Abstract
Growth of Fusarium sp. BVKT R2, a potential isolate of forest soils of Eastern Ghats on birchwood xylan in mineral salts medium (MSM) under un-optimized conditions of 30 °C, pH of 5.0, 150 rpm and inoculum size of 5 agar plugs for 7 days, yielded titer of 1290 U/mL of xylanase (EC 3.2.1.8). The effect of various operating parameters such as different substrates and their concentration, additional carbon and nitrogen sources, incubation temperature, initial pH, agitation and inoculum size on the production of xylanase by Fusarium sp. BVKT R2 was studied in shake flask culture by one factor at a time approach. The same culture exhibited higher production of xylanase (4200 U/mL) when grown on birch wood xylan in MSM under optimized conditions with an additional carbon source—sorbitol (1.5%) nitrogen source—yeast extract (1.5%) temperature of 30 °C, pH of 5.0, agitation of 200 rpm and inoculum of 6 agar plugs for only 5 days. There was enhancement in xylanase production under optimized conditions by 3.2 folds over yields under un-optimized conditions. Growth of BVKT R2 culture on locally available lignocelluloses—sawdust, rice straw and cotton stalk—in MSM for 5 days released soluble sugars to the maximum extent of 52.76% with respect to sawdust indicating its greater importance in saccharification essential for biotechnological applications.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0977-1) contains supplementary material, which is available to authorized users.
Keywords: Eastern Ghats, Fusarium sp., Optimization, Saccarification, Xylanase
Introduction
Hydrolytic enzymes play a pivotal role in industrial biotechnology, bioremediation, biotransformation, biodegradation and environmental protection. Xylanase is one of such enzymes involved in the hydrolytic process of hemicellulose (back bone xylan polymer) into its constituent carbohydrate monomer sugars which in turn are converted into various value-added products. Biodegradation of xylan is a complex procedure that requires the coordination of various xylanolytic enzymes that catalyze xylan and arabinoxylan polymers. This catalyst bunch includes endo-β1,4-xylanase (1,4-β-d-xylan xylanohydrolase, EC 3.2.1.8), which assault primary chain of xylans, β-d-xylosidase (1,4-β-xylan xylanohydrolase, EC 3.2.1.37), which hydrolyze xylo-oligosaccharides into d-xylose and an assortment of debranching enzymes, i.e., α-l-arabinofuranosidases, α-glucuronidases and acetyl esterases (Saha and Bothast 1999; Tony et al. 2005; Dodd and Cann 2009; Dodd et al. 2011; Despres et al. 2016).
Xylanases are secreted by a variety of microorganisms such as bacteria (Gilbert and Hazelwood 1999; Sunna and Antranikian 1997; Battan et al. 2006, Chakdar et al. 2016), fungi (Sunna and Antranikian 1997; Kuhad et al. 1998; Polizeli et al. 2005; Nair et al. 2008; Ramanjaneyulu and Rajasekhar Reddy 2016), actinomycetes (Ninawe et al. 2006; Yan et al. 2009; Altaf et al. 2016) and yeast (Lopes et al. 2011; Otero et al. 2015) which are cultivated under solid and submerged fermentations. Of above all, fungi are the most widely used in industrial xylanase production due to their wide range of temperature (40–60 °C) (Latif et al. 2006; Irfan et al. 2014). Xylanases have potential applications in different fields—saccharification of biomass, in biofuel industry (Dodd and Cann 2009; Uday et al. 2016), and as fading agents as a part of the pulp and paper industry (Raghukumar et al. 2004; Sridevi et al. 2016). For a majority of applications, they are utilized to hydrolyze the xylan segment from wood which facilitates eviction of lignin (Sun et al. 2012). It additionally helps in lighting up of the pulp to keep away from the chlorine free fading operations (Sridevi et al. 2016). In baking industries, the xylanase follows up on the gluten portion of the mixture and help in the even redistribution of the water substance of the bread (Wong and Saddler 1992; Beg et al. 2001; Garg et al. 2010). Xylanases additionally have the potential applications in animal feed industry (Damiano et al. 2003; Garg et al. 2010). They are utilized in the hydrolysis of non-starchy polysaccharides, for example, arabinoxylan in monogastric diets (Walsh et al. 1993). Xylanases likewise play a key part in the maceration of vegetable matter (Beck and Scoot 1974), protoplastation of plant cells, clarification of juices and wine (Biely 1985), liquefaction of espresso adhesive for making fluid espresso, recuperation of oil from subterranian mines, extraction of flavors and pigments, plant oils and starch (Mc Cleary 1986) and also to enhance the proficiency of farming silage generation (Wong and Saddler 1992).
