Abstract
Background
With the increased frequency of diagnostic imaging, pancreatic cysts are now detected in >3% of American adults. Most of these are intraductal papillary mucinous neoplasm (IPMN) with well-established but variable malignant potential. A biomarker that predicts malignant potential or dysplastic grade would help determine which IPMN require removal or can be safely observed. We previously reported that pancreatic fluid prostaglandin E2 (PGE2) levels may have promise as a predictor of IPMN dysplasia and seek to validate these results in the current study.
Study Design
Pancreatic cyst/duct fluid was prospectively collected from 100 patients with IPMN undergoing pancreatic resection. Surgical pathology revealed 47 low/moderate grade, 34 high grade, and 20 invasive IPMN. PGE2 levels were assessed by enzyme-linked immunoassay and correlated with IPMN dysplasia grade, demographics, clinical radiologic/pathologic variables, acute/chronic pancreatitis and non-steroidal anti-inflammatory drug use.
Results
Mean pancreatic cyst fluid PGE2 levels in high grade and invasive IPMN were significantly higher than low/moderate grade IPMN (3.5 and 4.4 respectively versus 1.2pg/µl, p<0.0016). At a threshold of 1.1pg/µl, PGE2 was 63% sensitive, 79% specific, and 71% accurate for detection of high grade/invasive IPMN. When tested in the subset of IPMN patients with preoperative pancreatic cyst fluid CEA >192ng/mL, PGE2 at a threshold of 0.5pg/µl demonstrated 78% sensitivity and 100% specificity, and 86% accuracy for detection of high grade/invasive IPMN.
Conclusions
Our results validate pancreatic cyst fluid PGE2 as an indicator of IPMN dysplasia especially in select patients with preoperative pancreatic cyst fluid CEA >192ng/mL. The inclusion of PGE2/CEA in a diagnostic biomarker panel may facilitate more optimal treatment stratification of IPMN patients.
Keywords: Prostaglandin E2, IPMN, Dysplasia, Biomarker, Pancreatic cyst, CEA
Introduction
Pancreatic cancer will be diagnosed in an estimated 53,070 Americans this year. In 2016, 41,780 died from pancreatic cancer. This makes pancreatic cancer the third leading cause of cancer-related death in the United States surpassing mortality from breast cancer(1). The aggressive biology and typical late discovery of pancreatic cancer contribute to these statistics(1). Most patients have inoperable disease and no chance for cure at the time of presentation(2). Thus, there is an urgent need for both improved systemic treatment options and earlier diagnostic strategies. Early detection and prevention of pancreatic cancer may be the answer for a unique subset of high-risk patients. Cystic lesions of the pancreas, some of which may progress to pancreatic cancer, provide the clinician with an opportunity to intervene prior to malignant transformation. While non-discriminatory resection of all pancreatic cysts would prevent many cancer cases, an unacceptable number of patients with low malignant potential pancreatic cysts would undergo unnecessary, potentially morbid pancreatic resection. Accurate preoperative diagnosis of pancreatic cyst type and malignant potential is therefore of utmost importance for optimal surgical risk stratification.
Intraductal papillary mucinous neoplasm (IPMN) is the most common premalignant mucinous cystic lesion of the pancreas. Most IPMN are accurately diagnosed based on radiographic features of multifocal ductal dilation alone. Unifocal (solitary) IPMN, however, must be distinguished from mucinous cystic neoplasm (MCN). MCN may be diagnosed by the presence of ovarian stroma on core biopsy or EUS-directed micro forceps biopsy. Unifocal IPMN may be diagnosed by the presence of a GNAS mutation. If neither are present, the patient falls into a category of the undifferentiated solitary mucinous cystic lesion of the pancreas (3).
Clinical management of IPMN, once diagnosed, is guided by an assessment of malignant potential. A fine needle aspiration consistent with high grade atypia or a core biopsy or EUS-directed micro-forceps biopsy consistent with high grade dysplastic or invasive IPMN may occasionally be available. An accurate dysplastic grade, however, is usually not known prior to IPMN surgical resection. Malignant potential of IPMN is linked to type of pancreatic ductal involvement. IPMN with branch-duct involvement alone (branch type) may often be observed while IPMN with main-duct involvement (with or without branch-duct involvement, i.e., mixed type and main type) are usually recommended for resection due to a higher risk of malignant transformation(4). Preoperative differentiation of IPMN ductal involvement is guided by cross-sectional imaging and endoscopic modalities. Nearly 30% of IPMN cases, however, are misclassified as having main duct involvement preoperatively that do not on surgical pathology (5). Conversely, 20% of IPMN thought to have branch-duct involvement alone preoperatively have main duct involvement on surgical pathology (5).
