Abstract
Attempts to characterize the neural differences between individuals with and without dyslexia generally point to reduced activation in and connectivity between brain areas in a reading network composed of the inferior frontal gyrus, the ventral occipito-temporal cortex, and the dorsal temporo-parietal circuit. However, developmental work on brain activity during reading has indicated that some brain areas show developmental decreases in activation with age. Thus, reading network connectivity may also show decreases that are positively associated with increases in reading ability. However, the developmental trajectory of reading network connectivity in typically developing readers is not yet well established. In the current study, we use a longitudinal design to determine how connectivity changes over time, and how these changes relate to changes in reading skill. We find that longitudinal increases in reading ability are associated with higher initial connectivity in the dorsal stream between fusiform and inferior parietal cortex, implicated in phonological decoding, followed by decreases in connectivity in this stream over time. We further find that increases in reading ability are supported by maintenance of connectivity in the ventral stream between inferior occipital and fusiform cortex, suggesting a more mature automatic orthographic recognition strategy. Readers who show little reading improvement over time do not attain high levels of connectivity in the dorsal stream at any time point, and their ventral stream connectivity decreases over time. These results together suggest that superior reading ability is initially supported by phonological decoding, with a decreased reliance on this strategy as reading becomes more automated. Our results indicate that development of the dorsal and ventral streams are closely linked, and support the hypothesis that a decrease in the dorsal stream is important for ventral stream development.
Keywords: reading network connectivity, reading development, dorsal stream, ventral stream, functional magnetic resonance imaging, structural equation modeling
Introduction
The process of reading is complex, relying on a number of brain regions that each play a different role in accessing linguistic information from written forms. While most people are able to successfully coordinate these brain regions to achieve fluid reading, about 5–10% of individuals are diagnosed with dyslexia, a disability in which a person has difficulty achieving fluid reading despite adequate instruction, motivation, and intelligence (Siegel, 2006). The extant neuroimaging literature examining differences between individuals with and without dyslexia has established that individuals with dyslexia tend to show reduced activation in key brain regions in the reading network (e.g. inferior frontal, temporo-parietal, and occipito-temporal cortex) compared to their typically reading peers (Pugh et al., 2001; Richlan, Kronbichler, & Wimmer, 2009, 2011; Shaywitz et al., 2002). Moreover, research using functional connectivity methods has also found that individuals with dyslexia differ in how well these regions work in tandem with one another (Cao, Bitan, & Booth, 2008; Finn et al., 2014; Horwitz, Rumsey, & Donohue, 1998; Koyama et al., 2013; Morken, Helland, Hugdahl, & Specht, 2017; Quaglino et al., 2008; van der Mark et al., 2011). Together, research suggests dyslexia involves more than an underactivation of key brain regions in typical left hemisphere reading networks, but also reduced functional connectivity between them.
The typical development of the reading network is not currently well described. This can make the interpretation of differences in connectivity between children with and without dyslexia difficult. For example, neuroimaging studies examining how activation in individual brain regions varies with age and skill have shown that decreases in activation over time can be a marker of better reading ability, depending on the brain region (Church, Coalson, Lugar, Petersen, & Schlaggar, 2008; McNorgan, Alvarez, Bhullar, Gayda, & Booth, 2011; Richlan et al., 2011). Thus, decreases in connectivity could, in some circumstances, be a marker of better reading, even in the left hemisphere. Specifically, one influential model of reading development proposes that there is a developmental shift from relying on dorsal stream processing, implicated in sound-symbol matching, to ventral stream processing, implicated in automatic recognition of word forms, that occurs as readers progress in age and skill (Pugh et al., 2001).
The dorsal, temporo-parietal circuit, including the left posterior superior temporal gyrus (L STG) and inferior parietal lobe (L IPL), is primarily involved in phonological processing and integrating visual (orthographic) and auditory (phonological) information, known as phonological decoding (Pugh et al., 2001). The dorsal route tends to be used for reading low frequency words and pronounceable pseudowords (Coltheart, 2006; Jobard, Crivello, & Tzourio-Mazoyer, 2003). The ventral, occipito-temporal circuit, including the fusiform gyrus (L FG) and inferior occipital gyrus (L IOG), is proposed to be critical for the fast, automatic processing of visual word forms (Pugh et al., 2001). In adults, a portion of the left ventral occipito-temporal cortex (L VOT), the putative visual word form area (Cohen et al., 2000; McCandliss, Cohen, & Dehaene, 2003), is thought to host neurons tuned to preferentially respond to real written words over pseudowords or consonant strings (Glezer, Jiang, & Riesenhuber, 2009). The ventral pathway tends to be used for words that are frequent or exception words whose pronunciation does not follow typical orthography to phonology mapping rules (Coltheart, 2006; Jobard et al., 2003). Thus, while both the dorsal and ventral streams are thought to be used throughout the lifespan depending on the type of word (i.e. exception versus unfamiliar words), the dorsal-to-ventral shift hypothesis proposes that children rely more on dorsal stream processing for all word types before shifting to reliance on the ventral stream for familiar words. This hypothesis has some support from cross-sectional studies of children and adults that have examined brain activation within these circuits (Richlan et al., 2011; Shaywitz et al., 2004; Simos et al., 2007). However, this model has not yet been directly tested with longitudinal data with either brain activation or functional connectivity analyses.
Research in both children and adults has indicated that functional connectivity within both ventral and dorsal reading circuits is related to reading skill, with more skilled readers showing greater connectivity compared to less skilled readers (Cao et al., 2008; Hampson et al., 2006; Horwitz et al., 1998; Levy et al., 2009; Quaglino et al., 2008; Shaywitz et al., 2004; van der Mark et al., 2011). Additional studies with adults have indicated that reading strategy may influence how the reading network is used (Kherif, Josse, Seghier, & Price, 2009; Seghier, Lee, Schofield, Ellis, & Price, 2008). In line with this idea, Levy et al (2009) found reading skill is related to using the ‘correct’ pathway for the type of word being read. That is, those readers who showed both higher connectivity between regions in the dorsal route during pseudoword reading and higher connectivity between regions in the ventral route during real word reading had better overall reading scores. Further, the same study demonstrated that individual differences in reading skill of either real or pseudowords were also related to individual differences in pathway connectivity. Adults who showed high dorsal route connectivity during real word reading showed relatively poor real word reading skill, presumably due to reliance on an incongruent pathway. The results of this study indicate that reading strategy (i.e., reliance on dorsal or ventral stream processing) is related to reading network connectivity and reading skill.
