Abstract
Background
Postoperative hypophosphatemia is common and is associated with a lower risk of liver failure after hepatectomy but higher morbidity after pancreatectomy. Whether different physiologic mechanisms underlie the hypophosphatemia associated with these very different clinical outcomes is unclear. This study aims to evaluate the underlying mechanism in postoperative hypophosphatemia.
Study designs
We prospectively enrolled 120 patients who underwent major hepatectomy (n=30), minor hepatectomy (n=30), pancreatectomy (n=30), and laparotomy without resection (control group, n=30). Preoperative and postoperative serum and urinary phosphorus, calcium, and creatinine, as well as phosphaturic factors, including serum nicotinamide phosphoribosyltransferase (NAMPT), fibroblast growth factor-23 (FGF-23), and parathyroid hormone (PTH) were measured. In addition, we evaluated urinary levels of nicotinamide catabolites, N-methyl-2-pyridone-5-carboxamide (2-PY) and N-methyl-4-pyridone-3-carboxamide (4-PY).
Results
We found that significant hypophosphatemia occurred from postoperative day (POD) to POD2 in all 4 groups were preceded by hyperphosphaturia from preoperative day to POD1. Phosphate level alterations were associated with a significant increase in NAMPT levels from preoperative day to POD2 in all 3 resected groups but not in the control group. FGF-23 levels were significantly decreased postoperatively in all 4 groups while PTH levels did not change in any of the 4 groups. Urine levels of 2-PY and 4-PY significantly decreased in all 4 groups postoperatively.
Conclusions
This study demonstrates that the mechanism of hypophosphatemia is the same for both liver and pancreas resections. Postoperative hypophosphatemia is associated with increased NAMPT. The mechanism that upregulates NAMPT and its role on disparate clinical outcomes in postoperative patients warrant further investigation.
Keywords: Postoperative hypophosphatemia, hyperphosphaturia, NAMPT, liver resection, pancreas surgery
INTRODUCTION
Hypophosphatemia has been reported after several types of operations and is associated with increased morbidity and mortality, but the underlying mechanisms involved remain uncertain1–3. Following partial hepatectomy, the absence of hypophosphatemia or lack of early nadir is associated with increased rates of liver insufficiency and increased 30-day mortality4–6. However, following pancreatectomy, the presence of hypophosphatemia is linked to higher rate of postoperative complications including pancreatic leaks and abscess formations7. Given their differences in clinical implication, the role of postoperative phosphate alteration and its underlying mechanism warrant investigation.
Phosphate participates in several critical biological processes, including signal transduction and energy transfer, and alterations in its homeostasis are often linked to serious complications such as respiratory failure, arrhythmias, renal insufficiency, and metabolic acidosis3. The pathophysiology of postoperative hypophosphatemia is likely multifactorial. Phosphate levels are regulated by a network of various organs, including bone, parathyroid glands, small intestine, liver, and kidneys8. For years, it was believed that in patients undergoing hepatectomy, liver regeneration and subsequent increased hepatic uptake of phosphate were responsible for the phosphate depletion9–11. However, increased urinary phosphate excretion is also observed, suggesting that renal mechanisms of phosphate handling play an important role in the development of postoperative hypophosphatemia3, 12, 13.
Several phosphaturic factors, including parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23), have been proposed as potential mediators of postoperative phosphaturia3, 12, 13. More recently, a novel phosphaturic factor, nicotinamide phosphoribosyltransferase (NAMPT), was also investigated as a mediator of hypophosphatemia after hepatectomy in rats14. NAMPT is the rate-limiting enzyme in the metabolic pathway converting nicotinamide (NAM) to nicotinamide adenine dinucleotide (NAD), an essential coenzyme in many cellular redox reactions such as those involved in DNA repair and regulation of cellular energy metabolism15–18. A 70% partial hepatectomy in rats results in an increased expression of NAMPT in the kidney cortex and systemic circulation14. These changes were associated with a decreased expression of sodium-dependent phosphate transporters in the renal proximal tubules, thereby causing hyperphosphaturia and hypophosphatemia14.
Based on the experimental finding, this prospective study was initiated to test the hypothesis that NAMPT is a key factor in the metabolic pathway responsible for hypophosphatemia in patients following hepatectomy, but a different mechanism in patients following pancreatectomy given different clinical outcomes. We characterized the effect of surgery on serum and urinary phosphate and several phosphaturic factors, including NAMPT, FGF-23, and PTH, as well as on urinary catabolites of nicotinamide, including N-methyl-2-pyridone-5- carboxamide (2-PY) and N-methyl-4-pyridoen-3-carboxamide (4-PY).
PATIENTS AND METHODS
Study patients
After approval by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC), 120 consecutive and eligible patients who underwent open operations for liver and pancreas tumors were prospectively enrolled in the study from August 2015 to September 2016. We obtained written informed consent from each patient, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The patients were divided in 4 groups of 30 patients each: major hepatectomy, minor hepatectomy, pancreatectomy, and control. Major hepatectomy was defined as a resection of ≥3 Couinaud's segments, whereas minor hepatectomy was defined as a resection of <3 segments. Pancreatectomy included patients who underwent pancreatoduodenectomy, central pancreatectomy, or distal pancreatectomy with splenectomy. Control patients were those who were deemed unresectable prior to or during exploratory laparotomy, and who had biopsies with or without placement of a hepatic artery infusion pump for regional, hepatic arterial chemotherapy. Control patients did not undergo any resection of liver or pancreas tissue other than biopsies in some cases. All patients in this study underwent elective open surgery. Laparoscopic and robotic operations were excluded to eliminate the potential effects of minimally invasive surgery on electrolyte alterations.
All patients enrolled in this study were ≥18 years of age and had preoperative glomerular filtration rates (GFR) of >60ml/min/1.73m2 as calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula19. All eligible patients, based on planned surgery, age, and GFR, were approached for consent to this prospective protocol. Patients were then placed into one of the 4 groups based on their actual surgical interventions.
All patients had blood and urine collected from the morning prior to surgery until postoperative day (POD) 4. Patients discharged prior to POD4 were excluded and replaced by additional patients. Those who remained hospitalized until POD7 had laboratory results documented for analysis but tests after POD4 were not required for inclusion in the study. Patients who required repeat operation for any reason during the study period were also excluded and replaced, given the likely confounding effects of additional surgery on electrolyte alterations.
After surgery, all patients received standard postoperative care and were advanced to a regular diet as tolerated prior to discharge. Intravenous phosphate supplementation was administered by the primary surgical team as needed to replace deficits noted on routine laboratory studies. In addition, intravenous fluids were given per routine care. Patients generally received lactated ringer’s solution postoperatively and were then switched to dextrose 5% in 1/2 normal saline + 20 mEq/L potassium chloride for maintenance fluid on POD1 onward. Neither solutions contained phosphate.
