Abstract
Objective
Bovine pericardium (BP) has been identified as a choice biomaterial for the development of surgical bioprosthetic heart valves (BHV) and transcatheter aortic valves (TAV). Porcine pericardium (PP) and younger BP have been suggested as candidates TAV leaflet biomaterials for smaller-profile devices due to their reduced thickness; however, their mechanical and structural properties remain to be fully characterized. This study characterized the material properties of chemically treated thick (PPK) and thin (PPN) PP, as well as fetal (FBP), calf (CBP) and adult (ABP) BP tissues in order to better understand their mechanical behavior.
Methods
Planar biaxial testing and uniaxial failure testing methods were employed to quantify tissue mechanical responses and failure properties. Fiber characteristics were examined using histological analysis.
Results
ABP and CBP tissues were significantly stiffer and stronger than the younger FBP tissues. Histological analysis revealed a significantly larger concentration of thin immature collagen fibers in the FBP tissues than in the ABP and CBP tissues. While PP tissues were thinnest, they were stiffer and less extensible than the BP tissues.
Conclusions
Due to comparable mechanical properties but significantly reduced thickness, CBP tissue may be a more suitable material for TAV manufacturing than ABP tissue. FBP tissue, despite its reduced thickness and higher flexibility, was weaker and should be studied in more detail. Although PP tissues are the thinnest, they were least extensible and failed at earlier strain than BP tissues. The differences between PP and BP tissues should be further investigated and suggest that they should not be used interchangeably in the manufacturing of TAV.
Keywords: Bovine pericardium, porcine pericardium, aging, biaxial testing, uniaxial testing, collagen, transcatheter aortic valve
Graphical abstract
1. Introduction
Biosprosthetic heart valves (BHV), designed to be implanted surgically or percutaneously, are commonly made from glutaraldehyde-treated (GL-treated) bovine pericardium (BP), porcine pericardium (PP) or porcine aortic valve tissues [1]. Due to adequate biological and mechanical properties, wide availability, and rigorous quality control related to intensive battery farming, adult BP (ABP) and calf BP (CBP) have been considered biological materials of choice to manufacture BHV leaflets since the 1980s [2]. Yet, it is unknown which BP tissue is preferred over the other one for BHV production, as commercial devices do not specify this information, but it is believed that tissue sorting is mainly guided by a tissue thickness threshold and structural integrity. Conversely, PP has only been used for BHV fabrication since the early days of transcatheter aortic valve (TAV) devices. Currently, the Edwards SAPIEN series (Edwards Lifesciences, Irvine, CA), made from BP tissue, along with the Medtronic CoreValve series (Medtronic, Minneapolis, MN), made from PP tissue, have dominated the TAV replacement (TAVR) market. According to a recent study by Grand View Research, Inc [3], the global cardiovascular and soft tissue patches market size was valued at US $2.5 billion in 2014, and is expected to reach nearly US $5.8 billion in 2022. In particular, BP is the most widely used biomaterial, owing to its superior biomechanical properties.
The first size 23 mm TAV SAPIEN device could be crimped into a 22-Fr sheath, which is equivalent to a vessel diameter of 7.33 mm. The newest generation size 23 mm SAPIEN 3 device is now crimped onto the lower-profile Edwards Commander delivery system, with a sheath size of 14 Fr, equivalent to a vessel diameter of 4.67 mm. This lower profile delivery system has expanded the patient population eligible for the safer transfemoral approach (compared to transapical) to include more patients who may have otherwise been excluded due to small vessel size. The smallest size 26 mm device of the first generation Medtronic Corevalve can be compressed within an 18 Fr sheath, while a 24 Fr sheath is needed for a size 26 mm SAPIEN valve. The latest Corevalve Evolut device [4] can be crimped within delivery sheaths as small as 14 Fr. In the rapidly growing field of minimally-invasive BHV replacement, it is clear that the industry is pushing towards lower-profile devices that minimize vascular complications, and simplify advancement of the catheter through small and/or tortuous arteries.
However, the size of the delivery catheter system hinges upon the minimum crimped diameter of the valve, which is in turn determined by the crimping profile of the stent and the thickness of the leaflet tissue. An attractive aspect of using GL-treated PP tissue for TAV leaflet fabrication lies in its lower thickness (~ 0.14–0.2 mm) compared to GL-treated BP (~ 0.32–0.42 mm). Similarly, younger BP has also been shown to be thinner than older BP [5], giving it a potential physical advantage for TAV production. It is important to note that the thickness of pericardium leaflet tissues are not only of crucial importance for TAV delivery, but also for the performance and long-term durability of the valve, where the use of thinner tissues may compromise the structural integrity of the device. A decade later after the first proof-of-concept TAVR case performed [6], with >200,000 procedures having been performed in >65 countries, 5-year valve durability findings [7, 8] with smaller experience reported up to 9 years show no significant signal of structural TAV deterioration. However, as the designs of the valves differ considerably, the long-term data for one valve may not necessarily equate to others. Therefore, understanding and comparing the mechanical properties of BP and PP tissues is critical for the design, manufacture, and assessment of TAV devices.
Although the mechanical properties of GL-treated BP tissue have been previously characterized through biaxial and uniaxial testing [9–14], the mechanical properties of BP and PP tissues have been compared primarily through uniaxial testing [11–13, 15]. To the authors’ knowledge, the only published study that has compared the mechanical response of younger, thinner BP with thicker, older BP tissue was performed by Sizeland et al. [5] through uniaxial testing. However, uniaxial mechanical testing is non-physiologic for soft tissues, as the coupling between the tissue’s directional responses is ignored. Additionally, variations in tissue treatment protocols and testing methodologies have resulted in a wide range of mechanical responses and parameters. For instance, the orientation of collagen fibers has been shown to significantly affect the mechanical properties of biological materials [16], yet, many of the published studies do not consider the alignment of the collagen fibers in the pericardium tissue prior to mechanical testing [17]. As native heart valve leaflets and pericardium tissue exhibit anisotropic behavior [18, 19], multiprotocol biaxial testing is needed to investigate the changes in coupling between the primary fiber and cross-fiber directions in these anisotropic tissues, while uniaxial failure testing is necessary to evaluate the mechanical strength (failure properties), especially in TAV applications, where tissue fatigue and durability need high attention.
Therefore, the objective of this study was to characterize and compare the mechanical responses of GL-treated BP and PP tissues of different thicknesses for the evaluation of their use in the context of BHV replacement. Multi-protocol biaxial testing and uniaxial failure testing were utilized to quantify the mechanical properties of fetal BP (FBP), CBP, and ABP, specifically chosen for their increasing thickness due to tissue maturation, as well as thin PP (PPN) and thick PP (PPK). An improved understanding and utilization of the BP and PP mechanical properties may ultimately result in TAV designs with more predictable performance and improved durability, as well as enable transcatheter valve technologies to be further developed with a smaller crimp profile required to improve the advancement of the catheter through small and/or tortuous arteries.
2. Material and Methods
2.1. Tissue preparation
Fresh pericardial sacs from 10 adult (12–24 months old), 11 calf (6–12 months old) and 11 fetal (third trimester of gestation) cattle, as well as from 22 6–9 months old pigs were obtained from Animal Technologies, Inc. (Tyler, TX). The fresh BP sacs were stored in cryopreservant solution (10% dimethyl sulfoxide and 90% culture medium RPMI 1640) at −80 °C until fixation could be performed. Prior to testing, thawed BP tissue [20] and fresh PP were cut into smaller sheets, and regions with defects such as abrupt cuts or tears and those in which the pericardial ligaments could not be clearly distinguished were excluded. Any adherent adipose tissue and loose surface fibers were also carefully removed.