The major parameters that influence microbial production of xylanase include choice of an appropriate substrate and microorganism, sort and size of the inoculum, control of temperature and pH of fermenting matter, time of incubation, etc. In the present study, we focused on optimization of xylanase production by Fusarium sp. BVKT R2, an isolate of forestry soil and evaluation of its efficiency in sachharification of different lignocellulosic substrates.
Materials and methods
Fungal culture and its maintenance
A fungal culture was isolated from population studies and subjected to primary and secondary screening for production of xylanase (Ramanjaneyulu et al. 2015, 2016) and the potential fungus was maintained on MSM amended with 0.1% of xylan at temperature of 30 °C and pH of 5 as previously described by Ramanjaneyulu et al. (2015).
Xylanase assay
Xylanase (E.C. 3.2.1.8) activity was measured using 1% birchwood xylan solution as substrate (Bailey et al. 1992). Xylanase activity was assayed in 3.0 mL of a reaction mixture consisting of 1.0 mL of crude extracellular enzyme source, 1 mL of 1% birch wood xylan (prepared in 0.05 M Na-citrate buffer, pH 5.3) and 1 mL of 0.05 M citrate buffer. The mixture was incubated at 55 °C for 10 min. The reaction was stopped by the addition of 3.0 mL of 3,5-dinitrosalicylic acid (DNS) and the contents were boiled for 15 min in a water bath (Miller 1959). After cooling, the color developed was read at 540 nm. The amount of xylose liberated was quantified using xylose as the standard. One unit of xylanase activity (U) is defined as the amount of enzyme liberating 1 µmol of xylose per min under standard conditions.
Extracellular protein
The content of the extracellular protein in the culture filtrate of fungi grown in different experiments was determined according to Folin’s method using bovine serum albumin as standard (Lowry et al. 1951).
Biomass measurement
The cultures of fungal isolates in the flasks were aseptically filtered through pre-weighed Whatman No. 1 filter paper to separate mycelial mat and culture filtrate. The filter paper along with mycelial mat was dried at 70 °C in an oven until constant weight was recorded. Difference between the weight of the filter paper bearing mycelial mat and weight of only filter paper represented biomass of fungal mat. Growth of fungal isolates was expressed in terms of mg/flask.
pH determination
pH of culture filtrate derived from growth of the fungal culture at different desired intervals of incubation was determined with the help of a pH meter (Elico).
Optimization of xylanase production
Production of xylanase depends on physical, biological and nutritional parameters. Therefore, these process parameters were optimized to achieve higher yields of xylanase by one variable (parameter) at a time approach. In this approach, only one parameter was altered keeping other parameters constant and the influence of altered parameter on xylanase production was examined.
Comparison of xylanase production on different xylan substrates
To compare production of xylanase by Fusarium sp. BVKT R2 on different xylan substrates in SmF, MSM was amended with birch wood xylan, oat spelt xylan and beech wood xylan at a concentration of 0.1%. Xylan-amended MSM was dispensed in 250 mL Erlenmeyer flasks at 50 mL per flask and inoculated with 5 agar plugs (0.5 mm) cut out from 5-day-old culture of Fusarium species on the plates. The flasks were incubated on a shaker (REMI) at 30 °C and 150 rpm. At desired intervals of incubation, flasks were processed for determination of growth, secretion of extracellular protein and xylanase.