Although branch duct versus main duct differentiation is an important distinction in assessing IPMN malignant risk, it is unreliable by itself. There are a number of clinical factors and imaging characteristics which may be useful in prediction of IPMN malignant potential, including age, symptoms/signs/conditions, imaging, cytology, DNA profiling, labs, family history, obesity(6), smoking(7), new onset diabetes(8), main duct size, and presence of mural nodules(4). However, no existing preoperative predictive factors are accurate enough to identify high-risk IPMN for surgical removal and/or low-risk IPMN that can be safely monitored. Highly accurate preoperative biomarkers are necessary to optimize preoperative risk stratification.
Prostaglandin E2 (PGE2) is a product of cyclooxygenase-2 (COX-2), a key enzyme in the inflammatory pathway(9). COX-2 overexpression and elevated PGE2 levels have been observed in multiple cancer types including pancreas(10–13). We previously reported that pancreatic cyst fluid PGE2 levels correlated with degree of IPMN dysplasia in a small cohort of IPMN patients (n=29)(14). In the present study, we aim to validate these findings with a larger cohort to evaluate the diagnostic utility of PGE2 as a biomarker for high risk IPMN.
Methods
Pancreatic cyst fluid or main duct fluid (in the case of isolated main duct IPMN) samples were obtained from IPMN patients at the time of endoscopic ultrasound guided fine needle aspiration and/or pancreatic resection at Indiana University Health University Hospital between June 2003 and August 2016. All patients provided informed consent in accordance with the Indiana University Institutional Review Board. Additional de-identified pancreatic fluid samples (n=10) were kindly provided by Johns Hopkins School of Medicine (Dr. Michael Goggins) under a material transfer agreement between Indiana University and Johns Hopkins University. After procurement, pancreatic cyst fluid aliquots were placed immediately on ice and then stored at −80° C. All pancreatic cyst fluid samples were from patients with diagnoses of IPMN. The diagnosis of IPMN was confirmed on surgical pathology by a University Hospital Staff pathologist and then reconfirmed by a pancreatic pathologist. Pathologic parameters including IPMN dimensions, main/branch duct involvement, and dysplasia grade (according to World Health Organization criteria) were assessed. A retrospective review of our prospectively collected database which records demographic and clinical data for these patients was also performed. This was supplemented through review of electronic (and paper) medical records. For this study, patients taking oral hypoglycemic medications and/or insulin at the time of preoperative evaluation were categorized as diabetic. Patients taking non-steroidal anti-inflammatory medications (NSAIDs) at the time of pre-operative evaluation were categorized as NSAID users.
PGE2 and CEA Analysis
Pancreatic cyst fluid (15–50 µl) PGE2 was analyzed using a PGE2 enzyme-linked immunosorbent assay (ELISA) (GE Healthcare Bio-Sciences Corp, Cranbury, NJ). In this assay, unlabeled PGE2 within pancreatic fluid samples competes with a fixed amount of peroxidase-labeled PGE2 for binding to a plate-bound, PGE2-specific antibody. With the addition of a substrate, bound PGE2 peroxidase is quantified and converted to PGE2 concentration in the samples (pg/ul). Carcinoembryonic antigen (CEA) was determined by a Beckman Coulter DxI 800 analyzer or ELISA (Sigma-Aldrich, St. Louis, MO). All patient samples underwent PGE2 analysis. A subset (n=63) had adequate fluid for concomitant CEA analysis.
Statistical Analysis
IBM Statistical Package for the Social Sciences (SPSS) 24 was used for all statistical analyses. Graphpad Prism 7.02 was used for graphical representation of data. Descriptive statistics including mean, standard deviation, and frequencies were calculated as appropriate. Baseline demographic and clinic-pathologic data were compared between low/moderate grade dysplasia (LGD/MGD) and high-grade dysplasia (HGD)/invasive IPMN using t test for continuous data and chi square for categorical data. Groups were further subdivided into LGD/MGD, HGD, and invasive IPMN and compared using ANOVA and chi square. Pearson correlation coefficient was calculated for determination of PGE2 association with other continuous variables. PGE2 was then analyzed based on each additional categorical demographic/clinicopathologic variable using t test and ANOVA. Univariate and multivariate analyses were conducted to compare PGE2 levels between IPMN dysplasia grades. P-values of <0.05 were considered statistically significant. The diagnostic utility of PGE2 as a biomarker for HGD/invasive IPMN was ascertained using sensitivity/specificity calculations and receiver operator characteristic analyses.
