Abstract
Pacific Islanders face many health disparities, including higher rates of cardiovascular disease, cancer, obesity, and diabetes compared to other racial and ethnic groups. Specifically, the Marshallese population suffers disproportionately from type 2 diabetes, with rates 400% higher than the general US population. As part of an ongoing community-based participatory research (CBPR) partnership, 148 participants were recruited for a study examining genetic variants to better understand diabetes. Participants provided a saliva specimen in an Oragene® DNA self-collection kit. Each participant provided approximately 2 mL volume of saliva and was asked qualitative questions about their experience. The study yielded a recruitment rate of 95.5%. Among the 148 persons who participated, 143 (96.6%) agreed to be contacted for future studies; 142 (95.9%) agreed to have their samples used for future IRB-approved studies; and 144 (97.3%) gave permission for the researchers to link information from this study to other studies in which they had participated. Qualitative responses showed that the majority of participants were willing to participate because of their desire to contribute to the health of their community and to understand the genetic influence related to diabetes. This study demonstrates willingness to participate in genetic research among Marshallese living in Arkansas. Willingness was likely enhanced because the feasibility study was part of a larger CBPR effort. This study is important to community stakeholders who have voiced a desire to collaboratively conduct genetic research related to diabetes, perinatal outcomes, and cancer.
Keywords: Community-based participatory research, Pacific Islanders, Genetics, Diabetes
Pacific Islanders are one of the fastest growing populations in the USA, with the most rapid growth in rural, southern states including Arkansas. In the continental US, Arkansas has the largest population of Marshallese, a Pacific Islander population from the Republic of the Marshall Islands (RMI) (United States Census Bureau 2010). While health data on Pacific Islanders are scarce, and much of this data is aggregated with data from Asian Americans, (Ahmad and Weller 2014; Ro and Yee 2010; Srinivasan and Guillermo 2000) available research shows that Pacific Islanders face higher rates of cardiovascular disease, cancer, obesity, and diabetes than other racial and ethnic groups (Hawley and McGarvey 2015; Mau et al. 2009; McElfish et al. 2016e; Minino 2013; Murphy et al. 2013; Okihiro and Harrigan 2005; Palafox and Tsark 2004; Schiller et al. 2012). Specifically, the Marshallese population suffers disproportionately from type 2 diabetes, with rates four times higher than the general US population (McElfish et al. 2016e). Health assessment data demonstrate a prevalence of diabetes at 38.4% and pre-diabetes at 32.6% among adults in Northwest Arkansas (McElfish et al. 2016e). In the RMI, diabetes prevalence is as high as 30% (Aitaoto and Ichiho 2013; Hawley and McGarvey 2015).
Disease incidence and the poor health outcomes associated with the most commonly observed chronic diseases such as diabetes, obesity, and heart disease are typically attributed to socioeconomic, demographic, and behavioral factors (LeDoux 2009; McElfish et al. 2016e; Minegishi et al. 2007; Woodall et al. 2011; Yamada et al. 2004). However, variations in underlying genetic architecture are important potential contributors to the observed variability in health outcomes within particular chronic diseases (Bouchard 2008; Keating and El-Osta 2015; Maes et al. 1997; Qi and Cho 2008; Stunkard et al. 1990; Sørensen et al. 1998). Rapid progress in understanding the genetic underpinnings of disease has occurred in recent years due to advances in new technologies for molecular genetics and the vast information obtained from the Human Genome Project (Hood and Rowen 2013; Lunshof et al. 2010; Wilson and Nicholls 2015). The emergence of new technologies and recent reductions in cost of genetic analysis have made genetic research more accessible for many health conditions, including type 2 diabetes. Type 2 diabetes is typically understood to result from interaction between environmental factors and a strong hereditary component (estimates of heritability range from 20 to 80% depending on the population studied) (Alberti et al. 2007; Ali 2013; Kaprio et al. 1992; Meigs et al. 2000; Poulsen et al. 2005). The magnitude of differences in diabetes prevalence between racial and ethnic groups when exposed to similar environments implies a significant genetic influence and thus increases the need for genetic research in diverse populations groups (Alberti et al. 2007).
To date, racial and ethnic minorities remain underrepresented in genetic research, potentially contributing to the further widening of health disparities (Bussey-Jones et al. 2010; Bustamante et al. 2011; James et al. 2008; Johnson et al. 2011; Ochs-Balcom et al. 2015; Popejoy and Fullerton 2016). Recent efforts to include racial and ethnic minorities in genetic research have focused on African American populations; there has been less research with other minority populations. In particular, very few genetic studies have included Pacific Islander populations. Recent research has started to assess obesity-related non-communicable diseases and their genetic risk factors among 19 Pacific Islander subgroups from Micronesia, Melanesia, and Polynesia not residing in the USA (n = 3040), (Hawley and McGarvey 2015) Samoan adults not residing in the USA (n = 3475), (Hawley et al. 2014) Pacific Islanders in California (n = 593), (Karter et al. 2013) and a small sample of Native Hawaiians (n = 16) (Townsend et al. 2015). Data collection in these studies included saliva and/or blood samples. Outside of the studies conducted in California and Hawaii, there is little to no genetic research focused on Pacific Islanders residing in the USA and no known genetic studies with Marshallese.
