Abstract
Background
High intensity care including hospitalizations, chemotherapy and other interventions at the end of life is costly and often of little value for cancer patients. Little is known about patterns of end of life care and resource utilization for women with uterine cancer.
Objective
We examined the costs and predictors of aggressive end of life care for women with uterine cancer.
Methods
In this observational cohort study the Surveillance, Epidemiology and End Results-Medicare linked database was used to identify women ≥65 who died from uterine cancer from 2000–2011. Resource utilization in the last month of life including ≥2 hospital admissions, >1 emergency department visit, ≥1 intensive care unit admission or use of chemotherapy in the last 14 days of life was examined. High intensity care was defined as the occurrence of any of the above outcomes. Logistic regression models were developed to identify factors associated with high intensity care. Total Medicare expenditures in the last month of life are reported.
Results
Of the 5,873 patients identified, the majority had stage IV (30.2%) cancer, were white (79.9%) and had endometrioid tumors (47.6%). High intensity care was rendered to 42.5% of women. During the last month of life, 15.0% had ≥2 hospital admissions, 9.0% had a hospitalization >14 days, 15.3% had >1 emergency department visits, 18.3% had an intensive care unit admission and 6.6% received chemotherapy in the last 14 days of life. The percentage of women who received high intensity care was stable over the study period. Characteristics of younger age, black race, higher number of comorbidities, stage IV disease, residence in the eastern US and more recent diagnosis were associated with high intensity care. The median Medicare payment during the last month of life was $7,645. Total per beneficiary Medicare payments remained stable from $9,656 (IQR, $3,190–$15,890) in 2000 to $9,208 (IQR, $3,309–$18,554) by 2011. The median healthcare expenditure was four times as high for those who received high intensity care compared to those who did not (median $16,173 vs $4,099).
Conclusion
Among women with uterine cancer, high intensity care is common in the last month of life, associated with substantial monetary expenditures, and does not appear to be decreasing.
Keywords: Uterine cancer, endometrial cancer, cost, end of life, palliative care
Introduction
Patients with advanced cancer often receive expensive, aggressive medical treatment including chemotherapy and intensive inpatient care when it is of questionable clinical value and has the potential to worsen quality of life.1–5 A review of claims in the last 30 days of life for cancer patients found that 52% of cancer patients had an acute care hospitalization, 27% an intensive care unit admission, 46% visited the emergency department and 11% were given intravenous chemotherapy.1 Healthcare spending at the end of life among patients with cancer diagnoses far exceeds that of those without cancer.2 Even among patients with cancer, those who die “from cancer” have higher healthcare expenditures at the end of life than those who die from causes of death other than cancer.3 End of life discussions have been associated with lower cost at the end of life. Zhang et al found 35% lower end of life costs among patients who reported having end of life discussion compared to those who did not.4 Similarly, those with documented do not resuscitate (DNR) orders also incur lower costs and report better quality of life compared to those who lack one.5
Estimates of healthcare spending during the end of of life for women with ovarian cancer show that inpatient care and chemotherapy account for a large portion of cost and that these costs are lower in those who elect to receive hospice care.6 Little data exists for healthcare costs at the end of life for gynecologic cancers other than ovarian cancer. Up to 24% of patients with a gynecologic malignancy including ovarian, uterine, cervical vaginal or vulvar cancer have been reported to receive “aggressive medical care” in the last 30 days of life including chemotherapy, hospital admission and intensive care unit admission.7 Patients who experience more intensive medical care at the end of life are more likely to report poorer quality of life.8
Limited data on the financial burden of end of life care is currently available for patients with uterine cancer. Identifying the patterns of care and resultant financial burden of patients with uterine cancer at the end of life is important. Allocating interest and resources to improve end of life care for patients with a gynecologic malignancy that is usually associated with a favorable prognosis requires defining the scope of the issue. The objective of our study was to quantify end of life healthcare spending among women with uterine cancer and identify associations with high intensity care and increased healthcare spending.
Methods
We used the Surveillance, Epidemiology, and End Results Program (SEER)-Medicare linked database for analysis. We identified women > 65 years of age who had uterine cancer diagnosed as their first or only cancer and confirmed with positive histology. We included women whose cause of death was uterine cancer from 2000–2011, and who had a valid date of death with agreement between SEER and Medicare within the month of death. We excluded women who were diagnosed by autopsy or death certificate, or who were eligible for Medicare because of end stage renal disease or disability. We also excluded women who did not have full coverage for both Medicare parts A and B or who were enrolled in a non-Medicare health maintenance organization from 12 months prior to death through time of death because of incomplete billing claims submitted to Medicare for reimbursement.
