95% CI) | p value | Effect size (95% CI) | p value | Effect size (95% CI) | p value | ||
| Paranoia (GPTS) outcome at week 10 | |||||||
| Insomnia at week 3 (SCI-8) | −2·27 (−3·03 to −1·51) | <0·0001 | −1·85 (−2·66 to 1·04) | <0·0001 | −0·67 (−0·86 to −0·48) | <0·0001 | 29·5% |
| Insomnia at week 10 (SCI-8) | −2·27 (−3·03 to −1·51) | <0·0001 | −0·97 (−1·80 to −0·14) | <0·0001 | −1·31 (−1·60 to −1·02) | <0·0001 | 57·8% |
| Hallucinations (SPEQ) outcome at week 10 | |||||||
| Insomnia at week 3 (SCI-8) | −1·60 (−2·00 to −1·20) | <0·0001 | −1·36 (−1·79 to −0·94) | <0·0001 | −0·33 (−0·43 to −0·23) | <0·0001 | 20·7% |
| Insomnia at week 10 (SCI-8) | −1·60 (−2·00 to −1·20) | <0·0001 | −0·90 (−1·34 to −0·46) | <0·0001 | −0·62 (−0·78 to −0·46) | <0·0001 | 38·6% |
Total n=1718. GPTS=Green et al Paranoid Thought Scales. SCI-8=Sleep Condition Indicator 8-item version. SPEQ=Specific Psychotic Experiences Questionnaire.
Outcome and mediators modelled by means of linear mixed effects models and the total, direct, and indirect effects determined using the Baron and Kenny31 approach. The effect size is the adjusted treatment difference (ie, non-standardised treatment difference).
The large improvement in insomnia is confirmed with the ISI assessment (table 4). The sleep treatment also led to improvements in depression, and improvements in anxiety, prodromal symptoms, nightmares, psychological wellbeing, and functioning, and all these improvements were maintained over time (Table 4, Table 5). Those participants randomised to the sleep treatment were also less likely to meet criteria over the course of the trial for a depressive episode, anxiety disorder, or ultra-high risk of psychosis (table 5). However, contact with mental health services did not differ between groups (table 5). Furthermore, the sleep treatment led to a small, sustained increase in symptoms of mania (table 5). With the sleep treatment, a greater risk also existed of meeting criteria for a manic episode (table 5). No adverse events were reported to the trial team.
Table 4.
Secondary outcome results
|
Unadjusted mean |
Adjusted difference*(95% CI), d† | p value | ||
|---|---|---|---|---|
| Control‡ | Treatment§ | |||
| Insomnia (ISI) | ||||
| Week 10 | 12·95 (5·27) | 9·23 (5·18) | −3·72 (−4·16 to −3·29), 0·94 | <0·0001 |
| Week 22‡ | 12·17 (5·29) | 8·62 (5·48) | −3·40 (−3·87 to −2·93), 0·86 | <0·0001 |
| Nightmares (DDNSI) | ||||
| Week 10 | 7·35 (7·85) | 5·47 (6·91) | −1·63 (−2·16 to −1·10), 0·20 | <0·0001 |
| Week 22‡§ | 7·32 (7·93) | 5·09 (6·66) | −1·84 (−2·41 to −1·26), 0·23 | <0·0001 |
| Prodromal psychosis (PQ-16) | ||||
| Week 10 | 4·35 (3·71) | 3·37 (3·29) | −0·81 (−1·03 to −0·60), 0·24 | <0·0001 |
| Week 22 | 4·05 (3·83) | 3·14 (3·24) | −0·74 (−0·98 to −0·51), 0·22 | <0·0001 |
| Depression (PHQ-9) | ||||
| Week 10 | 11·27 (6·72) | 8·44 (6·16) | −2·83 (−3·30 to −2·35), 0·48 | <0·0001 |
| Week 22 | 10·34 (6·79) | 8·00 (6·54) | −2·44 (−2·95 to −1·94), 0·42 | <0·0001 |
| Anxiety (GAD-7) | ||||