While a relationship between reading skill and reading network functional connectivity has been established in adults, the development of functional connectivity in left hemisphere reading circuits needs to be further understood, particularly for the English language. One study has examined changes in reading network connectivity longitudinally in Norwegian children with and without dyslexia (Morken et al., 2017). In this study, typically developing children were found to show primarily decreasing or stabilizing connectivity over time, while children with dyslexia showed aberrant patterns of development that may have reflected overcompensation or normalization relative to their baseline connectivity levels. However, the study by Morken et al (2017) does not examine skill within typically developing children, and does not examine the relationship between changes in connectivity to changes in reading skill. Further, Norwegian is a semi-transparent language, while English is opaque. That is, Norwegian orthographic to phonological mappings are fairly consistent and can be used to correctly pronounce unfamiliar words while mappings in English are not as consistent. The difference in consistency may result in differences in reading network use throughout development (Cao, Brennan, & Booth, 2015; Levy et al., 2009; Seghier et al., 2008).
While the extant research with children so far has indicated that the same positive relationship between current connectivity and current skill may hold true for English-speaking children (Cao et al., 2008; Quaglino et al., 2008; van der Mark et al., 2011), the dorsal-to-ventral shift hypothesis predicts that decreases in dorsal areas of the reading network are necessary for improvement of reading skill in children. This idea is supported by the general decreases in connectivity found in typical children in the Morken et al 2017 study. The current study builds on previous literature by taking a unique approach to examine the longitudinal changes of the reading network in typically developing children. Rather than understanding how current connectivity relates to current reading skill, we examine how changes in reading skill are related to changes in reading network connectivity. In line with the dorsal-to-ventral shift model of reading development, we expect increases in reading skill to be related to decreases in connectivity of the dorsal stream and reliance on phonological decoding, together with increases in connectivity in the ventral stream and reliance on orthographic recognition. Using data from children followed longitudinally, this study will investigate where in the reading network high connectivity and low connectivity is associated with better reading skill. This knowledge will be important in establishing a better benchmark that can be used to compare and understand children who struggle with reading.
Methods
This study was carried out in accordance with the recommendations of the Northwestern University Institutional Review Board with written informed consent from all the legal guardians of all participants and written assent of all participants. All participants and their guardians gave written informed consent or assent in accordance with the Declaration of Helsinki.
Participants
Data from a group of 59 healthy children (8 to 14 years-old at T1, 29 females) were selected from a group of 125 children that participated in a longitudinal study using inclusion criteria described below. All children were right handed, native and majority English speakers with normal or corrected to normal hearing and vision. Each child had no history of neurological or psychiatric disorders; 19 participants (32%) had a family history of learning problems as reported by parents. Participants were selected for the current analysis if they were of at least average intelligence and reading ability (see below). Further, all participants had acceptable quality MRI data at both time points (see below) and at least 40% accuracy on all conditions of the in-scanner experimental tasks.
Procedure
Study procedures took place over the course of several visits. During the first visit, participants completed a battery of standardized tests. In the second visit, participants completed a ‘mock scanning’ session in which they were familiarized with the scanner, trained to minimize head movement, and practiced the experimental task. The practice version of the experimental task had the same number of trials and timing as in the MRI session, but used different and comparable stimuli. Participants completed the T1 MRI session within one week of the mock scanning session. Approximately two to three years after the first session (range: 2.0 – 3.2 years, mean = 2.4 years, see Table 1), participants were invited back to complete a second standardized testing and T2 MRI session.
Table 1.
Mean (SD) parameter estimates for each pathway type included in the individual structural equation model for each participant at each time and mean percentage of participants for whom the paths were significant. Aside from auto-regressive terms, contemporaneous paths accounted for the most variance in the model as indicated by higher parameter estimates compared to other pathway types. Additionally, contemporaneous pathways were the path type most likely to be significant in individual participant models, aside from auto-regressive terms.
| Time 1 | Time 2 | |||
|---|---|---|---|---|
|
| ||||
| Pathway Type | Parameter Estimate | Percent Significant |
Parameter Estimate | Percent Significant |
|
|
||||
| Auto-regressive | 0.39 (0.18) | 92.8 | 0.40 (0.17) | 94.5 |
|
|
||||
| Contemporaneous | 0.25 (0.17) | 77.6 | 0.20 (0.19) | 73.2 |
|
|
||||
| Lagged | −0.03 (0.13) | 29.7 | −0.04 (0.11) | 26.7 |
|
|
||||
| Bilinear Interaction | −0.01 (0.08) | 7.1 | −0.01 (0.12) | 13.9 |
|
|
||||
| Input | 0.08 (0.11) | 13.6 | 0.07 (0.11) | 12.7 |
|
|
||||
| Lagged Input | −0.01 (0.10) | 27.5 | 0.00 (0.10) | 23.1 |
Standardized Tests
Participants completed a battery of standardized tests at both T1 and T2 to assess general intelligence and reading ability. Intelligence was measured by the full-scale IQ index from the full-scale Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), which includes both verbal and non-verbal components. All children had at least average intelligence (>85 standard score), per inclusionary criteria. Reading ability was assessed by the Phonemic Decoding Efficiency (PDE) and Sight Word Efficiency (SWE) subscales of the Test of Word Reading Efficiency (TOWRE; Torgeson, 1999). The TOWRE requires participants to read as many pseudo-words (PDE) or words (SWE) as possible in 45 seconds. All participants had at least average reading ability as measured by both the PDE and SWE subscales at T1 (>85 standard score), per inclusionary criteria.
Participants were categorized as either high or low reading skill change based on performance on the PDE subtest of the TOWRE. We chose this measure as it most closely captures mastery of the relationships between orthographic and phonological information in English (McNorgan et al., 2011). Additionally, while pseudoword and real word reading are highly correlated, pseudoword reading is not highly related to vocabulary (Ouellette & P., 2006; Ricketts, Nation, & Bishop, 2007). Reading skill change scores were determined by subtracting T1 from T2 raw (unstandardized) scores. To ensure improvement was not simply due to lower or higher initial performance, we calculated the residual variance in improvement on raw scores after T1 performance was accounted for. Participants were categorized as high or low improvement based on the median-split of the residual variance in performance change.
In-scanner Task
During the MRI sessions, participants completed a rhyming task as part of a series of tasks assessing reading or math. Participants were presented with a series of visual word pairs and were asked to indicate whether the words rhymed or not. Participants were asked to respond as quickly and as accurately as possible on a handheld keypad. Each word was presented for 800 ms separated by a 200 ms interstimulus interval. Participants could respond as soon as the second word was presented and up to the beginning of the next trial. A jittered response interval duration of between 2200 and 2800 ms was used to facilitate deconvolution of the signal associated with each trial. After 2200 ms, a red cross appeared signaling them to respond if they had not yet done so. The task took approximately 7 minutes to complete.
Word pairs were designed to manipulate orthographic and phonological similarity such that participants could not rely on spelling alone to accurately complete the task. There were two orthographically similar conditions in which word pairs had overlapping rimes with rhyming (e.g., cage-rage) and non-rhyming (e.g., smart-wart) pairs, and two orthographically dissimilar conditions with non-overlapping rimes with rhyming (e.g., grade-paid) and non-rhyming (e.g., trial-fall) pairs. Each condition had a total of 12 trials for a total of 48 reading trials. All words were monosyllabic, having neither homophones nor homographs and were matched across conditions for written word frequency in children (Zeno 1995) and the sum of their written bigram frequency (English Lexicon Project).