The clinical and demographic characteristics included year of diagnosis (1998–2011), year of death (2000–2011), age at diagnosis (65–69, 70–74, 75–79, ≥80 years), race (white, black, Hispanic, other/unknown), marital status (married, unmarried, unknown), location (metropolitan, non-metropolitan, unknown), and region of SEER registry (East, Midwest, West). An aggregate socioeconomic status score was derived from census tract data on education, poverty level, and income, as previously reported by Du and colleagues.9 The formula weighted education, poverty and income equally and ranked patients on a scale of 1–5 with 1 being the lowest value. We used the Klabunde adaptation of the Charlson10.11 comorbidity index to assess the presence of comorbid medical conditions, and grouped patients as 0, 1, or ≥2 comorbid conditions. Tumor histology was classified as endometrioid, serous, clear cell, carcinosarcoma, leiomyosarcoma, and other. Tumor grade was grouped as 1, 2, 3, and other/unknown. Stage was captured using American Joint Cancer Commission (AJCC) staging criteria and grouped as I, II, III, IV and unknown. Patients with early stage disease ultimately died of uterine cancer, as this was necessary for inclusion in the study. These patients were assumed to eventually have presented with recurrent “advanced” disease and therefore were an important population to include.
We examined utilization of hospital admissions including those with length of stay >14 days, emergency department visits, intensive care unit admissions, administration of chemotherapy and hospice services at the end of life in accordance with previously published indicators of aggressive medical care at the end of life.12 Hospitalization, emergency department visitation and intensive care unit admissions were analyzed during the last month of life. Hospice admission was categorized based on the first occurrence and analyzed as occurring in the last 3 days, 1, 3, and 6 months of life. Use of intravenous chemotherapy in the last 14 days of life was also examined.
Data on emergency department visits were extracted by searching the source and type codes of inpatient admission, the Healthcare Common Procedure Coding System, Berenson-Eggers type of service for procedure codes and revenue center codes from Medicare provider analysis and review (MEDPAR), carrier claims, and outpatient claims. One MEDPAR admission that had emergency department indicators in the last month of life was considered one emergency department visit. For emergency department visits identified from carrier and outpatient claims in the last month of life, claims occurred on the same day were considered one emergency department visit.12–14
Intensive care unit admission was defined as the presence of an intensive care unit indicator code, intensive care day count >0, or International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes for continuous invasive mechanical ventilation (96.7x) from MEDPAR within the last month of life as previously described.13,15,16 Data on use of intravenous chemotherapy was extracted by searching the Healthcare Common Procedure Coding System, ICD-9-CM diagnosis and procedure codes, and revenue center codes from MEDPAR, carrier, and outpatient claims. If a patient had at least one claim for chemotherapy within the last 14 days of life, she was coded as having received chemotherapy.
We developed a classification schema to identify high utilization of medical services at the end of life. High intensity care was defined as utilization of ≥2 hospital admissions, or ≥1 hospital admissions with LOS >14 days, or >1 emergency department visit or ≥1 intensive care unit admission in the last month of life or use of intravenous chemotherapy in the last 14 days, or first hospice admission in the last 3 days of life. We also examined total Medicare expenditures in the last month of life. Medicare expenditures were estimated by summing all payments from MEDPAR, carrier and outpatient claims, durable medical equipment (DME), and hospice in the last month of life. The total cost was adjusted for inflation to 2015 dollars and winsorized to the 3rd and 97th percentiles.17
Patient demographics were reported descriptively. High intensity care and the other outcomes were reported as number and percentage of patients who had the services stratified by year of death, and were compared using Cochran-Armitage test for linear trend in proportions. Medicare expenditures in the last month of life were reported as median and interquartile range (IQR) and were compared using Wilcoxon rank sums and Kruskal-Wallis tests. Logistic regression models were developed to identify clinical and demographic factors associated with high intensity care. All analyses were conducted with SAS, version 9.4 (SAS Institute, Cary, NC). All statistical tests were two-sided.