Serum and urine tests
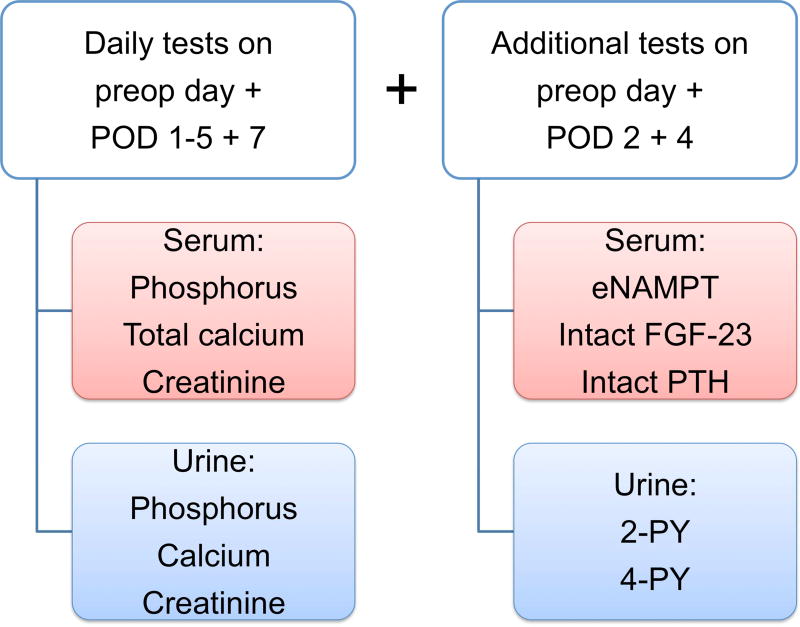
Serum and urine were collected and analyzed (Fig. 1A). On the day of the operation prior to incision, on POD 1 to 5, and POD 7, patients had daily serum phosphorus and calcium as well as spot urine phosphorus, calcium, and creatinine tested. Serum creatinine was tested any day within the 30 days prior to operation and also on POD 1 to 5, and POD7. In addition, prior to incision and on POD 2 and 4, patients had serum levels of intact PTH tested and additional blood and urine collected for further analysis.
Fig. 1.
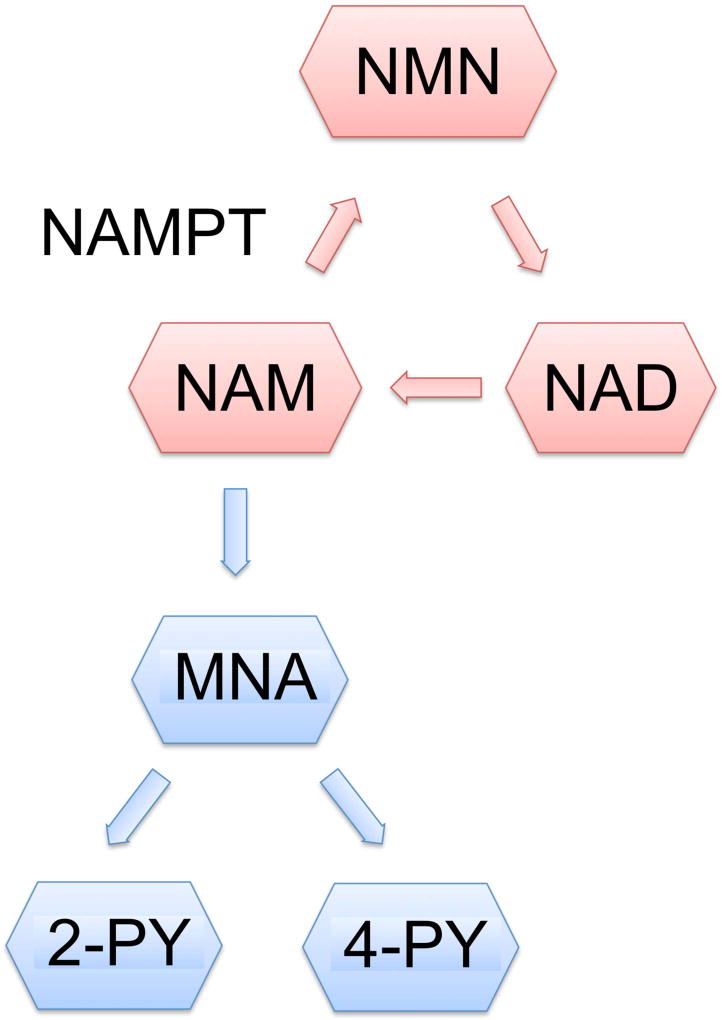
(A) Study tests were obtained from serum and urine. Nicotinamide phosphoribosyltransferase is the rate-limiting enzyme in the NAM metabolism whereas (B) 2-PY and 4-PY are urinary end products of the NAM metabolism. POD, postoperative day; eNAMPT, extracellular nicotinamide phosphoribosyltransferase; FGF-23, fibroblast growth factor-23; PTH, parathyroid hormone; 2-PY, N-methyl-2-pyridone-5-carboxamide; 4-PY, N-methyl-4-pyridone-3-carboxamide; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NAD, nicotinamide adenine dinucleotide; MNA, N1- methylnicotinamide.
Five milliliters (mL) of peripheral blood and 30mL of urine were collected on preoperative day, POD2, and POD4. Peripheral blood samples collected in plain tubes and then centrifuged at 1000g for 5 minutes, and 1 mL of serum from the supernatant was aliquoted to a new tube. Both serum and urine samples were stored at −80°C until further analysis.
NAMPT levels were analyzed from serum samples using an extracellular NAMPT (eNAMPT) enzyme-linked immunosorbent assay (ELISA) kit per manufacturer’s instructions (Adipogen Corp, San Diego, CA). FGF-23 levels in the serum were also analyzed using a full length FGF-23 ELISA kit according to the manufacturer’s protocol (Kainos Laboratories, Japan). Urine samples were used to measure the NAM metabolites, 2-PY and 4-PY, using a diethyl ether extraction method as previously described by Shibata20. After urinary metabolites were extracted, both 2-PY and 4-PY were simultaneously measured by high performance liquid chromatography (HPLC) in the MSKCC Cell Metabolism Core Laboratory. Additional details of 2-PY and 4-PY extraction and HPLC measurements are described (eDocument1), and an example of HPLC curves generated is shown (eFigure 1). The involvement of NAMPT in NAM metabolism and 2-PY and 4-PY as urinary end products are shown (Fig. 1B).
