Abstract
Purpose
The ability to predict mortality and admission to acute care hospitals, skilled nursing facilities (SNFs), and long-term care (LTC) facilities in the elderly and how it varies by activity of daily living (ADL) and instrumental ADL (IADL) status could be useful in measuring the success or failure of economic, social, or health policies aimed at disability prevention and management. We sought to derive and assess the predictive performance of rules to predict 3-year mortality and admission to acute care hospitals, SNFs, and LTC facilities among Medicare beneficiaries with differing ADL and IADL functioning levels.
Methods
Prospective cohort using Medicare Current Beneficiary Survey data from the 2001–2007 entry panels. In all, 23,407 community-dwelling Medicare beneficiaries were included. Multivariable logistic models created predicted probabilities for all-cause mortality and admission to acute care hospitals, SNFs, and LTC facilities, adjusting for sociodemographics, health conditions, impairments, behavior, and function.
Results
Sixteen, 22, 14, and 14 predictors remained in the final parsimonious model predicting 3-year all-cause mortality, inpatient admission, SNF admission, and LTC facility admission, respectively. The C-statistic for predicting 3-year all-cause mortality, inpatient admission, SNF admission, and LTC facility admission was 0.779, 0.672, 0.753, and 0.826 in the ADL activity limitation stage development cohorts, respectively, and 0.788, 0.669, 0.748, and 0.799 in the ADL activity limitation stage validation cohorts, respectively.
Conclusions
Parsimonious models can identify elderly Medicare beneficiaries at risk of poor outcomes and can aid policymakers, clinicians, and family members in improving care for older adults and supporting successful aging in the community.
Key works: outcomes, prediction, disability, Medicare, activity of daily living
1 Introduction
Americans age 65 years and older currently make up approximately 15% of the population, and will account for almost 22% by 2040 (US Department of Health & Human Services, 2016). Predicting events such as death and admission to acute care hospitals, skilled nursing facilities (SNFs), and long-term care (LTC) facilities in this large population is of great importance to healthcare planners and policymakers so that they can forecast and respond to resource needs by allocating appropriate funds. Predicting such events also provides important prognostic information to patients, family members, and clinicians by enabling interventions to delay such undesired events, and to allow people to prepare emotionally and/or financially for such events (Brodaty et al., 2014). The value of predictive rules to healthcare policymakers and healthcare planners can be increased if the included variables are derived from data that are routinely collected in the target population or a systematic sample. Similarly, the value of individual-level prognostic tools is enhanced if the tools are based on information that is readily accessible, such as responses to questions that can be asked of the patient or her/his proxy.
Several previous models have been developed to predict hospital admission or readmission among middle aged persons with a variety of health conditions (Billings et al., 2012; Kansagara et al., 2011; van Walraven et al., 2010). Robusto and colleagues developed and validated the drug derived complexity index, which stratified the general population aged 40 years and older according to one year and overall mortality and unplanned hospital admission derived from drug prescriptions (Robusto et al., 2016). McCormick and colleagues developed a predictive index for planned patient care in LTC settings among persons with HIV/AIDS (McCormick, Inui, Deyo, & Wood, 1991). Zhang and colleagues developed 1-, 5-, and 10-year mortality indices in those 70 years and older who participated in the Second Longitudinal Study on Aging (Zhang et al., 2012). However, these prior studies do not provide parsimonious models for predicting 3-year all-cause mortality, inpatient admission, SNF admission, and LTC facility admission in a nationally representative sample of Medicare Beneficiaries age 65 and older, nor has the prior work explored how patterns of disability relate to these risks. With the transition in causes of death from acute and infectious to chronic and degenerative illnesses (McKeown, 2009), the effective management of activity of daily living (ADL) and instrumental activity of daily living (IADL) limitations in the population will become increasingly important in preventing death, hospitalization, and the need for skilled nursing care or long-term care. The ability to predict the probability of these adverse events and how it varies by ADL and IADL status could be useful in measuring the success or failure of economic, social, or health policies aimed at disability prevention in and management of Medicare beneficiaries. Landi and colleagues found that after adjustment, disability was a stronger predictor of mortality among persons aged 80 years and older than multimorbidity (Landi et al., 2010). We therefore sought to derive and assess the predictive performance of rules to predict 3-year mortality and admission to acute care hospitals, skilled nursing facilities, and long-term care facilities among Medicare beneficiaries with differing levels of ADL and IADL functioning.
2 Methods
This study has approval by the Institutional Review Board at the University of Pennsylvania.
2.1 Data source
We used data from the Medicare Current Beneficiary Survey (MCBS), which is representative of the Medicare population and conducted by the Centers for Medicare and Medicaid Services (Health and Health Care of the Medicare Population, 2006; Kautter, Khatutsky, Pope, Chromy, & Adler, 2006; US Department of Health & Human Services, 2010). Beneficiaries, or their proxies when the sample person is unavailable, are interviewed about their health status and functioning during the autumn of the entry year into the survey and for the following three autumns, in addition to being surveyed about their healthcare utilization beginning January 1st after their autumn interview for a total of four years. To account for non-response and weighted sampling, survey weights are used, and those 80 years and older are oversampled because of their special needs (Kautter et al., 2006).
2.2 Study sample
The study cohort began with 33,934 community-dwelling adults aged 65 years and older who were part of the 2001–2007 MCBS entry panels, and each beneficiary was followed for three subsequent years. Beneficiaries enrolled in health maintenance organizations (n=10,047) were removed since we were uncertain if we had complete information about their utilization of healthcare services. Also, beneficiaries missing information on covariates (n=480) were excluded. Thus, 23,407 beneficiaries were included in the analyses.
2.3 Outcomes
Our study outcomes were all-cause mortality, inpatient hospital admission, SNF admission, and LTC facility admission, all ascertained during the three years after survey entry. Outcomes were obtained from the Cost and Use files. SNF admission was intended to capture short-term rehabilitation stays, while LTC facility admission was meant to ascertain more permanent placement. Since our focus was the burden of the adverse events, we treated death before inpatient hospital admission, SNF admission, and LTC facility admission as no event.
2.4 Predictor Variables
Predictor variables were categorized into different domains that included sociodemographics, health conditions, impairments, behavior, and function.