Tissue treatment and fixation were performed in two steps at room temperature. First, the tissues were pinned down to a rubber platform along the edges to maintain surface flatness. Care was taken during pinning to not stretch the pericardial sheets to avoid changes in fibril alignment [21]. The flat pericardia were then immersed in a solution of 0.625% glutaraldehyde. In the second step, the tissues were treated with a crosslinking anti-calcification solution composed of formaldehyde, ethanol, and Tween 80 (Sigma Aldrich, St. Louis, MO). Treated pericardia were then stored at 4°C in 0.25% glutaraldehyde for a minimum of 48 hours prior to testing.
Selection of testing samples was based on the orientation of macroscopically visible collagen fibers, uniform thickness and local homogeneity. First, visual inspection of the pericardium sheets under a light source was performed to identify a local preferred fiber direction. Samples were then cut into a 20 mm × 20 mm square biaxial testing samples such that the preferred fiber direction and the cross-preferred fiber direction aligned with the X1 and X2 directions of the device loading axes, respectively (Fig. 1A). Second, if an isotropic response was obtained from the sample after biaxial testing, mechanical alignment consisting of sample rotation was performed to acquire an anisotropic response. Initially, one biaxial sample was obtained per BP pericardial sac, while for PP tissue, more than one biaxial sample was obtained per PP pericardial sac, as tissue was sorted by thickness.
Fig. 1.
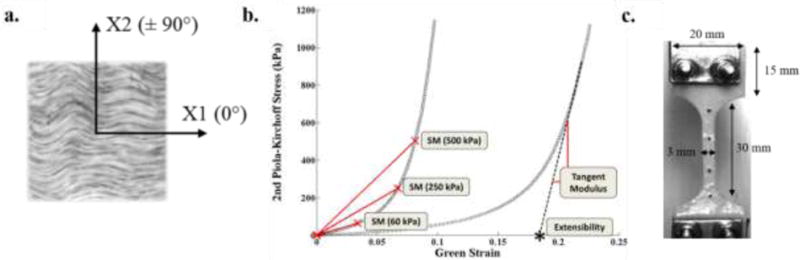
a) Orientation of biaxial samples, b) Representative equibiaxial stress-strain curve and calculated parameters, c) Dimensions of dog-bone uniaxial sample. SM60: Secant modulus at 60 kPa, SM250: Secant modulus at 250 kPa, SM500: Secant modulus at 500 kPa. Extensibility for both X1 and X2 directions.
Uniaxial test samples adjacent to the biaxial samples were carefully cut into a “dog-bone” shape (Fig. 1C) using a stencil with a central zone 30 mm long × 3 mm wide to ensure failure in the center region and to minimize grip effects. Each sample was cut in such a way as to ensure that the preferred fiber direction was parallel with the loading axis of the tensile device. Sample thickness was measured at five locations along each biaxial and uniaxial sample with a Mitutoyo 7301 rotating thickness gage (Aurora, IL) with an accuracy of ± 0.01 mm, and an average thickness was determined.
2.2. Planar biaxial mechanical testing
Planar biaxial testing was conducted following the methods detailed in Sacks and Sun (2003) [22]. Briefly, four graphite markers delimiting a square approximately 3 mm × 3 mm in size were glued to the central region of the tissue surface for optical strain measurements. Samples were then mounted in a trampoline fashion by sutures attached to barbless fish hooks, and tested in a controlled temperature (37 °C) 0.9% NaCl bath. In each specimen side, four barbless fish hooks (size 22) were punctured through the tissue thickness and spaced out evenly near the leaflet edge. A stress-controlled test protocol was utilized [23], wherein the ratio of the normal Lagrangian stress components T11:T22 was kept constant with T12 = T21 = 0. Tij is defined as the axial force per unit length over which the force is applied. Preconditioning of up to 40 cycles with T11:T12 = 1:1, and a rest period of 25 s between each set of 10 consecutive cycles was performed to reduce tissue hysteresis and achieve a stable tissue response. All specimens were tested at the maximum load possible without causing tissue damage. At the maximum, load, seven successive stress protocols were performed using the ratios T11:T12 = 1:0.75, 1:0.5, 1:0.3, 1:1, 0.75:1, 0.5:1, 0.3:1 with 10 cycles per run, and at a stress rate of about 50 kPa/s. This range was chosen for extensive coverage of the in-plane stress state [24]. Tissue samples were assumed to be incompressible and planar, and the unloaded reference markers were obtained using the post-preconditioning unloaded state, and used to analyze all the consecutive protocols.
Stiffness and extensibility in both the preferred fiber direction (X1) and cross-preferred fiber direction (X2) were computed from the equibiaxial stress-strain curves as material metrics. Tissue stiffness was quantified by means of the secant modulus at three equibiaxial stress values: 1) 60 kPa for tissue response under the high deformation/low force ‘toe region’, 2) 250 kPa to examine tissue response at a physiological level [23], and 3) 500 kPa for a high stress state, representing areas of the valve that may experience greater stress (i.e. attachment to stent, commissure regions). Linear regression was used to fit the high-modulus linear region of the stress-strain curve in order to calculate the extensibility (Fig. 1B). In addition, the degree of anisotropy (DA) was analyzed using the ratio of Green strains, , at maximum equibiaxial loading.
2.3. Uniaxial mechanical testing
The uniaxial test was conducted using a TestResources 100Q Universal Testing Machine (Shakopee, MN). Four graphite optical markers were placed on the narrow portion of the samples for optical strain measurements. The axial force was measured by means of a 4.4 N load cell (TestResources SM-500-294). The specimens were mechanically fixed to specially-designed anti-slip clamps, and special care was taken to avoid damage during clamping while avoiding slippage from the grips. The specimens were continuously hydrated with saline solution after mounting to allow for optimal tissue hydration during testing.
Prior to testing, all specimens were mechanically preconditioned by means of a series of 10 loading–unloading cycles to a peak load of approximately 30% of the maximum load [25] to remove the initial stress relaxation effect and to yield a stable response. The samples were then loaded at a rate of 50 mm/min until failure. A CCD camera was used to capture optical marker motion, while an in-house LabVIEW (National Instruments, Austin, TX, USA) program was used to simultaneously synchronize the markers locations and load information with each video frame. The ultimate tensile strength (UTS) and ultimate tensile strain (UTE) were determined from the uniaxial data, defined by the peak stress and maximum deformation withstood by the samples prior to failure, respectively. Tissue stiffness was also quantified by means of the tangent modulus defined as the slope of the stress-strain curve in the high-modulus linear region.
2.4. Histology
Histological analysis was conducted on the BP samples in order to evaluate the age-dependent structural differences. Ten samples for each age group were selected for quantitative histological analysis after biaxial testing. Samples were cut into strips parallel to the X1 direction of the biaxial test fixture, fixed in 10% neutral buffered formalin and embedded in paraffin. Sections cut at 5-μm thickness were stained in picrosirius red solution for one hour (reagents from Sigma Aldrich, St. Louis, MO). The slides were then washed in acidified water, three changes of 100% ethanol, and xylene before being mounted in a resinous medium and coverslipped. Picrosirius red staining was used in conjunction with circularly polarized light microscopy to assess collagen fiber thickness and organization. Fiber thickness determines the color, changing from green to yellow to orange to red with increasing thickness [26]. This principle can be used to quantify the relative proportion of collagen fibers at different maturity levels in the tissue. For example, yellow-orange strong birefringence would be assigned to mature thick collagen fibers, whereas newly-synthesized thin collagen fibers would display a weak birefringence associated with a slightly green color [27].
