Abstract
Background
A number of evidence-based interventions have been proposed to reduce post cesarean wound complications. Examples of such interventions include appropriate timing of preoperative antibiotics, appropriate choice of skin antisepsis, closure of the subcutaneous layer if subcutaneous depth is ≥ 2 cm, and subcuticular skin closure with suture rather than staples. However, the collective impact of these measures is unclear.
Objective
We sought to estimate the impact of a group of evidence-based surgical measures (prophylactic antibiotics administered prior to skin incision, chlorhexidine-alcohol for skin antisepsis, closure of subcutaneous layer, and subcuticular skin closure with suture) on wound complications after cesarean, and to estimate residual risk factors for wound complications.
Study Design
We conducted a secondary analysis of data from a randomized controlled trial of chlorhexidine-alcohol versus iodine-alcohol for skin antisepsis at cesarean from 2011–2015. The primary outcome for this analysis was a composite of wound complications, including surgical site infection (SSI), cellulitis, seroma, hematoma, and separation within 30 days. Risk of wound complications in women who received all four evidence-based measures (prophylactic antibiotics within 60 minutes of cesarean and prior to skin incision, chlorhexidine-alcohol for skin antisepsis with three minutes of drying time prior to incision, closure of subcutaneous layer if ≥ 2 cm of depth and subcuticular skin closure with suture) were compared to those who did not. We performed logistic regression analysis limited to patients who received all the evidence-based measures to estimate residual risk factors for wound complications and SSI.
Results
Of 1082 patients with follow-up, 349 (32.3%) received all the evidence-based measures and 733 (67.7%) did not. The risk of wound complications was significantly lower in patients who received all the evidence-based measures compared to those who did not (20.3% vs 28.1%; aRR 0.75, 95% CI 0.58, 0.95). The impact appeared to be largely driven by a reduction in surgical site infections. Among patients who received all the evidence-based measures, unscheduled cesarean was the only significant risk factor for wound complications (27.5% vs. 16.1%, aRR 1.71, 95% CI 1.12, 2.47) and SSI (6.9% vs. 1.6%, RR 3.74, 95% CI 1.18, 11.92). Other risk factors, including obesity, smoking, diabetes, chorioamnionitis, surgical experience, and skin incision type were not significant among patients who received all of the four evidence-based measures.
Conclusion
Implementation of evidence-based measures significantly reduces wound complications, but the residual risk remains high. This suggests the need for additional interventions, especially in patients undergoing unscheduled cesareans, who are at risk for wound complications even after receiving current evidence-based measures.
Keywords: Cesarean Delivery, Evidence-Based Measures, Infection, Surgical Site Infection, Wound Complications, Cesarean Antibiotic Prophylaxis, Cesarean Skin Antisepsis, Closure of Subcutaneous Tissue, Skin Closure
Introduction
Cesarean delivery is the most common major surgical procedure performed in the United States, with over 1.2 million performed per year1. Postoperative surgical site infections and wound complications are the most common and costly complications following cesarean delivery and affect approximately 10% of these deliveries.2 These complications represent a significant burden to the patient and contribute to rising healthcare costs.3 Thus, there is an urgent need to identify ways to reduce wound complications.
