Summary
In targeted proteomics, the development of robust methodologies is dependent upon the selection of a set of optimal peptides for each protein-of-interest. Unfortunately, predicting which peptides and respective product ion transitions provide the greatest signal-to-noise ratio in a particular assay matrix is complicated. Using in vitro synthesized proteins as analytical standards, we report here an empirically driven method for the selection of said peptides in a human plasma assay matrix.
Keywords: Targeted Proteomics, Selected Reaction Monitoring, In vitro Translation, Human Plasma Proteome, Proteotypic Peptides
Introduction
Mass spectrometry has emerged as the dominant technology for the characterization of proteins in biological matrices due, in part, to its unequivocal combination of speed, selectivity, and sensitivity. Most classical mass spectrometry based proteomic workflows have taken a shotgun approach in which the protein fraction is initially digested with a protease prior to analysis. The resulting peptides are then separated by nano-flow liquid chromatography, ionized, transferred to a mass spectrometer, and subjected to tandem mass spectrometry via data-dependent acquisition (DDA). In a DDA experiment, the mass information from periodic full-scan analyses of intact peptides is used to trigger subsequent tandem mass spectrometry (MS/MS) analyses of the most abundant precursor ions for sequence identification. The resulting peptide masses and fragment ions are then searched against protein sequence databases and ultimately used as a proxy for protein identification and/or relative abundance. This general discovery based approach has become extremely powerful for determining the protein content of moderately complex biological mixtures. However, the ability to accurately compare different samples is complicated by the semi-random sampling process of DDA. Some proteins of specific interest can go undetected in one or more compared samples. Furthermore, the immense dynamic range of relative protein concentration in clinically derived specimens usually necessitates laborious pre-analysis fractionation and chromatography protocols. These ultimately hinder the throughput of these methods and make them impractical for comprehensive studies with multiple biological and/or technical replicates.
Due to limitations of current discovery-based proteomic approaches, some laboratories have begun the development and application of technologies for the targeted analysis of proteins within complex mixtures. Numerous derivations of targeted mass spectrometry using the specific acquisition of tandem mass spectra of peptides predicted in silico have been reported. More recently these methods have been based on the use of selected reaction monitoring (SRM) on triple quadrupole mass spectrometers (1–3). These methods have high specificity within complex mixtures and can be performed in a fraction of the instrument time relative to discovery-based methods. In complex biological matrices, the chemical background of co-eluting analytes can often prohibit detection of a precursor ion in a DDA experiment. However, if the precursor ion m/z is known, a triple quadrupole mass spectrometer can be used to minimize the chemical interference using two orthogonal stages of mass analysis to selectively monitor a unique peptide. The combined specificity of chromatographic retention time, precursor ion mass, and product ion mass can enable the selective detection of a peptide within a complex matrix.
Targeted mass spectrometry measurements themselves are not necessarily quantitative. For an assay to be quantitative the analyte response needs to be assessed using protein or peptide standards of known abundance. These assays can provide absolute quantitative measurements if they are thoroughly vetted like any classical quantitative mass spectrometry measurement by assessing the measurement linearity, variance, accuracy, limit of detection (LOD), and limit of quantitation (LOQ) (4). The inter-laboratory consistency of these measurements can be robust between laboratories and across instrument platforms (5). Furthermore, the high duty cycle of modern triple quadrupole instrumentation enables multiplexing of targeted SRM assays to measure multiple peptides for an array of proteins in any given experiment (3,4,6).
While the power of SRM assays is undeniable, the development of robust methodology for the selection of peptides to use as a proxy of a translated gene product is not straight forward. Due to differences in their inherent physiochemical properties, equimolar peptides of different amino acid sequences can have drastically different responses in a mass spectrometer. A ‘proteotypic’ peptide for targeted proteomics is defined here as one that is 1) unique to a given gene product, 2) lacking in high frequency, non-synonymous, single nucleotide polymorphisms, 3) devoid of known post-translational modification sites, 4) has physiochemical properties amenable to a robust detection in the mass spectrometer, and 5) has salient features that generate characteristic MS/MS fragmentation patterns via collision-induced dissociation (CID). The selection of a set of best peptides for each protein of interest is a crucial step to the development of a targeted SRM assays because considerable amounts of time and resources are often spent to produce quantitative standards such as synthetic peptides (1,2), recombinant proteins from concatenated peptide sequences (3,7), or developing immunoaffinity reagents for the enrichment of low abundance tryptic peptides (6). Traditional approaches for selecting candidate peptides for SRM assays have relied on the mining of DDA spectral libraries (8,9), the use of prediction algorithms trained on previous DDA experimental results (10,11), or the costly synthesis of all in silico predicted tryptic peptides (12). The first two approaches are predicated on the assumption that the peptides most frequently identified in DDA experiments will produce peptides with the optimal signal-to-noise ratios for a targeted proteomic experiment. Unfortunately, this is not the case (13). There are numerous reasons why a peptide may not be selected for MS/MS during a DDA experiment. Therefore, a peptide that is not observed in this type of experiment should not be excluded in a targeted experiment. Conversely, a peptide that is routinely sampled in a DDA style experiment might not necessarily be a suitable peptide for an SRM experiment. For these reasons and more, an important step in the development of SRM assays is the use of an analytical protein standard to assess empirically which peptides provide a good proxy of the target protein.