The proportion of different colored fibers was assessed using published methods [28]. Briefly, images from four different locations in each sample were viewed with the use of a N-Achroplan 10× objective on an Axio Scope.A1 microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY) equipped with filters to provide circularly polarized illumination, recorded by a AxioCam MRc Rev.3 digital camera (Carl Zeiss Microscopy, LLC, Thornwood, NY) and analyzed using an in-house Matlab code (The Mathworks, Inc., Natick, MA). Images were resolved into their hue, saturation and value components. A histogram of hue frequency was obtained from the resolved 8-bit hue images, which contained 256 colors, defined as follows: red 2 to 9 and 230 to 256, orange 10 to 38, yellow 39 to 51, and green 52 to 128 [28]. The number of pixels within each hue range was determined and expressed as a percentage of the total number of pixels associated with collagen, which in turn was expressed as a percentage of the total number of pixels in the image analyzed.
2.5. Statistical analysis
All measurements are presented as a mean ± standard deviation. The One Way Analysis of Variance (ANOVA) test was implemented in SigmaPlot (Systat Software Inc., San Jose, CA). If statistical differences were found (p<0.05), pairwise multiple comparisons were performed using the Holm–Sidak or the Dunn`s methods. The Student’s t-test was used when two groups were compared. The Grubb’s test was utilized to remove outliers. Probability values p<0.001 were considered to indicate differences with high statistical significance.
3. Results
3.1. Tissue Morphology
The mean thickness for all biaxial and uniaxial samples was 0.427 ± 0.048 mm for ABP, 0.327 ± 0.046 mm for CBP, and 0.231 ± 0.017 mm for FBP. There was a high statistically significant difference in thickness between the three BP groups (Table 1). PPN and PPK samples had an average thickness of 0.142 ± 0.015 mm and 0.186 ± 0.01 mm, respectively. Porcine samples were significantly thinner than bovine samples, with the thin samples being significantly thinner than the thick samples of the same species. All material properties, morphological sample characteristics, and sample size information are presented in Table 1.
Table 1.
Mean ± SD values for material metrics calculated from the equibiaxial and uniaxial tests
| PPK | PPN | ABP | CBP | FBP | |
|---|---|---|---|---|---|
| Sample size (biaxial) | 12 | 12 | 10 | 11 | 11 |
| Sample size (uniaxial) | 20 | 13 | 10 | 10 | 10 |
| Thickness (mm) | 0.186 ± 0.010 | 0.142 ± 0.015 | 0.427 ± 0.048 | 0.327 ± 0.046 | 0.231 ± 0.017 |
| Extensibility (X1) | 0.049 ± 0.027 | 0.042 ± 0.023 | 0.094 ± 0.045 | 0.081 ± 0.031 | 0.099 ± 0.024 |
| Extensibility (X2) | 0.095 ± 0.022 | 0.103 ± 0.046 | 0.133 ± 0.038 | 0.166 ± 0.025 | 0.189 ± 0.029 |
| Stiffness at 60 kPa, X1 (MPa) | 2.417 ± 1.204 | 3.015 ± 1.281 | 1.903 ± 0.817 | 1.651 ± 0.734 | 0.897 ± 0.185 |
| Stiffness at 250 kPa, X1 (MPa) | 6.639 ± 2.831 | 8.626 ± 3.260 | 3.816 ± 1.815 | 4.031 ± 1.764 | 2.65 ± 0.571 |
| Stiffness at 500 kPa, X1 (MPa) | 11.037 ± 4.716 | 12.95 ± 5.190 | 6.041 ± 2.989 | 6.825 ± 2.946 | 4.626 ± 0.873 |
| Stiffness at 60 kPa, X2 (MPa) | 1.204 ± 0.294 | 1.281 ± 0.758 | 0.817 ± 0.243 | 0.733 ± 0.167 | 0.485 ± 0.129 |
| Stiffness at 250 kPa, X2 (MPa) | 2.831 ± 0.634 | 3.260 ± 1.688 | 1.815 ± 0.372 | 1.764 ± 0.312 | 1.358 ± 0.250 |
| Stiffness at 500 kPa, X2 (MPa) | 4.716 ± 1.083 | 5.190 ± 2.349 | 2.989 ± 0.539 | 2.946 ± 0.460 | 2.357 ± 0.348 |
| Degree of anisotropy | 0.492 ± 0.158 | 0.403 ± 0.127 | 0.541 ± 0.092 | 0.504 ± 0.181 | 0.520 ± 0.107 |
| Ultimate tensile strength (MPa) | 20.542 ± 7.109 | 17.46 ± 5.817 | 21.087 ± 4.144 | 22.438 ± 5.619 | 12.324 ± 4.352 |
| Ultimate tensile strain | 0.317 ± 0.086 | 0.237 ± 0.037 | 0.449 ± 0.062 | 0.46 ± 0.090 | 0.608 ± 0.070 |
| Uniaxial tangent modulus (MPa) | 123.521 ± 53.021 | 119.94 ± 58.108 | 110.714 ± 30.824 | 101.834 ± 34.104 | 46.001 ± 15.334 |
3.2. Biaxial mechanical response
The mean equibiaxial stress–strain curves in the preferred and cross-fiber directions for the five testing groups are shown in Fig. 2. In general, the curves exhibited the well-known nonlinear stress-strain response of soft tissues. The initial portion of the curves, known as the toe region, had a high deformation, low stress characteristic due to the un-crimping of collagen fibers and elasticity of elastin. Collagen fibers were then progressively recruited and dominated the stiff mechanical response at higher stress levels. A more gradual transition from low to high stiffness regions was observed in FBP, while ABP and CBP had a pronounced rapid stiffening effect at the transitional region in both directions. The transition region of both PPK and PPN samples occurred at earlier strains than BP samples. The average equibiaxial response was nearly identical for the PPN and PPK groups. From Fig. 2 it can also be seen that all of the tested tissues exhibited anisotropic behavior, being much less extensible in the preferred fiber direction.
Fig. 2.
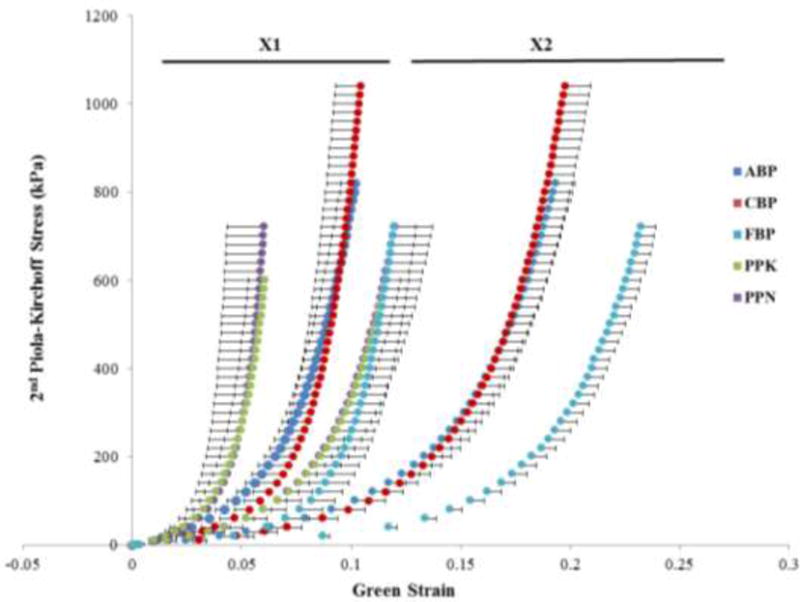
Equibiaxial response data for BP and PP testing groups, presented as mean ± standard error bars.