Effect of supplementation of additional carbon and nitrogen source on xylanase production
Effect of different simple and readily metabolizable sugars such as glucose, fructose, sucrose, maltose and sorbitol and, inorganic (ammonium nitrate and potassium nitrate) and organic (peptone, yeast extract, urea and beef extract) nitrogen sources on the production of xylanase by Fusarium species in SmF was studied. The sugars were additionally supplemented at 1% level to xylan-amended MSM. Fusarium sp. BVKT R2 was grown on MSM with xylan and one simple sugar in Erlenmeyer flasks in the manner as specified in earlier section. Further, to determine the optimal concentration the best carbon and nitrogen sources were studied at different levels (0.5, 1.0, 1.5 and 2.0%) for optimal production of xylanase.
Effect of inoculum size on xylanase production
In all the previous experiments, MSM with the appropriate amendments was inoculated with 5 agar plugs of 0.5 mm size cut out from 5-day-old culture of Fusarium sp. on MSM agar plate with a help of a cork borer. To examine the influence of inoculum size on xylanase production, 50 mL of MSM in 250-mL Erlenmeyer conical flasks was inoculated with 2, 4, 6, 8 and 10 agar plugs from 5-day-old culture of Fusarium sp. MSM agar plate and processed as described earlier.
Effect of initial pH of the medium on xylanase production
To find out the influence of initial pH on xylanase production, birchwood xylan–MSM with appropriate amendments was adjusted to different initial pH ranging from 4.0 to 6.0 with an increment of 0.5 units and inoculated with Fusarium sp. and incubated at 30 °C.
Effect of incubation temperature on xylanase production
To determine the optimum temperature for higher xylanase production, MSM with suitable amendments in 250 mL Erlenmeyer conical flasks was inoculated with Fusarium sp. and incubated at temperatures ranging from 25 to 40 °C with an increment of 5 °C.
Effect of agitation on xylanase production
To determine the optimum speed of agitation on xylanase production, 50 mL of the MSM formulated from the previous experiments was distributed in 250 mL Erlenmeyer conical flasks and inoculated with plugs of Fusarium sp. in the manner as specified in the earlier section and divided into four groups. Each group of flasks was agitated at different speed levels (100, 150, 200 or 250 rpm) in an incubator cum shaker (Remi/Scigenics) and incubated at 30 °C.
Saccharification of biomass
Different lignocellulosic feedstocks, viz., sawdust, rice straw and cotton stocks were collected, chopped and sieved through 1.0 mm sieve and used for saccharification. One gram of the biomass was added to 50 mL of optimized medium and autoclaved at 120 °C for 20 min. Fusarium sp. BVKT R2 was grown on medium containing lignocellulose at 30 °C and 200 rpm with 6 plugs of inoculum. Culture supernatants were withdrawn at 24 h intervals to monitor the release of reducing sugars. All experiments were performed in triplicates and the means of triplicates were used to estimate saccharification (%) with the following formula:
Statistical analysis
All experiments were carried out in triplicates and the means of data were subjected to Duncan’s multiple range (DMR) analysis.
Results and discussion
Optimization of xylanase production
In view of applications of xylanase in different industrial processes, mass production of xylanase by fermentation is gaining importance. Knowledge on nutrients and growth conditions is essential for large-scale cultivation of any organism in fermentation methods. Therefore, influence of different factors on production of xylanase by the potential culture Fusarium sp. BVKT R2 in submerged fermentation was assessed using ‘one variable at a time approach’.