| Week 10 | 8·35 (6·06) | 6·53 (5·40) | −1·86 (−2·29 to −1·43), 0·33 | <0·0001 |
| Week 22 | 7·67 (6·10) | 6·14 (5·41) | −1·56 (−2·01 to −1·10), 0·28 | <0·0001 |
| Mania (Altman) | ||||
| Week 10 | 2·97 (3·03) | 3·77 (3·33) | 0·93 (0·67 to 1·19), −0·31 | <0·0001 |
| Week 22 | 2·92 (3·06) | 3·57 (3·41) | 0·75 (0·46 to 1·03), −0·25 | <0·0001 |
| Functioning (WSAS) | ||||
| Week 10 | 15·92 (8·89) | 11·43 (8·37) | −4·36 (−5·03 to −3·69), 0·58 | <0·0001 |
| Week 22 | 14·92 (9·17) | 10·25 (8·30) | −4·33 (−5·05 to −3·62), 0·57 | <0·0001 |
| Wellbeing (WEMWBS) | ||||
| Week 10 | 38·73 (9·78) | 40·92 (9·63) | 2·47 (1·72 to 3·22), 0·29 | <0·0001 |
| Week 22 | 39·63 (10·19) | 42·12 (10·36) | 2·78 (1·97 to 3·60), 0·32 | <0·0001 |
Data are mean (SD). ISI=Insomnia Severity Index. DDNSI=Disturbing Dream and Nightmare Severity Index. PQ-16=Prodromal Questionnaire 16-item version. PHQ-9=Patient Health Questionnaire 9-item version. GAD-7=Generalised Anxiety Disorder 7-item version. WSAS=Work and Social Adjustment Scale. WEMWBS=Warwick–Edinburgh Mental Wellbeing Scale.
Linear mixed effects model adjusted for gender, student status, week, and interaction of week with randomisation, and including a random effect for student within university. Covariance matrix of within subject measurements was unstructured.
d is standardised effect size (Cohen's d).
1142 participants in the control group at week 10 and 971 participants at week 22, except for 970 participants for insomnia (ISI) and 963 participants for nightmares (DDNSI) at week 22.
733 participants in the treatment group at week 10 and 603 participants at week 22, except for 599 participants for nightmares (DDNSI) at week 22.
Table 5.
Secondary dichotomous outcome results
| Adjusted odds ratio*(95% CI) | p value | |
|---|---|---|
| Ultra-high risk of psychosis (PQ-16) | ||
| Week 10 | 0·26 (0·15 to 0·46) | <0·0001 |
| Week 22 | 0·33 (0·18 to 0·59) | 0·00026 |
| Mania (Altman) | ||
| Week 10 | 2·01 (1·48 to 2·73) | <0·0001 |
| Week 22 | 1·89 (1·34 to 2·66) | 0·00027 |
| Depressive disorder (PHQ-9) | ||
| Week 10 | 0·21 (0·14 to 0·32) | <0·0001 |
| Week 22 | 0·32 (0·21 to 0·48) | <0·0001 |
| Anxiety disorder (GAD-7) | ||
| Week 10 | 0·32 (0·21 to 0·48) | <0·0001 |
| Week 22 | 0·42 (0·27 to 0·64) | <0·0001 |
| Contacted mental health services | ||
| Week 10 | 1·19 (0·70 to 2·04) | 0·52 |
| Week 22 | 0·98 (0·55 to 1·75) | 0·94 |
| Mental health diagnosis | ||
| Week 10 | 1·33 (0·75 to 2·37) | 0·33 |
| Week 22 | 1·43 (0·78 to 2·63) | 0·25 |
| Psychiatric medication | ||
| Week 10 | 0·77 (0·47 to 1·26) | 0·30 |
| Week 22 | 0·96 (0·58 to 1·59) | 0·86 |
| Psychological therapy | ||
| Week 10 | 1·27 (0·48 to 3·35) | 0·63 |
| Week 22 | 0·41 (0·11 to 1·58) | 0·20 |
1142 participants in the control group at week 10 and 971 participants at week 22. 733 participants in the treatment group at week 10 and 603 participants at week 22. PQ-16=Prodromal Questionnaire 16-item version. PHQ-9=Patient Health Questionnaire 9-item version. GAD-7=Generalised Anxiety Disorder 7-item version.