Results
We identified 5,873 women who died of uterine cancer between 2000 and 2011 (Supplemental figure 1). Demographic descriptors of the cohort are displayed in Table 1. The largest proportions of the population were white (79.9%), unmarried (59.8%), and from metropolitan areas (89.9%). Women who died of uterine cancer in this cohort were most often older than 80 years (33.3%) and had the following characteristics: stage IV disease (30.2%), grade 3 (52.8%) endometrioid histology (47.6%) tumors.
Table 1.
Demographic characteristics of the cohort (n=5,873).
| N | % | |
|---|---|---|
| Year of diagnosis | ||
| 1998–2002 | 2,095 | 35.7 |
| 2003–2007 | 2,548 | 43.4 |
| 2008–2011 | 1,230 | 20.9 |
| Year of death | ||
| 2000–2003 | 1,620 | 27.6 |
| 2004–2007 | 2,118 | 36.1 |
| 2008–2011 | 2,135 | 36.4 |
| Age | ||
| 65–69 | 1,365 | 23.2 |
| 70–74 | 1,313 | 22.4 |
| 75–79 | 1,241 | 21.1 |
| ≥ 80 | 1,954 | 33.3 |
| Race | ||
| White | 4,693 | 79.9 |
| Black | 853 | 14.5 |
| Hispanic | 91 | 1.6 |
| Other/unknown | 236 | 4.0 |
| Marital status | ||
| Married | 2,093 | 35.6 |
| Unmarried | 3,513 | 59.8 |
| Unknown | 267 | 4.6 |
| Location | ||
| Metropolitan | 5,281 | 89.9 |
| Non-metropolitan | a | a |
| Unknown | a | a |
| Socioeconomic status | ||
| 1st Quintile (Lowest) | 1,743 | 29.7 |
| 2nd Quintile | 855 | 14.6 |
| 3rd Quintile | 1,154 | 19.7 |
| 4th Quintile | 1,039 | 17.7 |
| 5th Quintile | 628 | 10.7 |
| Unknown | 454 | 7.7 |
| Region of SEER Registry | ||
| Eastern | 1,496 | 25.5 |
| Midwest | 2,275 | 38.7 |
| West | 2,102 | 35.8 |
| Comorbidity | ||
| 0 | 2,519 | 42.9 |
| 1 | 1,510 | 25.7 |
| ≥ 2 | 1,844 | 31.4 |
| Histology | ||
| Endometrioid | 2,793 | 47.6 |
| Serous | 967 | 16.5 |
| Clear Cell | 201 | 3.4 |
| Carcinosarcoma | 931 | 15.9 |
| Leiomyosarcoma | 118 | 2.0 |
| Other | 863 | 14.7 |
| Stage | ||
| I | 1356 | 23.1 |
| II | 489 | 8.3 |
| III | 1,283 | 21.9 |
| IV | 1,775 | 30.2 |
| Unknown | 970 | 16.5 |
| Grade | ||
| 1 | 434 | 7.4 |
| 2 | 1,064 | 18.1 |
| 3 | 3,103 | 52.8 |
| Other/Unknown | 1,272 | 21.7 |
SEER: Surveillance, Epidemiology and End Results Program; NOS: Not otherwise specified; ESRD: End stage renal disease; HMO: Health maintenance organization
One of the cells with N <11, suppressed from reporting
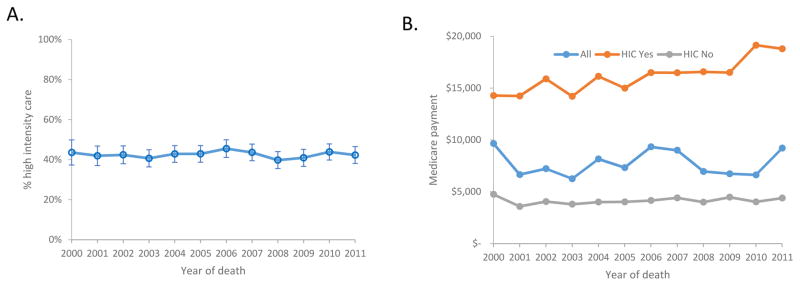
In the last month of life, 55.5% of decedents experienced ≥1 hospital admission, 15.0% were hospitalized ≥2 times, 9.0% had a hospitalization >14 days, 15.3% had at >1 emergency department visit, and 18.3% were admitted to the intensive care unit (Table 2). Intravenous chemotherapy was administered to 6.6% of women in the last 14 days of life. Overall, 3,858 (65.7%) of women received hospice services. Hospice care was initiated within the last 3 days of life in 10.3% of women in the total cohort, in the last month of life in 41.8%, in the last 3 months of life in 56.9%, and within 6 months in 62.1%. Overall, high intensity care was utilized by 42.5% of women. From 2000 to 2011 the rate of high intensity care use varied from a high of 45.5% in 2006 to a low of 39.8% in 2008 (P=1.00) (Figure 1A).