Comparison of xylanase production on different xylan substrates
Comparison of xylanase production on different commercially available xylan sources such as oat spelt xylan, beech wood xylan and birch wood xylan in SmF is given in Fig. 1. Among the substrates tested in this study, birch wood xylan supported maximum xylanase activity of 1185 U/mL after 7 days of incubation period followed by beech wood xylan (750 U/mL) and oat spelt xylan (690 U/mL). Maximum secretion of extracellular protein also occurred after 7 days of growth on birch wood xylan and beech wood xylan (Table 1). But, the maximum extracellular protein secretion was noticed on 4th day of incubation in case of oat spelt xylan. However, biomass production was maximum on 7th day of incubation with all the substrates and with little difference in quantity of biomass. There was no noticeable difference in pH of broth of the culture grown on different substrates during course of incubation. The present study is in agreement with Kumar et al. (2009) who used various pure polymeric substrates such as birchwood (519 ± 16 U/mL), beech wood (497 ± 13 U/mL), and oat spelt xylan (473 ± 18 U/mL) by Thermomyces lanuginosus MC 134 for production of xylanase. Pure xylan was found to be the most potent carbon source to induce endo-1,4-β-xylanase production by Penicillium janthinellum (Joshi and Khare 2012; Aditi and Ray 2014). Different kinds of commercially extracted xylan as well as xylan containing agricultural wastes were used in other studies for inducing xylanolytic enzyme production. Wahyuntari et al. (2009) observed cell growth and enzyme production in the medium containing oat spelt xylan and fruitless oil palm bunch (FPB) as carbon source. Knob and Carmona (2008) reported maximum xylanase production in 8- and 5-day-old cultures (27.21 U/mL and 65.29 U/mg of protein) by Penicillium sclerotium.
Fig. 1.
Comparison of production of xylanase on different xylan substrates in MSM by Fusarium sp. BVKT R2. The values are the means of triplicates with standard deviation (SD). The bars bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Table 1.
Biomass production, secretion of extracellular protein and pH changes upon growth of Fusarium sp. BVKT R2 on different xylan substrates
| Incubation time (days) | Beech wood xylan | Birch wood | Oat spelt xylan | |||
|---|---|---|---|---|---|---|
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 1.2a | 0.1a | 1.3a | 0.1a | 0.9a | 0.1a |
| II | 1.2a | 0.1a | 2.2b | 0.1a | 37.5c | 0.1a |
| III | 3.1a | 0.2a | 3.1a | 0.2a | 4.9b | 0.2a |
| IV | 4.2a | 0.3a | 3.9a | 0.3a | 6.5b | 0.2b |
| V | 4.5a | 0.3a | 4.4a | 0.3a | 5.2b | 0.3a |
| VI | 5.5a | 0.3a | 5.5a | 0.3a | 5.0a | 0.3a |
| VII | 6.1a | 0.3a | 5.8a | 0.4b | 4.8a | 0.3a |
| VIII | 4.8a | 0.3a | 4.8a | 0.3a | 4.6a | 0.3a |
| IX | 3.3a | 0.3a | 3.6a | 0.3a | 3.6a | 0.3a |
| X | 2.8a | 0.3a | 2.7a | 0.3a | 1.5b | 0.3a |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
Effect of supplementation of additional carbon on xylanase production
To study the effect of additional carbon sources like glucose, fructose, sucrose, maltose and sorbitol on secretion of xylanase in SmF, the prospective culture Fusarium sp. BVKTR2 was grown in the medium amended with an additional carbon source and xylanase secretion was monitored (Fig. 2). Xylanase yields reached peak on the 7th day of incubation in the medium with/without amendment of additional carbon sources.
Fig. 2.
Secretion of xylanase by Fusarium sp. BVKT R2 grown on xylan in the presence of additional carbon source. The values are the means of triplicates with standard deviation (SD). The bars bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Of all the carbon sources tested in the present study, sorbitol favored the maximum secretion of xylanase in SmF on 7th day of incubation followed by maltose. Glucose and sucrose reduced the secretion of xylanase in comparison to control. Low titers of xylanase enzyme on glucose medium in this study may be due to the catabolite repression (Fig. 2). Overall, release of extracellular protein in higher amounts occurred on 7th day of incubation in the medium amended with glucose, sorbitol, fructose, maltose and sucrose (Table 2). However, there is no significant difference in biomass production of the culture grown on different carbon sources during the course of incubation (Table 2). pH changes occurred in broth of the culture due to growth of the prospective fungus on different sugars as shown in Table ST 1.
Table 2.