Serum and urine levels of phosphorus, calcium, and creatinine as well as serum level of PTH were tested by the Department of Laboratory Medicine at MSKCC. Urinary levels of spot phosphorus, calcium, 2-PY, and 4-PY were adjusted for urinary creatinine to account for differences in urine volume. Fractional excretion of phosphorus (FEPi) as percentage was calculated by (urine phosphorus/serum phosphorus) × (serum creatinine/urinary creatinine) × 100 from daily spot collections12, 21. All tests were performed on all 120 patients except FGF-23 (Fig. 1A). Testing of only 33 patients for FGF-23 levels was deemed statistically adequate for the study.
Clinical and pathological variables
Patient information was obtained prospectively, including patient demographics, body mass index (BMI), preoperative chemotherapy status, perioperative laboratory results, and details of the surgical intervention and pathological diagnosis.
Statistical analysis
Categorical variables were presented using frequency and percentage, and compared using Fisher’s exact test. Continuous variables were presented using the median with the first and third interquartile range (IQR) for all laboratory results and the entire range for all other variables. The Wilcoxon matched-paired signed rank test was used to compare laboratory results at 2 time points whereas the Kruskal-Wallis test was used to compare other continuous variables between more than 2 groups. P values < 0.05 from two-sided tests were considered statistically significant. Statistical analyses and figures were generated using GraphPad Prism (GraphPad Software, CA) and R version 3.2.2 (cran.r-project.org).
RESULTS
A total of 120 patients who underwent open major hepatectomy, minor hepatectomy, pancreatectomy, or laparotomy without resection were enrolled. Patient demographics, operative and tumor diagnosis are reported (Table 1). Approximately half of the patients who underwent major and minor hepatectomy as well as those in the control group had colorectal liver metastasis. The majority of patients who underwent partial pancreatectomy had either pancreatic ductal adenocarcinoma or pancreatic neuroendocrine tumor. Use of preoperative chemotherapy, operative blood loss, Pringle time (hepatic pedicle occlusion during partial hepatectomy), operative time, and length of hospital stay were statistically different among 4 groups.
Table 1.
Study patients
| Major hepatectomy (n=30) |
Minor hepatectomy (n=30) |
Pancreatectomy (n=30) |
Control (n=30) | p Value | |
|---|---|---|---|---|---|
| Age, y, median (range) | 56 (35–84) | 64 (30–79) | 64 (48–83) | 61 (30–76) | 0.054 |
| Male, n (%) | 18 (60) | 11 (37) | 13 (43) | 16 (53) | 0.276 |
| BMI ≥ 25 kg/m2, n (%) | 19 (63) | 18 (60) | 22 (73) | 21 (70) | 0.682 |
| Diabetes mellitus, n (%) | 4 (13) | 5 (17) | 5 (17) | 6 (20) | 0.923 |
| Neoadjuvant chemotherapy, n (%) | 19 (63) | 19 (63) | 8 (27) | 23 (77) | <0.001* |
| Operations, n (%) | 15 (50) 3–4 segments; 15 (50) 4–5 segments | 13 (43) < 2 segments; 17 (47) 2–3 segments | 14 (47) Whipple; 3 (10) central pancreatectomy; 13 (43) distal pancreatectomy | 25 (83) HAIP; 5 (17) biopsies only | -- |
| Tumor diagnosis, n (%) | 16 (53) CRLM; 8 (27) ICC; 1 (3) HCC; 1 (3) GBC; 4 (13) others | 14 (47) CRLM; 1 (3) ICC; 3 (10) HCC; 6 (20) GBC; 6 (20) others | 10 (33) PDAC; 6 (20) PNET; 5 (17) IPMN; 4 (13) ampullary cancer; 5 (17) others | 15 (50) CRLM; 10 (33) ICC; 3 (10) PDAC; 2 (7) others | -- |
| Operative blood loss, mL, median (range) | 450 (100–2500) | 200 (20–800) | 175 (50–1600) | 63 (20–600) | <0.001* |
| Pringle time, min, median (range) | 25 (0–65) | 15 (0–64) | -- | -- | 0.042* |
| Operative time, min, median (range) | 230 (140–425) | 172 (77–401) | 145 (67–367) | 162 (104–281) | <0.001* |
| Length of stay, d, median (range) | 6 (4–56) | 6 (4–12) | 7 (4–7) | 5 (4–7) | 0.009* |
| Serum phosphate <2.4 on POD2, n (%) | 28 (93) | 23 (77) | 25 (83) | 24 (80) | 0.339 |
| Phosphate supplement on POD2, n (%) | 20 (67) | 18 (60) | 16 (53) | 16 (53) | 0.680 |
| Phosphate dosage on POD2, mmol, n (%) | 0.148 | ||||
| 15 | 14 (47) | 11 (37) | 11 (37) | 8 (27) | |
| 24 | 5 (16) | 5 (16) | 4 (13) | 6 (20) | |
| 39 | 1 (3) | 2 (7) | 1 (3) | 2 (7) |
Significant.
HAIP, hepatic artery infusion pump; CRLM, colorectal liver metastasis; ICC, intrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma; GBC, gallbladder carcinoma; PDAC, pancreatic ductal adenocarcinoma; PNET, pancreatic neuroendocrine tumor; IPMN, intraductal papillary mucinous neoplasm; POD, postoperative day.
Hypophosphatemia occurred in the majority of patients in all 4 groups on POD2, ranging from 77% in patients who underwent minor hepatectomy to 93% in patients who underwent major hepatectomy. Percent of patients who received phosphate supplements and dosage on POD2 were not significantly different among the 4 groups. In addition, patients who received phosphate supplements on POD2 did not result in significantly different POD3 phosphate level compared to those who did not receive phosphate supplements in all 4 groups (eFigure 2).