Sociodemographics included age (65–74, 75–84, or ≥85), sex, and race (non-Hispanic white, non-Hispanic black, Hispanic, or other). Education was grouped as less than high school, high school graduate, and some college and above. Living arrangement was categorized as lives alone, lives with spouse, lives with children, or lives with others. Dual eligibility was dichotomized as Medicare and Medicaid dual enrollment versus Medicare only. An indicator for proxy response versus self-response was included. Urban setting was compared to rural setting.
Health conditions were comorbidities that a doctor told the beneficiary he or she had or that occurred within the past year. The comorbidities were Alzheimer’s disease, amputation, angina pectoris or coronary artery disease, broken hip, cancer, chronic heart failure, complete or partial paralysis, depression, diabetes type 1, 2, or other or high blood sugar, emphysema/asthma/chronic obstructive pulmonary disease (COPD), hardening of the arteries, heart rhythm disease, heart valve disease, hypertension, incontinence, dialysis, or catheterization, mental or psychiatric disorder, mental retardation, myocardial infarction, non-rheumatoid arthritis, osteoporosis, Parkinson’s disease, rheumatoid arthritis, and stroke or brain hemorrhage.
Impairments included severe hearing impairment or being deaf, and severe vision impairment or having no usable vision.
The behavior domain was comprised of a variable for smoking (non-smoker, ever smoked, or current smoker).
Function at the baseline interview was measured by ADL and IADL activity limitation stages, which have been described previously (Kurichi et al., 2016; Stineman et al., 2014). Activity limitation stages were developed to specify clinically meaningful patterns of increasing difficulty with self-care items and to represent the severity and types of limitations experienced. Activity limitation stages improve on simple counts of limitation which only captures severity. Eating, toileting, dressing, bathing/showering, getting in or out of bed/chairs, and walking are ADL items. Using the telephone, managing money, preparing meals, doing light housework, shopping for personal items, and doing heavy housework are IADL items. There are 5 activity limitation stages within each of the ADL and IADL domains, ranging from 0–IV (no limitation to complete limitation) where greater disabilities are associated with higher numbered activity limitation stages. Activity limitation stage III was designed to account for patterns of limitation that are atypical of the hierarchy and is a non-fitting activity limitation stage.
All predictor variables were obtained from the baseline survey.
2.5 Statistical analyses
Beneficiaries were randomly divided into two groups. The development cohort included two-thirds of the sample (n=15,606), while the validation cohort included the remaining one-third of the sample (n=7,801). Beneficiaries from the development cohort were compared to those in the validation cohort with respect to each predictor. The absolute value of the standardized difference between the development and validation cohorts of each predictor was calculated (Yand D & Dalton JE, 2012).
Next, we examined bivariate associations between each predictor and each outcome (all-cause mortality, inpatient admission, SNF admission, and LTC facility admission) in the development cohort. Variables with p-value < 0.20 were entered in a multivariable logistic model for each outcome. Backward selection was then used to eliminate covariates with p-value > 0.05 to obtain a more parsimonious model. ADL and IADL activity limitation stages were entered into separate models because of co-linearity.
The C-statistic was obtained from the development cohort and was used to determine the goodness-of-fit of the model in the development cohort (Hanley & McNeil, 1982). Then the model coefficients in the parsimonious models obtained from the development cohort were applied to the validation cohort for each outcome, and the validation C-statistic was calculated for each outcome. The C-statistic obtained from the validation cohort was then compared to the one obtained from the development cohort for each outcome.
All statistical analyses used SAS 9.4 (SAS Institute, Inc.) (Lo A & A, 2005). All analyses accounted for complex sampling including weight, clustering, stratification, and sub-population.
3 Results
The average (standard deviation) age of Medicare beneficiaries in the development cohort was 76.4 (7.6) years. Most of the beneficiaries in the development cohort were female (56.7%), non-Hispanic white (83.7%), lived with their spouse (54.8%), and lived in an urban setting (71.9%) (table 1). Only a small portion was dually enrolled in Medicaid (11.5%) or had a proxy respond for them (7.1%). The most common self-reported health conditions were hypertension (58.0%), non-rheumatoid arthritis (44.8%), and incontinence, dialysis, or catheterization (26.8%). Approximately 6% of Medicare beneficiaries had either severe hearing impairment or deafness or severe vision impairment or no usable vision. Almost half the cohort had smoked (44.7%). The majority had no limitations in performing ADLs (72.5%) or IADLs (65.6%) at baseline. The characteristics of the validation cohort were, as expected, similar to those in the development cohort (table 1). Absolute standardized difference between the development and validation cohorts for all variables are very close to 0 which suggests that the two cohorts are similar (an absolute value >0.20 indicates imbalance).
Table 1.