Secretion of extracellular protein and biomass production by Fusarium sp. BVKT R2 grown on xylan in MSM in the presence of additional carbon source
| Incubation period (days) | Controlc | Carbon source | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Sucrose | Maltose | Sorbitol | ||||||||
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 1.3a | 0.1a | 1.0a | 0.1a | 1.0a | 0.1a | 1.2a | 0.1a | 1.4a | 0.1a | 1.5a | 0.1a |
| II | 2.2a | 0.1a | 2.0a | 0.1a | 1.7a | 0.1a | 1.6a | 0.1a | 1.6a | 0.1a | 2.4a | 0.2b |
| III | 3.1a | 0.2a | 2.7a | 0.1b | 2.4b | 0.2a | 2.2b | 0.2a | 2.6b | 0.2a | 3.1a | 0.2a |
| IV | 3.9a | 0.3a | 3.6a | 0.2b | 3.3b | 0.2b | 2.7b | 0.2b | 3.7a | 0.2b | 3.7a | 0.3a |
| V | 4.4a | 0.3a | 4.2a | 0.3a | 3.7b | 0.3a | 3.3b | 0.3a | 4.2a | 0.3a | 4.8c | 0.3a |
| VI | 5.5a | 0.3a | 5.4a | 0.3a | 4.8a | 0.3a | 2.4b | 0.3a | 4.8a | 0.3a | 5.1a | 0.3a |
| VII | 5.8a | 0.4a | 7.3b | 0.3a | 6.1c | 0.3a | 5.5a | 0.3a | 5.9c | 0.4b | 6.5c | 0.4b |
| VIII | 4.8a | 0.3a | 3.5b | 0.3a | 5.1a | 0.3a | 3.7b | 0.3a | 4.8a | 0.3a | 4.5a | 0.3a |
| IX | 3.6a | 0.3a | 1.1b | 0.3a | 2.1c | 0.3a | 1.9c | 0.3a | 2.1c | 0.3a | 2.1c | 0.3a |
| X | 2.7a | 0.3a | 1.7b | 0.3a | 0.5c | 0.3a | 5.7d | 0.3a | 0.5c | 0.3a | 1.7b | 0.3a |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
Control devoid of additional carbon source
In the present study, sorbitol served as the best additional carbon source for xylanase production by Fusarium sp. BVKTR2. Sorbitol at 1.5% level appeared to be the optimal concentration for xylanase production by the culture. In contrast, no appreciable production of xylanase by Aspergillus candidus on simple sugars except xylose was noticed (Garai and Kumar 2013).
Xylose was reported to be the best carbon source for xylanase production by Trichoderma harzianum 1073 D3 and Penicilluim sp. (Isil and Nilufer 2005; Murthy and Naidu 2012). But, Altaf et al. (2010) reported maximum production of xylanase by T. harzianum with maltose and starch as carbon source. Glucose, starch and sucrose could not simulate enzyme production but they supported the growth of Pleurotus eryngii and Flamulina velutipes. Cochliobolus sativus exhibited high activity of xylanase (1469.4, 1396.6 and 1116.7 U/g) after 8 days of incubation with xylan, starch and xylose, respectively (Arabi et al. 2011).
According to different reports (Biely 1985; Paul and Varma 1990; Nakamura et al. 1995; Oakley et al. 2003), the presence of simple sugars in medium lowered xylanolytic enzyme production by Bacillus circulans AB16. These reports also indicated that an inducer for certain microorganisms could be an inhibitor for others. As a consequence, it is important to choose a proper inducer for certain microorganisms. In the present study, sorbitol yielded higher production of xylanase by Fusarium sp. BVKT R2. Higher production of xylanase could be attributed to good growth and secretion of extracellular protein.
Effect of different concentrations of sorbitol on xylanase production
It is evident from the previous experiment that sorbitol is the best additional carbon source for xylanase production by Fusarium sp. BVKT R2 wherein only one and fixed concentration was tested. Therefore, another experiment was conducted to study the influence of sorbitol at different concentrations (0.5–2.0%) on production of xylanase by Fusarium sp. BVKT R2 (Fig. 3).
Fig. 3.