Within the rhyming task, participants completed 24 fixation baseline trials in which participants were instructed to press a button when the fixation cross changed from red to blue. There were 12 additional perceptual trials in which participants saw two nonalphabetic glyphs presented in increasing, decreasing, or constant height and determined whether the sequences matched (e.g., both increasing in height) or not (e.g., one decreasing and one increasing in height). Timing for the fixation and perceptual trials were the same as for lexical trials.
fMRI Data Acquisition
Participants were positioned in the MRI scanner with their head secured using foam pads. An optical response box was placed in the participant’s right hand to log responses. Visual stimuli were projected onto a screen, which participants viewed via a mirror attached to the inside of the head coil. Participants wore sound attenuating headphones to minimize the effects of the ambient scanner noise. Images were acquired using a 3.0-Tesla Siemens Trio scanner. The blood oxygen-level-dependent (BOLD) signal was measured using a susceptibility weighted single-shot echo planar imaging (EPI) method. Functional images were interleaved from bottom to top in a whole-brain acquisition. The following parameters were used: TE = 20 ms, flip angle = 80 degrees, matrix size = 128×120, field of view= 220×206.25 mm, slice thickness = 3 mm (0.48mm gap), number of slices = 32, TR = 2000 ms. Before functional image acquisition, a high resolution T1-weighted 3D structural image was acquired for each subject (TR = 1570 ms, TE = 3.36 ms, matrix size = 256×256, field of view = 240 mm, slice thickness = 1 mm, number of slices = 160).
fMRI Preprocessing and Analysis
fMRI data were analyzed using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ac.uk/spm). Slice timing was applied to minimize timing errors between slices and re-alignment was performed. Images were smoothed using a 4×4×8 nonisotropic Gaussian kernel. ArtRepair software (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) was used to correct for participant movement. Images were realigned in ArtRepair, which identified and replaced outlier volumes, associated with excessive movement (>4 mm in any direction) or spikes in the global signal, using interpolated values from the 2 adjacent non-outlier scans. No more than 10% of the volumes from each run and no more than 4 consecutive volumes were interpolated in this way. This preprocessing step is used in place of using motion parameters in further statistical analysis. Functional images were co-registered with the anatomical image, and normalized to the Montreal Neurological Institute (MNI) ICBM152 T1 template, which is an average of 152 normal adult MRI scans. Normalization to an adult template allows for comparison to fMRI research with adults and does not negatively affect functional image data (Darcy Burgund et al., 2002). All four lexical conditions, the fixation condition, and the perceptual condition were modeled within the general linear model. First-level (within-subjects) analyses determined the response to words by contrasting all words to the fixation condition. One-sample t-statistic maps comparing lexical trials to fixation were generated for each subject at T1 and T2.
Connectivity Analysis
Reading network connectivity was determined using extended unified structural equation modeling (euSEM) on the time series for regions of interest (ROIs) in the reading network (Gates, Molenaar, Hillary, & Slobounov, 2011). euSEM is a data-driven method for determining contemporary and lagged co-activation between brain regions selected as nodes of a network at the individual level of analysis. Further, euSEM is designed for use with event-related designs as it additionally determines the effect of a stimulus on the co-activation between regions through bilinear interaction terms. euSEM thus determines the temporal correlations between brain regions, but does not imply a causal relationship or influence of activity in one brain region on activity in another.
ROI Selection
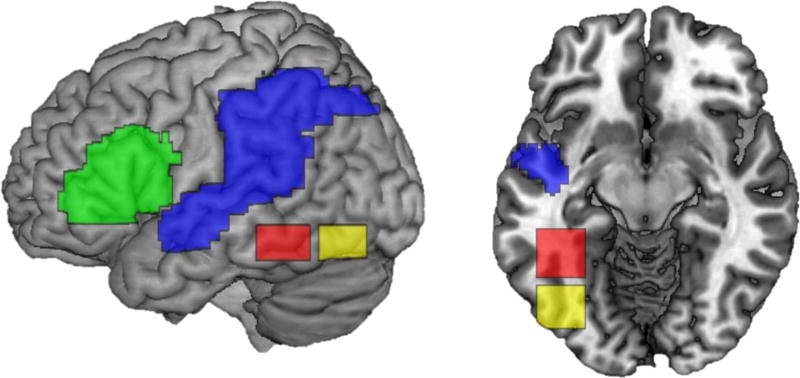
Based on previous literature, we chose to construct a model that included ROI’s implicated in four processing stages: phonological output computation, phonological decoding, orthographic processing, and visual processing (Levy et al., 2009). The location of each of these processes was first broadly determined using previous literature and then refined using data-driven methods, described below. Based on previous meta-analyses (Jobard et al., 2003; Taylor, Rastle, & Davis, 2012), the left inferior frontal gyrus (BA 44 and 45) is the area most likely to be involved in phonological output computation. Meta-analyses indicate a number of brain regions are potentially involved in phonological decoding. To ensure we did not bias selection of the phonological decoding node, we created a large ROI that consisted of inferior and superior parietal lobes, supramarginal gyrus, angular gyrus, and superior temporal gyrus. To select regions involved in orthographic processing, we used literature examining the posterior to anterior sensitivity gradient of the visual word form system to select an area sensitive to sub-lexical orthographic processing and an area sensitive to orthography at the word-level (Olulade, Flowers, Napoliello, & Eden, 2013; Taylor et al., 2012; van der Mark et al., 2009; Vinckier et al., 2007). Boxes were constructed using 20mm ranges in all three directions. Both ROIs used the same range of x and z coordinates (−30 to −50 and −10 to −20 respectively), but differed in their y coordinates; the middle portion ranged from −35 to −55 and the posterior portion ranged from −60 to −80 (see Figure 1).
Figure 1.
Search space for four regions of interest. Phonological output computing area is in green, phonological decoding in blue, word-level orthographic processing in red, and sublexical orthographic processing in yellow. Left shows a lateral view, right a ventral view at slice z = −10.