Table 2.
End of life measures by year of death among women with uterine cancer.
| Patients who had HIC | ≥1 hospital admissions in last month | ≥2 hospital admissions in last month | ≥1 hospital admissions (LOS > 14 days) in last month | 1st hospice admission in last 3 days | 1st hospice admission in last month | >1 ED visits in last month | ≥1 ICU admissions in last month | ≥1 IV chemotherapy in last 14 days | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| All | 2,496 | 42.5 | 3,260 | 55.5 | 881 | 15.0 | 527 | 9.0 | 607 | 10.3 | 2,456 | 41.8 | 897 | 15.3 | 1,076 | 18.3 | 388 | 6.6 |
| 2000 | 105 | 43.6 | 152 | 63.1 | 37 | 15.4 | 21 | 8.7 | 19 | 7.9 | 91 | 37.8 | 28 | 11.6 | 43 | 17.8 | 17 | 7.1 |
| 2001 | 166 | 41.9 | 218 | 55.1 | 56 | 14.1 | 39 | 9.9 | 35 | 8.8 | 142 | 35.9 | 48 | 12.1 | 58 | 14.7 | 19 | 4.8 |
| 2002 | 201 | 42.4 | 268 | 56.5 | 82 | 17.3 | 59 | 12.5 | 42 | 8.9 | 190 | 40.1 | 71 | 15.0 | 78 | 16.5 | 21 | 4.4 |
| 2003 | 207 | 40.7 | 270 | 53.1 | 72 | 14.2 | 42 | 8.3 | 55 | 10.8 | 196 | 38.5 | 61 | 12.0 | 79 | 15.5 | 36 | 7.1 |
| 2004 | 231 | 42.9 | 317 | 58.8 | 70 | 13.0 | 57 | 10.6 | 47 | 8.7 | 219 | 40.6 | 83 | 15.4 | 95 | 17.6 | 53 | 9.8 |
| 2005 | 235 | 42.9 | 305 | 55.7 | 82 | 15.0 | 46 | 8.4 | 68 | 12.4 | 229 | 41.8 | 101 | 18.4 | 88 | 16.1 | 35 | 6.4 |
| 2006 | 223 | 45.5 | 282 | 57.6 | 70 | 14.3 | 57 | 11.6 | 56 | 11.4 | 196 | 40.0 | 79 | 16.1 | 102 | 20.8 | 38 | 7.8 |
| 2007 | 236 | 43.6 | 306 | 56.6 | 83 | 15.3 | 52 | 9.6 | 58 | 10.7 | 245 | 45.3 | 91 | 16.8 | 110 | 20.3 | 35 | 6.5 |
| 2008 | 204 | 39.8 | 281 | 54.8 | 82 | 16.0 | 38 | 7.4 | 35 | 6.8 | 211 | 41.1 | 74 | 14.4 | 96 | 18.7 | 34 | 6.6 |
| 2009 | 210 | 40.9 | 269 | 52.4 | 71 | 13.8 | 41 | 8.0 | 56 | 10.9 | 235 | 45.8 | 77 | 15.0 | 92 | 17.9 | 30 | 5.9 |
| 2010 | 253 | 43.9 | 307 | 53.2 | 94 | 16.3 | 46 | 8.0 | 68 | 11.8 | 255 | 44.2 | 91 | 15.8 | 125 | 21.7 | 37 | 6.4 |
| 2011 | 225 | 42.3 | 285 | 53.6 | 82 | 15.4 | 29 | 5.5 | 68 | 12.8 | 247 | 46.4 | 93 | 17.5 | 110 | 20.7 | 33 | 6.2 |
| P | 1.00 | 0.03 | 0.67 | 0.002 | 0.03 | <0.001 | 0.02 | <0.001 | 0.97 | |||||||||
HIC was defined as any utilization of ≥2 hospital admissions, >1 hospital admission with LOS >14 days, >1 ED or ≥1 ICU admission in the last month of life, use of intravenous chemotherapy in the last 14 days of life or 1st hospice admission in the last 3 days of life
HIC: High intensity care; LOS: length of stay; ED: emergency department; ICU: intensive care unit; IV chemo: intravenous chemotherapy
Figure 1.