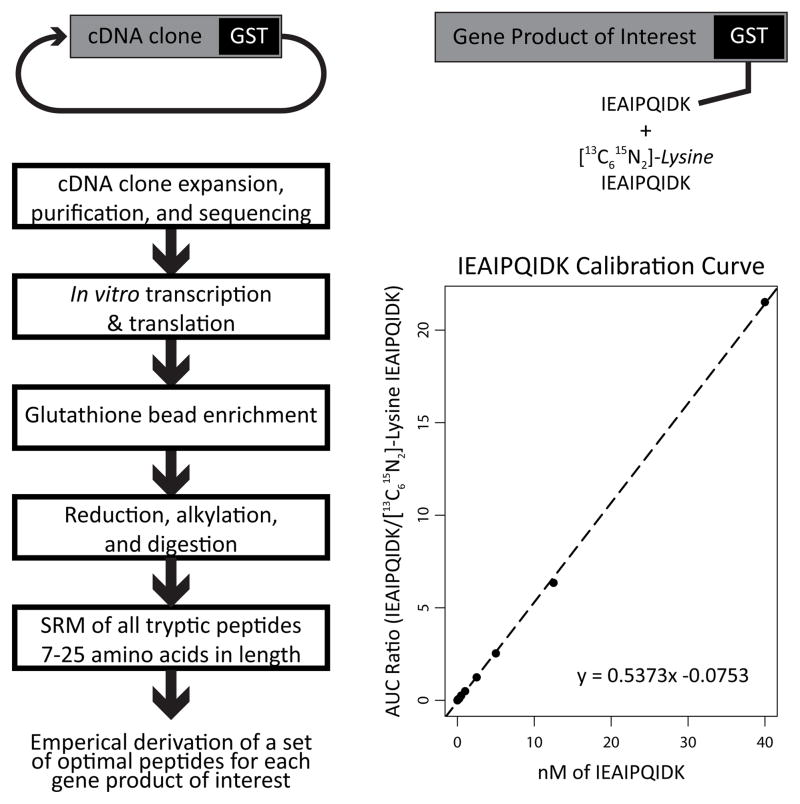
Here we demonstrate a general cost-effective strategy for the systematic selection of best peptides for use in an SRM assay for a protein of interest in a human plasma matrix.. We apply peptide selection criteria based on the sensitivity and specificity of a peptide’s SRM signal while also making considerations for matrix effects, chromatographic properties, digestion kinetics and post-digestion stability. Our strategy makes use of analytical standards expressed in vitro as N-terminal Schistosoma Japonicum Glutathione S-Transferase (SJ-GST) fusion proteins. Although discussed in the context of the human plasma proteome, this strategy is generalizable to other proteins and other biological matrices.
1. Materials
1.1 Preparation of c-terminal GST fusion proteins to use as analytical standards via in vitro protein expression
Full-length cDNA clones for human proteins from the pANT7_cGST clone collection. (Arizona State University Biodesign Institute Plasmid Repository, <https://dnasu.org/DNASU/Home.do>) (See Note 1).
Ampicillin Supplemented LB Culture Medium: 10 g/L Tryptone (Becton, Dickinson, and Company, Sparks, MD), 5 g/L Yeast Extract (Becton, Dickinson, and Company, Sparks, MD), and 10 g/L Sodium Chloride (Fisher Scientific, Fairlawn, NJ) supplemented with 100 μg ml−1 ampicillin (Invitrogen, Carlsbad, CA).
Molecular biology grade USP sterile purified water (Corning, Manassas, VA).
Sterile 14 mL polypropylene round-bottom tubes with snap-on lids (BD Biosciences, Durham, NC).
Allegra X-12R Centrifuge with SX4750 rotor (Beckman-Coulter Inc., Brea, CA).
QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA).
Bench-top centrifuge model 5417R (Eppendorf, Hauppauge, NY).
ND-1000 Spectrophotometer (Nanodrop, Wilmington, DE)
1-step Human Coupled in vitro protein synthesis kit (Pierce Biotechnology, Rockford, IL).
RiboLock RNAse inhibitor (Pierce Biotechnology, Rockford, IL).
Glutathione sepharose 4B beads (GE Healthcare Biosciences, Pittsburgh, PA).
Dulbecco’s phosphate-buffered saline without MgCl2 and CaCl2 (Gibco, Grand Island, NY)
Sepharose Bead Wash Solution #1 Dulbecco’s phosphate-buffered saline without MgCl2 and CaCl2 (Gibco, Grand Island, NY) supplemented with 863 mM sodium chloride (Fisher Scientific).
Sepharose Bead Wash Solution #2: 50 mM NH4HCO3, pH~7.8 (Fisher Scientific, Fair Lawn, NJ) dissolved in 18Ω water.
Sepharose bead reconstitution buffer: 0.1% PPS Silent Surfactant (Expedeon, San Diego, CA)/50mM Ammonium Bicarbonate (pH-7.8) supplemented with 5nM FasTrack crude ‘heavy’ [13C615N2] (L)-lysine–labeled LLLEYLEEK and IEAIPQIDK peptides (Life Technologies, Grand Island, NY).
1.2 SDS-PAGE/Western Blot Analysis of GST fusion proteins
Sample Loading Buffer: NuPage LDS 4X sample buffer (Life Technologies, Grand Island, NY).
SDS-PAGE Running Buffer: 20X NuPage MES SDS Running Buffer (Life Technologies, Grand Island, NY) diluted to 1X with deionized water.
Novex NuPage 4–12% bis-Tris mini gel, 10 well, 1.5 mm width (Life Technologies, Grand Island, NY).
Novex Sharp Pre-stained Protein Standards (Life Technologies, Grand Island, NY).
Invitrogen XCell SureLock mini-cell SDS-PAGE gel box (Life Technologies, Grand Island, NY).
PowerPac Basic Electrophoresis Power Supply (BioRad, Hercules, CA).
SilverQuest Staining kit (Life Technologies, Grand Island, NY).
XCell II Blot Module (Life Technologies, Grand Island, NY).
Blot Transfer Buffer: 20X NuPage transfer buffer (Life Technologies, Grand Island, NY) and Methanol (Fisher Scientific, Pittsburgh, PA) diluted to 1X and 10% (v/v), respectively, with deionized water.
PVDF filter paper sandwich (Life Technologies, Grand Island, NY).
Lab Rotator (Barnstead/Lab-Line).
Blot Blocking Buffer: Non-fat milk powder (Safeway Inc., Phoenix, AZ) and Tween-20 (Fisher Scientific, Pittsburgh, PA) diluted to 5% (w/v) and 0.1% (v/v), respectively, with Dulbecco’s phosphate-buffered saline without MgCl2 and CaCl2 (Gibco, Grand Island, NY).
Blot Washing Buffer: Tween-20 (Fisher Scientific, Pittsburgh, PA) diluted to 5% (w/v) and 0.1% (v/v), respectively, with Dulbecco’s phosphate-buffered saline without MgCl2 and CaCl2 (Gibco, Grand Island, NY).
Blot Primary Antibody Solution: Anti-GST Antibody (GE Healthcare Biosciences, Pittsburgh, PA) and Tween-20 (Fisher Scientific, Pittsburgh, PA) diluted 1000 fold and to 0.1% (v/v), respectively, with Dulbecco’s phosphate-buffered saline without MgCl2 and CaCl2 (Gibco, Grand Island, NY).
Blot Secondary Antibody Solution: Horseradish Peroxidase Anti-goat IgG Antibody (Pierce) and Tween-20 (Fisher Scientific, Fair Lawn, NJ) diluted 10000 fold and to 0.1% (v/v), respectively, with Dulbecco’s phosphate-buffered saline without MgCl2 and CaCl2 (Gibco, Grand Island, NY).
ECL Prime Western Blotting Kit (GE Healthcare Bio-Sciences, Pittsburgh, PA).
Autoradiography cassette (Fisher Scientific, Pittsburgh, PA).
BioMax Light Chemiluminescence Film (Kodak, Rochester, NY).
X-OMAT 2000A Film Developer (Kodak, Rochester, NY).