Baseline characteristics of the development and validation cohorts
| Covariates | Development cohort weighted % N=15,606 |
Validation cohort weighted % N=7,801 |
Absolute standardized difference between the development and validation cohorts |
|---|---|---|---|
| Sociodemographics | |||
| Age | 0.002 | ||
| 65–74 | 55.4 | 55.3 | |
| 75–84 | 34.0 | 34.1 | |
| ≥85 | 10.6 | 10.6 | |
| Sex | 0.003 | ||
| Female | 56.7 | 56.5 | |
| Race/Ethnicity | 0.038 | ||
| Non-Hispanic white | 83.7 | 83.4 | |
| Non-Hispanic black | 7.8 | 7.5 | |
| Hispanic | 5.2 | 6.0 | |
| Other | 3.3 | 3.1 | |
| Education | 0.015 | ||
| Less than high school | 26.2 | 26.2 | |
| High school graduate | 29.5 | 30.1 | |
| Some college and above | 44.4 | 43.7 | |
| Living arrangement | 0.028 | ||
| Lives alone | 31.5 | 30.5 | |
| Lives with spouse | 54.8 | 55.2 | |
| Lives with children | 8.8 | 9.5 | |
| Lives with others | 4.9 | 4.9 | |
| Medicare and Medicaid dual enrollee | 11.5 | 11.9 | 0.015 |
| Proxy responded | 7.1 | 7.2 | 0.005 |
| Urban setting | 71.9 | 73.2 | 0.029 |
| Presence of self-reported health conditions | |||
| Alzheimer’s disease | 2.1 | 2.1 | 0.004 |
| Amputation | 0.8 | 1.0 | 0.018 |
| Angina pectoris/coronary artery disease | 9.3 | 9.4 | 0.005 |
| Broken hip | 3.1 | 3.3 | 0.011 |
| Cancer | 17.0 | 16.8 | 0.005 |
| Chronic heart failure | 4.6 | 4.8 | 0.008 |
| Complete or partial paralysis | 2.4 | 2.6 | 0.012 |
| Depression | 8.5 | 9.2 | 0.026 |
| Diabetes type 1, 2, or other or high blood sugar | 14.2 | 15.1 | 0.025 |
| Emphysema/asthma/chronic obstructive pulmonary disease | 13.3 | 13.5 | 0.007 |
| Hardening of the arteries | 7.5 | 7.8 | 0.013 |
| Heart rhythm disease | 12.5 | 12.7 | 0.005 |
| Heart valve disease | 5.6 | 5.8 | 0.009 |
| Hypertension | 58.0 | 59.5 | 0.030 |
| Incontinence, dialysis, or catheterization | 26.8 | 27.2 | 0.009 |
| Mental or psychiatric disorder | 5.4 | 5.5 | 0.005 |
| Mental retardation | 0.4 | 0.3 | 0.010 |
| Myocardial infarction | 12.5 | 12.6 | 0.003 |
| Non-rheumatoid arthritis | 44.8 | 45.6 | 0.016 |
| Osteoporosis | 15.5 | 15.6 | 0.001 |
| Parkinson’s disease | 1.2 | 1.0 | 0.011 |
| Rheumatoid arthritis | 8.5 | 7.9 | 0.024 |
| Stroke or brain hemorrhage | 9.8 | 9.4 | 0.014 |
| Impairments | |||
| Severe hearing impairment or deaf | 6.8 | 6.4 | 0.014 |
| Severe vision impairment or no usable vision | 6.2 | 6.4 | 0.009 |
| Behavior | |||
| Smoking | 0.019 | ||
| Non-smoker | 43.7 | 44.6 | |
| Ever smoked | 44.7 | 43.7 | |
| Current smoker | 11.6 | 11.7 | |
| Function | |||
| Activity of Daily Living Stage | 0.024 | ||
| 0 | 72.5 | 71.7 | |
| I | 14.4 | 15.2 | |
| II | 7.1 | 7.1 | |
| III | 5.2 | 5.1 | |
| IV | 0.9 | 0.9 | |
| Instrumental Activity of Daily Living Stage | 0.008 | ||
| 0 | 65.6 | 65.6 | |
| I | 16.3 | 16.1 | |
| II | 7.3 | 7.4 | |
| III | 8.9 | 9.0 | |
| IV | 1.9 | 1.9 | |
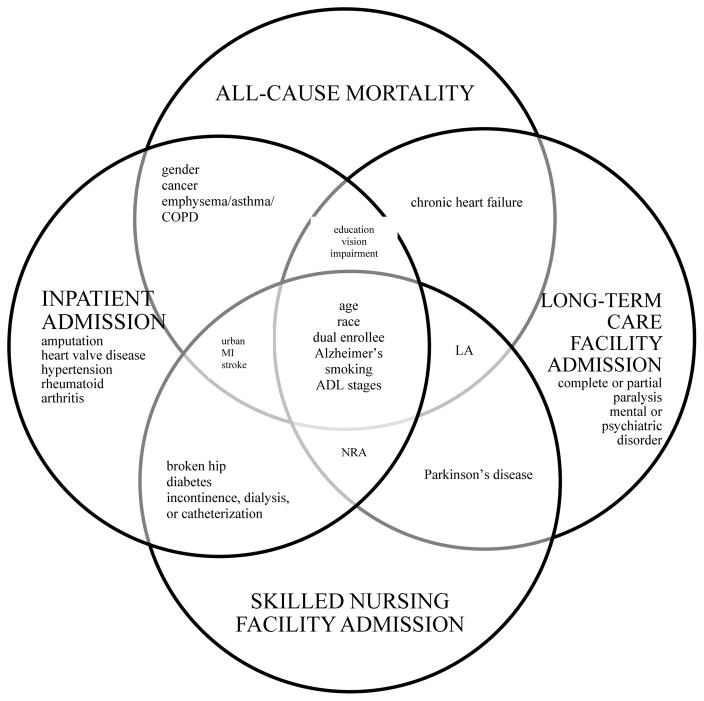
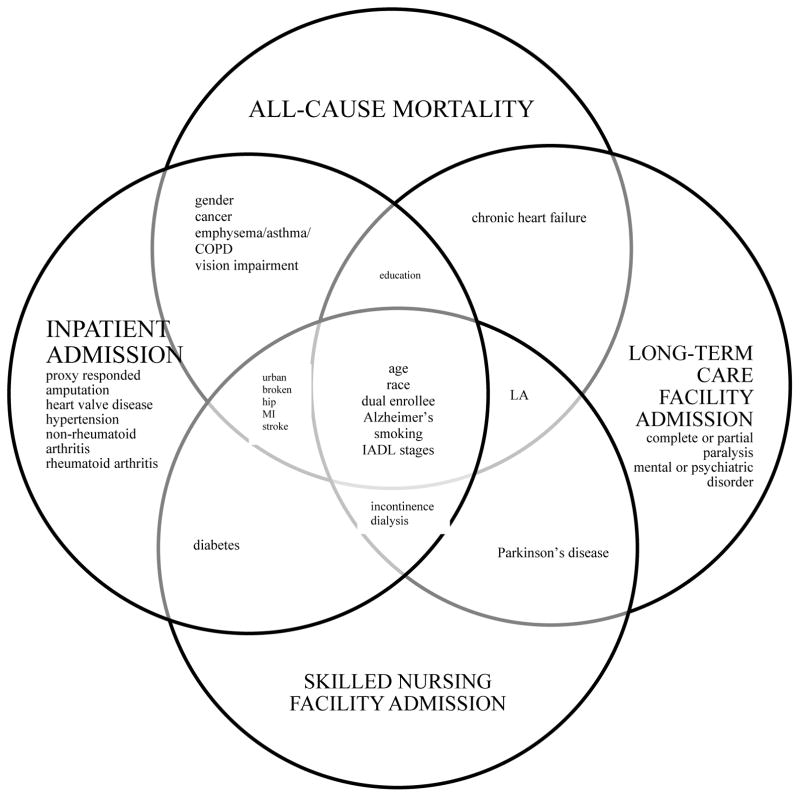
There were 33, 35, 32, and 28 predictors with p-value < 0.20 in the bivariate analysis for all-cause mortality, inpatient admission, SNF admission, and LTC facility admission, respectively, using ADL activity limitation stages as the measurement for function. The number of predictors remaining in the final parsimonious model predicting all-cause mortality, inpatient admission, SNF admission, and LTC facility admission (table 2) were 16, 22, 14, and 14, respectively (Figure 1). The predictors were similar when using IADL activity limitation stages, albeit with some minor differences (table 3). In predicting all-cause mortality, the IADL activity limitation stage parsimonious model included broken hip. Proxy-responded was included in the IADL activity limitation stage parsimonious inpatient admission model. Incontinence, dialysis, or catheterization was included in the IADL activity limitation stage parsimonious LTC facility model, while non-rheumatoid arthritis and severe vision impairment or having no usable vision were included in the ADL activity limitation stage parsimonious model.