Multiple risk factors for post cesarean infectious complications have been identified, some of which are modifiable and others that are not. Patient level risk factors which may be modifiable include obesity, previous cesarean delivery, hypertensive disorders of pregnancy, and tobacco use.4, 5 Pregnancy-related risk factors which are generally not modifiable include emergency or unscheduled cesarean delivery, presence of labor or rupture of membranes, and chorioamnionitis.5 Finally, surgical risk factors which are modifiable include operating time, surgeon experience, inappropriate timing or choice of antibiotic therapy, and operating room temperature.6–8
Several evidence-based measures have been shown to reduce the risk of post-cesarean wound infections.9 The administration of antibiotics within 60 minutes prior to skin incision decreased endometritis and post-cesarean infectious morbidities with no evidence of increase in neonatal sepsis work up in several studies and subsequent meta-analyses.10–13 Additionally, studies in both general surgery and obstetric patients have demonstrated that chlorhexidine-alcohol is superior to povidone-iodine based solutions for skin antisepsis and that the use of chlorhexidine-alcohol decreases the rate of surgical site infections and office visits for wound complications.14, 15 Suture closure of the subcutaneous layer at the time of cesarean delivery has been shown to reduce wound disruption in women with greater than two centimeters of subcutaneous tissue.16 Finally, several studies have examined the role of skin closure with suture compared with staples, with the most recent meta-analyses concluding that skin closure with suture decreases wound morbidity following cesarean. 17, 18
A limited number of studies have examined the impact of a combination of evidence-based measures to reduce post cesarean infectious complications. These studies have generally evaluated the implementation of a group of evidence-based bundles, and have shown that bundles may decrease surgical site infections and wound complications. 19–21 However, these studies are conducted in various size hospital settings with varying infection control policies prior to the studies. The heterogeneity in types of hospitals and types of preexisting infection control policies reduce the generalizability of the results. Further, these studies used bundles of surgical and educational interventions and measured improvement by comparing hospital rates of post cesarean complications before and after the implementation of the bundles. This makes it difficult to know the impact of the surgical steps used in these bundles. Additionally, no studies have evaluated residual risk factors for post cesarean infectious complications after evidence-based bundles have been implemented. These residual risk factors are important for targeting future interventions to reduce post cesarean complications.
We sought to estimate the impact of a group of evidence-based surgical measures on wound complications after cesarean delivery. We chose four evidence-based measures to evaluate as a bundle: appropriate antibiotic timing, chlorhexidine skin antisepsis, closure of the subcutaneous tissue if subcutaneous depth ≥ 2 cm, and subcuticular closure with suture. We also aimed to estimate residual risk factors for wound complications among women who received all of the evidence-based measures.
Materials and Methods
We conducted a secondary analysis of data from a randomized controlled trial in which women undergoing cesareans were randomly assigned to preoperative skin antisepsis with either chlorhexidine alcohol or iodine alcohol prior to cesarean. (Clinicaltrials.gov NCT01472549).15 The study was conducted with approval from the Washington University School of Medicine Human Research Protection Office. Pregnant women undergoing scheduled and nonscheduled cesarean delivery from September 2011 through June 2015 were eligible. Women with known allergy to chlorhexidine, alcohol, iodine, or shellfish or who had a skin infection near the operative site were excluded. Patients without follow-up after discharge were excluded from this analysis.
Institutional protocol recommends standard infection prevention measures including preoperative antibiotics, perioperative normothermia, clipping rather than shaving hair for removal, as well as appropriate aseptic technique. Additionally, our protocol recommends women receive 2 grams of cefazolin preoperatively for maternal weight < 120 kg and 3 grams for maternal weight > or = 120 kg. Patients with penicillin or cephalosporin allergy received 2 mg/kg gentamicin and 900 mg clindamycin. Choice of skin antisepsis was based on randomization. Subcutaneous tissue closure and subcuticular skin closure was at the discretion of the surgeon.
Patients were followed daily during their initial hospitalization and generally had a two week postoperative visit as well as a 4–6 week routine postpartum visit. They were contacted 30 days after delivery to assess symptoms of surgical site infection and wound complication and to ask about use of medical services for wound complications. Additionally, medical records were obtained from physician offices, emergency room visits, and hospital admissions. Medical records were reviewed by the principal investigator, who was blinded to the study arm, to assess for any diagnosis of surgical site infection or other wound complications. Demographic information, obstetric and medical history, and detailed description of the surgical procedure was collected by patient interview and supplemented with abstraction from patient charts. 15
The primary outcome for this analysis was a composite of wound complications that included surgical site infection, cellulitis, seroma, hematoma, and separation occurring within 30 days of cesarean. Surgical site infection included superficial, deep and organ-space surgical site infection based on the Centers for Disease Control and Prevention National Healthcare Safety Network definitions.22 Endometritis was considered the organ-space infection after cesarean. The diagnosis of the components of the composite were made by the treating physician and verified by chart review by the principal investigator of the original trial. Secondary outcomes for this analysis included individual components of the composite. Please see Appendix 1 for further definitions of study outcomes.