Researchers and minority participants identify ethical, legal, and social complexities associated with genetic research among racial and ethnic minority groups that can present challenges in recruitment (Beaton et al. 2016; Bussey-Jones et al. 2010; James et al. 2008; Johnson et al. 2011). Researchers’ lack of knowledge about cultural differences among racial and ethnic minorities can result in ineffective recruitment, enrollment, and retention in genetic studies (George et al. 2014). Potential racial and ethnic minority participants have given a range of reasons for refusal to participate, including mistrust of the scientific community, competing time demands, lack of access to information, stigma, language barriers, religious beliefs, health insurance status, and legal status (Bussey-Jones et al. 2010; James et al. 2008; Johnson et al. 2011; Ochs-Balcom et al. 2015).
Identified barriers to research recruitment among Pacific Islanders include fear, mistrust, and concern over misrepresentation of their community (George et al. 2014; Townsend et al. 2015). For the Marshallese, these barriers are influenced by the historical relationship between the USA and the RMI. The RMI is a US-affiliated Pacific Island jurisdiction in Micronesia (Barker 2012; Yamada et al. 2004). Between 1946 and 1958, the US military tested nuclear weapons on several of the Marshall Islands, (Barker 2012; Simon and Robison 1997; Yamada et al. 2004) and these tests, which were equivalent to 7200 Hiroshima-sized bombs, exposed the islanders to significant levels of nuclear radiation (Cronkite et al. 1995; Lessard et al. 1984; Pollock 2002; Robison et al. 1997; Simon 1997). People who inhabited the bombed islands were sent to other islands; however, the people living on nearby islands were not relocated, and they suffered from exposure to nuclear fallout. US scientists implemented Project 4.1 after the nuclear testing to study the effects of nuclear radiation on humans. Researchers conducted the studies without informed consent and without appropriate language translation (Barker 2012; Dobyns and Hyrmer 1992; Kroon et al. 2004; Lessard et al. 1984; Pollock 2002; Takahashi et al. 2003). Narrative health histories document health effects of the nuclear testing in the Marshallese community through their songs, art, and stories; in the creation of new words; and in Marshallese health beliefs and behaviors (Barker 2012). As a culture affected by historical trauma, the Marshallese exhibit distrust in academic researchers (Barker 2012). Therefore, specific attention must be given to overcome this historical trauma and distrust in research. One way to address historical trauma in the Marshallese community is through community-based participatory research (CBPR) on topics prioritized by the community and with full participation from the community. CBPR shares power and builds trust between academic researchers and the community and has demonstrated to be effective in engaging minority participants in genetics research studies (McCabe-Sellers et al. 2008; Minkler and Wallerstein 2008; Powell-Wiley et al. 2013).
In 2012, the research team engaged a community-based participatory research (CBPR) partnership with the Marshallese in Northwest Arkansas. Through a multiple-year engagement process described by McElfish et al. (2015a), (McElfish et al. 2015c) the Marshallese community chose diabetes as their primary health concern, and an interprofessional CBPR team began working with the community on this priority (Donoho et al. 2015; Hallgren et al. 2015; Long et al. 2016; McElfish 2016; McElfish et al. 2015a; McElfish et al. 2016a; McElfish et al. 2015b; McElfish et al. 2016b; McElfish et al. 2016c; McElfish et al. 2016d; McElfish et al. 2016e; McElfish et al. 2016f; Purvis et al. 2016; Scott et al. 2016; Yeary et al. 2016). In addition, the team implemented an interprofessional free clinic (the North Street Clinic) to treat uninsured Marshallese with diabetes. The CBPR team’s long-term goal is to reduce health disparities and increase health equity for broader Pacific Islander communities by developing new methods of preventing and treating diabetes and other cardio-metabolic disease.
The purpose of this study was to determine the feasibility of enrolling Marshallese in Northwest Arkansas in a genetic study that included the collection of a saliva sample to obtain DNA. Specifically, the genetic samples will be used to examine genetic variants that contribute to variation in estimated glomerular filtration rate (eGFR) and albumin/creatinine ratio to better understand diabetes among Marshallese and other Pacific Islanders. Therefore, we recruited from a clinic that focused on treating Marshallese community members with diabetes. We hypothesized that recruitment would be successful within the context of our larger CBPR partnership with the Marshallese community. This paper describes the recruitment results using a mixed method design.
Methods
Study setting
Participants for the genetic study were recruited from the University of Arkansas for Medical Sciences (UAMS) North Street Clinic, which serves Marshallese persons in Northwest Arkansas who have limited access to healthcare services. The majority of North Street Clinic patients have diabetes. Bilingual Marshallese staff are part of the clinic team, assisting patients and providers in assuring services are effectively communicated and culturally appropriate. These staff completed 15 h of study specific training that addressed consent procedures, saliva sample collection, quality control procedures, confidentiality, data security, and human subject protection.
Recruitment and consent
All patients who attended the North Street clinic between July 12, 2016 and February 2, 2017 were approached for participation in the study. Patients were approached during their regularly scheduled clinic visit, unless they had already been approached about the study at a previous clinic visit. Fifteen participants were not approached for participation because they were missed in the clinic flow. Patients meeting the study inclusion criteria (at least 18 years of age, self-reported Marshallese) were approached by a bilingual (English and Marshallese) research staff, provided information about the study and asked if they were interested in participating in the study. Those individuals agreeing to participate were provided the opportunity to join the study by providing written consent. Study staff went over the consent information verbally and provided a copy of the written consent document. Participants were provided the opportunity to have their questions answered. The consent information included a description of the DNA sample collection process and what the DNA would be used for. It also included language required from Genetic Information Nondiscrimination Act (GINA) to assure compliance with the federal requirements (Sarata et al. 2011). The consent included a Health Insurance Portability and Accountability Act (HIPAA) release for medical record abstraction and three additional consent options. The first option asked if participants would like to be contacted for future studies. The second option asked participants for permission to retain specimens for future Institutional Review Board (IRB)-approved studies. The third option asked participants for permission to link information from this study to other UAMS studies in which they had participated (Table 1). All materials, including consent, were provided in participants’ language of choice (English or Marshallese). The study was reviewed and approved by the UAMS IRB # 205419. Those who took part in the study were provided a US$20 gift card.