Results
A cohort of 100 patients preoperatively diagnosed with IPMN consented to participation in the study. Following pancreatic resection and subsequent pathological confirmation of IPMN, this cohort was classified by dysplastic grade into 47 with low/moderate grade (LGD/MGD), 34 with high grade (HGD), and 20 with invasive IPMN. Age and gender were not significantly different between grades of IPMN dysplasia. Among patients with LGD/MGD, HGD, and invasive IPMN, 39.1%, 55.2%, and 42.9% respectively were men (p=0.4). Mean age of LGD/MGD IPMN patients was 68.8 years compared to 65.5 and 70.1 years for those with HGD and invasive IPMN (p=0.3). Prevalence of diabetes and NSAID use did not vary according to degree of dysplasia. Although there was no difference in the rate of clinically diagnosed pancreatitis between individual dysplastic grades, the HGD/invasive combined IPMN cohort which is considered to be of higher risk was more commonly associated with pathologic diagnosis of chronic pancreatitis than lower risk LGD/MGD IPMN (82.1% vs. 61.4%, p=0.05). Consistent with published literature, the rate of main duct involvement was higher in HGD/invasive versus low/moderate grade IPMN (79.1% vs. 44.4%, p=0.001).
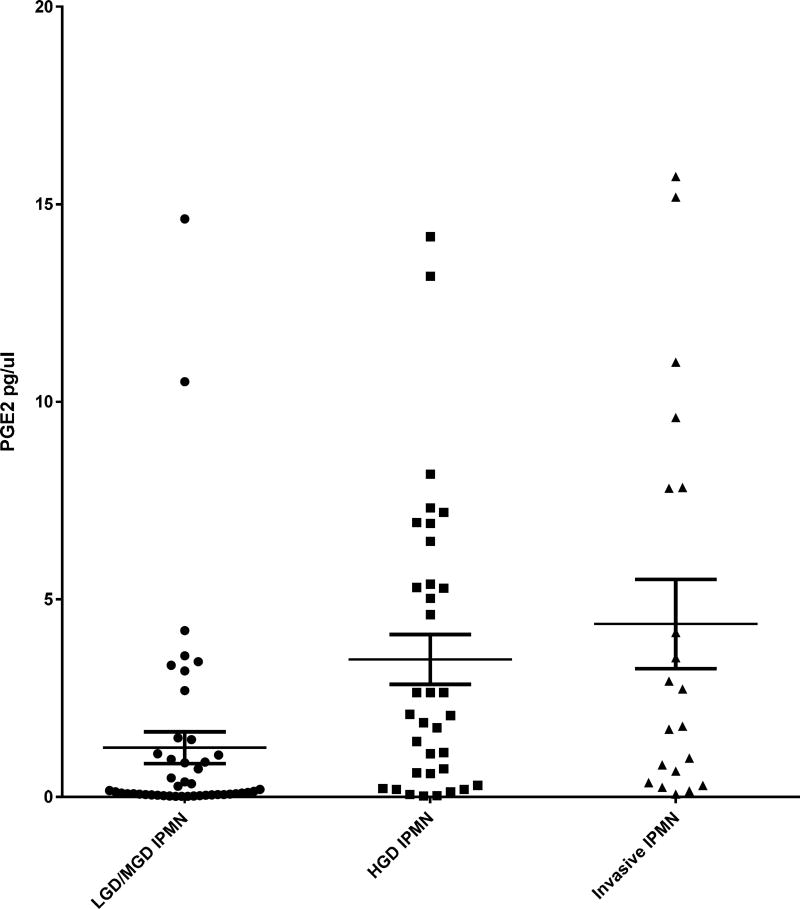
The concentration of PGE2 was determined in patient pancreatic cyst fluid and correlated with grade of IPMN dysplasia (Figure 1). Mean pancreatic cyst fluid PGE2 levels in LGD/MGD, HGD, and invasive IPMNs were significantly different, increasing in stepwise fashion from 1.2±2.7 to 3.5±3.7 and 4.4±5.1 pg/µl, respectively (p=0.002 for LGD/MGD vs. either HGD or invasive). Similarly, upon comparison of low-risk LGD/MGD versus high-risk HGD/invasive IPMN groups, the significant increase in PGE2 with dysplasia persisted (1.2±2.7 vs. 3.7±4.1 pg/µl, p<0.001). Pancreatic cyst fluid PGE2 levels were not affected by demographic or clinical variables such as gender, age, procedure used to obtain pancreatic fluid, presence of diabetes or pancreatitis, NSAID use, main or branch duct involvement, or cyst size (Table 1). Following multivariable analysis, including PGE2, duct involvement, and pathologic pancreatitis, only PGE2 and duct involvement demonstrated independently significant differences between IPMN grades of dysplasia.