Hypophosphatemia was preceded by hyperphosphaturia postoperatively
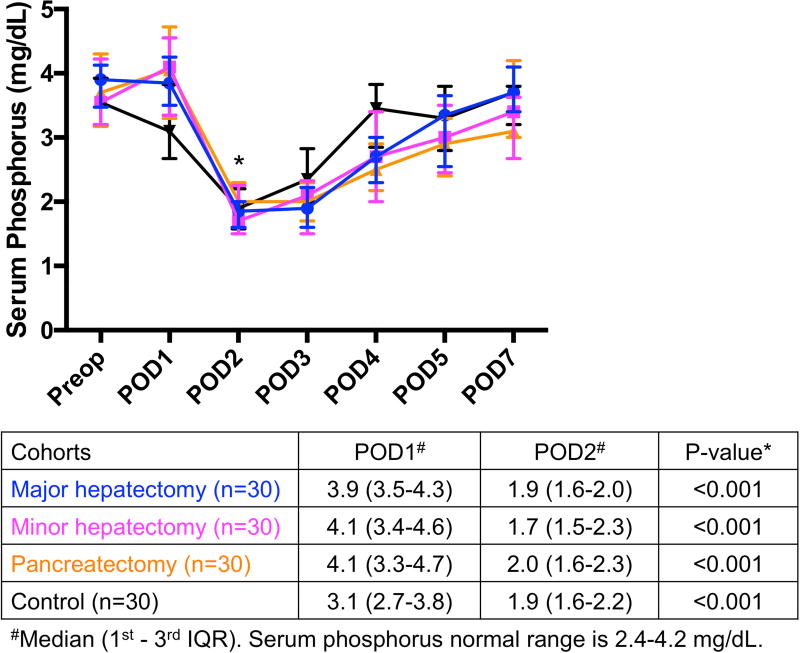
Serum phosphorus levels significantly decreased from POD1 to POD2 in major hepatectomy (3.9 to 1.9 mg/dL, p<0.001), minor hepatectomy (4.1 to 1.7 mg/dL, p<0.001), pancreatectomy (4.1 to 2.0 mg/dL, p<0.001), as well as in the control group (3.1 to 1.9 mg/dL, p<0.001) (Fig. 2A). The severity of hypophosphatemia among the 4 groups was not significantly different on POD2 (p=0.534). Serum phosphorus levels between POD1 and POD3 were also significantly different in all 4 groups (all p<0.001).
Fig. 2.
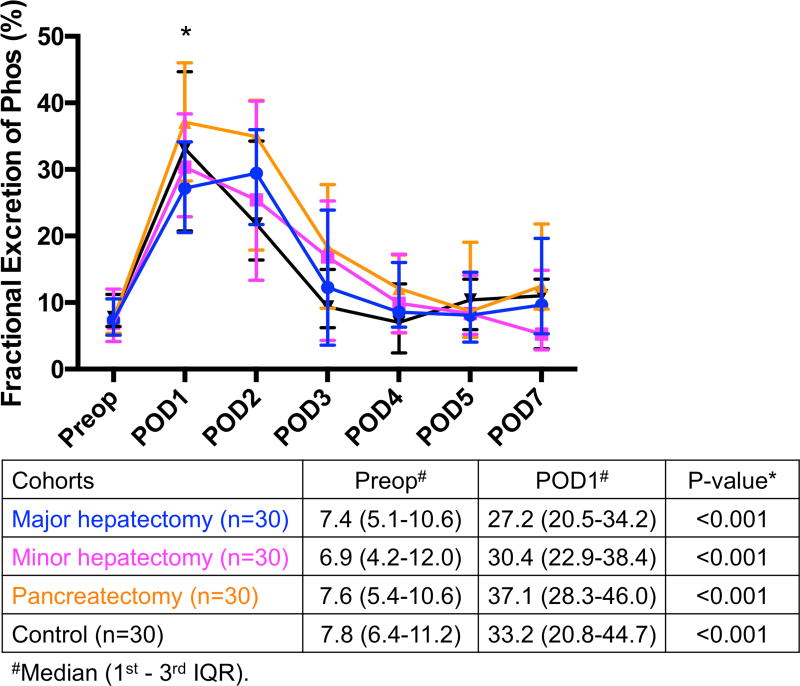
(A) Significant hypophosphatemia on POD2 was preceded by (B) significant hyperphosphaturia on POD1 in all 4 groups. *Significant. IQR, interquartile range; Phos, phosphorus; POD, postoperative day; Preop, preoperative.
Hypophosphatemia was preceded by a significant increase in fractional excretion of phosphorus from preoperative day to POD1 in major hepatectomy (7.4 to 27.2, p<0.001), minor hepatectomy (6.9 to 30.4, p<0.001), pancreatectomy (7.6 to 37.1, p<0.001), and the control cohort (7.8 to 33.2, p<0.001) (Fig. 2B). Patients who underwent pancreatectomy had the highest levels of hyperphosphaturia on POD1, which were significantly higher than both major and minor hepatectomy groups (p=0.007 and p=0.019, respectively).
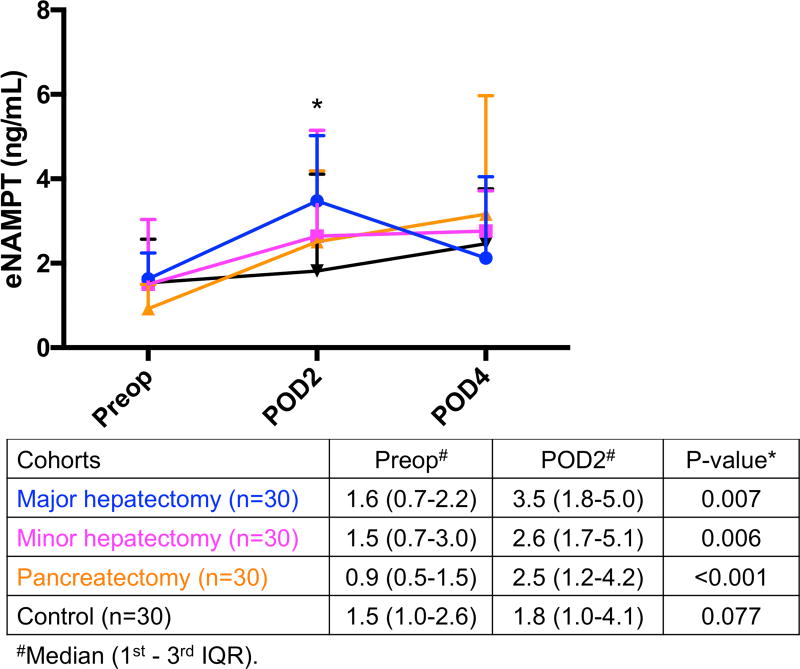
Hypophosphatemia was associated with increased NAMPT
Serum NAMPT levels were significantly increased from preoperative day to POD2 in major hepatectomy (1.6 to 3.5 ng/mL, p=0.007), minor hepatectomy (1.5 to 2.6, p=0.006), and pancreatectomy patients (0.9 to 2.5 ng/mL, p<0.001), but not in the control group (1.5 to 1.8 ng/mL, p=0.077) (Fig. 3A).
Fig. 3.