Logistic mixed effects model adjusted for gender, student status, week, and interaction of week with randomisation, and including a random effect for student within university. Covariance matrix of within subject measurements was unstructured.
Discussion
We aimed to investigate the effects on mental health of the reduction of sleep difficulties. The first necessary stage was for the intervention to reduce insomnia, which was achieved. A large effect size reduction was found with the digital CBT intervention in a large student population. But we designed the trial to establish the consequent effects on psychotic experiences. To our knowledge, the OASIS trial is the largest randomised controlled trial of a psychological intervention for a mental health problem. Students randomly assigned to the sleep intervention showed small, sustained reductions in paranoia and hallucinations, suggesting that disrupted sleep has a contributory causal role in the occurrence of these psychotic experiences in a specific population of young adults. The mediation analyses supported this interpretation—eg, improvements in sleep accounted for almost 60% of the change in paranoia after treatment. Insomnia might not be the largest cause of psychotic experiences but it is not an epiphenomenon. Hence, this study adds to our understanding of the causes of psychotic experiences and might indicate a promising route into the early treatment of some psychotic problems.
The focus on a sleep intervention in a young adult population is important. Young people with incipient disorders might be very reluctant to seek help for psychiatric problems. Trouble sleeping is a common complaint with little stigma. Hence, it provides a much more acceptable focus for a first step in a care pathway. The digital sleep treatment gave added benefits. Depression in particular, but also anxiety, psychological wellbeing, nightmares, and perceived functioning all improved. The effects on anxiety and depression are consistent with the results of a meta-analysis.33 Participants who received the sleep treatment in the trial were less likely to report symptoms at a level that met criteria for ultra-high risk of psychosis, depression, or anxiety disorder. At baseline, the frequency of positive screens for psychosis risk with the Prodromal Questionnaire was high, similar to the risk found with this questionnaire for adolescents referred to treatment services;34 this high rate will reflect the well established associations of sleep difficulties with psychotic experiences2, 3, 7, 8 (ie, that participants have been selected for insomnia and therefore will score higher on psychosis measures), and also the limitations of brief self-report questionnaires for assessing psychosis risk. However, in the trial, sleep treatment did not affect contact with mental health services. Most participants were not in contact with these services so a longer follow-up period might be needed to truly test such effects. Furthermore, manic symptoms associated with the sleep treatment increased. This outcome might be due to an actual increase in problematic manic symptoms or it might simply reflect the overall increase in psychological wellbeing in the sample since the questionnaire domains concern cheerfulness, self-confidence, reduced need for sleep, increase in amount of activity, and talkativeness. The Altman scale has been found to correlate poorly with self-ratings of elation.35
Are the study results generalisable beyond a student population? We consider that the results are likely to apply to the wider adult population. We used a treatment developed for adults, which was not modified for students. The large treatment reduction in insomnia for the students is very similar to that found in trials with general adult populations,10, 11, 36 while previous studies36, 37 with community samples have shown self-help sleep treatment to reduce anxiety and depression. Nonetheless, only a direct comparison in a trial can definitively determine the generalisablilty of our findings. Although not the primary objective of the study, the trial does indicate that the provision of internet-delivered CBT for insomnia to university students is likely to lead to reductions overall in insomnia, and smaller reductions in a number of other mental health symptoms, with benefits for positive psychological wellbeing too. Tailoring of the intervention specifically for this population could enhance engagement and outcome effects. Support to complete the intervention might well be helpful too.