Figure 1.
Prostaglandin E2 (PGE2) concentration in intraductal papillary mucinous neoplasm (IPNM) pancreatic fluid. PGE2 is plotted (y-axis) for each pancreatic cyst categorized by grade of IPMN dyplasia (x-axis). Mean PGE2 values are shown for each dysplasia grade with the middle horizontal lines. These are bordered by standard error of the mean bars. Mean PGE2 increases in a stepwise fashion from LGD/MGD, HGD, and invasive IPMN. This difference is statistically significant. LGD/MGD, low/moderate-grade dysplasia; HGD, high-grade dysplasia.
intraductal papillary mucinous neoplasm (IPNM) dysplasia. Pancreatic cyst fluid prostaglandin E2
Table 1.
Correlation of PGE2 Concentration with Demographics and Clinicopathologic Variables
| Mean PGE2 concentration |
p Value | |
|---|---|---|
| Sex | 0.6 | |
| Male | 2.7 | |
| Female | 2.3 | |
| Age* | −0.2 | 0.1 |
| Procedure | 0.1 | |
| Operating room | 2.7 | |
| Endoscopic | 1.7 | |
| Clinical AP/CP | 0.9 | |
| Yes | 2.5 | |
| No | 2.9 | |
| Pathologic CP | 0.4 | |
| Yes | 2.8 | |
| No | 2.7 | |
| DM | 0.7 | |
| Yes | 2.8 | |
| No | 2.4 | |
| NSAID use | 0.8 | |
| Yes | 2.3 | |
| No | 2.6 | |
| Duct involvement | 0.6 | |
| BD | 2.2 | |
| MD involved | 2.7 | |
| Cyst size* | −0.2 | 0.1 |
Continuous data analyzed with Pearson Correlation Coefficient.
AP, acute pancreatitis; CP, chronic pancreatitis; DM, diabetes mellitus.
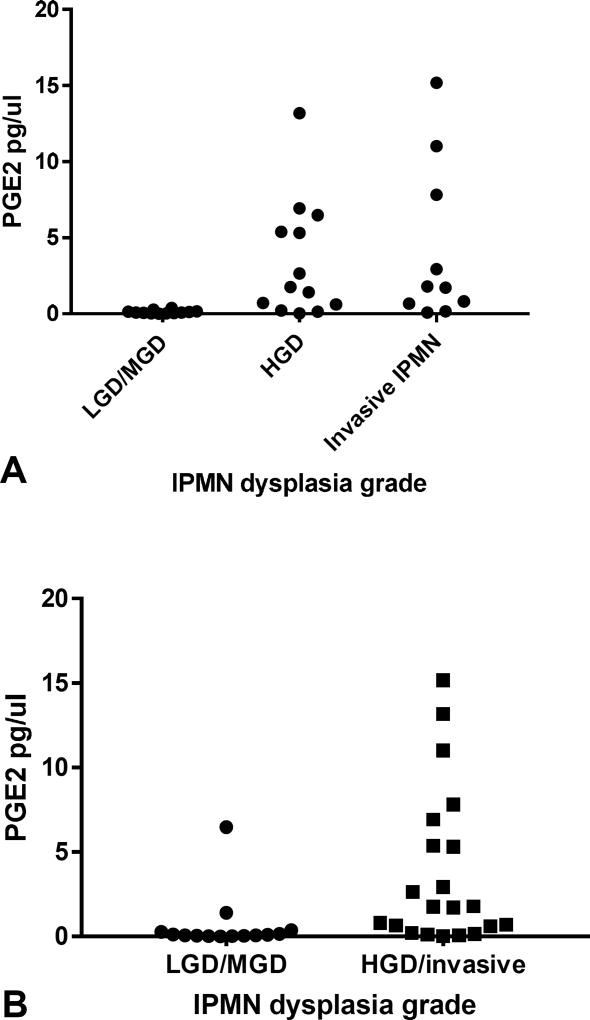
The diagnostic utility of PGE2 as a biomarker of IPMN dysplasia was assessed. Using Youden’s index, a PGE2 threshold of >1.1 pg/µl was chosen to indicate a positive test for HGD/invasive IPMN. This threshold yielded a sensitivity of 63.0% and specificity of 78.7% for detecting HGD/invasive IPMN, with a positive likelihood ratio of 3.0. The receiver operator curve revealed an area under the curve (AUC) of 0.77 (Figure 2). PGE2 was then evaluated in only those patients with likely pre-operative mucinous cyst diagnosis based on cyst fluid CEA of >192 ng/mL (n=35). Within this subset of patients, mean PGE2 in LGD/MGD IPMN was very low at 0.1±0.03 pg/µl, increasing to 3.4±1.1 and 4.2±1.7 pg/µl for HGD and invasive IPMN respectively (p=0.03) (Figure 3A) When comparing only two groups: LGD/MGD (0.1±0.03 pg/µl) and HGD/invasive (3.8±0.09 pg/µl) IPMN, this difference remains (p=0.001) (Figure 3B). At a threshold of 0.5 pg/µl chosen using Youden’s index, PGE2 more accurately diagnosed high-risk HGD/invasive IPMN with 78% sensitivity, 100% specificity, 86% accuracy, and an AUC of 0.91.