1.3 Sample Digestion
Sepharose bead dilution buffer: 0.1% PPS Silent Surfactant/50mM NH4HCO3 dissolved in 18Ω water.
Commercially Sourced Normal Plasma (Lampire Biological Laboratories, Pipersville, PA).
Plasma Dilution Buffer 1: 50mM NH4HCO3 dissolved in 18Ω water.
Plasma Dilution Buffer 2: 0.2% PPS Silent Surfactant/50mM NH4HCO3 dissolved in 18Ω water.
BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL).
500mM dithiothreitol (Sigma Aldrich, St. Louis, MO) dissolved in 18Ω water.
500mM iodoacetamide (Sigma Aldrich, St. Louis, MO) dissolved in 18Ω water.
Sequencing grade modified porcine trypsin (Promega, Madison, WI) reconstituted at 0.5 μg/μL with Plasma Dilution Buffer 1.
5N solution of Hydrochloric Acid (Fisher Scientific, Pittsburgh, PA) in 18Ω water.
Bench-top centrifuge model 5417R (Eppendorf, Hauppauge, NY).
1.4 Nano-flow Liquid Chromatography (nanoLC) Electrospray Ionization Tandem Mass Spectrometry of Digested Analytical Standards
Bench-top centrifuge model 5417R (Eppendorf, Hauppauge, NY)
In-house fritted trap columns: 200 μL of KASIL 1 potassium silicate (PQ Corporation, Malvern, PA) is mixed with 50 μL of formamide (Sigma Aldrich, St. Louis, MO), vortexed briefly, and centrifuged for 1 minute at 10K RPM in a bench-top centrifuge. Several 20 cm × 150 μm poly-amide coated fused silica capillaries (Polymicro Technologies, Phoenix, AZ) are submerged in the resulting supernatant for 2–3 seconds and then cured overnight at 80 °C in a laboratory oven.
A homemade pressure bomb interfaced with a high-pressure helium gas cylinder as described in references (14, 15).
Trap Column: 5 cm × 150 μm poly-amide coated fused silica capillary (Molex, Lisle, IL) fritted on one end with ~ 0.5 cm of polymerized potassium silicate and packed at 750 PSI with Jupiter Proteo 90Ǻ C12 4μ reversed-phase beads (Phenomenex, Ventura, CA).
Analytical Column: 20 cm × 75 μm polyamide-coated fused silica capillary pulled to 10 μm emitter tip with a Sutter P-2000 laser puller (Sutter Instruments, Novato, CA) and packed at 750 PSI with ReproSil-Pur 120Ǻ C18-AQ 3μ reversed-phase beads (Dr. Maisch GmbH, Germany).
Polypropylene auto-sampler vials with snap-on lids (National Scientific, Rockwood, TN).
Easy-nLC 1000 Liquid Chromatoraphy System (Thermo Fisher Scientific, San Jose, CA).
TSQ Quantiva Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific, San Jose, CA).
1.5 Quantification of in vitro expressed GST fusion proteins
Concentrated stock (1 uM each in 5% Acetonitrile/0.1% formic acid) of FasTrack crude ‘heavy’ [13C615N2] (L)-lysine–labeled LLLEYLEEK and IEAIPQIDK peptides (Life Technologies, Grand Island, NY)
Concentrated stock (5 uM each in 5% Acetonitrile/0.1% formic acid) of AQUA un–labeled LLLEYLEEK and IEAIPQIDK peptides (Life Technologies, Grand Island, NY)
1 nMole BSA Protein Digest Standard (Life Technologies, Grand Island, NY) reconstituted with 5% Acetonitrile/0.1% formic acid to 10 pmoles/μl.
1.6 Software for Method Editing and Analysis of Quantitative Proteomics Data
Skyline: see http://skyline.maccosslab.org
Panorama: see https://panoramaweb.org
2. Methods
2.1 Preparation of analytical standards as c-terminal GST fusion proteins via in vitro protein expression
Each bacterial cDNA clone is grown overnight in 5 ml of Ampicillin Supplemented LB Culture Medium. Bacterial cultures are performed in a floor shaker set to 200 RPM/37 °C.
Plasmid DNA is purified according to the manufacture’s mini-prep protocol with the slight modification of an additional “PE buffer” wash step to help facilitate removal of any residual RNAse.
The concentration of plasmid stocks is estimated via the A260/A280 ratio on a UV/Vis Spectrophotometer.
Plasmid stocks are Sanger sequenced using an M13 priming site upstream of the pANT7_cGST vector’s T7 promoter. Plasmid sequencing is performed for the purpose of confirming the identity of the cDNA insert and assessing plasmid purity.