Several limitations exist in the study. First, the study relied on self-report questionnaires, albeit validated in their development against clinical interviews. Similar change captured in rater-assessed measures would have strengthened confidence in the study results. Second, the samples tested were predominately in the non-clinical range of psychotic experiences, restricting the conclusions to the less severe end of the psychosis spectrum. Third, the participants were self-selecting in responding to the invitation, which will have affected the representativeness of the sample. However, access to the study was via an Internet webpage, which is a simpler process than obtaining treatment from clinical services. The whole study could be completed in the privacy of the home, which means that far fewer barriers existed to participation than conventional patient trials. Fourth, the extent to which the results will generalise to the rest of the population is not known. Even within the student population, we do not know the representativeness of the participants. Fifth, bias in the outcome results will have been introduced because of the high dropout rate, especially in the treatment group, which is similar to comparable online studies.12 The results did remain robust against conservative assumptions in the sensitivity analyses about those participants who dropped out, but it is notable that treatment effects were greater for those participants who completed the sleep treatment. Finally, the causal argument rests on the plausible assumption that the sleep treatment first changes the occurrence of insomnia, since that was the topic of the intervention, but the mediation analyses in this trial based on week 10 outcomes cannot fully capture the temporal order of changes or rule out reverse causation. We were able to show a significant amount of mediation based on the week 3 insomnia score as a mediator, while evidence for reverse causation was weak, which does follow the predicted temporal causal pathway. In reality, it is difficult in a clinical trial to capture potential temporal changes between mediator and outcomes, since improvements in paranoia and hallucinations are likely to closely parallel the improvement in sleep.
This work can be taken forward in several possible ways. Determination of the mechanisms linking insomnia to psychotic experiences will shed further light on the causes of psychosis and potentially enable treatment improvement.3, 13 Of great clinical interest will be the evaluation of the effects of improving sleep for patients attending clinical services with ultra-high risk of psychosis, or established clinical psychotic experiences, or at the early stages of relapse. Our experience is that patients with psychosis value their sleep difficulties being appropriately addressed, that this enhances engagement with other treatments, and that better sleep can contribute to a reduction in psychotic experiences. Furthermore, a gap exists in mental health services regarding intervention for early, relatively non-specific presentations, and proper sleep treatment might provide a sensible first response. Overall, this trial indicates the importance of sleep difficulties for mental health in the general population and the need for a reconsideration in clinical services of the priority given to improving sleep.
Acknowledgments
Acknowledgments
The study was done by the University of Oxford Sleep and Circadian Neuroscience Institute, with a grant from the Wellcome Trust (098461/Z/12/Z). The sleep treatment programme was provided to all the trial participants at no cost by Sleepio/Big Health Ltd. RGF and GMG are the principal investigators for the grant, and DFr and PJH are grant holders. BS is directly funded by the grant. The research in Nottingham (CHo, EBD, CG) was supported by the National Institute of Health Research (NIHR) MindTech Healthcare Technology Co-operative. DFr is supported by an NIHR Research Professorship. This study was funded by the Wellcome Trust and supported by the NIHR Oxford Health Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or Department of Health.
Contributors
DFr was the chief investigator, conceived the study, led the design, and drafted the paper. BS was the trial coordinator and contributed to the design. GMG conceived the study and contributed to the design. L-MY contributed to the design and was responsible for the main statistical outcome analyses. AN carried out the trial analyses. PJH conceived the study and contributed to the design. RE oversaw the statistical mediation analysis. AIL was responsible for the digital therapy programme. VW was responsible for the computer programming that carried out the screening, assessments, and links to the digital intervention. RGF contributed to the design of the trial. CHi was responsible for the development and coordination of the underlying True Colours platform. CAE conceived the study, contributed to the design, and was responsible for the digital therapy programme. Other authors led the research at their university site. All authors commented upon and approved the final manuscript.
Declaration of interests
DFr reports grants from Wellcome Trust, non-financial support (provision of the sleep treatment for the OASIS trial) from Sleepio, and grants from the UK National Institute of Health Research (NIHR), during the conduct of the study, and personal fees from Oxford Virtual Reality, a University of Oxford spin-out company, outside the submitted work. BS reports grants from Wellcome Trust, during the conduct of the study, and personal fees from Big Health Ltd, outside the submitted work. GMG reports personal fees from Servier, Lundbeck and Otsuka, Takeda, Merck, Medscape, Pfizer, P1vital, Compass Pathways, Shire, Angelini Spa, and Allergan, outside the submitted work. PJH reports grants from the Wellcome Trust and Medical Research Council, during the conduct of the study. The position of AIL at the University of Oxford is funded by Big Health Ltd. AIL reports non-financial support and other from Big Health Ltd, during the conduct of the study, and personal fees, non-financial support, and other from Big Health Ltd, outside the submitted work. CHi reports grants from Roche and Eli Lilly, outside the submitted work. AG reports grants from NIHR HTA Grant Number 13/15/04, outside the submitted work. PK reports research grant funding from NIHR and the Economic and Social Research Council. SJ reports research grant funding from NIHR outside the submitted work. PK is Vice President of the British Psychological Society. AMG reports grants from NIHR Maudsley Biomedical Research Centre, outside the submitted work, and is writing a book on sleep to be published by Bloomsbury Sigma. AMG has provided guidance and educational content for the Pediatric Sleep Council website; a freely available educational website providing tips to help with sleep in babies and young children. The website is partially supported by Johnson and Johnson, but they do not have any influence over content and do not advertise on it. AMG contributes to the British Broadcasting Corporation Focus magazine. CAE is a co-founder and Chief Medical Officer of the digital cognitive behavioural therapy for an insomnia programme (Sleepio/Big Health Ltd) and reports other from Big Health Ltd, during the conduct of the study, and other from Big Health Ltd and personal fees from Warnford Wellness, outside the submitted work. CAE has a US patent issued: serial number 14/172,347, Interactive System for Sleep Improvement; docket number HAME-001. All other authors declare no competing interests.