Table 2.
Factors predictive of all-cause mortality, inpatient admission, skilled nursing facility admission, and long-term care facility admission among Medicare beneficiaries between 2001 and 2007 using activities of daily living
| All-cause Mortality | Inpatient admission | Skilled nursing facility admission | Long-term care facility admission | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-coefficient | SE | p value | β-coefficient | SE | p value | β-coefficient | SE | p value | β-coefficient | SE | p value | |
| Sociodemographics | ||||||||||||
| Age (reference: 65–74) | ||||||||||||
| 75–84 | 0.78 | 0.07 | <.01 | 0.46 | 0.04 | <.01 | 0.98 | 0.07 | <.01 | 1.33 | 0.13 | <.01 |
| ≥85 | 1.52 | 0.09 | <.01 | 0.80 | 0.06 | <.01 | 1.44 | 0.09 | <.01 | 2.21 | 0.14 | <.01 |
| Sex (reference: female) | ||||||||||||
| Male | 0.53 | 0.07 | <.01 | 0.10 | 0.04 | 0.01 | ||||||
| Race/Ethnicity (reference: Non-Hispanic white) | ||||||||||||
| Non-Hispanic black | 0.081 | 0.11 | 0.47 | −0.18 | 0.07 | 0.01 | −0.13 | 0.12 | 0.27 | −0.34 | 0.18 | 0.05 |
| Hispanic | −0.41 | 0.16 | 0.01 | −0.21 | 0.09 | 0.02 | −0.92 | 0.19 | <.01 | −0.98 | 0.28 | <.01 |
| Other | −0.23 | 0.20 | 0.25 | −0.55 | 0.12 | <.01 | −0.67 | 0.22 | <.01 | −1.17 | 0.39 | <.01 |
| Education (reference: Some college and above) | ||||||||||||
| Less than high school | 0.40 | 0.08 | <.01 | 0.13 | 0.05 | 0.01 | 0.33 | 0.11 | <.01 | |||
| High school graduate | 0.29 | 0.08 | 0.01 | 0.05 | 0.05 | 0.29 | 0.25 | 0.11 | 0.03 | |||
| Living arrangement (reference: Lives with spouse) | ||||||||||||
| Lives alone | 0.23 | 0.08 | 0.03 | 0.48 | 0.07 | <.01 | 0.73 | 0.11 | <.01 | |||
| Lives with children | 0.31 | 0.11 | 0.04 | 0.14 | 0.11 | 0.19 | −0.08 | 0.17 | 0.64 | |||
| Lives with others | 0.23 | 0.14 | 0.01 | 0.55 | 0.13 | <.01 | 0.51 | 0.20 | 0.01 | |||
| Medicare and Medicaid dual enrollee (reference: Medicare only) | 0.29 | 0.09 | 0.02 | 0.26 | 0.06 | <.01 | 0.35 | 0.09 | <.01 | 0.51 | 0.136 | <.01 |
| Non-urban setting (reference: urban setting) | 0.18 | 0.07 | 0.06 | 0.18 | 0.04 | <.01 | 0.16 | 0.07 | <.01 | |||
| Presence of self-reported health conditions (reference: no) | ||||||||||||
| Alzheimer’s disease | 0.87 | 0.14 | <.01 | 0.31 | 0.12 | 0.01 | 0.56 | 0.14 | <.01 | 1.44 | 0.16 | <.01 |
| Amputation | 0.63 | 0.19 | <.01 | |||||||||
| Broken hip | 0.28 | 0.10 | <.01 | 0.43 | 0.13 | <.01 | ||||||
| Cancer | 0.54 | 0.07 | <.01 | 0.16 | 0.05 | <.01 | ||||||
| Chronic heart failure | 0.45 | 0.11 | <.01 | 0.38 | 0.15 | 0.01 | ||||||
| Complete or partial paralysis | 0.83 | 0.19 | <.01 | |||||||||
| Diabetes type 1, 2, or other or high blood sugar | 0.14 | 0.05 | <.01 | 0.27 | 0.08 | <.01 | ||||||
| Emphysema/asthma/chronic obstructive pulmonary disease | 0.24 | 0.08 | 0.03 | 0.31 | 0.05 | <.01 | ||||||
| Heart valve disease | 0.18 | 0.06 | <.01 | |||||||||
| Hypertension | 0.17 | 0.04 | <.01 | |||||||||
| Incontinence dialysis, or catheterization | 0.17 | 0.04 | <.01 | 0.17 | 0.07 | 0.01 | ||||||
| Mental or psychiatric disorder | 1.06 | 0.40 | <.01 | |||||||||
| Myocardial infarction | 0.27 | 0.08 | 0.01 | 0.28 | 0.06 | <.01 | 0.23 | 0.08 | 0.01 | |||
| Non-rheumatoid arthritis | 0.18 | 0.04 | <.01 | −0.26 | 0.09 | <.01 | ||||||
| Parkinson’s disease | 0.46 | 0.20 | 0.02 | 0.68 | 0.25 | <.01 | ||||||
| Rheumatoid arthritis | 0.15 | 0.06 | 0.02 | |||||||||
| Stroke or brain hemorrhage | 0.28 | 0.08 | 0.01 | 0.28 | 0.06 | <.01 | 0.26 | 0.09 | <.01 | |||
| Impairments (reference: no) | ||||||||||||
| Severe vision impairment or no usable vision | 0.28 | 0.10 | 0.05 | 0.31 | 0.07 | <.01 | 0.30 | 0.13 | 0.02 | |||
| Behavior | ||||||||||||
| Smoking (reference: non-smoker) | ||||||||||||
| Ever smoked | −0.01 | 0.04 | 0.98 | 0.01 | 0.03 | 0.97 | −0.14 | 0.05 | <.01 | −0.25 | 0.07 | <.01 |
| Current smoker | 0.36 | 0.06 | <.01 | 0.18 | 0.04 | <.01 | 0.26 | 0.06 | <.01 | 0.43 | 0.09 | <.01 |
| Function | ||||||||||||
| Activity of Daily Living Stage (reference: 0) | ||||||||||||
| I | 0.45 | 0.08 | <.01 | 0.38 | 0.05 | <.01 | 0.67 | 0.08 | <.01 | 0.40 | 0.13 | <.01 |
| II | 1.05 | 0.09 | <.01 | 0.60 | 0.07 | <.01 | 1.00 | 0.10 | <.01 | 1.11 | 0.13 | <.01 |
| III | 1.01 | 0.11 | <.01 | 0.38 | 0.08 | <.01 | 0.99 | 0.11 | <.01 | 1.10 | 0.15 | <.01 |
| IV | 2.01 | 0.20 | <.01 | 0.63 | 0.19 | 0.1 | 0.43 | 0.25 | 0.09 | 0.40 | 0.31 | 0.20 |
| All-cause Mortality | Inpatient admission | Skilled nursing facility admission | Long-term care facility admission | |||||