At 60 kPa, ABP and CBP samples were significantly stiffer than FBP, but not significantly different from PPN and PPK samples in the preferred fiber direction. The FBP samples were significantly more compliant than both PPN and PPK samples. Similarly, at 250 kPa in the preferred fiber direction ABP samples were significantly stiffer than FBP samples. Stiffness of CBP samples in the preferred fiber direction was higher than that of FBP samples, but the difference was not statistically significant. Comparing against PP tissues, CBP tissues were found to be significantly more compliant than only PPN tissues, and FBP tissues were significantly more compliant than both PPN and PPK samples. This trend was preserved at the 500 kPa level in the preferred fiber direction, although at this level, PPN tissues were found to be significantly stiffer than both ABP and CBP tissues. Although FBP could not be considered to be significantly more compliant than the other BP samples, they were more compliant than PPN and PPK tissues.
In the cross-preferred fiber direction, however, ABP and CBP tissues were significantly stiffer than FBP tissues at 60 kPa, 250 kPa and 500 kPa, see Fig. 3 and Table 1. When comparing BP and PP groups, PPK tissues were significantly stiffer than CBP at the three stress levels, as well as ABP samples at the 250 kPa and 500 kPa levels. PPN tissues were significantly stiffer than CBP at the 250 kPa and 500 kPa stress levels. Additionally, both PPN and PPK tissues were significantly stiffer than FBP tissues at the three stress levels. There was no statistically significant difference in tissue stiffness between the ABP and CBP groups, and between the PPK and PPN groups at the three stress values.
Fig. 3.
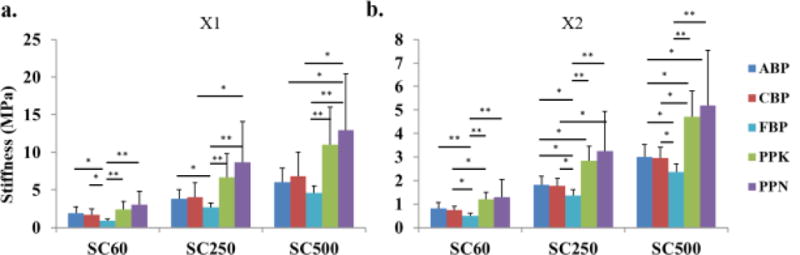
Mean and SD of stiffness measurements calculated as secant modulus at three stress levels for all testing groups. (*) indicates a statistically significant difference, (**) indicates a high statistically significant difference.
To further illustrate the differences in mechanical properties between the testing groups, linear regression was used to calculate the extensibility at the high-modulus linear region of the stress-strain curves (Fig. 4, Table 1). As expected, FBP tissues showed a higher extensibility than ABP and CBP tissue groups, with a statistically significant difference in the cross-preferred fiber direction. CBP samples exhibited a similar strain range to ABP samples. In addition, PPN and PPK samples were found to be significantly less extensible than ABP and CBP tissues, with high statistical significance against FBP. The mean DA values indicated that all specimens exhibited anisotropic behavior, where the cross-fiber direction almost doubled the strain value found in the preferred fiber direction (Table 1). Overall, PP samples were found to be the stiffest, followed by ABP and CBP samples, and finally FBP samples.
Fig. 4.
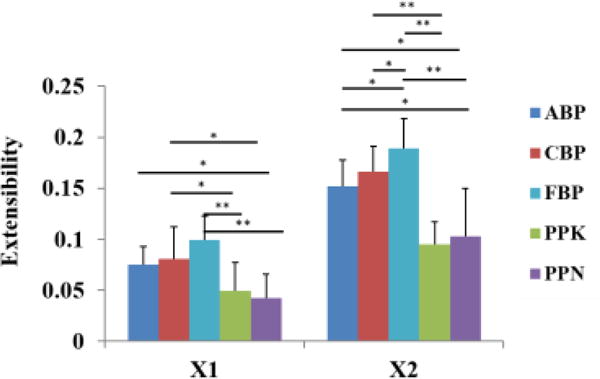
Mean and SD of extensibility measurements for all testing groups. (*) indicates a statistically significant difference, (**) indicates a high statistically significant difference.
3.3. Uniaxial failure
The UTS of ABP and CBP tissues were found to be much greater than that of FBP tissues, with a high statistical significance; both PPK and PPN tissues also had higher UTS values than FBP tissues, although only significant for PPK tissue (Fig. 5A). On the other hand, the UTE was significantly greater for FBP tissues than for ABP and CBP tissues, as well as for PPN and PPK tissues (Fig. 5B). The PP samples had a significantly lower strain at failure than the BP samples. The UTE was significantly smaller for PPN and PPK than for CBP and ABP samples. These tensile properties reflect the significantly higher tangent modulus of the ABP, CBP, and PP tissues compared with the FBP tissues (Table 1), and are in agreement with the biaxial data presented. There was no statistically significant difference in tensile properties between the ABP and CBP groups, and between the PPK and PPN groups.
Fig. 5.
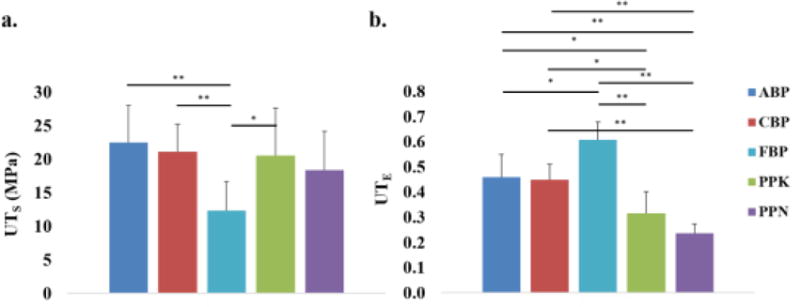
Mean and standard deviation of a) UTS measurements and b) UTE measurements for all testing groups. (*) indicates a statistically significant difference, while (**) indicates a high statistically significant difference.
3.4. Histological analysis
Histological images revealed that the FBP samples contained more thin immature collagen fibers than those of the ABP and CBP samples (Fig. 6). Collagen color (hue) quantitative examination of the images substantiated this finding, with a high statistically significant difference in the percentage of thin green fibers between ABP and FBP (1.969% versus 5.548%), and between CBP and FBP (2.629% versus 5.548%), where the proportion of thin newly synthesized fibers decreased with increasing age. The difference between the three BP groups for the yellow-orange thick collagen fibers did not achieve statistical significance.
Fig. 6.
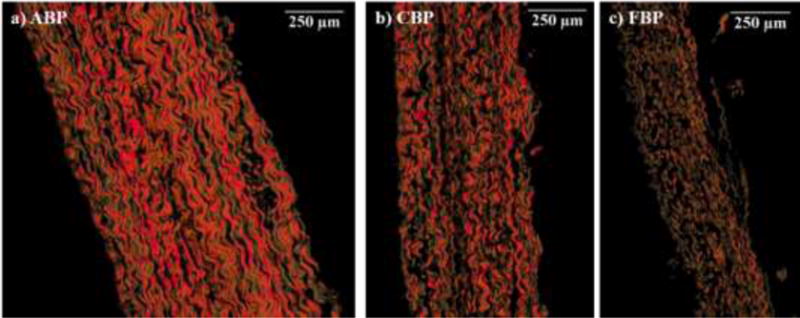
Representative picrosirius red-stained cross-sections for a) ABP, b) CBP and c) FBP.