We then used data driven methods to reduce each of these large anatomically-based ROIs to spheres with a 6mm radius. Within each of these four ROIs, we conducted a one-sample t-test using the lexical trials compared to fixation contrast from all subjects and all times. Group level analysis within each of the four ROIs revealed a cluster significant at the p < 0.05 cluster-level false discovery rate (FDR) corrected threshold at the 0.005 voxel-level threshold. The peak voxel in the phonological output ROI was located within BA 45 or the triangularis portion of the inferior frontal gyrus (IFG) at −48, 11, 26. The peak of the phonological decoding cluster was located within the inferior parietal lobe (IPL) close to the supramarginal gyrus (SMG) at −30, −59, 50. The word-level orthographic processing cluster showed a peak in the left fusiform gyrus (FG) at −44, −51, −14, corresponding with the location of the putative visual word form area reported in previous studies (Olulade et al., 2013; van der Mark et al., 2009; Vinckier et al., 2007). The sublexical orthographic processing cluster peak was located in the inferior occipital gyrus (IOG) at −40, −73, −10, consistent with previous reports of locations more sensitive to false fonts compared to words (see Figure 2). The group peak for each ROI was used as the center for each 6mm sphere. These 6mm spheres were further constrained to only include voxels within the pre-defined regions to ensure the sphere did not extend into regions not included in the broadly defined anatomical ROIs.
Figure 2.
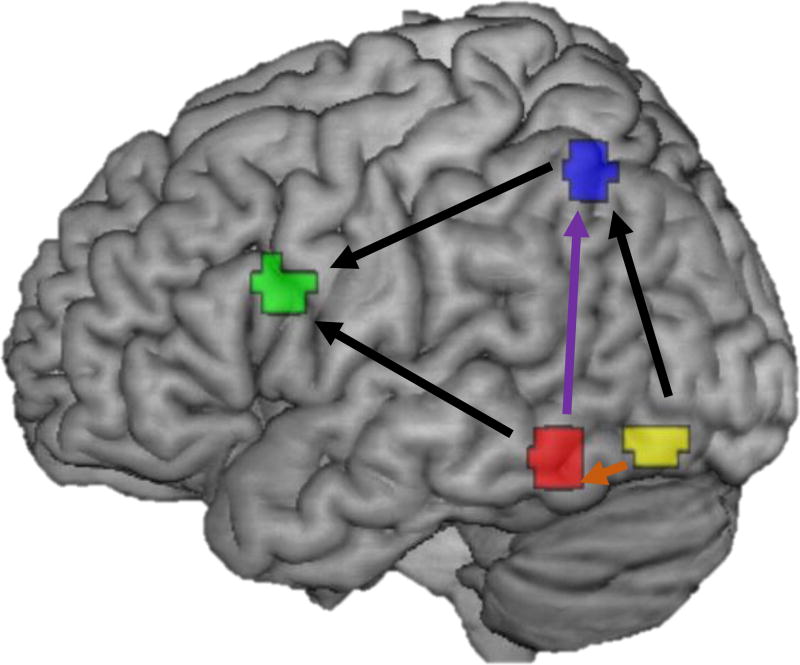
Illustration of the path model for contemporary connections between regions. Five forward feeding pathways were included. IOG (yellow) to both FG (red) and IPL (blue), FG to IPL, and FG and IPL to IFG (green). The dorsal pathway is highlighted in purple, the ventral in orange. Regions for each node reflect the 6mm sphere around the group peak found within the search space for each ROI, constrained to exclude voxels outside the search space.
Finally, within each of the 6mm spheres, we determined the peak voxel for the lexical trials minus fixation contrast for each participant at each time. This procedure allowed for individual variation across participants and time and ensured the number of informative voxels included in the analysis across participants was equated. The time series for these peak voxels were then extracted using the MarsBar tool within SPM (http://marsbar.sourceforge.net/) and used in the euSEM analysis.
euSEM Analysis
euSEM was conducted using a combination of Group Iterative Multiple Model Estimate (GIMME), a freely distributed MatLab based program (Gates & Molenaar, 2012) and traditional SEM pathway model construction. GIMME was used to center the time series for each subject individually at each of the two time points for each of 14 variables: four regions at time t, the lagged time series of each region at t+1, the time series of the direct effect of stimulus at time t, the time series of the lagged effect of stimulus at time t+1, and the bilinear interaction effect of stimulus on each concurrent pathway (the time series of each region at t times the input series at t). The model therefore examines the concurrent relationship between activation of brain regions (contemporary paths), a temporally causal relationship between brain regions (lagged path), the effect of stimulus on an individual brain region (direct effect of stimulus path), the lagged effect of stimulus, and the effect of stimulus on the relationship between activation in two brain regions (bilinear paths). The stimulus was convolved using a standard hemodynamic response function model to account for hemodynamic delay. The number of observations for each subject corresponds to the number of volumes minus one (to allow for time lagged observations). Each participant had 202 volumes collected and therefore 201 observations. Observations were considered to have a stimulus present if a volume took place during a word trial. Volumes collected during fixation and perceptual control trials were indicated as no stimulus.
Centered time series from each participant at each time were entered into a structural equation model using Mplus statistical program (Muthén & Muthén, 2012). The model was constructed in a confirmatory fashion based on previous literature showing the most parsimonious and best fitting model for reading is a feed-forward model that includes paths of IOG to IPL to IFG, from IOG to FG to IFG, and a path from FG to IPL (Levy et al., 2009; Richardson, Seghier, Leff, Thomas, & Price, 2011). This model thus had five unique pathways between each of the four ROIs (see Figure 2). We modeled the contemporary and lagged effects for each of these five pathways, the contemporary and lagged effect of stimulus on each ROI, the contemporary and lagged bilinear interaction between stimulus and each pathway, and the autoregressive term for each ROI. The focus of the analysis is on the paths between IOG and FG, most representative of orthographic processing in the ventral stream and the path between FG and IPL most representative of phonological decoding in the dorsal stream. However, including all of these theoretically possible paths in the model enables it to account for expected variance given our design. A model was estimated for each participant for each time.
Analysis of Person-Specific Connectivity Parameters
Finally, to determine how longitudinal changes in reading skill improvement relate to connectivity over time in the dorsal and ventral streams, unstandardized parameter estimates were extracted and entered into traditional ANOVA statistical analyses. We focused on contemporaneous pathways, as these paths were significant in a majority of the participants at each time (approximately 78% at T1 and 73% at T2) and accounted for the most variance in the model aside from auto-regressive effects. Table 1 provides mean parameter estimates for each path type and the percentage of participants for which each path type was significant. While all contemporaneous paths were not significant for all subjects, the focus of the current study is to determine the effect of individual differences and time on reading network connectivity as represented by parameter estimates generated by SEM analysis. Parameter estimates are generated regardless of significance and can thus be used in further analysis.
To determine whether there was an effect of time, skill change, or interaction of time and skill change on reading network connectivity and whether these effects differed depending on the path, a 2 (time: 1, 2) × 2 (skill change group: low, high) × 5 (path: IOG to FG, IOG to IPL, FG to IPL, FG to IFG, IPL to IFG) repeated measures ANOVA was conducted on unstandardized parameter estimates. We examined potential effects of time and skill change on all pathways so that the development of the reading network as a whole could be described. Planned follow-up tests included 2 (time: 1,2) × 2 (skill change: low, high) repeated measures ANOVAs for each path separately, and paired t-tests to establish whether a skill change group showed a significant effect of time for any pathway.