|---|---|---|---|---|---|---|---|---|
| Development Cohort | Validation cohort | Development cohort | Validation cohort | Development cohort | Validation cohort | Development cohort | Validation cohort | |
| C-statistics | 0.779 | 0.788 | 0.672 | 0.669 | 0.753 | 0.748 | 0.826 | 0.799 |
SE = Standard error
Figure 1.
Title for Figure 1a. Comparison of predictors for all-cause mortality, inpatient admission, skilled nursing facility admission, and long-term care facility admission using activity of daily living activity limitation stages
Key for figure 1a: COPD = chronic obstructive pulmonary disease; MI = myocardial infarction; LA = living arrangement; ADL = activity of daily living; NRA = non-rheumatoid arthritis
Title for Figure 1b. Comparison of predictors for all-cause mortality, inpatient admission, skilled nursing facility admission, and long-term care facility admission using instrumental activity of daily living activity limitation stages
Key for figure 1b: COPD = chronic obstructive pulmonary disease; MI = myocardial infarction; LA = living arrangement; IADL = instrumental activity of daily living
Table 3.
Factors predictive of all-cause mortality, inpatient admission, skilled nursing facility admission, and long-term care facility admission among Medicare beneficiaries between 2001 and 2007 using instrumental activities of daily living
| All-cause Mortality | Inpatient admission | Skilled nursing facility admission | Long-term care facility admission | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-coefficient | SE | p value | β-coefficient | SE | p value | β-coefficient | SE | p value | β-coefficient | SE | p value | |
| Sociodemographics | ||||||||||||
| Age (reference: 65–74) | ||||||||||||
| 75–84 | 0.76 | 0.07 | <.01 | 0.46 | 0.04 | <.01 | 0.98 | 0.07 | <.01 | 1.30 | 0.13 | <.01 |
| ≥85 | 1.47 | 0.09 | <.01 | 0.80 | 0.06 | <.01 | 1.45 | 0.09 | <.01 | 2.12 | 0.14 | <.01 |
| Sex (reference: female) | ||||||||||||
| Male | 0.55 | 0.07 | <.01 | 0.15 | 0.04 | <.01 | ||||||
| Race/Ethnicity (reference: non-Hispanic white) | ||||||||||||
| Non-Hispanic black | 0.09 | 0.11 | 0.43 | −0.17 | 0.07 | 0.02 | −0.12 | 0.12 | 0.32 | −0.30 | 0.18 | 0.09 |
| Hispanic | −0.37 | 0.16 | 0.02 | −0.21 | 0.09 | 0.02 | −0.91 | 0.19 | <.01 | −0.91 | 0.27 | <.01 |
| Other | −0.27 | 0.20 | 0.17 | −0.55 | 0.12 | <.01 | −0.70 | 0.22 | <.01 | −1.16 | 0.38 | <.01 |
| Education (reference: Some college and above) | ||||||||||||
| Less than high school | 0.37 | 0.08 | <.01 | 0.12 | 0.05 | 0.01 | 0.28 | 0.11 | 0.01 | |||
| High school graduate | 0.27 | 0.08 | <.01 | 0.04 | 0.05 | 0.34 | 0.23 | 0.11 | 0.04 | |||
| Living arrangement (reference: Lives with spouse) | ||||||||||||
| Lives alone | 0.22 | 0.07 | <.01 | 0.47 | 0.07 | <.01 | 0.73 | 0.11 | <.01 | |||
| Lives with children | 0.30 | 0.11 | <.01 | 0.15 | 0.11 | 0.17 | −0.08 | 0.17 | 0.64 | |||
| Lives with others | 0.21 | 0.14 | 0.15 | 0.55 | 0.13 | <.01 | 0.51 | 0.20 | <.01 | |||
| Medicare and Medicaid dual enrollee (reference: Medicare only) | 0.29 | 0.10 | <.01 | 0.25 | 0.06 | <.01 | 0.34 | 0.09 | <.01 | 0.49 | 0.13 | <.01 |
| Proxy responded (reference: no) | −0.16 | 0.08 | 0.04 | |||||||||
| Non-urban setting (reference: urban setting) | 0.17 | 0.07 | 0.01 | 0.17 | 0.04 | <.01 | 0.16 | 0.07 | 0.02 | |||
| Presence of self-reported health conditions (reference: no) | ||||||||||||
| Alzheimer’s disease | 0.78 | 0.14 | <.01 | 0.37 | 0.13 | <.01 | 0.58 | 0.15 | <.01 | 1.27 | 0.17 | <.01 |
| Amputation | 0.66 | 0.19 | <.01 | |||||||||
| Broken hip | 0.28 | 0.13 | 0.04 | 0.29 | 0.10 | <.01 | 0.46 | 0.13 | <.01 | |||
| Cancer | 0.53 | 0.07 | <.01 | 0.15 | 0.05 | <.01 | ||||||
| Chronic heart failure | 0.45 | 0.11 | <.01 | 0.37 | 0.15 | 0.02 | ||||||
| Complete or partial paralysis | 0.84 | 0.19 | <.01 | |||||||||
| Diabetes type 1, 2, or other or high blood sugar | 0.15 | 0.05 | <.01 | 0.29 | 0.08 | <.01 | ||||||
| Emphysema/asthma/chronic obstructive pulmonary disease | 0.22 | 0.08 | <.01 | 0.29 | 0.05 | <.01 | ||||||
| Heart valve disease | 0.17 | 0.06 | <.01 | |||||||||
| Hypertension | 0.16 | 0.04 | <.01 | |||||||||
| Incontinence dialysis, or catheterization | 0.17 | 0.04 | <.01 | 0.19 | 0.07 | <.01 | 0.19 | 0.10 | 0.04 | |||
| Mental or psychiatric disorder | 0.92 | 0.40 | 0.02 | |||||||||
| Myocardial infarction | 0.27 | 0.08 | <.01 | 0.26 | 0.06 | <.01 | 0.23 | 0.08 | <.01 | |||