Figure 2.
Receiver operator characteristic (ROC) curve analysis of prostaglandin E2 (PGE2) for the diagnosis of HGD/invasive intraductal papillary mucinous neoplasm (IPMN). ROC curve shown here demonstrates high rate of diagnostic accuracy of PGE2 for diagnosis of high-grade dysplasia and invasive IPMN.
Figure 3.
Pancreatic fluid prostaglandin E2 (PGE2) concentration in patients with cyst fluid CEA > 192 ng/mL. Pancreatic cyst fluid PGE2 is plotted (y-axis) for each grade of intraductal papillary mucinous neoplasm (IPMN) dysplasia (x-axis) in only those with preoperative pancreatic cyst fluid CEA >192 ng/mL. (A) Divides IPMN dysplasia grade into 3 groups, LGD/MGD, HGD, and invasive IPMN. Mean PGE2 values again increase in a stepwise fashion with increasing dysplasia grade. (B) Divides IPMN dysplasia into only two groups, LGD/MGD and HGD/invasive IPMN. The same significant difference between LGD/MGD and HGD/invasive IPMN is demonstrated. LGD/MGD, low/moderate-grade dysplasia; HGD, highgrade dysplasia.
To compare PGE2 performance with the ability of EUS-directed fine needle aspiration/cytology to detect high grade atypia, 81 of the 100 IPMN study patients with cytologic analysis of pancreatic cyst fluid were identified. Of these, 53% were either non-diagnostic for high grade/malignant cells (n=16) or incorrect diagnoses (n=27) upon final surgical pathology. After excluding patients with non-diagnostic cytology results, sensitivity was 23%, specificity was 89%, and accuracy was 58% for the identification of high grade/invasive IPMN by cytology (n=65). In the cytology cohort with cyst fluid CEA >192 ng/mL (n=27), sensitivity, specificity, and accuracy were 38%, 73%, and 52% respectively.
Discussion
For patients with pancreatic IPMN, an accurate assessment of malignant potential is critical to guide optimal clinical care. The gold standard for determination of malignant potential is dysplastic grade assessed on pathology. Few IPMN are feasible for core biopsy or EUS-directed micro-forceps biopsy with adequate sampling for dysplastic grade assessment. In the current series, no core biopsies were performed. EUS-directed fine-needle-aspiration is routine in larger IPMN but the resultant cytology is often inaccurate or non-diagnostic for IPMN dysplasia (53% in the current series). Other markers of malignant potential including demographic, clinical, laboratory, endoscopic, and radiographic parameters fall short of an accurate prediction of dysplastic grade. Thus, a significant challenge persists in preoperative determination of IPMN dysplastic grade. Due to the lack of reliable indicators of IPMN dysplasia, patients may be inappropriately risk stratified and either forego necessary or undergo unnecessary surgical resection. This may result in under-treatment of high-risk IPMN or over-treatment of low-risk IPMN, respectively. Thus, accurate biomarkers of IPMN dysplasia are needed for optimal clinical management.