4. Discussion
4.1 Comparison of mechanical properties with previous literature reports
BP and PP mechanical properties have been shown to differ as a result of the intrinsic tissue structural variability, selection and mounting of the samples into the testing device and/or due to altered treatment and testing protocols [12, 13, 15, 29–31]. Garcia Páez et. al investigated and compared the mechanical properties of GL-treated BP and PP tissues between 2001 and 2003 through the use of a hydraulic simulator, as well as uniaxial and biaxial loading [12, 13, 15, 31]. However, the biaxial testing conducted in these papers differs from the biaxial testing conducted in this study, resembling a membrane inflation test, in addition to lacking the multi-protocol aspect of the biaxial trials performed by our group. Garcia Páez et. al reported UTS values obtained by burst testing of 61.15–67.12 MPa and 49.93–61.18 MPa for PP and BP tissues, respectively, and uniaxially of 19.66–23.34 MPa and 25.70–28.72 MPa for PP and BP tissues, respectively). The same group also reported uniaxial UTS range of 15.80–34.36 MPa from CBP tissues in a later study [11]. Uniaxial results by this group match well with our UTS values of 17.46–20.54 MPa and 21.08–22.43 MPa for PP and ABP/CBP tissues, respectively.
Gauvin et. al [13] also investigated alterations in mechanical properties of BP and PP tissues as a result of parallel and orthogonal collagen fiber alignment with the uniaxial testing axis, in addition to random orientation. They found that the axially-aligned samples exhibited higher UTS values as compared to the perpendicularly-aligned samples. The randomly-aligned BP samples in the Gauvin et. al study were found to be stiffer than those included in this study, with a higher tangent modulus and lower strain at failure; however, UTS was preserved. The PP samples included in the Gauvin et. al study were found to be similarly stiff and extensible, but with lower UTS values than those obtained in this study.
As compared to previously conducted studies, the BP and PP UTE values found in this study correlated well to the results of previous studies by Crofts and Trowbridge [10], Pasquino et. al [32], and Oswal et. al [14] for BP tissues, and to the results of the Gauvin et. al [13] study for PP tissues. However, the Gauvin et. al study [13] found similar UTE values between BP and PP tissues, which were not found in this study. Although uniaxial tensile testing is advantageous for the determination of failure properties, biaxial testing can provide more complex insight into the coupling of the tissue directional responses.
There are several studies on the mechanical properties of chemically-treated or fresh BP using biaxial testing [21, 33–35], however, the biaxial mechanical response of thinner, younger BP tissues has not been investigated. To our knowledge, the only two studies that have used multi-protocol planar biaxial testing to characterize and compare the mechanical properties of BP and PP tissues were conducted by Li and Sun [36] and Labrosse et at. [37]. Li and Sun found PP tissues to be more compliant than BP tissues, differing from the results presented in this study. More recently, Labrosse et al. [37] conducted a study that characterized the biaxial mechanical response of fresh and fixed PP, commercial BP patches (chemically-treated), and fresh porcine aortic valve leaflets. In the Labrosse et al. study the GL-treated PP tissue was also more extensible than reported herein. We noted that these tests were performed under different chemical treatment and testing protocols to the current study: in Li and Sun’s study, the samples were left free-floating during fixation and anti-calcification solution was not used. Labrosse and colleagues used a cross-linking agent for 20 min and samples were left free-floating during fixation. In addition, Labrosse and colleagues adopted displacement-controlled testing protocols, and samples were preconditioned for 10 cycles and tested out of the saline bath. In this study, a more rigorous anti-calcification chemical treatment protocol was used, and during the chemical treatment the samples were pinned. It is known that during a cross-linking chemical treatment process, the geometry of samples may be changed, i.e., the in-plane dimensions may be reduced and the tissue thickness may increase. The pinning along the edges of the samples restricts such dimensional changes, thus, may lead to stiffer tissue responses.
4.2. Tissue properties related to BP collagen maturation and aging
The age-related changes that manifested in the increased stiffness and mechanical strength of the CBP and ABP tissues compared to the FBP tissues can be attributed primarily due to the intermolecular cross-linking of the collagen. The molecular mechanisms involved during growth, maturation and aging of collagenous tissues are well known and comprise two different mechanisms, a precise enzymatic process during development and maturation, and a subsequent non-enzymatic adventitious reaction with glucose and its oxidation products during aging, a process referred to as glycation [38]. Although both of these processes affect the mechanical properties of collagenous tissues, the most dramatic changes in the stiffness and mechanical strength of collagenous tissues occur between embryonic growth and maturity [39], as observed between the FBP group and the ABP/CBP groups in this study. This age-related stiffening of BP tissues is consistent with findings previously reported in other connective tissues such as cartilage [40] and tendons [41], as well as with human and porcine valves [42–44]. Nevertheless, to fully characterize the mechanical properties of GL-treated BP tissue, the long-term mechanical behavior of chemically-treated (exogenous cross-linkers) immature and mature BP needs to be quantified as well.
The histological analysis revealed a statistically significant difference in the percentage of green thin collagen fibers between the BP groups, where the proportion of thin fibers decreased with increasing age. Collagen’s ultimate tensile strength, in the range of 1 MPa, is directly proportional to the diameter of the fibrils [45]. Thus, thicker fibers in the mature collagen will be stronger than the thinner fibers observed in the younger collagen [46–48]. However, with increasing diameter, the flexibility of the older tissue also decreases [49]. A reduction in extensibility with age could be attributed to factors that impact collagen uncrimping, such as increased collagen crosslinking [50, 51] or elastic fiber fragmentation [41], which can lead to gradual permanent tissue stretch and less-crimped collagen fibers. These extensibility changes between FBP and ABP/CBP tissues motivate future studies of collagen crimp in BP tissues for BHV applications. Nevertheless, the finding that extensibility decreases with collagen maturation and aging is consistent with previous age-related studies in tendon collagen [52], human aortic valves [42, 53] and in porcine mitral and aortic valves [44].
To the authors’ knowledge, the only published study that has investigated and compared the mechanical properties of younger, thinner BP tissues with older BP tissues was performed by Sizeland et al. in 2014 [5]. GL-treated neonatal BP (4–7 days old) and ABP (18–24 months) samples were subjected to traditional uniaxial testing. The authors found that neonatal pericardium had a significantly higher elastic modulus and UTS than ABP. On the other hand, the UTE was significantly higher for ABP than for neonatal BP tissues. An opposite trend was observed in the uniaxial testing results of this study. It is important to note that the fetal tissues used in our study was obtained during the third trimester of cattle gestation, while the neonatal tissue used by Sizeland and colleagues was obtained from cattle between 4–7 days old. Additionally, it was not clear from their study what type of tissue presorting and preconditioning, if any, were carried out. Although this age difference and testing criteria could help to explain the discrepancies in the mechanical properties, current literature generally agrees that in collagenous tissues the stiffness and UTS increase with maturity [39, 44, 54].