We compared women who received all four evidence-based measures (prophylactic antibiotics within 60 minutes of cesarean and prior to skin incision, chlorhexidine-alcohol for skin antisepsis with three minutes of drying time prior to incision, closure of subcutaneous layer if ≥ 2cm of depth and subcuticular skin closure with suture) to those who did not. Baseline characteristics were compared between the two groups using the unpaired Student t test or Wilcoxon rank-sum test as appropriate for continuous variables, and the χ2 test or Fisher’s exact test as appropriate for categorical variables. We then calculated rates and unadjusted relative risk (RR) and 95% confidence intervals (CI) for the primary and secondary outcomes. Multivariable logistic regression was used to estimate the impact of evidence-based measures on outcomes. The final models adjusted for tobacco use, chorioamnionitis, and unscheduled cesarean delivery. Goodness-of-fit of the final models was tested using the Hosmer-Lemeshow test. The method proposed by Zhang et al was then used to estimate adjusted relative risks.23 We included all patients from the primary study in this analysis, and no a priori sample size estimation was performed.
We then conducted further analysis limited to women who received all four components of the evidence-based measures. Among these women, we performed multivariable logistic regression to estimate residual risk factors for the primary outcome composite outcome. The final model included unscheduled cesarean, obesity (BMI ≥30 kg/m2), tobacco use, diabetes (pregestational and gestational), chorioamnionitis, inexperienced surgeon (less than post graduate year 3), and vertical skin incision. The analysis was then repeated to estimate residual risk factors for the secondary outcome of surgical site infection.
All tests were two-tailed with a p<0.05 considered significant. Analyses were conducted using a software package (STATA, Version 12, Special Edition; Stata Corp, College Station, TX).
Results
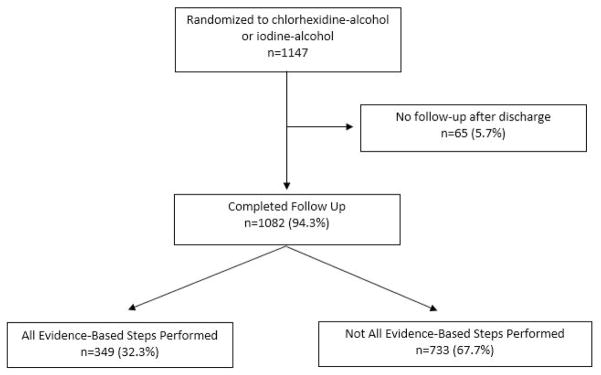
There were 1147 patients randomized in the primary trial, and 1082 (94.3%) had follow-up after discharge. Of the 1082 patients with follow-up, 349 (32.2%) had all four evidence-based measures and 733 (67.7%) did not (Figure 1). Of the four evidence based steps evaluated, 1076 (99.5%) had antibiotics given appropriately prior to skin incision, 538 (49.7%) received chlorhexidine alcohol skin antisepsis, 854 (78.9%) had appropriate subcutaneous closure with suture closure if subcutaneous depth was ≥2 cm, and 879 (81.2%) had skin closed with suture (Table 1).
Figure 1.
Flow Diagram of Study Participants
Table 1.
Evidence Based Measures Evaluated
| Evidence Based Measure (N=1082) | Patients Receiving Evidence Based Measure |
|---|---|
| All evidence based steps performed | 349 (32.3%) |
| Antibiotics given within 60 minutes and prior to skin incision | 1076 (99.5%) |
| Chlorhexidine alcohol skin antisepsis applied and allowed to dry appropriately | 521 (48.2%) |
| Appropriate subcutaneous closure if depth ≥ 2 cm | 854 (78.9%) |
| Subcuticular skin closure with Suture | 879 (81.2%) |
Baseline demographic and pregnancy characteristics were similar between women who did and did not have all four evidence-based measures performed, except that women who did not have all four measures applied were more likely to smoke and more likely to have chorioamnionitis (Table 2). They also had slightly higher blood loss, with no difference in postoperative transfusion rates between groups. Other surgical characteristics, including duration of surgery, depth of subcutaneous tissue, and unscheduled cesarean rates were similar between groups.
Table 2.