Newer pancreatic cyst fluid biomarkers are more commonly sufficient to distinguish cyst types. None, however, has consistently been a reliable indicator of IPMN dysplasia(15). Perhaps the most widely used pancreatic cyst fluid biomarker is CEA(16). CEA is able to differentiate mucinous from non-mucinous type cysts with an accuracy of 77% according to a large multi-institutional validation study of 1,861 patients(17). However, CEA alone lacks sufficient accuracy for routine identification of mucinous cysts and does not correlate with grade of dysplasia(18–20). A DNA mutation in KRAS is sufficient to diagnose mucinous cystic lesions (IPMN and MCN). A GNAS mutation is sufficient to diagnose IPMN and distinguish it from MCN and other non-mucinous pancreatic cyst types(21, 22). Neither the presence of KRAS, GNAS, or both mutations predict malignancy(18, 23). PancraGEN is a commercially available test that measures quantity and quality of DNA, KRAS, and GNAS oncogene mutations, LOH tumor suppressor mutations and DNA mutation copy number. Based on a proprietary combination of this molecular data and clinical variables, PancraGEN categorizes a pancreatic cyst as benign, statistically indolent, statistically higher risk, or aggressive(24). These categories guide a clinician’s recommendations for surveillance or surgery. This test is expensive but reports a higher accuracy than the 2012 algorithm as put forward by the revised International Consensus Guidelines. It too falls short of an optimal test to predict malignant potential.
We previously reported that pancreatic fluid prostaglandin E2 (PGE2) may be a promising biomarker of IPMN dysplasia(14). The present study seeks to validate this in a larger cohort of 100 IPMN patients, 53 with HGD or invasive IPMN. Pancreatic cyst fluid PGE2 levels were significantly higher in HGD/invasive IPMN compared to LGD/MGD. PGE2 alone demonstrated 70.8% accuracy in the detection of high-risk IPMN, compared to cytologic cyst fluid analysis with an accuracy of 58%. No correlation was found between PGE2 levels and the presence of chronic inflammation (chronic pancreatitis) other than pancreatitis associated with HGD or invasive IPMN. Similarly, pancreatic fluid PGE2 levels did not correlate with NSAID use. Importantly, when evaluated in a population of patients with CEA >192ng/mL, the accepted cutoff for mucinous versus non-mucinous cyst differentiation, diagnostic accuracy increased to 86% with 78% sensitivity and 100% specificity. This demonstrates that when utilized in a subset of patients with higher CEA production, the diagnostic accuracy of PGE2 as a biomarker of IPMN dysplasia substantially improves. Why a higher CEA would identify a subset of IPMN patients where PGE2, a marker of inflammation, would be more predictive is unclear. Finally, while cyst fluid PGE2 levels in other pancreatic cysts such serous cystadenomas (SCN) and MCN are low as in LGD/MGD IPMNs, PGE2 levels in a small number of pseudocysts tested in our laboratory were relatively high (unpublished observations). Thus, PGE2 would be most useful specifically in patients with pre-operative diagnosis of IPMN as a predictor of IPMN dysplasia.
The observed stepwise increase in mean PGE2 levels with IPMN dysplastic grade suggests that PGE2 production may be associated with malignant progression. To further evaluate this, a prospective study testing serial samples from individual patients under surveillance for possible IPMN should be performed. PGE2 is known to be involved in both angiogenesis and immunosuppression in the tumor microenvironment thus facilitating tumorigenesis(25). We have previously shown that the PGE2 pathway plays an important role in pancreatic tumorigenesis. Specifically, we demonstrated that COX-2, one of the enzymes that catalyzes the reaction producing prostaglandins, is over-expressed in precursor lesions known as pancreatic intraepithelial neoplasias (PanINs) and pancreatic adenocarcinomas(10, 11). Furthermore, NSAIDs that inhibit COX activity and block the inflammatory response may inhibit tumor growth in combination with other agents both in cell culture and animal models, including developmental models, of pancreatic cancer(10, 26–28).
Limitations of the current study include being retrospective with the majority of samples originating from a single institution. Validation of these results in a large, prospective multi-institution study would be optimal. Additionally, all patients included in this study underwent surgical resection and therefore may not be representative of the population overall population of surveillance and surgical patients in whom PGE2 testing would potentially be useful.