Purified plasmid DNA is used directly in the in vitro protein synthesis kit according to manufacturer protocol with a few minor modifications. Briefly, about 1 μg of plasmid DNA is used per 25 μL in vitro reaction mix supplemented with 12 Units of RNAse inhibitor. Protein synthesis reactions are carried out for 3.5 hours in a floor shaker set to 200 RPM/30 °C.
Completed protein synthesis reactions are combined with a 125 μL aliquot of a 3% slurry of glutathione sepharose 4B beads previously washed and equilibrated with DPBS.
Bead/protein mixture is rocked end-over-end for 16–18 hours at 4 ° C.
Bead/protein mixture is centrifuged at 500g/4 °C for 5 minutes in a bench-top centrifuge.
Supernatant is removed and saved for SDS-PAGE/Western Blot analysis to ensure efficient recombinant protein capture.
Sepharose bead pellets are washed twice with Sepharose Bead Wash Solution #1 and twice with Sepharose Bead Wash Solution #2.
Washed sepharose bead pellets are reconstituted with 50 μL of Sepharose Bead Reconstitution Buffer containing the heavy isotope-labeled peptides LLLEYLEEK and IEAIPQIDK from SJ-GST.
2.2 SDS-PAGE/Western Blot Analysis of GST fusion proteins
A 5 μL aliquot of the undigested bead/protein mixture is combined with 1.7 μL aliquot of SDS-PAGE Sample Loading Buffer.
Mixtures are incubated for 5 minutes at 95°C to facilitate protein denaturation.
Denatured protein extracts are resolved for 60 minutes on a pre-cast Novex NuPage 4–12% bis-Tris mini gel using an XCell SureLock mini-cell gel box interfaced with a Power-Pac Basic Electrophoresis Power Supply set to 150 V.
Confirmation of protein expression can be performed by subjecting gels to either silver staining or immunoblotting against a polyclonal anti-GST antibody.
Silver staining, when applicable, is performed according to manufacturer’s instructions.
Immunoblotting, when applicable, is performed by transferring SDS-PAGE resolved proteins onto a PVDF membrane for 1 hour at 30V using the XCell II Blot Module according to manufacturer’s instructions.
PVDF blots are rinsed briefly with deionized water and incubated in Blot Blocking Buffer while shaking for 60 minutes at room temperature or overnight at 4 °C.
PVDF blots are washed 2X with Blot Washing Buffer while shaking for 5 minutes each at room temperature.
PVDF blots are incubated in Blot Primary Antibody Solution with shaking for 60 minutes at room temperature.
PVDF blots are washed 3X with shaking for 5 minutes each at room temperature with Blot Washing Buffer.
PVDF blots are incubated in Blot Secondary Antibody Solution with shaking for 60 minutes at room temperature.
PVDF blots are washed 3X with shaking for 5 minutes each at room temperature with Blot Washing Buffer.
PVDF blots are visualized using the ECL Prime Western Blotting Kit with the BioMax Light Chemiluminescence Film according to manufacturer’s instructions.
2.3 Sample Digestion
For each analytical standard, a 25 μL aliquot of enriched, bead-bound GST fusion proteins is diluted back out to 50 μL with Sepharose Bead Dilution Buffer.
For plasma samples, a 5 μL aliquot of Plasma is diluted out to 500 μL with Plasma Dilution Buffer 1.
Protein concentration of the diluted plasma is estimated via a Bovine Serum Albumin calibrated BCA assay according to manufacturer’s protocol.
A 25 μL aliquot of diluted plasma is combined with 25 μL of Plasma Dilution Buffer 2.
Diluted bead-bound GST fusion proteins and twice diluted plasma samples are incubated for 5 minutes at 95°C to facilitate protein denaturation.
Denatured proteins are reduced with the addition of 500 mM dithiothreitol to a concentration of 5 mM and incubation at 60 °C for 30 minutes.
Reduced samples are alkylated via the addition of 500 mM iodoacetamide to a final concentration of 15 mM and incubation at room temperature (22–25°C) for 30 minutes in the dark.
Alkylation reactions are quenched via the addition of an additional aliquot of 500 mM dithiothreitol to bring the final concentration to 15 mM.
Each reduced and alkylated bead-bound protein mixture is digested with 1 μg of sequencing grade modified porcine trypsin for 2 hours at 37°C with mixing at 1200 RPM.
Digestion progress is quenched by the addition of 2.5 μl of 5 M HCl.