Finally, to compare the current findings with those of previous developmental studies examining the relationship between current reading network connectivity and current reading ability, we conducted correlations between raw scores on the standardized test of reading ability and unstandardized parameter estimates for the dorsal and ventral pathways. T1 parameter estimates were correlated with T1 reading skill and T2 parameter estimates with T2 reading skill. To determine whether connectivity could be used to predict reading skill change, we also correlated T1 connectivity with skill change for both the dorsal and ventral paths of interest.
Results
Behavioral Performance
Mean standardized scores and accuracy on the in-scanner task for each group at T1 and T2 are reported in Table 2. The average time between scans was 2.40 years, and improvement groups did not differ in time between scans (high: 2.35, low: 2.46; t (53) = 1.12, p = 0.27). A 2 (time: 1, 2) × 2 (skill change group: low, high) repeated measures ANOVA showed that there was no main effect of skill, and the skill groups did not differ on the reading skill metric at T1 (F (1, 57) = 3.02, p = 0.088). A significant skill change by time interaction indicates high improvers do show greater improvement on raw score performance (F (1, 57) = 36.94, p < 0.001). This finding is expected, given that participants were categorized on improvement, but not guaranteed, as participants were categorized based on residual improvement after accounting for T1 performance. The same repeated measures ANOVA conducted on the Sight Word Efficiency subtest of the TOWRE revealed that while all groups improved over time as indicated by a significant main effect of time, (F (1,57) = 80.17, p < 0.001), the high improvers made greater gains in raw scores (F (1,57) = 4.70, p = 0.034). Age was not significantly correlated with residual improvement scores (r (57) = −0.24, p = 0.71). However, while age is not expected to affect gains in raw score over time, age could affect the relationship between change in connectivity and change in skill. For example, skill change in older children could be related to different patterns of connectivity change as compared to younger children. We therefore included age as a covariate in subsequent analyses, yet age did not have a significant effect in any comparison. Subsequent results are thus reported without including age as a covariate.
Table 2.
Mean (SD) demographics of improvement group. Skill group was determined using the Phonemic Decoding subtest of the Test of Word Reading Efficiency. IQ was measured by the full-scale IQ index from the Wechsler’s Abbreviated Scale of Intelligence. Improvement groups start out equivalent at T1, but high improvers score significantly higher than their low improving counterparts at T2. Both groups performed well on the in-scanner rhyme judgment task at both times. Both groups improved similarly on task performance over time.
| Age at T1 |
IQ | Phonemic Decoding Efficiency Raw Score |
Sight Word Efficiency Raw Score | In-Scanner Task Accuracy |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| T1 | T2 | Change | T1 | T2 | Change | T1 | T2 | |||
|
|
||||||||||
| Low | 11.3 (1.4) | 116.6 (13.1) | 42.3 (10.9) | 41.3 (9.5) | −1.0 (4.9) | 75.6 (9.3) | 81.6 (8.9) | 6.0 (5.8) | 85.2 (0.1) | 90.5 (0.1) |
|
|
||||||||||
| High | 11.1 (1.7) | 116.7 (12.7) | 42.4 (10.5) | 49.5 (6.5) | 7.1 (5.3) | 76.9 (11.0) | 86.7 (8.9) | 9.8 (7.6) | 86.0 (0.1) | 91.0 (0.1) |
All children performed well on the in-scanner rhyme judgment task; average performance across all conditions for both skill change groups was approximately 85% at both times. Per inclusionary criteria, all participants attained accuracy of at least 40% on all conditions. Performance did not differ between skill change groups at either time, as revealed by a 2 (time: 1, 2) ×2 (skill change group: low, high) repeated measures ANOVA (F (1, 57) = 0.11, p = 0.745). A significant main effect of time shows that all participants regardless of skill change group improved on the rhyme judgment task over time (F (1, 57) = 30.71, p < 0.0001).
euSEM Analysis
We used Comparative Fit Index (CFI) and standardized root mean square residual (SRMR) to determine model fit. CFI compares fit of the target model to a null model in which it is assumed all variables are uncorrelated. CFI scores range between 0 and 1, with 1 indicating the best fit. SRMR is an absolute measure of fit that indicates the difference between the residuals of the observed and predicted correlations between variables with values ranging from 0 to 1, and 0 indicating a perfect fit on the target model. Traditionally, a CFI > 0.90 and SRMR < 0.08 is considered good model fit. CFI values between 0.80 and 0.90 and SRMR values between 0.08 and 0.10 are generally considered acceptable but suboptimal (Hooper, Coughlan, & Mullen, 2008). The mean CFI at T1 was 0.935 (range: 0.832 – 1.000) and 0.914 at T2 (range: 0.756 – 0.999). The mean SRMR value at T1 was 0.037 (range: 0.015 – 0.066) and 0.043 at T2 (range: 0.018 – 0.088). While the range of fit indices indicate that the model fit acceptably for most individuals, analyses in which participants with suboptimal model fit (CFI < 0.80, SRMR > 0.08) at either T1 or T2 were excluded produced a similar pattern of results reported below at statistically significant levels. Therefore, the following results are reported with all participants included.
Path Estimate Analysis
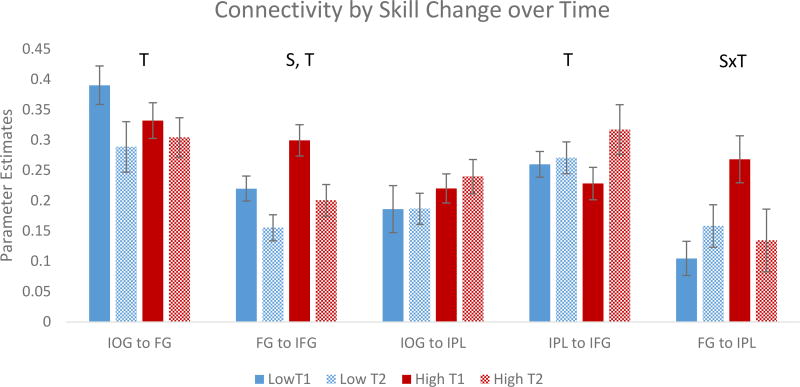
The 2×2×5 repeated measures ANOVA revealed main effects of skill change group (F (1, 57) = 4.03, p < 0.049), time (F (1, 57) = 6.27, p = 0.015), and path (F (4, 228) = 13.02, p < 0.001), illustrated in Figure 3. Contrary to previous developmental studies of reading network connectivity, children generally showed a reduction in connectivity over time. However, a significant time × path interaction (F (4, 228) = 3.38, p = 0.010), indicates this was not the case for all pathways, and an additional skill change group × time × path interaction (F (4, 228) = 3.39, p = 0.010) indicate that these longitudinal changes in connectivity differ depending on changes in skill. Mauchly’s Test for Sphericity showed that the assumption for sphericity was violated in the main effect of path and both interactions, yet Greenhouse-Geisser corrected level p-values remained significant (p < 0.001, p = 0.018, and p = 0.018).