| Non-rheumatoid arthritis | 0.17 | 0.04 | <.01 | |||||||||
| Parkinson’s disease | 0.49 | 0.20 | 0.01 | 0.66 | 0.24 | <.01 | ||||||
| Rheumatoid arthritis | 0.14 | 0.06 | 0.03 | |||||||||
| Stroke or brain hemorrhage | 0.31 | 0.08 | <.01 | 0.29 | 0.06 | <.01 | 0.28 | 0.09 | <.01 | |||
| Impairments (reference: no) | ||||||||||||
| Severe vision impairment or no usable vision | 0.26 | 0.10 | <.01 | 0.30 | 0.07 | <.01 | ||||||
| Behavior | ||||||||||||
| Smoking (reference: non-smoker) | ||||||||||||
| Ever smoked | 0.01 | 0.04 | 0.93 | 0.01 | 0.03 | 0.92 | −0.12 | 0.05 | <.01 | −0.25 | 0.07 | <.01 |
| Current smoker | 0.35 | 0.06 | <.01 | 0.17 | 0.04 | <.01 | 0.26 | 0.06 | <.01 | 0.44 | 0.10 | <.01 |
| Function | ||||||||||||
| Instrumental Activity of Daily Living Stage (reference: 0) | ||||||||||||
| I | 0.66 | 0.09 | <.01 | 0.43 | 0.05 | <.01 | 0.61 | 0.08 | <.01 | 0.47 | 0.13 | <.01 |
| II | 1.08 | 0.10 | <.01 | 0.66 | 0.07 | <.01 | 1.11 | 0.10 | <.01 | 1.02 | 0.14 | <.01 |
| III | 0.91 | 0.09 | <.01 | 0.46 | 0.07 | <.01 | 0.78 | 0.10 | <.01 | 1.08 | 0.13 | <.01 |
| IV | 1.53 | 0.16 | <.01 | 0.50 | 0.14 | <.01 | 0.67 | 0.18 | <.01 | 1.08 | 0.22 | <.01 |
| All-cause Mortality | Inpatient admission | Skilled nursing facility admission | Long-term care facility admission | |||||
|---|---|---|---|---|---|---|---|---|
| Development cohort | Validation cohort | Development cohort | Validation cohort | Development cohort | Validation cohort | Development cohort | Validation cohort | |
| C-statistics | 0.781 | 0.786 | 0.673 | 0.666 | 0.751 | 0.747 | 0.825 | 0.802 |
SE = Standard error
The C-statistic for predicting all-cause mortality, inpatient admission, SNF admission, and LTC facility admission was 0.779, 0.672, 0.753, and 0.826 in the ADL activity limitation stage development cohorts, respectively, and 0.788, 0.669, 0.748, and 0.799 in the validation cohorts, respectively. The C-statistics were also similar when using IADL activity limitation stages in the development and validation cohorts. For all-cause mortality, inpatient admission, SNF admission, and LTC facility admission, the C-statistic was 0.781, 0.673, 0.751, and 0.825 in the IADL activity limitation stage development cohorts, respectively, and 0.786, 0.666, 0.747, and 0.802 in the validation cohorts, respectively.
4 Discussion
We found that routinely collected survey data can be used to predict older Medicare beneficiaries’ three-year risk of mortality and admission to an inpatient hospital, SNF, or LTC facility with reasonably high predictive accuracy (C-statistics in the validation cohort ranging from 0.669 to 0.799). Such models can be used on an aggregate basis to forecast and plan for population-level health services’ needs, and as a basis for risk adjustment in the context of evaluating quality of care, costs, and medical effectiveness. Our findings build and expand on previously developed models (Billings et al., 2012; Kansagara et al., 2011; McCormick et al., 1991; Robusto et al., 2016; van Walraven et al., 2010), particularly for those with limitations in performing ADLs and IADLs. The potential utility of these models for such purposes is enhanced by their basis in variables routinely ascertained in the MCBS. Such models may also be useful to aid in identifying high-risk persons who may benefit from interventions to reduce disparities in people at high risk of these particular outcomes, and to benefit individual patients and caregivers in planning for adverse life events. Although the predictors used in this study were retrieved from the MCBS, similar information at the individual level might instead be obtained from an electronic medical record or directly from patients’ responses.
Policymakers and clinicians are challenged with developing and providing services with limited resources and needing to focus on high impact problems. Developing prediction rules is one way to aid policymakers and clinicians in forecasting patient outcomes. Creating predictive models can help policymakers plan and develop services and can help identify groups at highest risk of poor outcomes. Knowledge of predictors can help guide future research efforts and clinicians to provide extra care to those sub-groups at risk in efforts to improve the quality of care provided to patients attempting to delay or reduce the risk of mortality, or admission to an inpatient hospital, SNF, or LTC facility.