Other potential cyst fluid biomarkers for the determination of IPMN dysplasia have been reported in the literature. The cytokine interleukin-1β (IL1β) was shown to be 92% accurate in detecting HGD/invasive IPMN in a sample size of 40 cyst fluids(29). This report of IL1 and ours of PGE2, both markers of inflammation elevated in high-risk IMPN, provide evidence for the involvement of inflammation with malignant progression – either as a cause or consequence. Regardless, such immune response indicators may serve as potential biomarkers of dysplasia and warrant further testing. Telomerase activity has also been recently reported to be higher in invasive than non-invasive mucinous lesions with an accuracy of 88.2% in the identification of HGD/invasive IPMN(30, 31). Several microRNA (miRNA)-based biomarkers have shown promise in detecting high-risk IPMN(32, 33). Matthaei et al. reported a panel of several miRNAs with a sensitivity and specificity of 89% and 100% for differentiating HGD from LGD IPMN(34). Mucins, specifically, MUC2 and MUC4 were elevated in pancreatic cyst fluid of HGD/invasive IPMN as compared to LGD/MGD IPMN in a study by Maker et al. (35). A later publication reported 89.7% accuracy of a panel of mucins for detecting HGD and invasive IPMN (36). One or more of these markers may fulfill the clinical need for a marker of IPMN dysplasia, but all await more extensive validation.
Conclusions
We have validated PGE2 as a promising pancreatic fluid biomarker able to distinguish low/moderate grade from high/invasive grade IPMN. Pending future prospective evaluation, such a biomarker would optimize clinical management of pancreatic cyst patients. In patients suspected of IPMN, a diagnostic panel including pancreatic fluid PGE2 may facilitate risk stratification by identifying high-risk (high/invasive grade) lesions for surgical resection and low-risk (low/moderate grade) lesions appropriate for monitoring.
Acknowledgments
Support: This project received support from the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Abbreviations
- IPMN
intraductal papillary mucinous neoplasm
- MCN
mucinous cystic neoplasm
- PGE2
prostaglandin E2
- COX-2
cyclooxygenase-2
- NSAID
non-steroidal anti-inflammatory medications
- CEA
carcinoembryonic antigen
- LGD
low grade dysplasia
- MGD
moderate grade dysplasia
- HGD
high grade dysplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the American Hepatopancreatobiliary Association, Miami, FL, April 2017.
References
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: 2016. [accessed 16 January]. http://seer.cancer.gov/statfacts/html/pancreas.html. [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Roch AM, Bigelow K, Schmidt CM, 2nd, et al. Management of undifferentiated solitary mucinous cystic lesion of the pancreas: a clinical dilemma. J Am Coll Surg. 2017 doi: 10.1016/j.jamcollsurg.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt CM. Is surgical intervention for cystic neoplasms of the pancreas being underutilized? J Gastrointest Surg. 2014;18:184–186. doi: 10.1007/s11605-013-2396-x. [DOI] [PubMed] [Google Scholar]
- 6.Sturm EC, Roch AM, Shaffer KM, et al. Obesity increases malignant risk in patients with branch-duct intraductal papillary mucinous neoplasm. Surgery. 2013;154:803–808. doi: 10.1016/j.surg.2013.07.011. discussion 808–809. [DOI] [PubMed] [Google Scholar]
- 7.Carr RA, Roch AM, Shaffer K, et al. Smoking and IPMN malignant progression. Am J Surg. 2016 doi: 10.1016/j.amjsurg.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Mimura T, Masuda A, Matsumoto I, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol. 2010;44:e224–229. doi: 10.1097/MCG.0b013e3181d8fb91. [DOI] [PubMed] [Google Scholar]
- 9.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunology (Orlando, Fla.) 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Yip-Schneider MT, Barnard DS, Billings SD, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139–146. doi: 10.1093/carcin/21.2.139. [DOI] [PubMed] [Google Scholar]
- 11.Crowell PL, Schmidt CM, Yip-Schneider MT, et al. Cyclooxygenase-2 expression in hamster and human pancreatic neoplasia. Neoplasia (New York, N.Y.) 2006;8:437–445. doi: 10.1593/neo.04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juuti A, Louhimo J, Nordling S, et al. Cyclooxygenase-2 expression correlates with poor prognosis in pancreatic cancer. J Clin Pathol. 2006;59:382–386. doi: 10.1136/jcp.2005.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsubayashi H, Infante JR, Winter J, et al. Tumor COX-2 expression and prognosis of patients with resectable pancreatic cancer. Cancer Biol Ther. 2007;6:1569–1575. doi: 10.4161/cbt.6.10.4711. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt CM, Yip-Schneider MT, Ralstin MC, et al. PGE(2) in pancreatic cyst fluid helps differentiate IPMN from MCN and predict IPMN dysplasia. J Gastrointest Surg. 2008;12:243–249. doi: 10.1007/s11605-007-0404-8. [DOI] [PubMed] [Google Scholar]