Table 1.
Additional consent options
| Agreed | Declined | Incomplete *did not respond to the question |
|
|---|---|---|---|
| I would like to be contacted about opportunities to participate in additional research projects. | 143 (96.6%) | 1 (<1%) | 4 (2.7%) |
| I give permission for UAMS to keep my saliva sample to use in future studies that are approved by the UAMS Institutional Review Board. | 142 (95.9%) | 3 (2.0%) | 3 (2.0%) |
| I give permission for UAMS to link information from this study to other UAMS studies I have participated in. | 144 (97.3%) | 1 (<1%) | 3 (2.0%) |
Data collection
Participants provided a saliva specimen in an Oragene® DNA self-collection kit for DNA extraction (DNA Genotek Website: Technical Support: FAQ 2016). Each participant was informed of the manufacturer’s instructions for providing the saliva sample and donated approximately 2 mL volume of saliva into the collection tube. The saliva collection took approximately 2–10 min with a mean of approximately 5 min. In addition to the saliva specimen, the participants were asked to respond to three questions immediately after the sample collection. (1) How do you feel about researchers examining your DNA to better understand diabetes in Pacific Islanders? (2) How do you feel about providing a spit sample? (3) How would you like for us to update you on the study’s progress? Data collectors explained to participants that they would be provided study updates but would not be provided with personalized results. The three questions were developed with input from Marshallese stakeholders to inform future studies.
Data analysis
Records of potential participants’ decisions of whether or not to participate were systematically collected by the research manager and used to calculate recruitment rates. Descriptive analyses were conducted on potential participants’ reasons for refusal, when available. For the three open-ended questions asked immediately after the sample collection, responses were translated from Marshallese to English, coded, and summarized by the principal investigator (lead author) and research manager and then confirmed by two co-investigators. Descriptive analyses of participants’ preferred method of contact for receiving the study’s overall results were conducted.
Results
Participant rate
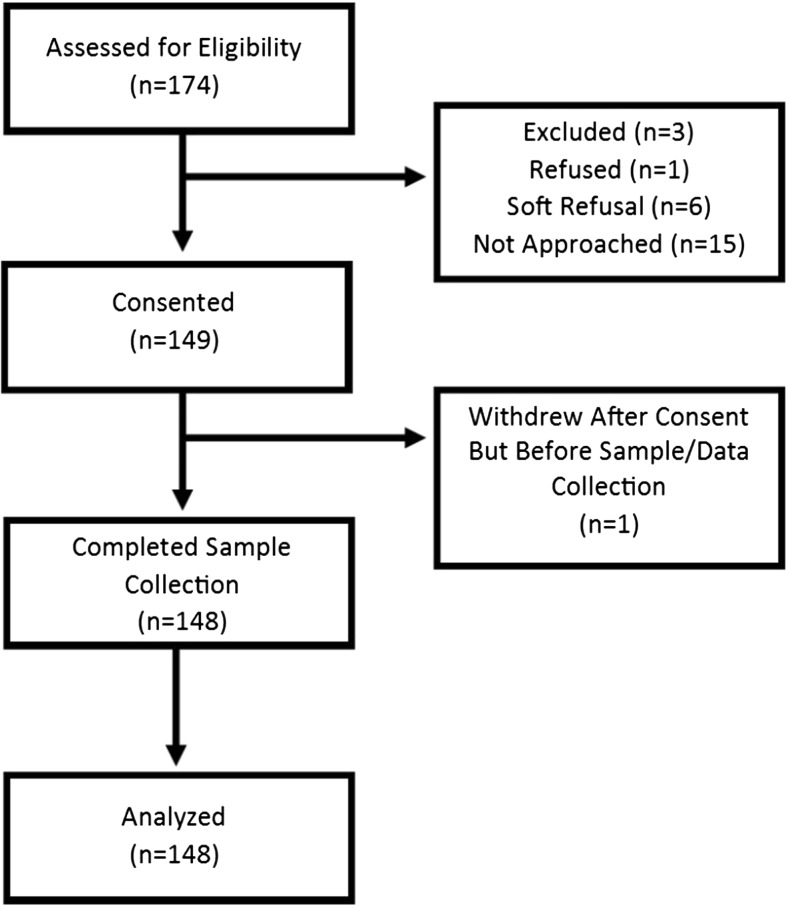
A total of 174 people were assessed for eligibility. Three patients did not meet eligibility criteria and were excluded. Of the 171 eligible patients, 156 were approached during their regularly scheduled clinic visit and offered information about the study and the opportunity to consent and participate. Fifteen eligible patients were not approached due to patient flow constraints and a lack of staff availability. Of the 156 patients approached for participation, one participant refused, six gave soft refusals (e.g., “not this time,” “too busy today”), and 149 (95.5%) patients agreed to participate. One participant decided not to continue and did not provide a sample after consent was given. One-hundred forty-eight participants were included in the study. See Fig. 1.
Fig. 1.