Our earlier work demonstrates that higher baseline ADL activity limitation stage is associated with functional deterioration (Bogner et al., 2017), institutionalization (Bogner et al., 2017; Stineman, Xie, Streim, et al., 2012), and mortality (Bogner et al., 2017; Stineman, Xie, Pan, et al., 2012; Stineman, Xie, Streim, et al., 2012; Zhang et al., 2012) among older persons. We found in our current study that all ADL activity limitation stages (I–IV), i.e., beneficiaries with some level of difficulty performing the activities, were highly predictive of mortality and inpatient admissions compared to being classified at stage 0 where beneficiaries have no difficulty performing any ADL (p<0.01). For SNF and LTC facility admissions, only ADL activity limitation stages I–III compared to stage 0 were highly predictive (p<0.01). Beneficiaries categorized at higher, more disabled activity limitation stages may need institutionalization because of their increased care burden, or are at higher risk of death because the burden may be too severe. Creating advance directives and advance care planning are important in this high risk group.
Because of the importance given to the high increase in the oldest old group of the population (National Institute on Aging, 2015), we conducted a post hoc analysis to determine if similar variables were predictive of the four outcomes (mortality and admission to an inpatient hospital, SNF, and LTC facility) after we stratified the cohort by age group (65–74, 75–84, and ≥85). For mortality, sex, cancer, smoking, and ADL activity limitation stages were common predictors in all three models. Specifically for the 85 and older age group, the predictors of mortality also included education, Alzheimer’s disease, chronic heart failure, incontinence, dialysis, or catheterization, and vision impairment. For inpatient admissions, the common predictors for all three age groups were urban setting, myocardial infarction, and ADL activity limitation stages. Among those ≥85, broken hip and mental or psychiatric disorder were also predictors. Only race and ADL activity limitation stages were common predictors of SNF admissions regardless of age. For the oldest old group, living arrangement, education, urban setting, mental or psychiatric disorder, and heart valve disease were also predictors. Finally, for LTC facility admissions, living arrangement, Alzheimer’s disease, and ADL activity limitation stages were common predictors among all three age groups. Race, education, heart rhythm disease, complete or partial paralysis, and mental or psychiatric disorder were also predictors among those ≥85 years and older. These factors have also been found in other studies (Andersen-Ranberg K, Petersen I, Robine J, & Christensen K, 2011; National Institute on Aging, 2015; US Census Bureau, 2011).
Medicare provides health insurance for 43 million elderly and disabled people, accounting for 14% of the federal budget (Kennedy & Tuleu, 2007). The effective management of ADL and IADL limitations in the population will become increasingly essential. Beyond the impact of comorbid diagnoses, activity limitation is an independent risk factor for increasing healthcare costs (Chan et al., 2002). Costs increase more directly with the number of ADL limitations and health service use events rather than with the intensity of treatment received during discrete events (Chan et al., 2002). Thus, if more becomes known about the possibility of postponing the onset of activity limitations or stopping further deterioration, then the economic impacts of the growth of the older adult population and the growing number of younger beneficiaries entering Medicare because of disabilities can be mitigated. Therefore, the parsimonious models that we developed to predict mortality and admission to an inpatient hospital, SNF, or LTC facility can be used in real world applications to screen beneficiaries at the highest risk of these adverse outcomes and develop interventions at the individual-, family-, and community-levels within medical homes, accountable care organizations, and programs of all-inclusive-care for elders (PACE) targeted to reduce adverse events and health service use costs among disabled beneficiaries. The ADL and IADL limitation information ascertained from activity limitation stages could further aid in targeting interventions and population health initiatives to sub-groups of people with similar types and severities of disabilities.
Several strengths of this study are noteworthy. First, the MCBS is an ongoing survey of nationally representative Medicare beneficiaries, which enhances the utility of the predictive models. Second, the models had reasonably good predictive ability, as noted by the high C-statistics. Third, the models performed as well in the validation cohorts as it did in the development cohorts, suggesting that they are not over-fit.
There are some limitations of the study that deserve mention. The activity limitation stages were derived based on self-or proxy-reported responses, which may not be an accurate portrayal of which activities are truly difficult to perform. There may be response bias due to imperfect recall since survey questions ask the respondent to recall events during the past year. However, we felt that it was important to include proxy responses even though proxies may not have answered the same way as the sample person. Conversely, bias may have been introduced if proxy responses were excluded (Stineman, Ross, & Maislin, 2005). Additional variables, such as self-advocacy, prior falls, attitudinal barriers (i.e., fear), or unmet needs at the site of medical care (i.e., lack of interpreter availability) may have been associated with the outcomes, but were not available in our dataset. Our study results can only be applied to the elderly, community-dwelling Medicare population since beneficiaries less than 65 years of age were excluded, since those less than 65 years of age have much different rates of death and healthcare services utilization than older Medicare beneficiaries.
5 Conclusions
Parsimonious models can identify elderly Medicare beneficiaries at risk of poor outcomes and can aid policymakers, clinicians, and family members in improving care for older adults and supporting successful aging in the community. Results from this study that identified predictors for poor patient outcomes among elderly Medicare beneficiaries may have utility in developing guidelines to enable beneficiaries to dwell in the community longer. Prediction models may empower the patient, family members, and care providers to tailor care plans to the specific individual’s needs and possibly prevent or delay poor outcomes. Being able to understand these predictors will help clinicians educate patients and families about prevention strategies and approaches to care that reduce the risk of poor outcomes. Identifying beneficiaries who will benefit most from special attention may be key to improving outcomes and could also inform targeted interventions to reduce disparities for people with disabilities. Understanding the link between sub-groups of people most vulnerable to death, inpatient admission, SNF admission, and LTC facility admission and gaps in healthcare can help guide the development and implementation of interventions. Careful surveillance could enable disability management that reduces risks of adverse outcomes and improves the quality of life among elderly Medicare beneficiaries with disabilities.
Highlights.
Predictive models can be useful to patients, caregivers, and policymakers in caring for those who are older and disabled.
We found different variables that predicted 3-year death, and admission to a hospital, skilled nursing facility, and long-term care facility.
These models can identify elderly beneficiaries who are at risk of these poor health outcomes and the information can help support successful aging in the community.
Acknowledgments
We would like to thank Dr. Margaret G. Stineman for the foundation of the activity limitation staging systems.