1A. Percentage of women receiving high intensity care. 1B. Median health care costs at the end of life for the cohort.
The median overall healthcare expenditure during the last month of life was $7,645 (IQR, $3,217 – $16,246) (Table 3). Year by year median healthcare expenditures can be found in Figure 1B and the healthcare spending across the study period was stable (Table 3). Among those who received high intensity care, healthcare expenditures were four times higher ($16,173 vs $4,099) than for those who did not receive high intensity care (P<0.001).
Table 3.
Medicare expenditure during the last month of life
| Median | (IQR) | |
|---|---|---|
| Overall | $7,645 | ($3,217–$16,246) |
| Presence of HIC | ||
| No | $4,099 | ($2,121–$8,218) |
| Yes | $16,173 | ($10,079–$25,064) |
| Year of death | ||
| 2000 | $9,656 | ($3,190–$15,890) |
| 2001 | $6,653 | ($2,740–$13,875) |
| 2002 | $7,228 | ($2,956–$15,562) |
| 2003 | $6,256 | ($2,921–$14,201) |
| 2004 | $8,159 | ($3,426–$16,424) |
| 2005 | $7,331 | ($3,212–$15,138) |
| 2006 | $9,335 | ($3,403–$17,140) |
| 2007 | $9,004 | ($3,317–$16,667) |
| 2008 | $6,960 | ($3,254–$15,954) |
| 2009 | $6,738 | ($3,339–$17,390) |
| 2010 | $6,629 | ($3,329–$17,897) |
| 2011 | $9,208 | ($3,309–$18,554) |
71 patients had no claims from MEDPAR, NCH, OUTSAF, DME, HOSPICE in the last month of life and were not included in the analysis of payment.
HIC: high intensity care; IQR: interquartile range.
In a multivariable model, more recent year of diagnosis (aOR=1.04; 95% CI, 1.02–1.06) black race (aOR=1.70; 95% CI, 1.45–2.01), a greater number of medical comorbidities (aOR=1.65; 95% CI, 1.45–1.87) and the presence of a stage IV neoplasm (aOR=1.32; 95% CI, 1.14–1.54) were associated with high intensity care (Table 4). In contrast, less older women and residents of the midwest (compared to eastern) United States received high intensity care. There was no association between socioeconomic status and receipt of high intensity care.
Table 4.
Multivariate regression for associations with high intensity care
| Adjusted OR | |
|---|---|
| Year of diagnosis (continuous) | 1.04 (1.02–1.06)a |
| Age | |
| 65–69 | Referent |
| 70–74 | 0.76 (0.65–0.89)a |
| 75–79 | 0.71 (0.61–0.84) a |
| >=80 | 0.45 (0.39–0.53) a |
| Race | |
| White | Referent |
| Black | 1.70 (1.45–2.01) a |
| Hispanic | 1.28 (0.83–1.97) |
| Other/unknown | 1.38 (1.05–1.82) a |
| Marital status | |
| Married | Referent |
| Unknown | 0.88 (0.67–1.15) |
| Unmarried | 0.96 (0.86–1.09) |
| Location | |
| Metropolitan | Referent |
| Non-metropolitan | 1.02 (0.84–1.24) |
| Unknown | –b |
| Socioeconomic status | |
| 1st Quintile (Lowest) | Referent |
| 2nd Quintile | 0.84 (0.70–1.00) |
| 3rd Quintile | 0.97 (0.82–1.14) |
| 4th Quintile | 0.85 (0.71–1.02) |
| 5th Quintile | 0.89 (0.73–1.10) |
| Unknown | 0.83 (0.67–1.03) |
| Region of SEER Registry | |
| Eastern | Referent |
| Midwest | 0.77 (0.66–0.89) a |
| West | 0.94 (0.82–1.09) |
| Comorbidity | |
| 0 | Referent |
| 1 | 1.29 (1.13–1.48) a |
| >=2 | 1.65 (1.45–1.87) a |
| Histology | |
| Endometrioid | Referent |
| Serous | 1.00 (0.85–1.17) |
| Clear Cell | 0.83 (0.61–1.13) |
| Carcinosarcoma | 1.17 (0.99–1.37) |
| Leiomyosarcoma | 0.82 (0.55–1.22) |
| Other | 1.02 (0.87–1.21) |
| Stage | |
| I | Referent |
| II | 1.00 (0.81–1.25) |
| III | 1.00 (0.85–1.18) |
| IV | 1.32 (1.14–1.54) a |
| Unknown | 0.99 (0.82–1.20) |
Logistic regression model was fitted including year of diagnosis, age, race, marital status, location, socioeconomic status, region of SEER registry, comorbidity, histology and stage. OR: Odds ratio; SEER: surveillance, epidemiology and end results program
P-value < 0.05
Non-estimable
Discussion
Among women with uterine cancer, aggressive therapy at the end of life is common. Despite increased availability of palliative care services, hospitalization, emergency department visits and use of chemotherapy in the last month of life have remained frequent. Importantly, while we observed some year-to-year variation in the costs of care during the last month of life, overall costs have remained relatively stable over time and have not declined significantly.