Baseline Characteristics of Study Participants
| Type of Complication N=1082 |
All evidence-based measures1 applied n=349 (32.3%) | Not all evidence-based measures applied n=733 (67.7%) | p |
|---|---|---|---|
| Maternal age, y | 28.0 ± 5.7 | 28.6 ± 5.8 | 0.16 |
| Gestational age at delivery, wk | 37.4 ± 2.8 | 37.3 ± 3.1 | 0.63 |
| Race | |||
| African American | 192 (55.0%) | 400 (54.6%) | 0.57 |
| Caucasian | 139 (39.8%) | 302 (41.2%) | |
| Other | 18 (5.2%) | 31 (4.2%) | |
| BMI kg/m2 | 34.1 ± 8.3 | 34.7 ± 9.9 | 0.28 |
| Obese (BMI>30 kg/m2) | 234 (67.0%) | 511 (69.7%) | 0.38 |
| Public Insurance | 218 (62.5%) | 451 (61.5%) | 0.77 |
| Current tobacco use | 44 (12.6%) | 132 (18.0%) | 0.02 |
| Diabetes mellitus | 39 (11.2%) | 78 (10.6%) | 0.79 |
| Chronic hypertension | 36 (10.3%) | 75 (10.2%) | 0.97 |
| Pregnancy induced hypertension | 40 (11.5%) | 102 (13.9%) | 0.26 |
| Primiparous | 90 (25.8%) | 185 (25.2%) | 0.85 |
| Chorioamnionitis | 6 (1.7%) | 34 (4.6%) | 0.02 |
| Unscheduled Cesarean | 131 (37.5%) | 311 (42.4%) | 0.13 |
| Primary Cesarean | 206 (59.0%) | 434 (59.2%) | 0.95 |
| Duration of surgery, min | 56 (44, 69.5) | 55 (42,70) | 0.91 |
| Depth of subcutaneous layer, cm | 2.11 ± 1.32 | 2.20 ± 1.15 | 0.21 |
| Estimated blood loss, mL | 821.8 ± 237.3 | 868.9 ± 266.6 | 0.01 |
| Postoperative blood transfusion | 5 (1.4%) | 18 (2.3%) | 0.33 |
Women who received all four evidence-based steps were less likely to have the primary composite outcome of wound complications after cesarean (20.3% vs 28.1%; aRR 0.75, 95% CI 0.58, 0.95) after controlling for chorioamnionitis, smoking, and unscheduled cesarean delivery (Table 3). This was largely driven by a reduction in surgical site infection, which was significantly less common in those receiving all evidence-based measures (3.7% vs 9.3%, aRR 0.43, 95% CI 0.24, 0.76). Rates of wound cellulitis, hematoma, seroma, or wound separation were not different between groups. Wound separation was the most common wound complication encountered in both groups.
Table 3.
Impact of evidence-based interventions on wound complications after cesarean
| Type of Complication N=1082 |
All evidence-based measures1 applied n=349 (32.3%) | Not all evidence-based measures applied n=733 (67.7%) | aRR (95% CI)2 |
|---|---|---|---|
| Any Wound Complication n=277 (25.6%) | 71 (20.3%) | 206 (28.1%) | 0.75 (0.58, 0.95) |
| Surgical Site Infection n=81 (7.5 %) | 13 (3.7%) | 68 (9.3%) | 0.43 (0.24, 0.76) |
| Cellulitis n=15 (1.4%) | 2 (0.5%) | 13 (1.8%) | 0.34 (0.08, 1.51)3 |
| Hematoma n=12 (1.1%) | 6 (1.6%) | 6 (0.8%) | 2.33 (0.74, 7.01)3 |
| Seroma n=52 (4.8%) | 14 (4.0%) | 38 (5.2%) | 0.79 (0.43, 1.43) |
| Wound Separation n=131 (12.1%) | 37 (10.6%) | 94 (12.8%) | 0.85 (0.58, 1.20) |
Evidence-base measures: chlorhexidine-alcohol for skin antisepsis, prophylactic antibiotics within 60 minutes of cesarean given at skin incision, closure of subcutaneous layer if ≥ 2cm of depth and subcuticular skin closure with suture
Adjusted for tobacco use, chorioamnionitis, and unscheduled cesarean
Unadjusted given small number of outcomes
When analysis was limited to the 349 women who received all the evidence-based measures, unscheduled cesarean delivery was the only significant residual risk factor for wound complications (27.5% vs. 16.1%, aRR 1.71, 95% CI 1.12, 2.47) (Table 4). Other traditional risk factors for wound complications, including obesity, smoking, diabetes, chorioamnionitis, surgical experience, and skin incision type were not significantly associated with wound complications in women who had received all evidence-based measures. Unscheduled cesarean delivery was also the only significant residual risk factor for surgical site infection (6.9% vs. 1.6%, RR 3.74, 95% CI 1.18, 11.92).