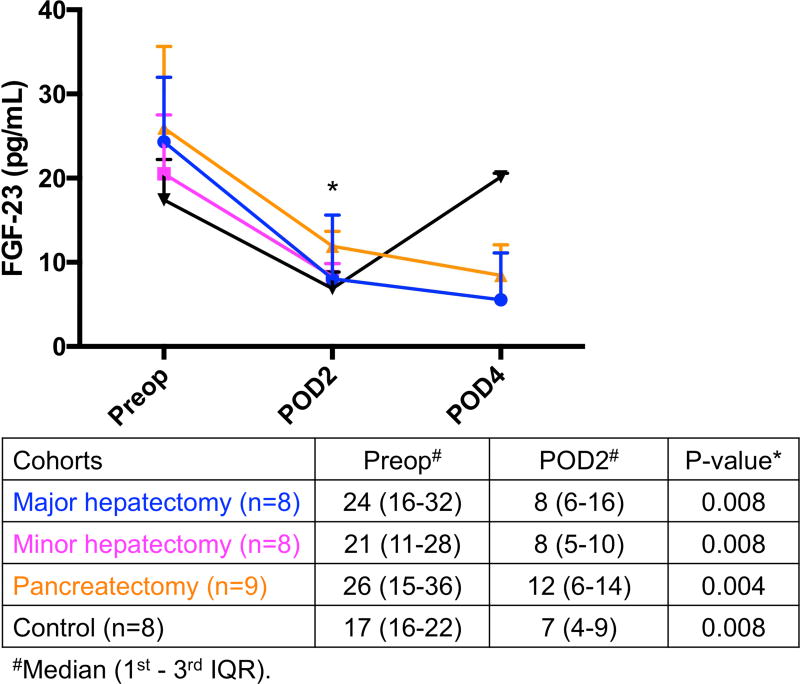
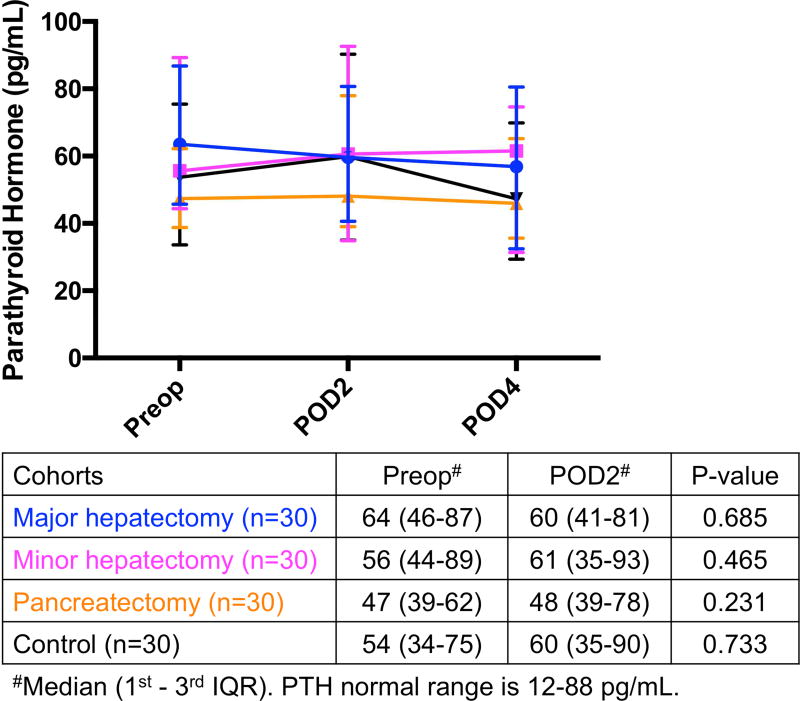
(A) Significant increase in eNAMPT levels on POD2 compared to preoperative levels occurred in all 3 resected groups, whereas (B) FGF-23 levels were significantly decreased postoperatively and (C) PTH were not significantly affected by operation in all 4 groups. *Significant. eNAMPT, extracellular nicotinamide phosphoribosyltransferase; FGF-23, fibroblast growth factor-23; IQR, interquartile range; POD, postoperative day; Preop, preoperative; PTH, parathyroid hormone.
FGF-23 levels were measured in a representative sampling of all groups (n=33) since the levels were all significantly decreased postoperatively in a pilot analysis (Fig. 3B). FGF-23 decreased from preoperative day to POD2 in major hepatectomy (24 to 8, p=0.008, n=8), minor hepatectomy (21 to 8, p=0.008, n=8), pancreatectomy (26 to 12, p=0.004, n=9), and control group (17 to 7, p=0.008, n=8). No additional measurements were performed because the decreased level of the phosphaturic factor FGF-23 on POD2 suggested that it was not involved in the development of hypophosphatemia in these patients.
PTH did not appear to be involved in postoperative hypophosphatemia, as the levels did not significantly change perioperatively (Fig. 3C). Transient postoperative hypocalcemia (eFigure 3A) may cause secondary hyperparathyroidism that would cause phosphaturia, but PTH levels did not increase postoperatively. Postoperative hypocalcemia resulted in physiologically appropriate reduction in postoperative urinary calcium/creatinine levels, leading to decreased urinary calcium losses (eFigure 3B).