Prior studies in women with gynecologic cancers have shown that intensive treatment, including hospitalizations and use of chemotherapeutic agents, is common at the end of life. Wu and colleagues reported that 30% of patients with a gynecologic malignancy received a therapeutic intervention (chemotherapy, radiation therapy or surgery) in the last month of life.18 Zakhour and colleagues studied the effect of documented end of life discussions on healthcare utilization and reported similar measures of healthcare utilization during the last 30 days of life (40% emergency department visit in last 30 days, 49% hospital admission in last 30 days, etc.)19 We observed that 6.6% of patients received chemotherapy during the last 14 days of life, which is similar to 4% reported in a single institution retrospective review of gynecologic oncology patients and 4.8 – 12.7% in a multi country retrospective study of patients with multiple tumor types during the last 30 days of life. 1,20 Our data from a large cohort of elderly women with uterine cancer revealed similar trends; the use of hospital services and chemotherapy are common at the end of life.
A number of patient, physician, and systems factors likely influence the intensity of care at the end of life for cancer patients.6 Regional variation appears to be an important factor in the intensity of end of life care. Our data suggest high intensity care in the last month of life is more common in the eastern United States compared to the midwest region, further supporting that practice patterns can have a large influence of healthcare cost. Race has also been associated with receiving aggressive end of life care. In a report by Wright and colleagues, black patients with stage IV cancer were over three times more likely to receive high intensity care at the end of life. 21 We similarly found that black race was associated with receiving high intensity end of life care. Black patients are also less likely to have a documented DNR order or advance directive and be less likely to receive opiates and benzodiazapines while hospitalized compared to white patients.22 Ramey and Chin demonstrated that black patients with cancer (all sites) use hospice services at a lower rate compared to white patients with cancer, which is in line with our observation of their likelihood to receive high intensity care at the end of life.23 Possible explanations for this observation include poor access to care, lack of appropriate referral to hospice, cultural beliefs and preference for aggressive treatments. The higher incidence and mortality of black patients with endometrial cancer makes addressing quality of end of life care in this population specifically an important issue that should be prioritized.24 While we did not find data on end of life healthcare costs among black patents with gynecologic malignancies, black patients with prostate cancer had higher costs during the last year of life compared to non-Hispanic white patients.25
Healthcare costs during the last month of life for patients with uterine cancer demonstrated some year-to-year variation, but were relatively stable over the course of our study period. Limited data exist to provide a comparison regarding trends in overall end of life costs over time. In line with the cost data that we noted, high intensity care, hospitalizations, and use of chemotherapy were relatively stable over the course of the study. Despite the recent focus on incorporation of palliative care into routine oncology practice and its intended effect on reducing aggressive care and healthcare costs at the end of life these data are not supportive of decreasing costs or high intensity care. This data is in contrast with a review of cancer decedents from Canada that reported increases in systemic therapy cost and use during the last year of life from 2002 – 2007.26 It is possible that we are observing a lag time between the dissemination of guidelines promoting routine incorporation of palliative care into oncology care for patients with advanced cancer and widespread incorporation of these recommendations into clinical practice that would produce a measureable effect.
The immense financial burden of cancer care on the United States healthcare system, especially at the end of life, has sparked interest in how to improve patient care while reducing cost. Transitioning to appropriate end of life care starts with the “end of life discussion.” Zhang et al demonstrated that patients who reported having an end of life discussion had less costly care (35% less costly) and a higher quality of life in the last week of life.4 Ideally, end of life conversations occur far before the last week of life and allow for timely intervention of palliative care. Timely palliative care consultation for inpatients (>30 days before death) has been associated with lower cost of care and less aggressive interventions.27 Other sources suggest the optimal timing of palliative care involvement is even earlier in the disease course for patients with advanced cancer, although the exact timing is not universally agreed upon.