4.3. Implications for TAV manufacturing
In this study, four main findings can be highlighted in relation to the identification of an optimal pericardial candidate biomaterial for manufacturing TAV leaflets. First, there were no statistically significant differences in the mechanical properties between the ABP and CBP tissues, indicating that CBP tissue is as strong and extensible but significantly thinner than ABP. These findings suggest that CBP may be a better tissue source in the manufacturing of TAV technologies. Second, although FBP tissues were significantly thinner, they were also significantly more extensible and more compliant than the adult and calf BP tissues. One could argue that the decreased thickness and higher flexibility of the FBP tissue would enable TAV to develop smaller profile configurations, provided the tissue meets the strength criteria. However, FBP— having the mechanical properties and characteristics described herein—should be used with caution and additional tissue characterization is needed before it can be used as a suitable biomaterial for TAV design.
Third, the mechanical properties of PP tissues were found to significantly differ from those of BP tissues. PP tissues were found to be significantly stiffer and less extensible than ABP and CBP tissues, but with similar ultimate tensile strength. PP tissues were found to be stronger and stiffer than FBP tissues, albeit much less extensible. Interestingly, their thickness, on average, was favorably thin. The impact of these mechanical properties differences on TAV function should be further investigated. These findings suggest that BP and PP tissues should not be used interchangeably in the manufacturing of surgical and transcatheter BHV.
Lastly, biaxial mechanical testing revealed that the mechanical behavior of the pericardium was quantitatively consistent with the specimen’s local fiber architecture. The high compliance of the native aortic valve leaflet along its radial direction allows the leaflet to be stretched in the diastolic cycle, while the circumferential stiffness is important for supporting the high transvalvular pressure [55]. Such anisotropic properties of the valve leaflets are important for proper valve function [56]; however, there is no evidence that the current process of fabrication of pericardium TAV leaflet tissue considers the direction of the tissue fibers with respect to the flow. The findings of this study suggest that in order to mimic the native response of the native valve leaflets, specimens with good structural uniformity should be oriented in a way such that the preferred fiber direction aligns with the circumferential direction of the leaflet, thus increasing the stiffness in that direction; while the cross-preferred fiber direction should be aligned along the radial direction, enhancing leaflet apposition.
4.4. Limitations
There are some limitations to this study. First, BP sacs were cryopreserved prior to fixation while PP sacs were chemically treated while still fresh. However, cryopreservation has been shown to retain the native tissue’s structural integrity and mechanical properties [20, 57]. Vinci et al. (2013) [58] investigated the mechanical properties of decellularized cryopreserved human pericardium. Uniaxial tensile loading tests revealed equivalent elastic modulus, UTS and UTE of the decellularized tissues, before and after the cryopreservation, in comparison with the fresh tissue.
Considering the effects of age on the mechanical properties of biomaterials, another limitation was the relatively narrow age range between the CBP and the ABP tissues compared to the broader age range between the FBP and the CBP tissues. A larger sample size including BP tissue between birth and 6 months of age would facilitate a deeper understanding and comparison of the age-dependent mechanical properties of the BP. Finally, flexure is also a major mode of deformation in the native heart valves [59] and pericardial BHV [60]. Yet, uniaxial and biaxial tensile experiments do not fully represent the flexure/bending deformation of the soft tissue. Thus, a better understanding of the flexural behavior of BP and PP tissues, especially when comparing young/older and thick/thin tissues, is of importance and should be examined in future studies.
5. Conclusions
By providing the first complete set of multi-protocol biaxial and uniaxial failure testing data for GL-treated PP and BP tissues using the same testing equipment and protocols, this study provided an important comparison with respect to the use of PP and BP tissues in the context of BHV and TAV fabrication. Planar biaxial testing was able to characterize the similar anisotropy of the two tissue species, in addition to the higher stiffness and reduced extensibility of PP tissues. Uniaxial failure testing was able to characterize the similar UTS between ABP, CBP and PP tissues, in addition to the higher UTE of BP tissues, compared to PP tissues, correlating with the biaxial results. While PP tissues were thinnest, potentially being more favorable for making a smaller crimped profile of TAV devices, they were found to be twice as stiff as the BP tissues, in addition to being less extensible. Overall, these results suggest that PP and BP tissues may not be used interchangeably in the manufacturing of TAV devices.
When comparing between BP tissues, ABP and CBP tissues demonstrated a significantly higher stiffness and UTs than FBP tissues, while FBP tissues were significantly thinner and showed a significant higher extensibility and UTE than ABP and CBP tissues. The results of this study suggest that although the decreased thickness and higher flexibility could give the FBP tissue a potential advantage for TAV production, additional biomechanical characterization is needed before it can used as a viable tissue candidate. From a mechanistic perspective, this study also highlights the potential advantage of considering the pericardium tissue anisotropy during the design and fabrication process of TAV.
Highlights.
Complete set of biaxial and uniaxial data for bovine-BP and porcine-PP pericardium
Calf BP has a similar mechanical response than Adult BP but is thinner
Fetal BP is thinner and more extensible than older BP but weaker
While PP is thinnest, it is stiffer and less extensible than BP
Acknowledgments
This work was supported in part by the R21HL108240 grant. Andrés Caballero is in part supported by a Fulbright-Colciencias fellowship. We would like to thank Michael Moon, Erica Shin, and Narae Kim for experimental and data analysis support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vesely I. The evolution of bioprosthetic heart valve design and its impact on durability. Cardiovascular Pathology. 2003;12(5):277–286. doi: 10.1016/s1054-8807(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 2.Aguiari P, Fiorese M, Iop L, Gerosa G, Bagno A. Mechanical testing of pericardium for manufacturing prosthetic heart valves. Interactive CardioVascular and Thoracic Surgery. 2016;22(1):72–84. doi: 10.1093/icvts/ivv282. [DOI] [PubMed] [Google Scholar]
- 3.I. Grand View Research. Cardiovascular and Soft Tissue Repair Patches Market Analysis by Application (Cardiac Repair, Vascular Repair, Pericardial Repair, Dural Repair, Soft Tissue Repair), by Raw Material (ePTFE, Biomaterial, Tissue Engineered Material) and Segment Forecasts To 2022. 2016 [Google Scholar]