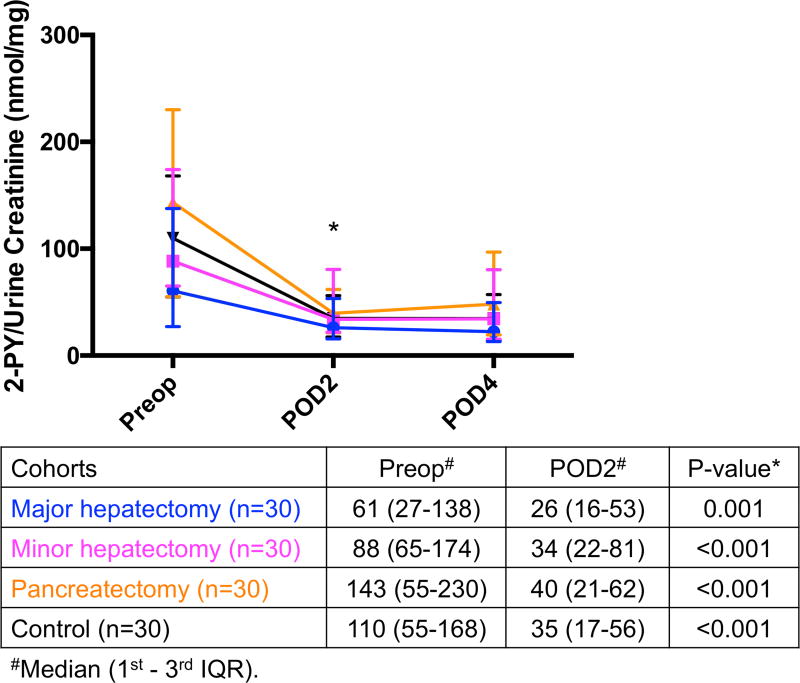
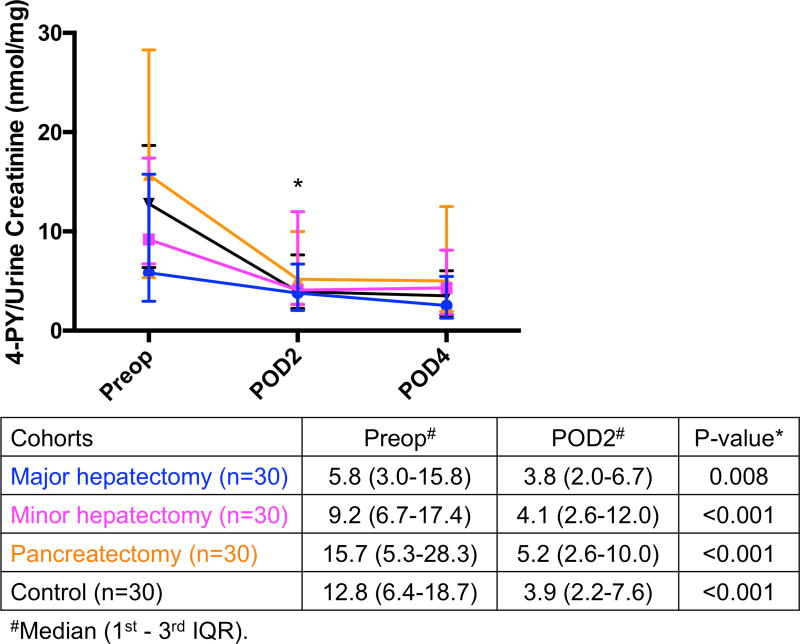
Decreased urinary levels of nicotinamide catabolites after surgery
Metabolites of nicotinamide are normally excreted in urine in the form of 2-PY and 4-PY. Both urinary levels of 2-PY and 4-PY decreased postoperatively in all 4 groups (Fig. 4A and Fig. 4B, 13 respectively). 2-PY decreased from preoperative day to POD2 in major hepatectomy (61 to 26, p=0.001), minor hepatectomy (88 to 34, p<0.001), pancreatectomy (143 to 40, p<0.001), and in the control group (110 to 35, p<0.001). Similarly, 4-PY decreased from preoperative day to POD2 in major hepatectomy (5.8 to 3.8, p=0.008), minor hepatectomy (9.2 to 4.1, p<0.001), pancreatectomy (15.7 to 5.2, p<0.001), and in the control cohort (12.8 to 3.9, p<0.001).
Fig. 4.
Significant decrease in (A) urinary 2-PY and (B) 4-PY excretions on POD2 compared to their preoperative levels in all 4 groups. * Significant. 2-PY, N-methyl-2-pyridone-5-carboxamide; 4-PY, N-methyl-4-pyridone-3-carboxamide; IQR, interquartile range; POD, postoperative day; Preop, preoperative.
Most patients in all 4 groups had a serum creatinine within the normal range of 0.6–1.3 mg/dL during their hospitalization (eFigure 4). Only 4 patients (3%) had elevated postoperative serum creatinine, ranging from 1.4 to 2.1 mg/dL. Thus, postoperative electrolyte alterations were not likely secondary to acute kidney injury.
DISCUSSION
In this study, we observed that hypophosphatemia occurred in majority of patients following liver and pancreas resection, as well as those with open laparotomy without resection. Our prior large retrospective studies showed that hypophosphatemia was associated with improved outcome following hepatectomy but worse outcome following pancreatectomy4, 7. Given the striking differences in the clinical implications, we hypothesized that the underlying mechanism of hypophosphatemia in hepatectomy would be different from that in pancreatectomy. Thus, we initiated this prospective study to characterize the alterations in serum and urine phosphate as well as potential phosphaturic factors in patients following different operations. However, the results of the present study show that the mechanism of hypophosphatemia was the same for both liver and pancreas resection. Postoperative hypophosphatemia was preceded by hyperphosphaturia in all 4 cohorts, and that hypophosphatemia was associated with increased serum levels of NAMPT postoperatively in resected cohorts.
NAMPT is a novel phosphaturic factor that downregulates expression of sodium-dependent phosphate transporters type IIa and IIc in the renal tubule following major hepatectomy in rats14. NAM also decreased phosphate uptake in both kidneys and intestines14, 22. In addition to being a phosphaturic factor, NAMPT also catalyzes the biosynthesis of NAD+ from NAM, and NAD+ is an essential coenzyme in many metabolic pathways such as those involved in DNA repair and immune modulation15–18, 23. A recent study showed that NAMPT and NAD biosynthesis were crucial in liver regeneration after resection in mice24. NAMPT has also been implicated in several metabolic disorders. Increased levels of NAMPT have been associated with obesity, type 2 diabetes mellitus, nonalcoholic fatty liver disease, Parkinson’s disease, and several cancers, including breast, gastric, and colorectal cancer16, 25–29.
Most of the patients in our study underwent hepatectomy or pancreatectomy for cancer. NAMPT levels associated with increased cancer metabolism would expect to decrease after resection of the tumor, but their increased levels postoperatively suggest a greater impact of increased cellular metabolism and inflammation from the stress of open surgery. NAMPT expression and NAD metabolism have been shown to be upregulated with immune cell activation and synthesis of inflammatory cytokines including tumor necrosis factor, and the introduction of NAMPT inhibitor can reduce inflammatory response30. We speculate that inflammatory changes and increased cellular metabolism associated with surgery, independent of type of resection, may be the initial event that leads to the postoperative increase in NAMPT. NAMPT then not only stimulates NAM metabolism but also acts as a phosphaturic factor, leading to hyperphosphaturia and hypophosphatemia14, 15. The underlying mechanism that upregulates NAMPT postoperatively and its source remained to be elucidated. The expression of NAMPT in renal cortex increases in rats following partial hepatectomy14. However, we were not able to test whether the kidney is also the source of NAMPT in humans since it would not be feasible or ethical to obtain healthy kidney biopsies perioperatively to assess its cortical levels.
Patients in the control group also had an increasing trend in the NAMPT levels. However, this did not reach statistical significance and therefore the increased phosphaturia and hypophosphatemia observed in this group is unlikely to be the result of altered NAM metabolism. Additional mechanisms not examined here may be responsible for hypophosphatemia in the control group. We believe that the open surgical intervention itself, even without liver or pancreas resection, may be sufficient to trigger similar inflammatory and metabolic changes leading to hypophosphatemia. Other proinflammatory cytokines such as tumor necrosis factor and interleukin-6 have found to correlate with hypophosphatemia during early sepsis, and this similar inflammatory response may also occur with open surgery31.