An important goal of palliative care involvement is to help transition appropriate patients to hospice care and avoid high intensity care at the end of life. Trends in patterns of hospice use over time generally support either gradual improvements in hospice use or stability. Fairfield et al showed that among decedents with ovarian cancer, hospice usage increased from 49.7% in 2001 to 74.9% in 2005.28 They also showed less hospice usage among black women, lower income women and Medicare fee for service women. A separate study of ovarian cancer patients showed a more modest increase in hospice discharges from an inpatient unit from 2006–2011 of 9.2% to 11.1%.27 Similar trends have been noted for other disease sites.30
While this work represents one of the first studies to examine end of life care in women with uterine cancer, we recognize a number of important limitations. First, our data represent reimbursements and may not capture the true cost of care. While Medicare claims data likely do not represent the entirety of healthcare costs at the end of life, these data have been widely used as a surrogate for medical spending. Second, as with any study of claims data, there may be the undercapture of some services as well as use of hospice care. However, we examined only major interventions likely to generate a billing code and this dataset has been used in a number of other studies to examine hospice services. Third, our data derive from a cohort of women age ≥65 due to the nature of the database we used and therefore our results might not be applicable to a population of women age <65. Fourth, by selecting only those with documented cause of death as gynecologic malignancy we significantly reduced our sample size. This was done to avoid including patients who died of other causes but ultimately is subject to variations in reporting. For example, someone who dies of extensive thromboembolic disease secondary to their hyper-coagulable state caused by metastatic endometrial cancer would not be captured in this study if their cause of death was not listed as gynecologic malignancy. Finally, due to the retrospective nature of the study, associations identified cannot be assumed to be causal. As with any observational study, we cannot assess individual patient and physician attitudes and beliefs that undoubtedly influenced decision-making and ultimately resource utilization and cost.
While care at the end of life should be individualized, treatment goals for women with advanced uterine cancer should focus on preserving quality of life and palliation of symptoms. Ideally, such care would result in avoidance of procedures and systemic therapies, and minimization of hospitalization and emergency room visits. Our data show that this ideal is not the reality for many patients who die of uterine cancer. Further efforts should be made to improve the transition away from intensive medical care to end of life care.
Supplementary Material
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants from the National Cancer Institute. Dr. Hershman is the recipient of a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation. Dr. Tergas is a recipient of an NCI Diversity Supplement (CA197730).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
The authors have no conflicts of interest or disclosures.
Work presented in poster format at the Society of Gynecologic Oncology annual meeting in National Harbor Maryland March 2017.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of Site of Death, Health Care Utilization, and Hospital Expenditures for Patients Dying With Cancer in 7 Developed Countries. Jama. 2016;315(3):272. doi: 10.1001/jama.2015.18603. [DOI] [PubMed] [Google Scholar]
- 2.Tangka FK, Subramanian S, Sabatino SA, et al. End-of-Life Medical Costs of Medicaid Cancer Patients. Health Services Research. 2014;50(3):690–709. doi: 10.1111/1475-6773.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langton JM, Reeve R, Srasuebkul P, et al. Health service use and costs in the last 6 months of life in elderly decedents with a history of cancer: a comprehensive analysis from a health payer perspective. British journal of cancer. 2016;114:1293–302. doi: 10.1038/bjc.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Archives of internal medicine. 2009;169:480–8. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrido MM, Balboni TA, Maciejewski PK, Bao Y, Prigerson HG. Quality of Life and Cost of Care at the End of Life: The Role of Advance Directives. Journal of pain and symptom management. 2015;49:828–35. doi: 10.1016/j.jpainsymman.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewin SN, Buttin BM, Powell MA, et al. Resource utilization for ovarian cancer patients at the end of life: how much is too much? Gynecologic oncology. 2005;99:261–6. doi: 10.1016/j.ygyno.2005.07.102. [DOI] [PubMed] [Google Scholar]