Funding: This research was supported by the National Institutes of Health (AG040105). There are no personal conflicts of interest of any of the authors, and no authors reported disclosures beyond the funding source. Neither the National Institutes of Health nor the Centers for Medicare and Medicaid Services (CMS) (which provided the data) played a role in the design or conduct of the study, in the analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen-Ranberg K, Petersen I, Robine J, Christensen K. Who are the oldest-old? 2011 [Google Scholar]
- Billings J, Blunt I, Steventon A, Georghiou T, Lewis G, Bardsley M. Development of a predictive model to identify inpatients at risk of re-admission within 30 days of discharge (PARR-30) BMJ Open. 2012;2(4) doi: 10.1136/bmjopen-2012-001667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner HR, de Vries McClintock HF, Kurichi JE, Kwong PL, Xie D, Hennessy S, … Stineman MG. Patient Satisfaction and Prognosis for Functional Improvement and Deterioration, Institutionalization, and Death Among Medicare Beneficiaries Over 2 Years. Arch Phys Med Rehabil. 2017;98(1):1–10. doi: 10.1016/j.apmr.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodaty H, Connors MH, Xu J, Woodward M, Ames D group Ps. Predictors of institutionalization in dementia: a three year longitudinal study. J Alzheimers Dis. 2014;40(1):221–226. doi: 10.3233/JAD-131850. [DOI] [PubMed] [Google Scholar]
- Chan L, Beaver S, Maclehose RF, Jha A, Maciejewski M, Doctor JN. Disability and health care costs in the Medicare population. Arch Phys Med Rehabil. 2002;83(9):1196–1201. doi: 10.1053/apmr.2002.34811. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Health and Health Care of the Medicare Population. Technical Documentation for the Medicare Current Beneficiary Survey- Appendix A. 2006:2. [Google Scholar]
- Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, Kripalani S. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautter J, Khatutsky G, Pope GC, Chromy JR, Adler GS. Impact of nonresponse on Medicare Current Beneficiary Survey estimates. Health Care Financ Rev. 2006;27(4):71–93. [PMC free article] [PubMed] [Google Scholar]
- Kennedy J, Tuleu IB. Working age Medicare beneficiaries with disabilities: population characteristics and policy considerations. J Health Hum Serv Adm. 2007;30(3):268–291. [PubMed] [Google Scholar]
- Kurichi JE, Streim JE, Bogner HR, Xie D, Kwong PL, Hennessy S. Comparison of predictive value of activity limitation staging systems based on dichotomous versus trichotomous responses in the Medicare Current Beneficiary Survey. Disabil Health J. 2016;9(1):64–73. doi: 10.1016/j.dhjo.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Liperoti R, Russo A, Capoluongo E, Barillaro C, Pahor M, … Onder G. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. J Clin Epidemiol. 2010;63(7):752–759. doi: 10.1016/j.jclinepi.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Lo A, AG Variance Estimation and the Components of Variance for the Medicare Current Beneficiary Survey Sample. American Statistical Association Section on Survey Research Methods. 2005:3333–3341. [Google Scholar]
- McCormick WC, Inui TS, Deyo RA, Wood RW. Long-term care preferences of hospitalized persons with AIDS. J Gen Intern Med. 1991;6(6):524–528. doi: 10.1007/BF02598221. [DOI] [PubMed] [Google Scholar]
- McKeown RE. The Epidemiologic Transition: Changing Patterns of Mortality and Population Dynamics. Am J Lifestyle Med. 2009;3(1 Suppl):19S–26S. doi: 10.1177/1559827609335350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Aging. Trend 3: Rising Numbers of the Oldest Old. Why Population Aging Matters: A Global Perspective. 2015 Retrieved from https://www.nia.nih.gov/publication/why-population-aging-matters-global-perspective/trend-3-rising-numbers-oldest-old.
- Robusto F, Lepore V, D’Ettorre A, Lucisano G, De Berardis G, Bisceglia L, … Nicolucci A. The Drug Derived Complexity Index (DDCI) Predicts Mortality, Unplanned Hospitalization and Hospital Readmissions at the Population Level. PLoS One. 2016;11(2):e0149203. doi: 10.1371/journal.pone.0149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stineman MG, Ross RN, Maislin G. Functional status measures for integrating medical and social care. Int J Integr Care. 2005;5:e07. doi: 10.5334/ijic.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stineman MG, Streim JE, Pan Q, Kurichi JE, Schussler-Fiorenza Rose SM, Xie D. Activity Limitation Stages empirically derived for Activities of Daily Living (ADL) and Instrumental ADL in the U.S. Adult community-dwelling Medicare population. PM R. 2014;6(11):976–987. doi: 10.1016/j.pmrj.2014.05.001. quiz 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stineman MG, Xie D, Pan Q, Kurichi JE, Zhang Z, Saliba D, … Streim J. All-cause 1-, 5-, and 10-year mortality in elderly people according to activities of daily living stage. J Am Geriatr Soc. 2012;60(3):485–492. doi: 10.1111/j.1532-5415.2011.03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stineman MG, Xie D, Streim JE, Pan Q, Kurichi JE, Henry-Sanchez JT, … Saliba D. Home accessibility, living circumstances, stage of activity limitation, and nursing home use. Arch Phys Med Rehabil. 2012;93(9):1609–1616. doi: 10.1016/j.apmr.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Census Bureau. Sisty-five plus in the United States. 2011 Retrieved from https://www.census.gov/population/socdemo/statbriefs/agebrief.html.
- US Department of Health & Human Services. Medicare Current Beneficiary Survey (MCBS) 2010 Retrieved from http://www.cms.hhs.gov/MCBS/
- US Department of Health & Human Services. Administration on Aging (AoA) Aging Statistics. 2016 Retrieved from https://aoa.acl.gov/Aging_Statistics/Index.aspx.
- van Walraven C, Dhalla IA, Bell C, Etchells E, Stiell IG, Zarnke K, … Forster AJ. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557. doi: 10.1503/cmaj.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yand D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS(R) 2012 Retrieved from http://support.sas.com/resources/papers/proceedings12/335-2012.pdf.
- Zhang Z, Xie D, Kurichi JE, Streim J, Zhang G, Stineman MG. Mortality predictive indexes for the community-dwelling elderly US population. J Gen Intern Med. 2012;27(8):901–910. doi: 10.1007/s11606-012-2027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]