Acidified digests are incubated for one hour at room temperature to facilitate hydrolysis of the PPS surfactant.
Digested standards are centrifuged at 13000g/4 °C for 5 minutes in a bench-top centrifuge to pellet the sepharose bead fraction.
Supernatants are transferred to polypropylene auto-sampler vials with snap-on lids and stored a 4 °C while queued for injection.
2.4 Nano-flow Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry of Digested Analytical Standards
A 3 μL aliquot of each digest is loaded from a 20 μL sample loop onto an in-house prepared trap column at a flow rate of 2 μL/min for 3 minutes.
Peptides are resolved on an Analytical Column using a 30 minute linear gradient from 5% acetonitrile in 0.1% formic acid to 40% acetonitrile in 0.1% formic acid at a flow rate of 300 nL/min. The initial gradient was followed by a steeper 5 minute gradient from 40% acetonitrile in 0.1% formic acid to 60% acetonitrile in 0.1% formic acid also at 300 nL/min. The column was then washed for 5 minutes with 95% acetonitrile in 0.1% formic acid at 500 nL/min. Prior to the next injection, the trapping column is re-equilibrated with 5 μL of 5% acetonitrile in 0.1% formic acid and the analytical column is re-equilibrated with 3 μL of 5% acetonitrile in 0.1% formic acid. All re-equilibration steps are performed at 250 bar.
Eluting peptides are ionized and emitted into a triple quadrupole mass spectrometer for tandem mass spectrometry analysis.
2.5 Empirical Selection of Optimal Peptides for Targeted Proteomic Workflows
For each GST fusion protein-of-interest, tryptic peptides and their respective fragment ions are chosen using Skyline, an open source document editor for building targeted proteomic methods and analyzing the ensuing mass spectrometry data.
-
Prior to beginning any experiments, all peptide and transition settings are configured in Skyline to match experimental design.
Settings>Peptide SettingsSettings>Transition Settings For the current protocol, we monitor monoisotopic masses for all fully tryptic peptides from 7 to 25 amino acids in length in their (+2) charge state with all cysteines monitored as carbamidomethylated residues. For tandem MS analysis, we monitor singly charged y3 to yn − 1 fragment ions (See Note 2).
-
Amino acid sequences for each protein-of-interest are imported as FASTA files and digested in silico.
File>Import>FASTA In the initial round of MS/MS analysis, all peptides and respective MS/MS transitions that fulfill the criterion detailed above are considered.
Transition list are exported from Skyline as instrument/vendor specific .csv files (See Note 3).
Exported transition lists are used to generate instrument/vendor specific SRM methods.
For nanoLC-SRM analysis, each analytical standard is injected separately. Data is acquired using a dwell time of 2 milliseconds with both mass-filtering quadrupoles set to 0.7 FWHM resolution. Fragmentation is performed at 1.5 mTorr using optimized instrument-specific calculated peptide collision energies (16).
-
Results are imported into Skyline.
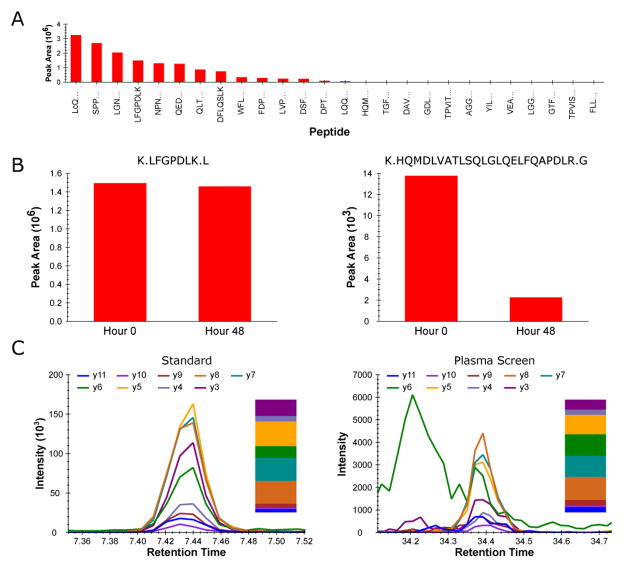
File>Import>Results (Add single-injection replicates in files) Chromatographic data for each peptide are manually inspected. Peptides not observed in these initial experiments are annotated and omitted from further consideration. For every other precursor, the peak area for each co-eluting transition is integrated and the relative distribution of y-ion intensities is noted for each peptide. An example of a relative y-ion distribution for an individual peptide from recombinant SERPINF2 (Also commonly referred to as Alpha-2-Antiplasmin, Accession # P08697) is shown in figure 2C. Integrated peak areas from all monitored y-ion fragments for a given peptide are summed. The sum of each peptide’s MS/MS intensities is ranked against the summed intensities of all other peptides derived from the same parent protein. Figure 2A represents an example of a relative peptide ranking for SERPINF2.