- 4.Taramasso M, Pozzoli A, Latib A, La Canna G, Colombo A, Maisano F, Alfieri O. New devices for TAVI: technologies and initial clinical experiences. Nat Rev Cardiol. 2014;11(3):157–167. doi: 10.1038/nrcardio.2013.221. [DOI] [PubMed] [Google Scholar]
- 5.Sizeland KH, Wells HC, Higgins J, Cunanan CM, Kirby N, Hawley A, Mudie ST, Haverkamp RG. Age Dependent Differences in Collagen Alignment of Glutaraldehyde Fixed Bovine Pericardium. BioMed research international 2014. 2014 doi: 10.1155/2014/189197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis. Circulation. 2002;106(24):3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 7.Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. The Lancet. 2015;385(9986):2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 8.Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. The Lancet. 2015;385(9986):2485–2491. doi: 10.1016/S0140-6736(15)60290-2. [DOI] [PubMed] [Google Scholar]
- 9.Hulsmann J, Grun K, El Amouri S, Barth M, Hornung K, Holzfuss C, Lichtenberg A, Akhyari P. Transplantation material bovine pericardium: biomechanical and immunogenic characteristics after decellularization vs. glutaraldehyde-fixing. Xenotransplantation. 2012;19(5):286–97. doi: 10.1111/j.1399-3089.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 10.Crofts CE, Trowbridge EA. The tensile strength of natural and chemically modified bovine pericardium. Journal of biomedical materials research. 1988;22(2):89–98. doi: 10.1002/jbm.820220202. [DOI] [PubMed] [Google Scholar]
- 11.Garcia Paez JM, Carrera A, Jorge E, Millan I, Cordon A, Rocha A, Maestro M, Castillo-Olivares JL. Hysteresis of a biomaterial: influence of sutures and biological adhesives, Journal of materials science. Materials in medicine. 2007;18(5):715–24. doi: 10.1007/s10856-006-0009-x. [DOI] [PubMed] [Google Scholar]
- 12.Garcia Paez JM, Jorge Herrero E, Carrera Sanmartin A, Millan I, Cordon A, Martin Maestro M, Rocha A, Arenaz B, Castillo-Olivares JL. Comparison of the mechanical behaviors of biological tissues subjected to uniaxial tensile testing: pig, calf and ostrich pericardium sutured with Gore-Tex. Biomaterials. 2003;24(9):1671–9. doi: 10.1016/s0142-9612(02)00536-7. [DOI] [PubMed] [Google Scholar]
- 13.Gauvin R, Marinov G, Mehri Y, Klein J, Li B, Larouche D, Guzman R, Zhang Z, Germain L, Guidoin R. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. Journal of biomaterials applications. 2013;28(4):552–65. doi: 10.1177/0885328212465482. [DOI] [PubMed] [Google Scholar]
- 14.Oswal D, Korossis S, Mirsadraee S, Wilcox H, Watterson K, Fisher J, Ingham E. Biomechanical characterization of decellularized and cross-linked bovine pericardium. The Journal of heart valve disease. 2007;16(2):165–74. [PubMed] [Google Scholar]
- 15.Garcia Paez JM, Jorge E, Rocha A, Castillo-Olivares JL, Millan I, Carrera A, Cordon A, Tellez G, Burgos R. Mechanical effects of increases in the load applied in uniaxial and biaxial tensile testing. Part II. Porcine pericardium. Journal of materials science Materials in medicine. 2002;13(5):477–83. doi: 10.1023/a:1014710504963. [DOI] [PubMed] [Google Scholar]
- 16.Sellaro TL, Hildebrand D, Lu Q, Vyavahare N, Scott M, Sacks MS. Effects of collagen fiber orientation on the response of biologically derived soft tissue biomaterials to cyclic loading. Journal of biomedical materials research Part A. 2007;80(1):194–205. doi: 10.1002/jbm.a.30871. [DOI] [PubMed] [Google Scholar]
- 17.Aguiari P, Fiorese M, Iop L, Gerosa G, Bagno A. Mechanical testing of pericardium for manufacturing prosthetic heart valves. Interactive cardiovascular and thoracic surgery. 2016;22(1):72–84. doi: 10.1093/icvts/ivv282. [DOI] [PubMed] [Google Scholar]
- 18.Billiar KL, Sacks MS. Biaxial Mechanical Properties of the Natural and Glutaraldehyde Treated Aortic Valve Cusp—Part I: Experimental Results. Journal of biomechanical engineering. 1999;122(1):23–30. doi: 10.1115/1.429624. [DOI] [PubMed] [Google Scholar]
- 19.Zioupos P, Barbenel JC, Fisher J. Anisotropic elasticity and strength of glutaraldehyde fixed bovine pericardium for use in pericardial bioprosthetic valves. Journal of biomedical materials research. 1994;28(1):49–57. doi: 10.1002/jbm.820280107. [DOI] [PubMed] [Google Scholar]
- 20.Bia D, Pessana F, Armentano R, Perez H, Graf S, Zocalo Y, Saldias M, Perez N, Alvarez O, Silva W, Machin D, Sueta P, Ferrin S, Acosta M, Alvarez I. Cryopreservation procedure does not modify human carotid homografts mechanical properties: an isobaric and dynamic analysis. Cell Tissue Bank. 2006;7(3):183–94. doi: 10.1007/s10561-005-0655-0. [DOI] [PubMed] [Google Scholar]
- 21.Sacks MS, Chuong CJ. Orthotropic Mechanical Properties of Chemically Treated Bovine Pericardium. Annals of Biomedical Engineering. 1998;26(5):892–902. doi: 10.1114/1.135. [DOI] [PubMed] [Google Scholar]
- 22.Sacks MS, Sun W. Multiaxial mechanical behavior of biological materials. Annu Rev Biomed Eng. 2003;5:251–84. doi: 10.1146/annurev.bioeng.5.011303.120714. [DOI] [PubMed] [Google Scholar]
- 23.Sun W, Sacks MS, Sellaro TL, Slaughter WS, Scott MJ. Biaxial mechanical response of bioprosthetic heart valve biomaterials to high in-plane shear. J Biomech Eng. 2003;125(3):372–80. doi: 10.1115/1.1572518. [DOI] [PubMed] [Google Scholar]
- 24.Sun W, Sacks MS, Scott MJ. Effects of boundary conditions on the estimation of the planar biaxial mechanical properties of soft tissues. Journal of biomechanical engineering. 2005;127(4):709–715. doi: 10.1115/1.1933931. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Herrera CM, Atienza JM, Rojo FJ, Claes E, Guinea GV, Celentano DJ, Garcia-Montero C, Burgos RL. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med Biol Eng Comput. 2012;50(6):559–66. doi: 10.1007/s11517-012-0876-x. [DOI] [PubMed] [Google Scholar]
- 26.Junqueira LC, Montes GS, Sanchez EM. The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry. 1982;74(1):153–6. doi: 10.1007/BF00495061. [DOI] [PubMed] [Google Scholar]
- 27.Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22(2):97–104. [Google Scholar]
- 28.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113(10):1344–52. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 29.Arbeiter D, Grabow N, Wessarges Y, Sternberg K, Schmitz KP. Suitability of porcine pericardial tissue for heart valve engineering: biomechanical properties. Biomedical Engineering/Biomedizinische Technik. 2012;57(SI-1 Track-S):882–883. doi: 10.1515/bmt-2013-4095. [DOI] [PubMed] [Google Scholar]
- 30.Garcia Paez JM, Jorge-Herrero E, Carrera A, Millan I, Rocha A, Calero P, Cordon A, Salvador J, Sainz N, Mendez J, Castillo-Olivares JL. Chemical treatment and tissue selection: factors that influence the mechanical behaviour of porcine pericardium. Biomaterials. 2001;22(20):2759–67. doi: 10.1016/s0142-9612(01)00019-9. [DOI] [PubMed] [Google Scholar]
- 31.Garcia Paez JM, Jorge-Herrero E, Carrera A, Millan I, Rocha A, Salvador J, Mendez J, Tellez G, Castillo-Olivares JL. Porcine pericardial membrane subjected to tensile testing: preliminary study of the process of selecting tissue for use in the construction of cardiac bioprostheses, Journal of materials science. Materials in medicine. 2001;12(5):425–30. doi: 10.1023/a:1011201104225. [DOI] [PubMed] [Google Scholar]