Table 4.
Residual risk factors for wound complications among those with all evidence based steps performed
| Residual Risk Factor N=349 |
Wound Complication n=71 (20.3%) | aRR1 (95% CI) |
|---|---|---|
| Unscheduled Cesarean n=131 | 36 (27.5%) | 1.71 (1.12, 2.47) |
| Scheduled Cesarean n=218 | 35 (16.1%) | |
| Obese2 n=233 | 48 (20.5%) | 1.03 (0.63, 1.63) |
| Non-obese n=116 | 23 (20.0%) | |
| Tobacco use n=44 | 13 (29.6%) | 1.46 (0.80, 2.42) |
| No Tobacco use n=305 | 58 (19.0%) | |
| Diabetes n=39 | 7 (18.0%) | 0.83 (0.37, 1.69) |
| No Diabetes n=310 | 64 (20.7%) | |
| Chorioamnionitis n=6 | 2 (33.3%) | 1.13 (0.23, 3.52) |
| No Chorioamnionitis n=343 | 69 (20.1%) | |
| Inexperienced Surgeon (<PGY 3)3 n=271 | 57 (21.0%) | 0.80 (0.43, 1.39) |
| Experienced Surgeon n=78 | 14 (18.0%) | |
| Vertical Skin Incision n=3 | 1 (33.3%) | 2.20 (0.28, 5.36) |
| Pfannenstiel Incision n=346 | 70 (20.2%) |
Adjusted for all other risk factors in this column.
Obese defined as BMI ≥30 kg/m2
PGY 3 =postgraduate year 3.
Comment
The use of a combination of four evidence-based surgical measures resulted in a 25% reduction in the risk of wound complications and a 57% reduction in the risk of surgical site infections in women undergoing cesarean delivery. The baseline rate of wound complications was high and the residual risk among women who received all evidence-based measures remained high. Unscheduled cesarean delivery was the only significant residual risk factor for both wound complications and surgical site infection.
Several previous studies have shown institutional improvement in post cesarean infectious outcomes when a bundle of evidence-based surgical and educational measures were implemented. A study by Rauk conducted at an academic medical center with 400 deliveries a year implemented a bundle that included preoperative preparation using chlorhexidine gluconate no-rinse cloths, added chlorhexidine alcohol for skin antisepsis, a comprehensive staff training and education program, and modified instrument sterilization techniques over 6 months. They found a decrease in the rate of surgical site infection by 85%, with sustained improvement for 6 months.21 A similar study conducted by Ng et al at an academic hospital performing 2500 deliveries a year used five years of phased implementation of education to optimize timing of antibiotics prophylaxis, decrease pre-hospital hair shaving, and implement hair clipping rather than shaving in the hospital. Additionally, the institution started using chlorhexidine alcohol for skin antisepsis. These changes reduced the rate of surgical site infection by 50%, with sustained improvement over the last two years of the study.19
Hsu and colleagues used phased implementation of a bundle of hospital infection control policies applied to employees and staff as well as surgical interventions. The surgical interventions included chlorhexidine for skin antisepsis, antibiotics administered before incision with increased dosages of antibiotics for obese patients as well as the addition of azithromycin to standard cephalosporin antibiotics, cord traction for removal of placenta, and closure of deep subcutaneous layer > 2 cm. The phased implementation of these interventions resulted in a 98% reduction in surgical site infections with a rate of 0.1% in the year following implementation of all phases.20
Our study built on these previous studies in important ways. It was conducted at an institution where standard infection-control policies are already in place, with steps such as hair clipping rather than shaving and perioperative normothermia already being performed. Our analysis focused on evidence-based surgical measures only, in order to target the question of whether a group of surgical measures improved outcomes. By using details about surgical steps collected in the original trial, we were able to evaluate the impact of evidence-based measures on women who received them, rather than using an observational design comparing rates before and after implementation of a bundle as had been done in previous studies. This allowed us to control for patient specific confounders in analysis. Unlike previous studies, we evaluated residual risk factors for post cesarean wound complications when women received all evidence-based measures.