Participation rates
Among the 148 persons who did participate, 143 (96.6%) also agreed to be contacted for future studies, one (<1%) declined to be contacted for future studies, and four (2.7%) did not respond. Of the participants, 142 (95.9%) agreed to have their samples used for future IRB-approved studies, three participants (2.0%) declined to have their samples used for future studies, and three (2.0%) did not respond. Similarly, 144 (97.3%) gave permission for UAMS to link information from this study to other UAMS studies in which they have participated, one participant (<1%) declined, and three (2%) did not respond. One participant declined all three of the consent options, and three participants’ information was incomplete for additional consent options.
Participant characteristics
More females than males (64.9% vs. 35.1%) participated in the study. The mean age was 55 years (SD ± 11). More than eight out of ten participants (84%) did not have health insurance. Clinical data were gathered from participants’ medical records. The average weight was 170.9 lb (77.5 kg, SD ± 16.2). Body Mass Index (BMI, kg/m2) was collected for 115 patients. Height measurement was missing in 34 individuals. None of the participants was underweight, and only 21 participants (18.3%) had a healthy weight. Thirty participants (26.1%) were overweight and 64 were obese, with 36 (31.3%), 20 (17.4%), and 8 (7%) classified as having class I, II, or III obesity, respectively.
The vast majority of participants had HbA1C greater than 6.5%, which is indicative of diabetes, and half of participants had levels ranging between 8.3% and 12.5%, which is indicative of poorly controlled diabetes. More than seven out of ten patients (71.9%) had blood glucose greater than or equal to 200 mg/dL, which is indicative of diabetes. However, on the date of sample collection, only two participants reported fasting, and non-fasting glucose as less reliable. For urine albumin/creatinine ratio, 87 participants (62.6%) had an elevated urinary albumin excretion (i.e., ≥30 mg/g creatinine), which is indicative of kidney damage. Among the participants, there was a wide variability in urine albumin/creatinine ratio as 50% of the sample had ratios ranging from 18.5 to 191.4. Based on the American Diabetes Association’s standards (Tuttle et al. 2014) for stages of chronic kidney disease, 103 patients (73.6%) were classified as having kidney damage with mild decreased eGFR, and two had kidney failure. Participants’ demographic and health information are described in Table 2.
Table 2.
Participants’ demographic and health information: descriptive statistics
| Socio-demographic characteristics | Frequency | Percenta
(%) |
Mean (SD) |
Medianb
(IQR) |
|---|---|---|---|---|
| Gender | ||||
| Males | 52 | 35.1 | ||
| Females | 96 | 64.8 | ||
| Age | 55 (11) |
55 (46–63) |
||
| 25–34 | 6 | 4.1 | ||
| 35–44 | 26 | 17.6 | ||
| 45–54 | 33 | 22.3 | ||
| 55–64 | 57 | 38.5 | ||
| 65+ | 26 | 17.6 | ||
| Health insurance coverage | ||||
| Yes | 23 | 15.6 | ||
| No | 124 | 84.4 | ||
| Clinical measures | ||||
| Weight (kilograms) | 77.52 (16.24) |
77.11 (65.59–87.09) |
||
| Body Mass Index (BMI), kg/m2 |
31.1 (6.0) |
30.8 (27.1–34.6) |
||
| Weight status(Centers for Disease Control and Prevention 2017) | ||||
| Underweight (<18.5) | 0 | 0 | ||
| Healthy weight (18.5 to <25.0) | 21 | 18.3 | ||
| Overweight (25.0 to <30.0) | 30 | 26.1 | ||
| Obese-Class 1 (30 to <35) | 36 | 31.3 | ||
| Obese-Class 2: (35 to <40) | 20 | 17.4 | ||
| Obese-Class 3: (> 40) | 8 | 7.0 | ||
| HBA1C | 10.2 (2.6) |
9.9 (8.3–12.5) |
||
| Diabetes classification(American Diabetes Association 2017b) | ||||
| Normal (≤5.6%) | 5 | 3.4 | ||
| Prediabetes (5.7%–6.4%) | 5 | 3.4 | ||
| Diabetes (≥6.5%) | 137 | 93.2 | ||
| Glucose | 258.6 (97.5) |
252.0 (188.0–320.0) |
||
| Diabetes classification(American Diabetes Association 2017b) | ||||
| <200 mg/dL | 41 | 28.1 | ||
| ≥200 mg/dL | 105 | 71.9 | ||
| Urine albumin/creatinine ratio | 141.0 (190.1) |
49.3 (18.5–191.4) |
||
| Abnormalities in albumin excretion(American Diabetes Association 2015) | ||||
| Normal (<30 mg/g creatinine) | 52 | 37.4 | ||
| Increased urinary albumin excretion (≥30 mg/g creatinine) |
87 | 62.6 | ||
| Estimated glomerular filtration rate (eGFR) | 56.0 (13.5) |
60.1 (58.5–60.1) |
||
| Stages of chronic kidney disease based on eGFR (mL/min/1.73 m2)(American Diabetes Association 2017a) | ||||
| Failure (<15) | 2 | 1.4 | ||
| Severe decrease in eGFR (15–29) | 7 | 5.0 | ||
| Moderate decrease in eGFR (30–59) | 27 | 19.3 | ||
| Kidney damage with mild decrease in eGFR (60–89) | 103 | 73.6 | ||
| Kidney damage with normal or increased eGFR (≥90) | 1 | 0.7 | ||
aValid percentages of observations out of the total number of non-missing responses are reported. bMedian and Interquartile range (IQR) are displayed as additional measures of central tendency and dispersion as data were skewed for some of the clinical variables
Interview responses
When asked “How do you feel about researchers examining your DNA to better understand diabetes in Pacific Islanders?” all but one participant gave a response indicating that they felt positive about it. Responses included “It is a good purpose in order to find out more health issues in Pacific Islanders.” Others stated “I would like to know more about our illnesses so providing what is needed is okay” and “I think it is a good idea because we need to know the real reason why there is diabetes in Pacific Islanders.” One participant stated “I don't know how I feel.” Participants also discussed that the positive feelings were based upon the long-term CBPR relationships with the community. As one participant responded, “Good, because I trust the researcher.”