- 15.Thiruvengadam N, Park WG. Systematic review of pancreatic cyst fluid biomarkers: the path forward. Clin Translational Gastroenterol. 2015;6:e88. doi: 10.1038/ctg.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Gaddam S, Ge PS, Keach JW, et al. Suboptimal accuracy of carcinoembryonic antigen in differentiation of mucinous and nonmucinous pancreatic cysts: results of a large multicenter study. Gastrointest Endosc. 2015;82:1060–1069. doi: 10.1016/j.gie.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–1102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 19.Nagula S, Kennedy T, Schattner MA, et al. Evaluation of cyst fluid CEA analysis in the diagnosis of mucinous cysts of the pancreas. J Gastrointest Surg. 2010;14:1997–2003. doi: 10.1007/s11605-010-1281-0. [DOI] [PubMed] [Google Scholar]
- 20.Brugge WR. Cyst fluid: moving beyond the carcinoembryonic antigen. Gastrointest Endosc. 2015;82:1070–1071. doi: 10.1016/j.gie.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Khalid A, McGrath KM, Zahid M, et al. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol. 2005;3:967–973. doi: 10.1016/s1542-3565(05)00409-x. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Science Translational Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Research. 2014;20:4381–4389. doi: 10.1158/1078-0432.CCR-14-0513. [DOI] [PubMed] [Google Scholar]
- 24.Al-Haddad MA, Kowalski T, Siddiqui A, et al. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy. 2015;47:136–142. doi: 10.1055/s-0034-1390742. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Dubois RN. Eicosanoids and cancer. Nature reviews. Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yip-Schneider MT, Nakshatri H, Sweeney CJ, et al. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Molecular Cancer Ther. 2005;4:587–594. doi: 10.1158/1535-7163.MCT-04-0215. [DOI] [PubMed] [Google Scholar]
- 27.Yip-Schneider MT, Wu H, Ralstin M, et al. Suppression of pancreatic tumor growth by combination chemotherapy with sulindac and LC-1 is associated with cyclin D1 inhibition in vivo. Molecular Cancer Ther. 2007;6:1736–1744. doi: 10.1158/1535-7163.MCT-06-0794. [DOI] [PubMed] [Google Scholar]
- 28.Yip-Schneider MT, Wu H, Hruban RH, et al. Efficacy of dimethylaminoparthenolide and sulindac in combination with gemcitabine in a genetically engineered mouse model of pancreatic cancer. Pancreas. 2013;42:160–167. doi: 10.1097/MPA.0b013e318254f455. [DOI] [PubMed] [Google Scholar]
- 29.Maker AV, Katabi N, Qin LX, et al. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Research. 2011;17:1502–1508. doi: 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh TS, Cheng AJ, Chen TC, et al. Telomerase activity is a useful marker to distinguish malignant pancreatic cystic tumors from benign neoplasms and pseudocysts. J Surg Research. 1999;87:171–177. doi: 10.1006/jsre.1999.5699. [DOI] [PubMed] [Google Scholar]
- 31.Hata T, Dal Molin M, Suenaga M, et al. Cyst fluid telomerase activity predicts the histologic grade of cystic neoplasms of the pancreas. Clin Cancer Research. 2016;22:5141–5151. doi: 10.1158/1078-0432.CCR-16-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caponi S, Funel N, Frampton AE, et al. The good, the bad and the ugly: a tale of miR-101, miR-21 and miR-155 in pancreatic intraductal papillary mucinous neoplasms. Ann Oncol. 2013;24:734–741. doi: 10.1093/annonc/mds513. [DOI] [PubMed] [Google Scholar]
- 33.Farrell JJ, Toste P, Wu N, et al. Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer. Am J Gastroenterol. 2013;108:1352–1359. doi: 10.1038/ajg.2013.167. [DOI] [PubMed] [Google Scholar]
- 34.Matthaei H, Wylie D, Lloyd MB, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Research. 2012;18:4713–4724. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maker AV, Katabi N, Gonen M, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2011;18:199–206. doi: 10.1245/s10434-010-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabbar KS, Verbeke C, Hyltander AG, et al. Proteomic mucin profiling for the identification of cystic precursors of pancreatic cancer. J Nat Cancer Inst. 2014;106:djt439. doi: 10.1093/jnci/djt439. [DOI] [PMC free article] [PubMed] [Google Scholar]