Supplementary Material
References
- 1.Harvey AG, Murray G, Chandler R, Soehner A. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev. 2011;31:225–235. doi: 10.1016/j.cpr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman D, Pugh K, Vorontsova N, Southgate L. Insomnia and paranoia. Schizophr Res. 2009;108:280–284. doi: 10.1016/j.schres.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeve S, Sheaves B, Freeman D. The role of sleep dysfunction in the occurrence of delusions and hallucinations. Clin Psychol Rev. 2015;42:96–115. doi: 10.1016/j.cpr.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendler KS, Campbell J. Interventionist causal models in psychiatry: repositioning the mind-body problem. Psychol Med. 2009;39:881–887. doi: 10.1017/S0033291708004467. [DOI] [PubMed] [Google Scholar]
- 5.Dunn G, Emsley R, Liu H. Evaluation and validation of social and psychological markers in randomised trials of complex interventions in mental health: a methodological research programme. Health Technol Assess. 2015;19:1–115. doi: 10.3310/hta19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–270. doi: 10.1177/0962280209105014. [DOI] [PubMed] [Google Scholar]
- 7.Taylor M, Gregory A, Freeman D, Ronald A. Do sleep disturbances and psychotic experiences in adolescence share genetic and environmental influences? J Abnorm Psychol. 2015;124:674–684. doi: 10.1037/abn0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheaves B, Porcheret K, Tsanas A. Insomnia, nightmares and chronotype as markers of risk for severe mental illness. Sleep. 2016;39:173–181. doi: 10.5665/sleep.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qaseem A, Kansagara D, Forciea M, Cooke M, Denberg T. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 10.Ritterband L, Thorndike F, Ingersoll K. Effect of a web-based cognitive behaviour therapy for insomnia intervention with a 1-year follow-up: a randomized clinical trial. JAMA Psychiatry. 2017;74:68–75. doi: 10.1001/jamapsychiatry.2016.3249. [DOI] [PubMed] [Google Scholar]
- 11.Espie C, Kyle S, Williams C. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35:769–781. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen H, Batterham P, Gosling J. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3:333–341. doi: 10.1016/S2215-0366(15)00536-2. [DOI] [PubMed] [Google Scholar]
- 13.Freeman D, Waite F, Startup H. Efficacy of cognitive behavioural therapy for sleep improvement in patients with persistent delusions and hallucinations (BEST): a prospective, assessor-blind, randomised controlled pilot study. Lancet Psychiatry. 2015;2:975–983. doi: 10.1016/S2215-0366(15)00314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilderbeck A, Atkinson L, McMahon H. Psychoeducation and online mood tracking for patients with bipolar disorder. J Affect Disord. 2016;205:245–251. doi: 10.1016/j.jad.2016.06.064. [DOI] [PubMed] [Google Scholar]
- 15.Freeman D, Sheaves B, Goodwin G. Effects of cognitive behavioural therapy for insomnia on the mental health of university students. Trials. 2015;16:236. doi: 10.1186/s13063-015-0756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espie CA, Kyle SD, Hames P, Gardani M, Fleming L, Cape J. The sleep condition indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open. 2014;4:e004183. doi: 10.1136/bmjopen-2013-004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt CE, Torgerson DJ. Is restricted randomisation necessary? Br Med J. 2006;332:1506–1508. doi: 10.1136/bmj.332.7556.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luik A, Bostock S, Chisnall L. Treating depression and anxiety with digital cognitive behavioural therapy for insomnia: a real world NHS evaluation using standardized outcome measures. Behav Cogn Psychother. 2017;45:91–96. doi: 10.1017/S1352465816000369. [DOI] [PubMed] [Google Scholar]