When asked “How do you feel about providing a spit sample?,” all but one of the participants gave an answer that indicated they felt good or fine about the saliva sample because they believed in the purpose of the study. Participants replied: “I feel good. I didn't know that my spit can be included in studies about diabetes but now I know;” “If it’s for a good purpose, I will give any sample needed;” and “Very good, I'm happy to provide my spit.” Others stated “I'm happy to provide you and the study with a sample because it is important.” However, one participant stated. “It’s disgusting because it’s spit.” Only three of the participants noted that they found the saliva collection challenging. These participants stated: “It was kind of hard for me to do, but I can do it;” “It was hard to spit but I managed it;” and “It was kind of a hard thing to do, but I am fine.”
When asked “How would you like for us to update you on the study’s progress?,” all participants stated that they would like to be updated about the study’s overall results, with phone updates (41.2%) the most common preference. An additional 16.9% said they would like to be updated by phone and a second method (e.g., e-mail or text), 9.5% requested updates by mail, and only 2% requested updates by e-mail. Others requested a home visit (2%), text (1.4%), or Facebook (<1%) as the method for updating them on the study’s results. The remaining 26.4% stated that they would like to be updated about the study results, but did not list a preferred method.
Discussion
The study team hypothesized that, despite documented challenges in recruiting minority communities into genetics studies, recruitment would be successful within the context of our larger CBPR partnership with the Marshallese community. This study demonstrates willingness to participate in genetic research among Marshallese living in Arkansas, with more than 95% of patients approached agreeing to participate in the study and the vast majority of those participants agreeing to have their samples saved in a biobank and used for other IRB-approved studies (95.9%), to be contacted for future studies (96.6%), and to have their genetic data linked to other studies in which they have participated at UAMS (97.3%). Importantly, all participants reported a desire to receive study updates, with the majority requesting phone call updates. While the request for study updates was not surprising, the desire to receive those through a phone call rather than in written format was not expected and will require additional staff time to fulfill the requests. Phone updates will allow participants to ask questions and allow the study team to better explain the results.
Qualitative responses showed that a majority of the participants had positive feelings about the study and about the saliva sample as the method for collecting the DNA specimen. Participants discussed their desire to participate as a way of contributing to the health of their community and to understanding the genetic influence related to diabetes in their community. This was likely enhanced because the study focused on diabetes, a topic prioritized by the community through a CBPR process (McElfish et al. 2015c). Participants also noted that their decision to participate was based on pre-existing relationships with the study team. Therefore, this study’s success was likely enhanced because it was conducted in the context of a larger CBPR partnership, which helped the study team overcome historical trauma that has constrained genetic research with Pacific Islanders.
An additional contributor to successful recruitment was the involvement of bilingual Marshallese staff who took time to fully describe the rationale for the study to participants and who explained DNA and genetics studies to participants. It is important to note that while the bilingual Marshallese study staff took care to explain the study, DNA and genetics are terms that are not part of the Marshallese language and translating the concepts was difficult.
Integration of the genetic study into ongoing clinical operations helped facilitate recruitment with minimal added cost. These factors are consistent with prior genetic research with other racial and ethnic minority communities that showed successful recruitment strategies may include initial face-to-face contact, bilingual and bicultural recruitment team, community-engaged research methods, transparency of how the research will be used, and monetary incentives (James et al. 2008; Johnson et al. 2011; Townsend et al. 2015).
Limitations and strengths
This study is limited by the nonprobability sample and the small sample size (n = 148). Furthermore, only Marshallese patients of the North Street Clinic in Arkansas were recruited. Many of these patients had diabetes which may have affected their motivation; therefore, the study may not be generalizable to other Pacific Islander communities living outside Arkansas or who seek care in another clinic. While the qualitative responses were overwhelmingly positive, it is important to note that the questions were only asked of participants who consented to the study and likely do not represent the responses of the person who declined to participate. Despite these limitations, the study is important because it contributes to the very limited number of genetic studies with Pacific Islanders in the USA. The study contributes significantly to the literature as the first published study to examine feasibility of recruiting Marshallese in the USA for genetic research.
Conclusion
Among Marshallese living in Arkansas, a community affected by historical trauma and exhibiting distrust in academic researchers, researchers were able to collect samples for genetic research in 95.5% of those approach for a study related to diabetes. This study is important to CBPR community stakeholders who have voiced a desire to collaboratively conduct genetic research related to diabetes, birth defects and other perinatal outcomes, and cancer. The genetic samples collected in this study will be used to examine genetic variants that may contribute to variation in eGFR and albumin/creatinine ratio to better understand diabetes among Marshallese. Other genetic studies with the Marshallese community are being planned in collaboration with community stakeholders.
Acknowledgements and source of funding
This pilot study was funded in part by a grant from the Sturgis Foundation. In addition, the community-based participatory research work is supported by the Translational Research Institute supported the project described, grant UL1TR000039, through the United States National Institutes of Health (NIH) National Center for Research Resources and National Center for Advancing Translational Sciences.