Figure 3.
Mean parameter estimates with standard error bars for each path for skill change groups at each time. Significant skill change x time interaction is indicated by SxT, main effects of time by T, and main effects of skill change group by S. The only path to show an interaction between skill change and time was the dorsal decoding FG to IPL pathway; the high skill change group showed a significant decrease over time, but connectivity in the low skill change group remained similar. Connectivity between the orthographic, ventral path IOG and FG as well as the FG and IFG path decreased over time across all participants, while connectivity between IPL and IFG increased over time. The only path to show a main effect of skill change was the FG to IFG pathway with the high skill change group showing greater connectivity compared to the low skill change group.
The ventral, orthographic processing pathway of IOG to FG showed a significant main effect of time (F (1, 57) = 4.50, p = 0.038), but no relationship with skill change (F (1,57) = 0.33, p = 0.568). However, paired two-sample t-tests on the high and low improving groups separately indicated that while the high improvers showed consistent levels of connectivity over time (t (29) = 0.65, p = 0.520), the low improvers showed a decrease in connectivity over time (t (28) = 2.33, p = 0.027). In the dorsal phonological decoding path of FG to IPL, there was an interaction between skill change group and time (F (1, 57) = 6.09, p = 0.017). Follow-up tests on this path revealed high improvers showed higher connectivity at T1 (t (50) = 3.42, p = 0.001) followed by a decrease over time (t (29) = 2.29, p = 0.030). The low improving group, however, showed no difference in connectivity over time (t (28) = 1.12, p = 0.274). This pathway did not show main effects of time (F (1, 57) = 1.12, p = 0.290) or skill change group (F (1, 57) = 2.89, p = 0.090).
In other pathways of the reading network, only the FG to IFG pathways showed a main effect of skill (F (1, 57) = 6.42, p = 0.014) with the high skill change group showing greater connectivity between these regions at both times. Both this pathway and the IPL to IFG pathway showed main effects of time (F (1, 57) = 12.73, p < 0.001 and F (1, 57) = 4.10, p = 0.047 respectively). However, while connectivity tended to decrease over time in the IOG to IFG pathway, it increased over time in the IPL to IFG pathway. Connectivity between the IOG and IPL remained relatively stable over time (F (1, 57) = 0.20, p = 0.66) and between skill change groups (F (1, 57) = 1.54, p = 0.220).
In correlational analyses, connectivity in the dorsal FG to IPL and ventral IOG to FG paths were not correlated with current raw or standardized test score performance at either T1 or T2 (all p > 0.100), indicating connectivity change is related to reading change, not contemporary connectivity to contemporary skill. Further, T1 ventral pathway connectivity was not correlated with skill change (r (57) = −0.03, p = 0.805). T1 connectivity in the dorsal pathway was correlated with reading skill change (r (57) = 0.26, p = 0.043), but this correlation does not survive false discovery rate (FDR) correction for multiple comparison (FDR corrected for 6 analyses p = 0.259).
Discussion
The goal of the current study was to determine the developmental trajectory of the reading network in typical children, and whether network connectivity changes would support the dorsal-to-ventral shift hypothesis of reading development. Overall, we found decreases in connectivity for most connections from the first (T1) to the second (T2) time point about 2–3 years apart, regardless of changes in reading skill. This result is somewhat surprising, considering previous developmental studies of English reading network connectivity indicating that increased connectivity is generally associated with better reading skill (Cao et al., 2008; Quaglino et al., 2008; van der Mark et al., 2011). However, these previous studies on reading network connectivity in children focused on comparing good and poor readers at one time point, generally at about age 11. Yet, Quaglino et al (2008) reported that age-matched controls showed decreased connectivity between the fusiform gyrus (FG, BA 37) and the inferior frontal gyrus (IFG, BA 44/45) compared to children with dyslexia and younger reading-level matched controls. The current results also agree with Morken and colleagues’ 2017 study that showed general decreases in reading network connectivity over time, but extend the results to a more opaque language of English. While we do not replicate the finding that current connectivity is related to current reading skill, this may be due to differences in population. Previous studies compared typical children to those with dyslexia. In contrast, no child had difficulty with reading in the current study, as all participants scored within one standard deviation of the standardized mean performance for their age group. Therefore, in the case of typical readers, we find that changes in skill, not current skill, are most related to reading network connectivity.
When examining the case for the dorsal-to-ventral shift, we found a significant decrease in the dorsal, decoding processing pathway from FG to inferior parietal lobule (IPL) for the group who improved more from the first to the second time point. This finding supports the hypothesis that readers should show a decreased reliance on the dorsal pathway as they increase in skill. While the high and low improving groups did not differ in behavioral performance at T1, they did differ in connectivity; high improvers showed greater connectivity between FG and IPL at T1 compared to the low improvers. This pattern of results for the dorsal stream is consistent with the hypothesis put forth by Pugh et al (2001) that strong phonological decoding skills are necessary for the development of the ventral stream for reading. Children who went on to improve their reading abilities had a sufficiently strong connection between orthographic word forms and their relationships with sounds before they made a shift away from using dorsal stream processing. This interpretation is also supported by the correlation between T1 dorsal stream connectivity and reading improvement. An alternative interpretation is that decreases in the dorsal stream are not due to decreased reliance, but increased efficiency. This interpretation is consistent with the cross-sectional studies on brain activity in the reading network that suggest the dorsal stream is important early on and has a role in the reading process through adulthood (Church et al., 2008; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003).
In terms of the ventral stream, we found decreases in connectivity from inferior occipito-temporal gyrus (IOG) to the FG in the sample overall. However, this effect was primarily driven by the low improving readers; high improving readers maintained their level of connectivity in the ventral stream over time. While this result is inconsistent with our hypothesis that decreases in the dorsal stream would be accompanied by increases in the ventral stream, it is not incompatible with the dorsal-to-ventral shift. Previous cross sectional studies have suggested that rather than the ventral stream being added to the reading network as skill increases, the ventral stream becomes increasingly left lateralized, and the right hemisphere becomes less involved as skill progresses (Brown et al., 2005; Church et al., 2008; Turkeltaub et al., 2003). The current study did not examine the connectivity amongst and between regions in the right hemisphere homologue regions. The results, therefore, cannot support decreased involvement of the right hemisphere over increases in involvement of the left. However, our results are consistent with the idea that the ventral stream is not added to the reading network, but rather is an important part of the reading network across development. This finding may indicate that the dorsal-to-ventral shift is not a sequential process with the dorsal stream developing before the ventral stream, but rather they both may develop simultaneously. The finding that low improving readers fail to show any changes in the dorsal stream while at the same time exhibiting decreases in the ventral stream suggests that the development of these two processing streams are tied together. It is also further support for the hypothesis that decreases in the dorsal stream are important for the continued development of the ventral stream. It seems these low improving readers showed a failure to fully engage in the dorsal stream that was accompanied by a lack of maintenance of the ventral stream.