- 32.Pasquino E, Pascale S, Andreon M, Rinaldi S, Laborde F, Galloni M. Bovine pericardium for heart valve bioprostheses: in vitro andin vivo characterization of new chemical treatments. Journal of Materials Science: Materials in Medicine. 1994;5(12):850–854. [Google Scholar]
- 33.Langdon SE, Chernecky R, Pereira CA, Abdulla D, Lee JM. Biaxial mechanical/structural effects of equibiaxial strain during crosslinking of bovine pericardial xenograft materials. Biomaterials. 1999;20(2):137–153. doi: 10.1016/s0142-9612(98)00142-2. [DOI] [PubMed] [Google Scholar]
- 34.Páez JG, Jorge E, Rocha A, Maestro M, Castillo-Olivares J, Millan I, Carrera A, Cordon A, Tellez G, Burgos R. Mechanical effects of increases in the load applied in uniaxial and biaxial tensile testing: Part I. Calf pericardium. Journal of Materials Science: Materials in Medicine. 2002;13(4):381–388. doi: 10.1023/a:1014388618649. [DOI] [PubMed] [Google Scholar]
- 35.Páez JMG, Carrera A, Cordón A, Jorge-Herrero E, Rocha A, Salvador J, Méndez J, Castillo-Olivares JL, Milláan I, Sainz N. Uniaxial and biaxial tensile strength of calf pericardium used in the construction of bioprostheses: biomaterial selection criteria. Journal of biomaterials applications. 2000;15(1):47–64. doi: 10.1106/77V6-PMK9-DUH4-LLR8. [DOI] [PubMed] [Google Scholar]
- 36.Li K, Sun W. Simulated thin pericardial bioprosthetic valve leaflet deformation under static pressure-only loading conditions: implications for percutaneous valves. Annals of biomedical engineering. 2010;38(8):2690–2701. doi: 10.1007/s10439-010-0009-3. [DOI] [PubMed] [Google Scholar]
- 37.Labrosse MR, Jafar R, Ngu J, Boodhwani M. Planar biaxial testing of heart valve cusp replacement biomaterials: Experiments, theory and material constants. Acta biomaterialia. 2016;45:303–320. doi: 10.1016/j.actbio.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 38.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mechanisms of Ageing and Development. 1998;106(1–2):1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 39.Bailey AJ, Paul RG. The mechanisms and consequences of the maturation and ageing of collagen. Proceedings of the Indian Academy of Sciences-Chemical Sciences, Springer. 1999:57–69. [Google Scholar]
- 40.Charlebois M, McKee MD, Buschmann MD. Nonlinear tensile properties of bovine articular cartilage and their variation with age and depth. Journal of biomechanical engineering. 2004;126(2):129–137. doi: 10.1115/1.1688771. [DOI] [PubMed] [Google Scholar]
- 41.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mechanisms of Ageing and Development. 2001;122(7):735–755. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 42.van Geemen D, Soares AL, Oomen PJ, Driessen-Mol A, Janssen-van den Broek MW, van den Bogaerdt AJ, Bogers AJ, Goumans MJ, Baaijens FP, Bouten CV. Age-Dependent Changes in Geometry, Tissue Composition and Mechanical Properties of Fetal to Adult Cryopreserved Human Heart Valves. PLoS One. 2016;11(2):e0149020. doi: 10.1371/journal.pone.0149020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin C, Pham T, Sun W. Significant differences in the material properties between aged human and porcine aortic tissues. European Journal of Cardio-Thoracic Surgery. 2011;40(1):28–34. doi: 10.1016/j.ejcts.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephens EH, de Jonge N, McNeill MP, Durst CA, Grande-Allen KJ. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Engineering Part A. 2009;16(3):867–878. doi: 10.1089/ten.tea.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtani O, USHIKI T, TAGUCHI T, KIKUTA A. Collagen fibrillar networks as skeletal frameworks: a demonstration by cell-maceration/scanning electron microscope method. Archives of histology and cytology. 1988;51(3):249–261. doi: 10.1679/aohc.51.249. [DOI] [PubMed] [Google Scholar]
- 46.Curwin SL, Roy RR, Vailas AC. Regional and age variations in growing tendon. Journal of morphology. 1994;221(3):309–320. doi: 10.1002/jmor.1052210306. [DOI] [PubMed] [Google Scholar]
- 47.Woo SLY, Buckwalter JA. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18–20, 1987. Journal of Orthopaedic Research. 1988;6(6):907–931. doi: 10.1002/jor.1100060615. [DOI] [PubMed] [Google Scholar]
- 48.Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. Journal of Applied Physiology. 1990;68(3):1033–1040. doi: 10.1152/jappl.1990.68.3.1033. [DOI] [PubMed] [Google Scholar]
- 49.Parry DAD. The molecular fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophysical Chemistry. 1988;29(1–2):195–209. doi: 10.1016/0301-4622(88)87039-x. [DOI] [PubMed] [Google Scholar]
- 50.Angrist A. Aging Heart Valves and a Unitary Pathological Hypothesis for Sclerosis 1 2. Journal of gerontology. 1964;19(2):135–143. doi: 10.1093/geronj/19.2.135. [DOI] [PubMed] [Google Scholar]
- 51.Bashey RI, Torii S, Angrist A. Age-related collagen and elastin content of human heart valves. Journal of gerontology. 1967;22(2):203–208. doi: 10.1093/geronj/22.2.203. [DOI] [PubMed] [Google Scholar]
- 52.Diamant J, Keller A, Baer E, Litt M, Arridge R. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proceedings of the Royal Society of London B: Biological Sciences. 1972;180(1060):293–315. doi: 10.1098/rspb.1972.0019. [DOI] [PubMed] [Google Scholar]
- 53.Christie GW, Barratt-Boyes BG. Age-dependent changes in the radial stretch of human aortic valve leaflets determined by biaxial testing. The Annals of thoracic surgery. 1995;60:S156–S159. doi: 10.1016/0003-4975(95)00219-b. [DOI] [PubMed] [Google Scholar]
- 54.LaCroix AS, Duenwald-Kuehl SE, Brickson S, Akins TL, Diffee G, Aiken J, Vanderby R, Lakes RS. Effect of Age and Exercise on the Viscoelastic Properties of Rat Tail Tendon. Annals of Biomedical Engineering. 2013;41(6):1120–1128. doi: 10.1007/s10439-013-0796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasan A, Ragaert K, Swieszkowski W, Selimović Š, Paul A, Camci-Unal G, Mofrad MR, Khademhosseini A. Biomechanical properties of native and tissue engineered heart valve constructs. Journal of Biomechanics. 2014;47(9):1949–1963. doi: 10.1016/j.jbiomech.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Merryman WD, Huang HYS, Schoen FJ, Sacks MS. The effects of cellular contraction on aortic valve leaflet flexural stiffness. Journal of biomechanics. 2006;39(1):88–96. doi: 10.1016/j.jbiomech.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Gerson CJ, Goldstein S, Heacox AE. Retained structural integrity of collagen and elastin within cryopreserved human heart valve tissue as detected by two-photon laser scanning confocal microscopy. Cryobiology. 2009;59(2):171–9. doi: 10.1016/j.cryobiol.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Vinci MC, Tessitore G, Castiglioni L, Prandi F, Soncini M, Santoro R, Consolo F, Colazzo F, Micheli B, Sironi L. Mechanical compliance and immunological compatibility of fixative-free decellularized/cryopreserved human pericardium. PloS one. 2013;8(5):e64769. doi: 10.1371/journal.pone.0064769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugimoto H, Shimada K, Sacks M. Dynamic Geometry of the native aortic heart valve leaflet. Journal of Biomechanics, submitted [Google Scholar]
- 60.Iyengar AK, Sugimoto H, Smith DB, Sacks MS. Dynamic in vitro quantification of bioprosthetic heart valve leaflet motion using structured light projection. Annals of Biomedical Engineering. 2001;29(11):963–973. doi: 10.1114/1.1415523. [DOI] [PubMed] [Google Scholar]