Acknowledgments
Authors Contributions
All authors contributed to this work. P.M., S.K, and J.S. conceptualized the project, conducted data analyses, and wrote the paper. S.I. collected data, assisted with data analysis, and edited the paper. T.S. and R.W. collected data and edited the paper. R.N., C.L., and B.A. wrote sections and edited the paper. N.H., S.R., and N.A. provided extensive editing to the paper. All authors discussed the results and implications and commented on the manuscript at all stages. N.A. and S.R. are members of the Pacific Islander Community.
Compliance with ethical standards
Conflicts of interest
No authors have conflicts of interest to report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. The Institutional Review Board (IRB) at the University of Arkansas for Medical Science Northwest reviewed and approved the study procedures described in the article #205419.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
This article is part of the Topical Collection on Inclusion of Diverse Populations In Genomics Research and Health Services: A Scientific and Health Equity Imperative
Contributor Information
Pearl A. McElfish, Phone: 479.713.8680, Email: pamcelfish@uams.edu
Marie-Rachelle Narcisse, Email: narcissem@uams.edu.
Christopher R. Long, Email: Crlong2@uams.edu
Britni L. Ayers, Email: blayers@uams.edu
Nicola L. Hawley, Email: nicola.hawley@yale.edu
Nia Aitaoto, Email: Nia.aitaoto@gmail.com.
Sheldon Riklon, Email: sricklon@uams.edu.
L. Joseph Su, Email: ljsu@uams.edu.
Shumona Z. Ima, Email: shumonatazz06@gmail.com
Ralph O. Wilmoth, Email: rowilmoth@uams.edu
Thomas K. Schulz, Email: TKSchulz@uams.edu
Susan Kadlubar, Email: sakadlubar@uams.edu.
References
- Ahmad F, Weller C (2014) Reading between the data the incomplete story of Asian Americans, Native Hawaiians, and Pacific Islanders
- Aitaoto N, Ichiho H. Assessing the health care system of services for non-communicable diseases in the US-affiliated Pacific Islands: a Pacific regional perspective Hawaii. J Med Public Health. 2013;72:106–114. [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med. 2007;24:451–463. doi: 10.1111/j.1464-5491.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- Ali O. Genetics of type 2 diabetes. World J Diabetes. 2013;4:114–123. doi: 10.4239/wjd.v4.i4.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (9) Microvascular complications and foot care. Diabetes Care. 2015;38(Suppl):S58–S66. doi: 10.2337/dc15-S012. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association 10. Microvascular complications and foot care. Diabetes Care. 2017;40:S88–S98. doi: 10.2337/dc17-S013. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2017b) Diagnosing diabetes and learning about prediabetes http://www.diabetes.org/diabetes-basics/diagnosis/
- Barker H. Bravo for the Marshallese: regaining control in a post-nuclear, post-colonial world. Belmont: Cengage Learning; 2012. [Google Scholar]
- Beaton A et al (2016) Engaging Māori in biobanking and genomic research: a model for biobanks to guide culturally informed governance, operational, and community engagement activities. Genet Med. doi:10.1038/gim.2016.111 [DOI] [PubMed]
- Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity (Silver Spring) 2008;16(Suppl 3):S5–S10. doi: 10.1038/oby.2008.528. [DOI] [PubMed] [Google Scholar]
- Bussey-Jones J, Garrett J, Henderson G, Moloney M, Blumenthal C, Corbie-Smith G. The role of race and trust in tissue/blood donation for genetic research. Genet Med. 2010;12:116–121. doi: 10.1097/GIM.0b013e3181cd6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante CD, Burchard EG, De la Vega FM. Genomics for the world. Nature. 2011;475:163–165. doi: 10.1038/475163a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2017) About adult BMI https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/
- Cronkite E, Bond V, Conard R. Medical effects of exposure of human beings to fallout radiation from a thermonuclear explosion. Stem Cells (Dayton, Ohio) 1995;13(Suppl 1):49–57. [PubMed] [Google Scholar]
- DNA Genotek Website: Technical Support: FAQ. (2016) http://www.dnagenotek.com/techsupport_faq.htm
- Dobyns B, Hyrmer B. The surgical management of benign and malignant thyroid neoplasms in Marshall Islanders exposed to hydrogen bomb fallout. World J Surg. 1992;16:126–139. doi: 10.1007/BF02067128. [DOI] [PubMed] [Google Scholar]
- Donoho G, McElfish P, Avants R, Hallgren E. A novel recruiting and surveying method: participatory research during a Pacific Islander community’s traditional cultural event. Gateways Int J Commun Res Engagement. 2015;8:150–159. doi: 10.5130/ijcre.v8i1.4227. [DOI] [Google Scholar]
- George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104:e16–e31. doi: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren E, McElfish P, Rubon-Chutaro J. Barriers and opportunities: a community-based participatory research study of health beliefs related to diabetes in a US Marshallese community. Diab Educ. 2015;41:86–94. doi: 10.1177/0145721714559131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley N, McGarvey S. Obesity and diabetes in Pacific Islanders: the current burden and the need for urgent action. Curr Diab Rep. 2015;15:29. doi: 10.1007/s11892-015-0594-5. [DOI] [PubMed] [Google Scholar]