In other pathways of the reading network involving the IFG, we show conflicting main effects of time. While the IPL to IFG pathway increased over time, particularly for the high improvers group, the FG to IFG pathway decreased across all readers. This conflicting finding may reflect task demands. While the design of the task ensured that participants could not rely on either orthography or phonology alone, ultimately a rhyming decision had to be made for each word pair. Participants’ increases in accuracy on this rhyming task may be a result of their increased reliance on a more phonologically related path (IPL to IFG) and decreased reliance on the more orthographically related path (FG to IFG). Indeed, a post hoc analysis indicated that change in connectivity between IPL and IFG is negatively correlated with change in connectivity between FG and IFG (r (57) = −0.39, p = 0.002). Previous research has found that the FG is less sensitive to phonological familiarity effects (Kronbichler et al., 2007), which may make the phonologically related path better suited for solving a rhyme judgment task, particularly in the face of conflicting phonological and orthographic information. Research comparing brain activation of children and adults suggest adults are more likely to activate decoding brain areas such as the IPL, even when only either phonology or orthography information is necessary (Booth et al., 2004). The increase in connectivity between the IPL and IFG may thus reflect the reading network reaching a more mature, adult-like pattern of activity.
The final path, IOG to IPL, was the only path to show no changes over time or skill. This result contrasts that of Levy et al. (2009) who reported that connectivity between visual (middle occipital gyrus) and phonological decoding (left parietal lobe) regions was predictive of adults’ real word reading ability. The difference in results is likely due to differences in how the visual regions in particular were chosen in the two studies. The visual processing region selected by Levy et al. (2009) was chosen to reflect general visual processing and the search space was defined using data driven methods from studies within their own lab. Our visual processing region search space, in contrast, was guided by previous reports of areas involved in sublexical processing. The visual region used by Levy et al. (2009) was more posterior compared to the one used in the current study, x=−32, y=−91, z=10 and x=−40, y=−73, z=−10 respectively. The Levy et al (2009) region is likely to reflect processing orientation and local contours, whereas the region in our study is likely to reflect letter processing (Dehaene, Cohen, Sigman, & Vinckier, 2005). Given the gradient of feature sensitivity in the occipito-temporal area to word forms (Dehaene et al., 2005), the pathways in the two studies are likely not reflecting the same neural processes making it difficult to compare across them.
Interestingly, the pattern of reported results was related to changes in reading skill independent of age. While we did not expect gains in reading skill to be related to age (e.g. younger children showing greater gains than older children), age could have played a role in how changes in skill related to changes in reading network connectivity. One potential manifestation of the dorsal-to-ventral shift could be a staged process with changes in the dorsal stream being completed before changes in the ventral stream could take place. This account would predict that younger children’s skill change should be associated with changes in the dorsal stream and older children’s skill change should be associated with changes in the ventral stream. In contrast, our results suggest that a simultaneous change in dorsal and ventral streams is related to changes in skill, regardless of age. That there was no relationship between dorsal or ventral stream connectivity and concurrent raw score at either time further indicates that these changes in connectivity are specifically related to skill change. The lack of evidence for age related changes in connectivity does not rule-out the possibility that age could have subtler effects in different circumstances. Future research should continue to examine how age is related to the development of the reading network.
Finally, the current study examined reading network connectivity in English. The stimuli used in this study were purposefully constructed such that all words could not be read relying solely on high frequency sound-to-symbol relationships, and knowledge of more opaque or whole word correspondences was necessary to complete the task with high accuracy. However, more transparent languages may show different trajectories. For example, Morken et al (2017) examined reading network development in the semi-transparent language Norwegian. While the overall results across the two studies are similar, there are some differences, particularly the increase in connectivity between the IPL and IFG found in the current study. Previous cross-sectional reports of reading connectivity in English have additionally reported developmental increases in the connection between IPL and IFG (Bitan et al., 2007; Bitan, Cheon, Lu, Burman, & Booth, 2009). While the differences between studies may be due to differences in the models (precise locations of brain regions vary across studies), the results may be due to differences in strategies used across languages. Just as activation differences between individuals with dyslexia and typical controls is thought to differ across orthographic depth (Richlan, 2014), so too might skill related differences in reading network connectivity.
Conclusion
The results of the current study provide a model of reading network development in typical readers. We found that readers who show larger gains in reading skill show decreases in dorsal stream connectivity while maintaining connectivity in the ventral stream. In contrast, low improving readers failed to show changes in the dorsal stream and failed to maintain ventral stream connectivity, indicating failed development of the dorsal stream may be detrimental to ventral stream development. This pattern of results indicates that the development of the dorsal and ventral paths are linked. We hypothesize that it is only with a strong dorsal stream that reductions in the dorsal stream can be attained without cost to the ventral stream. Future research would benefit from investigating whether there is a sensitive period for the development of the dorsal stream and whether interventions aimed at encouraging the development of the dorsal stream in readers who are still struggling can lead to long-term improvements even in older readers.
Acknowledgments
Funding Source
This research was supported by National Institute of Child Health and Human Development Grant (grant number HD042049 to J.R.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bitan T, Cheon J, Lu D, Burman DD, Booth JR. Developmental increase in top-down and bottom-up processing in a phonological task: an effective connectivity, fMRI study. Journal of Cognitive Neuroscience. 2009;21(6):1135–45. doi: 10.1162/jocn.2009.21065. http://doi.org/10.1162/jocn.2009.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam M-M, Booth JR. Developmental changes in activation and effective connectivity in phonological processing. NeuroImage. 2007;38(3):564–575. doi: 10.1016/j.neuroimage.2007.07.048. http://doi.org/10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. Journal of Cognitive Neuroscience. 2004;16(7):1234–49. doi: 10.1162/0898929041920496. http://doi.org/10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. Retrieved from file://localhost/Users/matthieu/Documents/Papers/article/Brown2005CerebCortex.pdf%5Cnpapers://e6449990-22e5-4886-8c14-f32018591362/Paper/p5299. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Booth JR. Effective brain connectivity in children with reading difficulties during phonological processing. Brain and Language. 2008;107(2):91–101. doi: 10.1016/j.bandl.2007.12.009. http://doi.org/10.1016/j.bandl.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Brennan C, Booth JR. The brain adapts to orthography with experience: evidence from English and Chinese. Developmental Science. 2015;18(5):785–98. doi: 10.1111/desc.12245. http://doi.org/10.1111/desc.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex. 2008;18(9):2054–65. doi: 10.1093/cercor/bhm228. http://doi.org/10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff M-A, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(2):291–307. doi: 10.1093/brain/123.2.291. http://doi.org/10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Coltheart M. Dual route and connectionist models of reading: an overview. London Review of Education. 2006;4(1):5–17. http://doi.org/10.1080/13603110600574322. [Google Scholar]
- Darcy Burgund E, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The Feasibility of a Common Stereotactic Space for Children and Adults in fMRI Studies of Development. NeuroImage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. http://doi.org/10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words : a proposal. 2005;9(7) doi: 10.1016/j.tics.2005.05.004. http://doi.org/10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Finn ES, Shen X, Holahan JM, Scheinost D, Lacadie C, Papademetris X, Constable RT. Disruption of functional networks in dyslexia: A whole-brain, data-driven analysis of connectivity. Biological Psychiatry. 2014;76(5):397–404. doi: 10.1016/j.biopsych.2013.08.031. http://doi.org/10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates KM, Molenaar PCM. Group search algorithm recovers effective connectivity maps for individuals in homogeneous and heterogeneous samples. NeuroImage. 2012;63(1):310–9. doi: 10.1016/j.neuroimage.2012.06.026. http://doi.org/10.1016/j.neuroimage.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Gates KM, Molenaar PCM, Hillary FG, Slobounov S. Extended unified SEM approach for modeling event-related fMRI data. NeuroImage. 2011;54(2):1151–8. doi: 10.1016/j.neuroimage.2010.08.051. http://doi.org/10.1016/j.neuroimage.2010.08.051. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “visual word form area”. Neuron. 2009;62(2):199–204. doi: 10.1016/j.neuron.2009.03.017. http://doi.org/10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity–behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. NeuroImage. 2006;31 doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Hooper D, Coughlan J, Mullen M. Structural equation modelling: Guidelines for determining model fit. Articles. 2008 Retrieved from http://arrow.dit.ie/cgi/viewcontent.cgi?article=1001&context=buschmanart.