This study is a secondary analysis of a randomized trial conducted at an academic institution with high surgical volume. Data regarding surgical steps and outcomes were collected prospectively. The use of active surveillance for postoperative complications through the medical record and with telephone calls insured a low rate of patients lost to followup. The original study had broad inclusion criteria, and the inclusion of both scheduled and unscheduled cesareans in the analysis makes the results generalizable.
There are potential limitations to the study that should be considered. Because this is a secondary analysis of a randomized trial, it is essentially a cohort study and subject to selection bias and unmeasured confounders. While it is reassuring that nearly all patient and pregnancy characteristics were similar between groups, women who did not receive all evidence-based measures were more likely to smoke and to have chorioamnionitis, which are known risk factors for our primary outcome. We adjusted for these confounders in our analysis. Additionally, while the use of chlorhexidine-alcohol was prescribed by the randomization in the original trial and the use of antibiotics is standard and occurred in 99.5% of patients, the remaining two measures, subcutaneous and subcuticular closure with suture, were based on the surgeon’s choice at the time of cesarean. We cannot control for the confounding by indication that may be associated with surgical choices and an increase in wound complications. In addition, the sample size for this analysis was fixed and may have been underpowered to evaluate each evidence-based measure alone, individual components of the composite outcome, and the residual risk factors. Finally, the high baseline rate of wound complications in this high-risk cohort means our findings may not be applicable to clinical settings with lower risk patients.
In conclusion, the use of a combination of evidence-based surgical measures significantly reduced post-cesarean wound complications and surgical site infections. However, even when women received all-evidence based surgical steps, the risk of complications remained high and was unexplained by most traditional risk factors. These findings highlight the need for additional innovative interventions to reduce post cesarean infectious morbidity, especially in patients undergoing unscheduled cesareans, who remain at risk for wound complications even after receiving current evidence-based measures.
Acknowledgments
This work was supported by a Women’s Reproductive Health Research Career Development grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (1K12HD063086-01, to Drs. Tuuli and Macones). Dr. Temming is supported by a NIH T32 training grant (5T32HD055172-07). This publication was also made possible by Grant Number UL1 TR000448 from the NIH National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Appendix 1: Outcome Definition
| Outcome1 | Definition |
|---|---|
| Surgical Site Infection (SSI:) | Centers for Disease Control and Prevention National Healthcare Safety Network Definition: 22 Infection occurs within 30 days after operative procedure AND |
| Superficial SSI | Involves only skin and subcutaneous tissue of the incision; AND patient has at least one of the following:
|
| Deep Incisional SSI | Involves deep soft tissues of the incision (eg, fascial and muscle layers; AND patient has at least one of the following:
|
| Organ/Space SSI | The infection appears to be related to the operation and the infection involves any part of the anatomy (organs or spaces), other than the incision, which was opened or manipulated during an operation and at least one of the following :
|
| Cellulitis | Redness or induration around the cesarean incision, diagnosed and treated as cellulitis by the surgeon, attending physician, or outpatient physician |
| Seroma | Collection of serous fluid at the cesarean incision, diagnosed and treated by the surgeon, attending physician, or outpatient physician |
| Hematoma | Collection of bloody fluid at the cesarean incision, diagnosed and treated by the surgeon, attending physician, or outpatient physician |
| Wound Separation | Any separation of the wound necessitating intervention, diagnosed by the surgeon, attending physician, or outpatient physician |
All outcomes were validated by the principal investigator in the original trial who was blinded to study arm of participants.
Footnotes
The authors report no conflict of interest.