- Hawley NL, et al. Prevalence of adiposity and associated cardiometabolic risk factors in the Samoan genome-wide association study. Am J Hum Biol. 2014;26:491–501. doi: 10.1002/ajhb.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L, Rowen L. The human genome project: big science transforms biology and medicine. Genome Med. 2013;5:79. doi: 10.1186/gm483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RD, Yu JH, Henrikson NB, Bowen DJ, Fullerton SM, Group HDW Strategies and stakeholders: minority recruitment in cancer genetics research. Commun Genet. 2008;11:241–249. doi: 10.1159/000116878. [DOI] [PubMed] [Google Scholar]
- Johnson VA, Powell-Young YM, Torres ER, Spruill IJ. A systematic review of strategies that increase the recruitment and retention of African American adults in genetic and genomic studies. ABNF J. 2011;22:84–88. [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, et al. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35:1060–1067. doi: 10.1007/BF02221682. [DOI] [PubMed] [Google Scholar]
- Karter AJ, Schillinger D, Adams AS, Moffet HH, Liu J, Adler NE, Kanaya AM. Elevated rates of diabetes in Pacific Islanders and Asian subgroups The Diabetes Study of Northern California (DISTANCE) Diabetes Care. 2013;36:574–579. doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating ST, El-Osta A. Epigenetics and metabolism. Circ Res. 2015;116:715–736. doi: 10.1161/CIRCRESAHA.116.303936. [DOI] [PubMed] [Google Scholar]
- Kroon E, et al. Cancer in the Republic of the Marshall Islands Pacific. Health Dialog. 2004;11:70–77. [PubMed] [Google Scholar]
- LeDoux C (2009) Challenges encountered reaching the Marshallese in Arkansas. Arkansas Assessment Initiative
- Lessard E, Miltenberger R, Cohn S, Musolino S, Conard R. Protracted exposure to fallout: the Rongelap and Utirik experience. Health Phys. 1984;46:511–527. doi: 10.1097/00004032-198403000-00002. [DOI] [PubMed] [Google Scholar]
- Long C, Stewart M, Cunningham T, Warmack T, McElfish P. Health research participants’ preferences for receiving research results. Clin Trials. 2016;13:582–591. doi: 10.1177/1740774516665598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunshof JE, et al. Personal genomes in progress: from the human genome project to the personal genome project. Dialogues Clin Neurosci. 2010;12:47–60. doi: 10.31887/DCNS.2010.12.1/jlunshof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–351. doi: 10.1023/A:1025635913927. [DOI] [PubMed] [Google Scholar]
- Mau M, Sinclair K, Saito E, Baumhofer K, Kaholokula J. Cardiometabolic health disparities in native Hawaiians and other Pacific Islanders. Epidemiol Rev. 2009;31:113–129. doi: 10.1093/ajerev/mxp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe-Sellers B, et al. Personalizing nutrigenomics research through community based participatory research and OMICS technologies. OMICS. 2008;12:263–272. doi: 10.1089/omi.2008.0041. [DOI] [PubMed] [Google Scholar]
- McElfish P (2016) Health policies creating inequality for Marshallese migrants in Arkansas. J Ark Med Soc [PMC free article] [PubMed]
- McElfish P, Bridges M, Hudson J, Purvis R, Bursac Z, Kohler P, Goulden P. Family model of diabetes education with a Pacific Islander community. Diab Educ. 2015;41:706–715. doi: 10.1177/0145721715606806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfish P, Hallgren E, Yamada S. Effect of US health policies on health care access for Marshallese migrants. Am J Public Health. 2015;105:637–643. doi: 10.2105/AJPH.2014.302452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfish P, et al. Community-driven research agenda to reduce health disparities. Clin Transl Sci. 2015;8:690–695. doi: 10.1111/cts.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfish P, Hallgren E, Henry L, Ritok M, Rubon-Chutaro J, Kohler P. Health beliefs of Marshallese regarding type 2 diabetes. Am J Health Behav. 2016;40:248–257. doi: 10.5993/AJHB.40.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfish P, et al. Social ecology and diabetes self-management among Pacific Islanders in Arkansas. J Fam Med Dis Prev. 2016;2:026. doi: 10.23937/2469-5793/1510026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfish P, Post J, Rowland B. A social ecological and community-engaged perspective for addressing health disparities among Marshallese in Arkansas. Int J Nurs Clin Pract. 2016;3:1–6. doi: 10.15344/2394-4978/2016/191. [DOI] [Google Scholar]
- McElfish P, et al. Interpretive policy analysis: Marshallese COFA migrants and the Affordable Care Act. Int J Equity Health. 2016;15:91. doi: 10.1186/s12939-016-0381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfish P et al (2016e) Diabetes and hypertension in Marshallese adults: results from faith-based health screening events. J Racial Ethn Health Disparities. doi:10.1007/s40615-016-0308-y [DOI] [PMC free article] [PubMed]
- McElfish PA et al (2016f) Engagement practices that join scientific methods with community wisdom: designing a patient-centered, randomized control trial with a Pacific Islander community. Nurs Inq. doi:10.1111/nin.12141 [DOI] [PMC free article] [PubMed]
- Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham offspring study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- Minegishi M, et al. Diabetes mellitus and obesity among participants receiving screening for cancer in the Republic of the Marshall Islands. J Int Health. 2007;22:133–141. [Google Scholar]
- Minino A (2013) Death in the United States, 2011. National Vital Statistics System, Mortality
- Minkler M, Wallerstein N. Community-based participartory research for health: from process to outcomes. San Francisco: Jossey-Bass Publishers; 2008. [Google Scholar]
- Murphy S, Xi J, Kochanek K (2013) National Vital Statistics Reports vol 61. CDC/NCHS, National Vital Statistics System, Mortality