- 19.Green CEL, Freeman D, Kuipers E. Measuring ideas of persecution and social reference: the Green et al. Paranoid Thought Scales (GPTS) Psychol Med. 2008;38:101–111. doi: 10.1017/S0033291707001638. [DOI] [PubMed] [Google Scholar]
- 20.Ronald A, Sieradzka D, Cardno A, Haworth C, McGuire P, Freeman D. Characterization of psychotic experiences in adolescence using the Specific Psychotic Experiences Questionnaire (SPEQ): findings from a study of 5000 16 year old twins. Schizophr Bull. 2014;40:868–877. doi: 10.1093/schbul/sbt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 22.Krakow BJ, Melendrez DC, Johnston LG. Sleep dynamic therapy for Cerro Grande fire evacuees. J Clin Psychiatry. 2002;63:673–684. doi: 10.4088/jcp.v63n0804. [DOI] [PubMed] [Google Scholar]
- 23.Ising HK, Veling W, Loewy RL. The validity of the 16-item version of the Prodromal Questionnaire (PQ-16) to screen for ultra high risk of developing psychosis in the general help-seeking population. Schizophr Bull. 2012;38:1288–1296. doi: 10.1093/schbul/sbs068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JBW, Löwe B. The Patient Health Questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 26.Altman EG, Hedeker D, Peterson JL, Davis JM. The Altman self-rating mania scale. Biol Psychiatry. 1997;42:948–955. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- 27.Tennant R, Hiller L, Fishwick R. The Warwick–Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5:63. doi: 10.1186/1477-7525-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mundt JC, Marks IM, Shear MK, Greist JM. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- 29.Freeman D, Lister R, Evans N. The use of intuitive and analytic reasoning styles by patients with persecutory delusions. J Behav Ther Exp Psychiatry. 2014;45:454–458. doi: 10.1016/j.jbtep.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Gueorguieva R, Krystal J. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 31.Baron R, Kenny DA. The moderator-mediator variable distinction in social psychological research. J Pers Soc Psychol. 1986;5:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 32.Freeman D, Dunn G, Startup H. Effects of cognitive behaviour therapy for worry on persecutory delusions in patients with psychosis (WIT): a parallel, single-blind, randomised controlled trial with a mediation analysis. Lancet Psychiatry. 2015;2:305–313. doi: 10.1016/S2215-0366(15)00039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye YY, Zhang YF, Chen J. Internet-based cognitive behavioral therapy for insomnia (ICBT-i) improves comorbid anxiety and depression—a meta-analysis of randomized controlled trials. PLoS One. 2015;10:e0142258. doi: 10.1371/journal.pone.0142258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jong Y, Mulder C, Boon A, Deen M, van't Hof M, van der Gaag M. Screening for psychosis risk among adolescents in Child and Adolescent Mental Health Services: a description of the first step with the 16-item version of the Prodromal Questionnaire (PQ-16) Early Interv Psychiatry. 2016 doi: 10.1111/eip.12362. DOI:10.1111/eip.12362 published online Nov 9. [DOI] [PubMed] [Google Scholar]
- 35.Tsanas A, Saunders K, Bilderbeck A. Daily longitudinal self-monitoring of mood variability in bipolar disorder and borderline personality disorder. J Affect Disord. 2016;205:225–233. doi: 10.1016/j.jad.2016.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zachariae R, Lyby M, Ritterband L, O'Toole M. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia— a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. doi: 10.1016/j.smrv.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 37.van Straten A, Cuijpers P. Self-help therapy for insomnia: a meta-analysis. Sleep Med Rev. 2009;13:61–71. doi: 10.1016/j.smrv.2008.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.