In a second round of nanoLC-MS/MS analysis, peptide stability is assessed and retention time calibration is performed. Each analytical standard digest is spiked with iRT calibration standards (See Note 4) and incubated at 4 °C in the auto-sampler for 48 hours prior to re-injection. Integrated peak areas are compared for each peptide at the 0 hour time point (initial nanoLC-MS/MS) and at the 48 hour time point (post autosampler incubation) to assess peptide degradation/modification.
Results are imported back into the original Skyline document and are again manually inspected. Relative retention times are calculated for each remaining peptide (see Note 5) and SRM signal intensities are compared to those from the initial round of LC-MS/MS analysis. Peptides with sub-optimal stability profiles (See Note 6) are annotated as such and omitted from further consideration.
Edited Skyline files are then uploaded to Panorama for the purpose of creating a chromatogram library (see Note 7). Chromatogram libraries provide a way to store results from previous curated targeted proteomic experiments by capturing peptide physiochemical properties such as relative product ion distribution, chromatographic peak shape, and relative retention time information.
In a third round of MS/MS analysis, a pooled plasma digest is spiked with iRT calibration standards and screened for each peptide that survived the initial two stages of selection. The combined specificity of peptide retention time and MS/MS fragmentation pattern provides a relatively straightforward way of confirming peptide detectability and selecting SRM transitions with the optimal signal-to-noise directly in the human plasma assay matrix.
Figure 2.
(A) Relative SRM signal intensities for tryptic peptides from recombinant SERPINF2 (Also commonly referred to as Alpha-2-antiplasmin, Accession # P08697) as an N-terminal SJ-GST fusion protein. (B) Comparison of two peptides from SERPINF2 SJ-GST fusion protein before and after 48 hour autosampler incubation. (C) Co-eluting fragment ions for a peptide from SERPINF2 SJ-GST fusion protein (Left panel) and co-eluting fragment ions for that same peptide from native SERPINF2 in human plasma (Right panel). The relative contribution of each co-eluting fragment ion is displayed as a bar graph next to each respective chromatogram.
2.6 Quantification of in vitro expressed GST fusion proteins
Absolute quantification of SJ-GST fusion proteins is facilitated by spiking the [13C615N2]-lysine–labeled LLLEYLEEK and IEAIPQIDK peptides from SJ-GST into each into each in vitro protein synthesis reaction.
The unlabeled to labeled integrated peak area ratio is measured via nanoLC-SRM.
Observed peak area ratios are converted to absolute concentrations using an external calibration curve comprised of varying amounts of un-labeled LLLEYLEEK and IEAIPQIDK peptides spiked into a constant amount of the corresponding heavy isotope–labeled peptides (See Note 8).
Figure 1.
General strategy for empirical SRM method development with in vitro synthesized SJ-GST fusion proteins as analytical standards.
Acknowledgments
This work was supported in part by National Institutes of Health grants P41 GM103533, R01 GM107142, and R01 GM107806.
Footnotes
The in vitro translation kit utilized in this protocol is also compatible with full-length cDNA clones in the pT7CFE-CHis expression vector.
Peptides longer that 12–15 amino acid residues tend to have singly charged fragments with m/z’s that exceed the functional mass range of a triple quadrupole mass spectrometer. Thus, we find it useful to set the mass range in skyline to match instrument capabilities.
Settings>Transition Settings>Instrument Tab
Methods are designed to include both the light and heavy isotope-labeled SJ-GST peptides and such that no more than 500 transitions are monitored in a single run. In the event that multiple injections are required for full protein coverage, the SJ-GST peptides are used to normalize signals across injections.
Multiple vendors now offer sets of peptides that can be used as retention time standards. It is also possible to use individual protein digests as a source of iRT peptides provided that there are at least 10 stable reference peptides that span most of one’s retention time range. For the current protocol, all peptides have been calibrated relative to the Biognosys iRT peptide standards (17).
Link to Skyline tutorial for iRT retention time calibration and prediction - https://skyline.gs.washington.edu/labkey/wiki/home/software/Skyline/page.view?name=tutorial_irt.
For the current protocol, we eliminate peptides that dropped in intensity more than 15% from the first injection to the second injection following incubation in the 4°C autosampler.