One limitation of this study is that we measured the extracellular NAMPT (eNAMPT) in the serum, but the intracellular NAMPT (iNAMPT) was previously identified to downregulate the sodium-dependent phosphate transporter14. Since it was not feasible to obtain postoperative kidney biopsies in patients, we used eNAMPT as a surrogate marker. NAMPT is ubiquitously expressed in nearly all organs and tissues but mostly concentrated intracellularly in the cytoplasm and nucleus16, 32. However, small amounts of NAMPT are released extracellularly and may serve as an enzyme or cytokine, although a specific receptor has not been identified16, 33. It has been shown that eNAMPT is secreted following cellular stress and inflammatory stimuli, and that many clinical studies on the role of NAMPT in different cancers and metabolic disorders also utilized eNAMPT as a surrogate marker32. The relative concentration of eNAMPT and iNAMPT is also unclear. In one study, it was shown that melanoma release eNAMPT that corresponds to only 1% of the levels of iNAMPT34, and that both eNAMPT and iNAMPT are increased in chronic lymphocytic leukemia35. Thus, the concentration of eNAMPT likely correlates to that of iNAMPT; and therefore, could be used as a surrogate for the relative concentration of iNAMPT over the perioperative period.
In the rat study by Nomura et al, increased NAMPT levels in the kidney following partial hepatectectomy were associated with increased urinary excretion of the NAM catabolites, 2-PY and 4-PY14. In our study, however, we observed a significant decrease in urinary 2-PY and 4-PY in all 4 of the groups studied. 2-PY and 4-PY are end products from the metabolism of NAM into N1- methylnicotinamide (MNA), which then is catalyzed by MNA oxidase into 2-PY and 4-PY18, 36. 2-PY and 4-PY were thought to increase with NAD utilization such as during tissue stress and increased energy expenditure36. Physical stress such as cold exposure has been shown to increase the excretion of 2-PY and 4-PY in humans36. On the other hand, depression and excess fatigue in rats result in decreased excretion of 2-PY and 4-PY in rats37.
There are some possibilities to explain our findings in 2-PY and 4-PY excretion. It has been proposed that excess NAM and its catabolites are released from the liver and then excreted into the kidney in rats after partial hepatectomy14. One possibility is that humans may have a greater reserve than rats to continue biosynthesis of NAD from NAM, while NAD continues to be utilized with increasing postoperative metabolic demands. Thus, there would not be an excess of NAM for renal excretion in human, unlike in rats14. Another possibility, which is a limitation of this study, is that all of our tests were performed from one time point collection of blood or urine each day. Shibata et al showed that there was a diurnal variation in excretion of 2-PY and 4-PY, such that urinary output was higher during the day than at night36. The time of the day at which when we obtained specimens from our patients may have affected our results, but this possibility is unlikely a major factor since most of our patients had their blood and urine collected in the morning each day during routine clinical collections.
In addition to evaluating the role of abnormal NAM metabolism in postoperative hypophosphatemia, we also examined other phosphaturic factors. We found that FGF-23 and PTH obtained on POD2 were not responsible for postoperative hypophosphatemia, but we are limited in our conclusion given lack of tests on POD1. Prior smaller studies also were not able to conclude the participation of FGF-23 and PTH on postoperative hypophosphatemia12–14. Aside from the effects of phosphaturic factors, hypophosphatemia may also be secondary to intravenous glucose infusion, which can shift phosphate intracellularly3. In our study, patients received routine intravenous fluid per primary surgical team, which are generally dextrose 5% in 1/2 normal saline + 20 mEq/L potassium chloride as maintenance fluid on POD1 onward while they were off diet. However, this transcellular shift would be transient and would not cause excess phosphate in the circulation to be excreted into urine. In contrast, glucose infusion would reduce phosphaturia, hence it would not be a likely mechanism of postoperative hyperphosphaturia and hypophosphatemia that we observed38.
In conclusion, we found that urinary phosphate wasting contributed to postoperative hypophosphatemia in patients following liver and pancreas resection, as well as those with open laparotomy without resection. Postoperative hypophosphatemia in the resected cohorts was associated with alterations in NAMPT and NAM catabolites. The involvement of NAM metabolism in postoperative hypophosphatemia and associated complications warrant additional investigation. Further understanding of this mechanism and potential targeting of NAMPT may mitigate hypophosphatemia related complications.
Supplementary Material
Acknowledgments
We thank Kevin Staton and Michael McDevitt of the Radiochemistry & Molecular Imaging Probes in Memorial Sloan Kettering Cancer Center for their expertise in high performance liquid chromatography. We also thank Paula Garcia and Danielle Casella, Clinical Research Coordinators of the Hepatopancreatobiliary Service, for their administrative support.
Support: This work was supported by NIH/NCI P30 CA008748 Cancer Center Support Grant. Dr Jaimes received Byrne Research Fund from Memorial Sloan Kettering Cancer Center.
Abbreviations
- POD
postoperative days
- NAMPT
nicotinamide phosphoribosyltransferase
- FGF-23
fibroblast growth factor-23
- PTH
parathyroid hormone
- 2-PY
N-methyl-2-pyridone-5-carboxamide
- 4-PY
N-methyl-4-pyridone-3-carboxamide
- NAM
nicotinamide
- NAD
nicotinamide adenine dinucleotide
- GFR
glomerular filtration rates
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- ELISA
enzyme-linked immunosorbent assay
- HPLC
high performance liquid chromatography
- BMI
body mass index
- IQR
interquartile range
- MNA
N1-methylnicotinamide.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Poster presentation at American Society of Nephrology meeting, Chicago, IL, November 2016.