This paper was presented in part as poster #158 at the 37th annual meeting of the Society for Maternal and Fetal Medicine, Las Vegas, NV, January 23–27, 2017.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Hamilton BE, Osterman MJ. Births in the United States, 2015. NCHS data brief. 2016:1–8. [PubMed] [Google Scholar]
- 2.Conner SN, Verticchio JC, Tuuli MG, Odibo AO, Macones GA, Cahill AG. Maternal obesity and risk of postcesarean wound complications. American journal of perinatology. 2014;31:299–304. doi: 10.1055/s-0033-1348402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infection control and hospital epidemiology. 2010;31:276–82. doi: 10.1086/650755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Reviews in obstetrics & gynecology. 2012;5:69–77. [PMC free article] [PubMed] [Google Scholar]
- 5.Krieger Y, Walfisch A, Sheiner E. Surgical site infection following cesarean deliveries: trends and risk factors. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2017;30:8–12. doi: 10.3109/14767058.2016.1163540. [DOI] [PubMed] [Google Scholar]
- 6.Olsen MA, Butler AM, Willers DM, Devkota P, Gross GA, Fraser VJ. Risk factors for surgical site infection after low transverse cesarean section. Infection control and hospital epidemiology. 2008;29:477–84. doi: 10.1086/587810. discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 7.Menderes G, Athar Ali N, Aagaard K, Sangi-Haghpeykar H. Chlorhexidine-alcohol compared with povidone-iodine for surgical-site antisepsis in cesarean deliveries. Obstetrics and gynecology. 2012;120:1037–44. doi: 10.1097/aog.0b013e31826f3bd9. [DOI] [PubMed] [Google Scholar]
- 8.ACOG Practice Bulletin No. 120: Use of prophylactic antibiotics in labor and delivery. Obstetrics and gynecology. 2011;117:1472–83. doi: 10.1097/AOG.0b013e3182238c31. [DOI] [PubMed] [Google Scholar]
- 9.Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: an updated systematic review. American journal of obstetrics and gynecology. 2013;209:294–306. doi: 10.1016/j.ajog.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. American journal of obstetrics and gynecology. 2007;196:455, e1–5. doi: 10.1016/j.ajog.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Baaqeel H, Baaqeel R. Timing of administration of prophylactic antibiotics for caesarean section: a systematic review and meta-analysis. BJOG: an international journal of obstetrics and gynaecology. 2013;120:661–9. doi: 10.1111/1471-0528.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. American journal of obstetrics and gynecology. 2008;199:301, e1–6. doi: 10.1016/j.ajog.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 13.Tita AT, Szychowski JM, Boggess K, et al. Adjunctive Azithromycin Prophylaxis for Cesarean Delivery. The New England journal of medicine. 2016;375:1231–41. doi: 10.1056/NEJMoa1602044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darouiche RO, Wall MJ, Jr, Itani KM, et al. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. The New England journal of medicine. 2010;362:18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 15.Tuuli MG, Liu J, Stout MJ, et al. A Randomized Trial Comparing Skin Antiseptic Agents at Cesarean Delivery. The New England journal of medicine. 2016;374:647–55. doi: 10.1056/NEJMoa1511048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstetrics and gynecology. 2004;103:974–80. doi: 10.1097/01.AOG.0000124807.76451.47. [DOI] [PubMed] [Google Scholar]
- 17.Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. American journal of obstetrics and gynecology. 2015;212:621, e1–10. doi: 10.1016/j.ajog.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, Macones GA, Odibo AO. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstetrics and gynecology. 2011;117:682–90. doi: 10.1097/AOG.0b013e31820ad61e. [DOI] [PubMed] [Google Scholar]
- 19.Ng W, Brown A, Alexander D, et al. A multifaceted prevention program to reduce infection after cesarean section: Interventions assessed using an intensive postdischarge surveillance system. American journal of infection control. 2015;43:805–9. doi: 10.1016/j.ajic.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CD, Cohn I, Caban R. Reduction and sustainability of cesarean section surgical site infection: An evidence-based, innovative, and multidisciplinary quality improvement intervention bundle program. American journal of infection control. 2016;44:1315–20. doi: 10.1016/j.ajic.2016.04.217. [DOI] [PubMed] [Google Scholar]
- 21.Rauk PN. Educational intervention, revised instrument sterilization methods, and comprehensive preoperative skin preparation protocol reduce cesarean section surgical site infections. American journal of infection control. 2010;38:319–23. doi: 10.1016/j.ajic.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infection control and hospital epidemiology. 1999;20:250–78. doi: 10.1086/501620. quiz 79–80. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]