Link to Panaroma tutorial for creating chromatogram libraries - https://panoramaweb.org/labkey/wiki/home/page.view?name=chromatogram_libraries
Calibration points used are dictated by the sensitivity of the mass spectrometer. For the current protocol, calibration points are measured at 0.1 nM, 0.25 nM, 0.5 nM, 1 nM, 2.5 nM, 5 nM, 12.5 nM, and 40 nM of of the un-labeled SJ-GST peptides spiked into a solution containing 5 nM of the heavy isotope-labeled SJ-GST peptides and 5 μM Bovine serum albumin digest in 5% acetonitrile and 0.1% formic acid.
References
- 1.Barnidge DR, Goodmanson MK, Klee GG, et al. Absolute quantification of the model biomarker prostate-specific antigen in serum by LC-MS/MS using protein cleavage and isotope dilution mass spectrometry. J Proteome Res. 2004;3:644–652. doi: 10.1021/pr049963d. (2004) [DOI] [PubMed] [Google Scholar]
- 2.Gerber SA, Rush J, Stemman O, et al. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson LN, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5(4):573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez H, Tezak Z, Mesri M, et al. Analytical validation of protein-based multiplex assays: a workshop report by the NCI-FDA interagency oncology task force on molecular diagnostics. Clin Chem. 2010;56:237–243. doi: 10.1373/clinchem.2009.136416. [DOI] [PubMed] [Google Scholar]
- 5.Addona TA, Abbatiello SE, Schilling B, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27:633–641. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson NL, Anderson NG, Haines LR, et al. Mass spectrometric quantitation of peptides and proteins using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA) J Proteome Res. 2004;3:235–244. doi: 10.1021/pr034086h. [DOI] [PubMed] [Google Scholar]
- 7.Beynon RJ, Doherty MK, Pratt JM, et al. Multiplexed absolute quantification in proteomics using artificial QCAT proteins of concatenated signature peptides. Nat Methods. 2005;2:587–589. doi: 10.1038/nmeth774. [DOI] [PubMed] [Google Scholar]
- 8.Picotti P, Lam H, Campbell D, et al. A database of mass spectrometric assays for the yeast proteome. Nat Methods. 2008;5:913–914. doi: 10.1038/nmeth1108-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash A, Tomazela DM, Frewen B, et al. Expediting the Development of Targeted SRM Assays: Using Data from Shotgun Proteomics to Automate Method Development. J Proteome Res. 2009;8:2733–2739. doi: 10.1021/pr801028b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallick P, Schirle M, Chen SS, et al. Computational prediction of proteotypic peptides for quantitative proteomics. Nat Biotechnol. 2007;25:125–131. doi: 10.1038/nbt1275. [DOI] [PubMed] [Google Scholar]
- 11.Fusaro VA, Mani DR, Mesirov JP, et al. Prediction of high-responding peptides for targeted protein assays by mass spectrometry. Nat Biotechnol. 2009;27:190–198. doi: 10.1038/nbt.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picotti P, Rinner O, Stallmach R, et al. High-throughput generation of selected reaction-monitoring assays for proteins and proteomes. Nat Methods. 2009;7:43–46. doi: 10.1038/nmeth.1408. [DOI] [PubMed] [Google Scholar]
- 13.Stergachis AB, MacLean B, Lee K, et al. Rapid empirical discovery of optimal peptides for targeted proteomics. Nat Methods. 2011;8:1041–1046. doi: 10.1038/nmeth.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Haller P. Packing capillary columns and pre-columns (traps) University of Washington Proteomics Resource document. 2013 http://proteomicsresource.washington.edu/docs/protocols05/Packing_Capillary_Columns.pdf.
- 15.Yates JR, III, McCormack AL, Link AL, et al. Future prospects for the analysis of complex biological systems using, micro-column liquid chromatography-electrospray tandem mass spectrometry. Analyst. 1996;121:65R–76R. doi: 10.1039/an996210065r. [DOI] [PubMed] [Google Scholar]
- 16.MacLean B, Tomazela DM, Abbatiello SE, et al. Effect of collision energy optimization on the measurement of peptides by selected reaction monitoring (SRM) mass spectrometry. Anal Chem. 2010;82:10116–10124. doi: 10.1021/ac102179j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escher C, Reiter L, MacLean B, et al. Using iRT, a normalized retention time for more targeted measurement of peptides. Proteomics. 2012;12(8):1111–1121. doi: 10.1002/pmic.201100463. [DOI] [PMC free article] [PubMed] [Google Scholar]