References
- 1.Rasmussen A, Kimose HH, Hessov I. Severity of postoperative hypophosphatemia in relation to glucose administration and renal handling of phosphate. Acta Chir Scand. 1988;154:617–621. [PubMed] [Google Scholar]
- 2.Cohen J, Kogan A, Sahar G, et al. Hypophosphatemia following open heart surgery: incidence and consequences. Eur J Cardiothorac Surg. 2004;26:306–310. doi: 10.1016/j.ejcts.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Datta HK, Malik M, Neely RD. Hepatic surgery-related hypophosphatemia. Clin Chim Acta. 2007;380:13–23. doi: 10.1016/j.cca.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Herbert GS, Prussing KB, Simpson AL, et al. Early trends in serum phosphate and creatinine levels are associated with mortality following major hepatectomy. HPB (Oxford) 2015;17:1058–1065. doi: 10.1111/hpb.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squires MH, 3rd, Dann GC, Lad NL, et al. Hypophosphataemia after major hepatectomy and the risk of post-operative hepatic insufficiency and mortality: an analysis of 719 patients. HPB (Oxford) 2014;16:884–891. doi: 10.1111/hpb.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margonis GA, Amini N, Buettner S, et al. Impact of perioperative phosphorus and glucose levels on liver regeneration and long-term outcomes after major liver resection. J Gastrointest Surg. 2016;20:1305–1316. doi: 10.1007/s11605-016-3147-6. [DOI] [PubMed] [Google Scholar]
- 7.Sadot ERL, McIntyre CA, Allen PJ, et al. Early post-operative hypophosphatemia as a novel predictor of anastomotic failure after pancreas resection: a risk-prediction tool. Oral Presentation at the Annual Meeting of Americas Hepato-Pancreato-Biliary Association; Miami Beach, FL. 2015. [Google Scholar]
- 8.Tatsumi S, Miyagawa A, Kaneko I, et al. Regulation of renal phosphate handling: interorgan communication in health and disease. J Bone Miner Metab. 2016;34:1–10. doi: 10.1007/s00774-015-0705-z. [DOI] [PubMed] [Google Scholar]
- 9.Corbin IR, Buist R, Volotovskyy V, et al. Regenerative activity and liver function following partial hepatectomy in the rat using (31)P-MR spectroscopy. Hepatology. 2002;36:345–353. doi: 10.1053/jhep.2002.34742. [DOI] [PubMed] [Google Scholar]
- 10.Kooby DA, Zakian KL, Challa SN, et al. Use of phosphorous-31 nuclear magnetic resonance spectroscopy to determine safe timing of chemotherapy after hepatic resection. Cancer Res. 2000;60:3800–3806. [PubMed] [Google Scholar]
- 11.Assy N, Gong Y, Zhang M, et al. Use of proliferating cell nuclear antigen as a marker of liver regeneration after partial hepatectomy in rats. J Lab Clin Med. 1998;131:251–256. doi: 10.1016/s0022-2143(98)90097-x. [DOI] [PubMed] [Google Scholar]
- 12.Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241:343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nafidi O, Lapointe RW, Lepage R, et al. Mechanisms of renal phosphate loss in liver resection-associated hypophosphatemia. Ann Surg. 2009;249:824–827. doi: 10.1097/SLA.0b013e3181a3e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomura K, Tatsumi S, Miyagawa A, et al. Hepatectomy-related hypophosphatemia: a novel phosphaturic factor in the liver-kidney axis. J Am Soc Nephrol. 2014;25:761–772. doi: 10.1681/ASN.2013060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garten A, Petzold S, Korner A, et al. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garten A, Schuster S, Penke M, et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 17.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 18.Shibata K, Taguchi H. Chapter 7 Nicotinic Acid and Nicotinamide (325–364), Modern chromatographic analysis of vitamins. New York, NY: Marcel Dekker, Inc.; 2001. [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata K, Kawada T, Iwai K. Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide, by high-performance liquid chromatography. J Chromatogr. 1988;424:23–28. doi: 10.1016/s0378-4347(00)81072-5. [DOI] [PubMed] [Google Scholar]
- 21.Tan SJ, Smith ER, Cai MM, et al. Relationship between timed and spot urine collections for measuring phosphate excretion. Int Urol Nephrol. 2016;48:115–124. doi: 10.1007/s11255-015-1149-z. [DOI] [PubMed] [Google Scholar]
- 22.Katai K, Tanaka H, Tatsumi S, et al. Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant. 1999;14:1195–1201. doi: 10.1093/ndt/14.5.1195. [DOI] [PubMed] [Google Scholar]
- 23.Rongvaux A, Galli M, Denanglaire S, et al. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008;181:4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee S, Chellappa K, Moffitt A, et al. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology. 2017;65:616–630. doi: 10.1002/hep.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan B, Young DA, Lu ZH, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells: metabolic basis and potential clinical implications. J Biol Chem. 2013;288:3500–3511. doi: 10.1074/jbc.M112.394510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santiago JA, Littlefield AM, Potashkin JA. Integrative transcriptomic meta-analysis of Parkinson's disease and depression identifies NAMPT as a potential blood biomarker for de novo Parkinson's disease. Sci Rep. 2016;6:34579. doi: 10.1038/srep34579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju HQ, Zhuang ZN, Li H, et al. Regulation of the Nampt-mediated NAD salvage pathway and its therapeutic implications in pancreatic cancer. Cancer Lett. 2016;379:1–11. doi: 10.1016/j.canlet.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Hong SM, Park CW, Kim SW, et al. NAMPT suppresses glucose deprivation-induced oxidative stress by increasing NADPH levels in breast cancer. Oncogene. 2015;35:3544–3554. doi: 10.1038/onc.2015.415. [DOI] [PubMed] [Google Scholar]
- 29.Penke M, Larsen PS, Schuster S, et al. Hepatic NAD salvage pathway is enhanced in mice on a high-fat diet. Mol Cell Endocrinol. 2015;412:65–72. doi: 10.1016/j.mce.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Van Gool F, Galli M, Gueydan C, et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15:206–210. doi: 10.1038/nm.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barak V, Schwartz A, Kalickman I, et al. Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines. Am J Med. 1998;104:40–47. doi: 10.1016/s0002-9343(97)00275-1. [DOI] [PubMed] [Google Scholar]
- 32.Carbone F, Liberale L, Bonaventura A, et al. Regulation and function of extracellular nicotinamide phosphoribosyltransferase/visfatin. Compr Physiol. 2017;7:603–621. doi: 10.1002/cphy.c160029. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Zhang Y, Dorweiler B, et al. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008;283:34833–34843. doi: 10.1074/jbc.M805866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grolla AA, Torretta S, Gnemmi I, et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is a tumoural cytokine released from melanoma. Pigment Cell Melanoma Res. 2015;28:718–729. doi: 10.1111/pcmr.12420. [DOI] [PubMed] [Google Scholar]
- 35.Audrito V, Serra S, Brusa D, et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125:111–123. doi: 10.1182/blood-2014-07-589069. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto H, Ishikawa A, Yoshitake Y, et al. Diurnal variations in human urinary excretion of nicotinamide catabolites: effects of stress on the metabolism of nicotinamide. Am J Clin Nutr. 2003;77:406–410. doi: 10.1093/ajcn/77.2.406. [DOI] [PubMed] [Google Scholar]
- 37.Zhang F, Jia Z, Gao P, et al. Metabonomics study of urine and plasma in depression and excess fatigue rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Mol Biosyst. 2010;6:852–861. doi: 10.1039/b914751a. [DOI] [PubMed] [Google Scholar]
- 38.Rodgers A, Bungane N, Allie-Hamdulay S, et al. Calciuria, oxaluria and phosphaturia after ingestion of glucose, xylitol and sorbitol in two population groups with different stone-risk profiles. Urol Res. 2009;37:121–125. doi: 10.1007/s00240-009-0184-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.