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(15):8939–44. doi: 10.1073/pnas.95.15.8939. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=21181&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. http://doi.org/10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kherif F, Josse G, Seghier ML, Price CJ. The main sources of intersubject variability in neuronal activation for reading aloud. Journal of Cognitive Neuroscience. 2009;21(4):654–68. doi: 10.1162/jocn.2009.21084. http://doi.org/10.1162/jocn.2009.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, Schwartz SJ, Milham MP. Cortical signatures of dyslexia and remediation: an intrinsic functional connectivity approach. PloS One. 2013;8(2):e55454. doi: 10.1371/journal.pone.0055454. http://doi.org/10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. taksi: on orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2007;19(10):1584–94. doi: 10.1162/jocn.2007.19.10.1584. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2989180&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Pernet C, Treserras S, Boulanouar K, Aubry F, Démonet JF, Celsis P. Testing for the dual-route cascade reading model in the brain: An fMRI effective connectivity account of an efficient reading style. PLoS ONE. 2009 doi: 10.1371/journal.pone.0006675. http://doi.org/10.1371/journal.pone.0006675. [DOI] [PMC free article] [PubMed]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. http://doi.org/10.1016/S1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McNorgan C, Alvarez A, Bhullar A, Gayda J, Booth JR. Prediction of reading skill several years later depends on age and brain region: implications for developmental models of reading. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2011;31(26):9641–8. doi: 10.1523/JNEUROSCI.0334-11.2011. http://doi.org/10.1523/JNEUROSCI.0334-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morken F, Helland T, Hugdahl K, Specht K. Reading in dyslexia across literacy development: A longitudinal study of effective connectivity. NeuroImage. 2017;144:92–100. doi: 10.1016/j.neuroimage.2016.09.060. http://doi.org/10.1016/j.neuroimage.2016.09.060. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Los Angeles: Muthen and Muthen; 2012. [Google Scholar]
- Olulade Oa, Flowers DL, Napoliello EM, Eden GF. Developmental differences for word processing in the ventral stream. Brain and Language. 2013;125(2):134–45. doi: 10.1016/j.bandl.2012.04.003. http://doi.org/10.1016/j.bandl.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette GP, P G. What’s meaning got to do with it: The role of vocabulary in word reading and reading comprehension. Journal of Educational Psychology. 2006;98(3):554–566. http://doi.org/10.1037/0022-0663.98.3.554. [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34(6):479–492. doi: 10.1016/s0021-9924(01)00060-0. http://doi.org/10.1016/S0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Quaglino V, Bourdin B, Czternasty G, Vrignaud P, Fall S, Meyer ME, de Marco G. Differences in effective connectivity between dyslexic children and normal readers during a pseudoword reading task: an fMRI study. Neurophysiologie Clinique. 2008;38(2):73–82. doi: 10.1016/j.neucli.2007.12.007. http://doi.org/10.1016/j.neucli.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Richardson FM, Seghier ML, Leff AP, Thomas MSC, Price CJ. Multiple Routes from Occipital to Temporal Cortices during Reading. Journal of Neuroscience. 2011;31(22) doi: 10.1523/JNEUROSCI.6519-10.2011. Retrieved from http://www.jneurosci.org/content/31/22/8239.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F. Functional neuroanatomy of developmental dyslexia: the role of orthographic depth. Frontiers in Human Neuroscience. 2014 May;8:347. doi: 10.3389/fnhum.2014.00347. http://doi.org/10.3389/fnhum.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–308. doi: 10.1002/hbm.20752. http://doi.org/10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56(3):1735–42. doi: 10.1016/j.neuroimage.2011.02.040. http://doi.org/10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Ricketts J, Nation K, Bishop DVM. Vocabulary Is Important for Some, but Not All Reading Skills. Scientific Studies of Reading. 2007;11(3):235–257. http://doi.org/10.1080/10888430701344306. [Google Scholar]
- Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. NeuroImage. 2008;42(3):1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. http://doi.org/10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman Ba, Pugh KR, Fulbright RK, Skudlarski P, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically- based intervention. Biological Psychiatry. 2004;55(9):926–33. doi: 10.1016/j.biopsych.2003.12.019. http://doi.org/10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. http://doi.org/10.1016/S0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Siegel LS. Perspectives on dyslexia. Paediatrics & Child Health. 2006;11(9):581–7. doi: 10.1093/pch/11.9.581. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Denton C, Papanicolaou AC. Altering the brain circuits for reading through intervention: a magnetic source imaging study. Neuropsychology. 2007;21(4):485–496. doi: 10.1037/0894-4105.21.4.485. http://doi.org/10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Taylor JSH, Rastle K, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin. 2012;139(4):766–791. doi: 10.1037/a0030266. http://doi.org/10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6(7):767–73. doi: 10.1038/nn1065. http://doi.org/10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmüller J, Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage. 2009;47(4):1940–9. doi: 10.1016/j.neuroimage.2009.05.021. http://doi.org/10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Brandeis D. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. NeuroImage. 2011;54(3):2426–36. doi: 10.1016/j.neuroimage.2010.10.002. http://doi.org/10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word-form system. Neuron. 2007;55(1):143–156. doi: 10.1016/j.neuron.2007.05.031. http://doi.org/10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]