- Ochs-Balcom H, Jandorf L, Wang Y, Johnson D, Ray V, Willis M, Erwin D. “It takes a village”: multilevel approaches to recruit African Americans and their families for genetic research. J Community Genet. 2015;6:39–45. doi: 10.1007/s12687-014-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okihiro M, Harrigan R. An overview of obesity and diabetes in the diverse populations of the Pacific. Ethn Dis. 2005;15(S5):71–80. [PubMed] [Google Scholar]
- Palafox N, Tsark J. Cancer in the US associated Pacific Islands (UASPI): history and participatory development. Pacific Health Dialog. 2004;11:8–13. [PubMed] [Google Scholar]
- Pollock N. Health transitions, fast and nasty: exposure to nuclear radiation. Pac Health Dialog. 2002;9:275–282. [PubMed] [Google Scholar]
- Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538:161–164. doi: 10.1038/538161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P, Levin K, Petersen I, Christensen K, Beck-Nielsen H, Vaag A. Heritability of insulin secretion, peripheral and hepatic insulin action, and intracellular glucose partitioning in young and old Danish twins. Diabetes. 2005;54:275–283. doi: 10.2337/diabetes.54.1.275. [DOI] [PubMed] [Google Scholar]
- Powell-Wiley TM, et al. Churches as targets for cardiovascular disease prevention: comparison of genes, nutrition, exercise, wellness and spiritual growth (GoodNEWS) and Dallas County populations. J Public Health (Oxf) 2013;35:99–106. doi: 10.1093/pubmed/fds060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis R et al. (2016) Community health warriors: Marshallese community health workers’ perceptions and experiences with CBPR and community engagement under review by Progress in Community Health Partnerships: Research, Education, and Action [DOI] [PubMed]
- Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–694. doi: 10.1111/j.1753-4887.2008.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro M, Yee A. Out of the shadows: Asian Americans Native Hawaiians, and Pacific Islanders. Am J Public Health. 2010;100:776–778. doi: 10.2105/AJPH.2010.192229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison W, et al. The Northern Marshall Islands radiological survey: data and dose assessments. Health Phys. 1997;73:37–48. doi: 10.1097/00004032-199707000-00004. [DOI] [PubMed] [Google Scholar]
- Sarata A, DeBregh J, Staman J (2011) U.S. Congressional Research Service. The Genetic Information Nondiscrimination Act of 2008 and the Patient Protection and Affordable Care Act of 2010: overview and legal analysis of potential interactions
- Schiller J, Lucas J, Peregoy J. Summary health statistics for U.S. adults: National Health Interview Survey, 2011 vol 10(256) Washington, DC: Centers for Disease Control and Prevention; 2012. [PubMed] [Google Scholar]
- Scott A, Shreve M, Ayers B, McElfish PA. Breast-feeding perceptions, beliefs and experiences of Marshallese migrants: an exploratory study. Public Health Nutr. 2016;19:1–10. doi: 10.1017/S1368980016001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S, Robison W. A compilation of nuclear weapons test detonation data for U.S. Pacific ocean tests. Health Phys. 1997;73:258–264. doi: 10.1097/00004032-199707000-00022. [DOI] [PubMed] [Google Scholar]
- Simon SL. A brief history of people and events related to atomic weapons testing in the Marshall Islands. Health Phys. 1997;73:5–20. doi: 10.1097/00004032-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Guillermo T. Toward improved health: disaggregating Asian American and Native Hawaiian Pacific Islander data. Am J Public Health. 2000;90:1731–1734. doi: 10.2105/AJPH.90.11.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322:1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- Sørensen TI, Holst C, Stunkard AJ. Adoption study of environmental modifications of the genetic influences on obesity. Int J Obes Relat Metab Disord. 1998;22:73–81. doi: 10.1038/sj.ijo.0800548. [DOI] [PubMed] [Google Scholar]
- Takahashi T, et al. The relationship of thyroid cancer with radiation exposure from nuclear weapon testing in the Marshall Islands. J Epidemiol / Jpn Epidemiol Assoc. 2003;13:99–107. doi: 10.2188/jea.13.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend C et al. (2015) Community-based participatory research integrates behavioral and biological research to achieve health equity for Native Hawaiians. Int J Environ Res Public Health 13 [DOI] [PMC free article] [PubMed]
- Tuttle K et al (2014) Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. doi:10.2337/dc14-1296 [DOI] [PMC free article] [PubMed]
- United States Census Bureau (2010) 2010 Census interactive population search http://www.census.gov/2010census/popmap/ipmtext.php. Accessed 15 May 2013
- Wilson BJ, Nicholls SG. The Human Genome Project, and recent advances in personalized genomics risk. Manag Health Policy. 2015;8:9–20. doi: 10.2147/RMHP.S58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall P, Scollard D, Rajan L. Hansen Disease among Micronesian and Marshallese persons living in the United States. Emerg Infect Dis. 2011;17:1202–1208. doi: 10.3201/eid1707.102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Dodd A, Soe T, Chen T, Bauman K. Diabetes mellitus prevalence in out-patient Marshallese adults on Ebeye Island, Republic of the Marshall Islands. Hawaii Med J. 2004;63:45–51. [PubMed] [Google Scholar]
- Yeary K et al. (2016) Cultural adaptation of diabetes self-management education for Marshallese residing in the US: lessons learned in curriculum development under review by progress in Community Health Partnerships: Research, Education, and Action [DOI] [PMC free article] [PubMed]