- 7.Taylor JS, Brown AJ, Prescott LS, Sun CC, Ramondetta LM, Bodurka DC. Dying well: How equal is end of life care among gynecologic oncology patients? Gynecologic oncology. 2016;140:295–300. doi: 10.1016/j.ygyno.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–9. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 10.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG., Jr Assessing illness severity: does clinical judgment work? J Chronic Dis. 1986;39:439–52. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 12.Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:1133–8. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Accordino MK, Wright JD, Vasan S, et al. Use and Costs of Disease Monitoring in Women With Metastatic Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:2820–6. doi: 10.1200/JCO.2016.66.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:315–21. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 15.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Medical care. 2004;42:801–9. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 16.Cooke CR, Feemster LC, Wiener RS, O'Neil ME, Slatore CG. Aggressiveness of intensive care use among patients with lung cancer in the Surveillance, Epidemiology, and End Results-Medicare registry. Chest. 2014;146:916–23. doi: 10.1378/chest.14-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ly DP, Jha AK, Epstein AM. The association between hospital margins, quality of care, and closure or other change in operating status. Journal of general internal medicine. 2011;26:1291–6. doi: 10.1007/s11606-011-1815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu E, Rogers A, Ji L, et al. Escalation of oncologic services at the end of life among patients with gynecologic cancer at an urban, public hospital. Journal of oncology practice / American Society of Clinical Oncology. 2015;11:e163–9. doi: 10.1200/JOP.2014.001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakhour M, LaBrant L, Rimel BJ, et al. Too much, too late: Aggressive measures and the timing of end of life care discussions in women with gynecologic malignancies. Gynecologic oncology. 2015;138:383–7. doi: 10.1016/j.ygyno.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JS, Brown AJ, Prescott LS, Sun CC, Ramondetta LM, Bodurka DC. Dying well: How equal is end of life care among gynecologic oncology patients? Gynecologic oncology. 2016;140:295–300. doi: 10.1016/j.ygyno.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loggers ET, Maciejewski PK, Paulk E, et al. Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5559–64. doi: 10.1200/JCO.2009.22.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgio KL, Williams BR, Dionne-Odom JN, Redden DT, Noh H, Goode PS, … Bailey FA. Racial differences in processes of care at end of life in VA medical centers: planned secondary analysis of data from the BEACON trial. Journal of palliative medicine. 2016;19(2):157–163. doi: 10.1089/jpm.2015.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramey SJ, Chin SH. Disparity in hospice utilization by African American patients with cancer. The American journal of hospice & palliative care. 2012;29:346–54. doi: 10.1177/1049909111423804. [DOI] [PubMed] [Google Scholar]
- 24.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The growing burden of endometrial cancer: a major racial disparity affecting black women. Cancer Epidemiology and Prevention Biomarkers. 2015;24(9):1407–1415. doi: 10.1158/1055-9965.EPI-15-0316. [DOI] [PubMed] [Google Scholar]
- 25.Chhatre S, Bruce Malkowicz S, Sanford Schwartz J, Jayadevappa R. Understanding the Racial and Ethnic Differences in Cost and Mortality Among Advanced Stage Prostate Cancer Patients (STROBE) Medicine (Baltimore) 2015;94:e1353. doi: 10.1097/MD.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pataky RE, Cheung WY, de Oliveira C, et al. Population-based trends in systemic therapy use and cost for cancer patients in the last year of life. Current oncology. 2016;23:S32–41. doi: 10.3747/co.23.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nevadunsky NS, Gordon S, Spoozak L, et al. The role and timing of palliative medicine consultation for women with gynecologic malignancies: association with end of life interventions and direct hospital costs. Gynecologic oncology. 2014;132:3–7. doi: 10.1016/j.ygyno.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairfield KM, Murray KM, Wierman HR, et al. Disparities in hospice care among older women dying with ovarian cancer. Gynecologic oncology. 2012;125:14–8. doi: 10.1016/j.ygyno.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 29.Uppal S, Rice LW, Beniwal A, Spencer RJ. Trends in hospice discharge, documented inpatient palliative care services and inpatient mortality in ovarian carcinoma. Gynecologic oncology. 2016;143:371–8. doi: 10.1016/j.ygyno.2016.08.238. [DOI] [PubMed] [Google Scholar]
- 30.Huo J, Du XL, Lairson DR, et al. Utilization of surgery, chemotherapy, radiation therapy, and hospice at the end of life for patients diagnosed with metastatic melanoma. American journal of clinical oncology. 2015;38:235–41. doi: 10.1097/COC.0b013e31829378f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.