Secretion of xylanase by Fusarium sp. BVKT R2 grown on xylan in the presence of sorbitol. The values are the means of triplicates with standard deviation (SD). The line graph bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Among the different concentrations of sorbitol tested, 1.5% concentration showed maximum production of xylanase on 7th day of incubation (Fig. 3). Secretion of extracellular protein and biomass production is also very high at this concentration (Table 3). No significant difference in pH change upon growth of the prospective culture was observed (ST 2). At lower concentration of sorbitol, production of enzyme was less which may be due to insufficient concentrations of sorbitol to support the growth of fungal culture while higher concentrations may cause inhibition of the growth of the organism.
Table 3.
Secretion of extracellular protein and biomass production by Fusarium sp. BVKT R2 grown on xylan in the presence of sorbitol
| Incubation period (days) | Sorbitol (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | |||||
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 1.4a | 0.1a | 1.5a | 0.1a | 2.1b | 0.2b | 1.3a | 0.1a |
| II | 1.9a | 0.2a | 2.3a | 0.2a | 2.7b | 0.2a | 1.9a | 0.1b |
| III | 3.2a | 0.2a | 3.0a | 0.2a | 3.4b | 0.2a | 2.6a | 0.2a |
| IV | 3.7a | 0.2b | 3.5a | 0.2b | 4.1b | 0.2b | 3.4a | 0.2b |
| V | 4.1a | 0.2a | 4.6b | 0.3b | 4.6b | 0.3b | 4.2a | 0.3b |
| VI | 4.9a | 0.3a | 5.3b | 0.3a | 5.2b | 0.3a | 4.9a | 0.3a |
| VII | 6.4a | 0.3a | 6.3a | 0.3a | 6.5a | 0.4a | 6.2a | 0.3a |
| VIII | 5.3a | 0.3a | 4.1b | 0.3a | 4.8b | 0.3a | 5.3a | 0.3a |
| IX | 4.2a | 0.3a | 1.7b | 0.3a | 2.6c | 0.3a | 4.7a | 0.3a |
| X | 0.6a | 0.3a | 0.8a | 0.3a | 2.0a | 0.3a | 2.2b | 0.3a |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
Effect of supplementation of additional nitrogen sources on xylanase production
Nitrogen is another important factor which influences the production of enzymes in culture medium. In the present study, the effect of different organic and inorganic nitrogen sources like peptone, beef extract, yeast extract, urea, NH4 NO3 and KNO3 on production of xylanase by Fusarium sp. BVKT R2 was evaluated in SmF. Yeast extract (YE) yielded maximum enzyme on 7th day of incubation followed by beef extract, urea peptone, NH4 NO3 and KNO3 (Fig. 4). The pattern of secretion of extracellular protein and biomass production followed the same trend as observed with enzyme production (Table 4). There is no significant difference in pH changes in medium with nitrogen sources upon growth of the fungus (ST 3).
Fig. 4.
Production of xylanase by Fusarium sp. BVKT R2 as influenced by inclusion of additional nitrogen source in MSM. The values are the means of triplicates with standard deviation (SD). The bars bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Table 4.
Secretion of extracellular protein and biomass production by Fusarium sp. BVKT R2 grown on xylan in the presence of additional nitrogen source
| Incubation period(days) | Controlc | Nitrogen source | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptone | Beef extract | Yeast extract | Urea | NH4NO3 | KNO3 | |||||||||
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 1.3a | 0.1a | 2.2b | 0.1a | 2.1b | 0.1a | 2.5b | 0.2b | 2.0b | 0.1a | 2.1b | 0.1a | 1.7a | 0.15b |
| II | 2.2a | 0.1a | 2.8b | 0.1a | 2.4a | 0.6b | 3.1b | 0.2a | 2.3a | 0.1a | 2.6b | 0.2c | 2.2a | 0.2c |
| III | 3.1a | 0.2a | 3.6b | 0.2a | 3.0a | 0.2a | 3.8b | 0.2a | 2.7a | 0.2a | 3.2b | 0.2a | 2.8a | 0.2a |
| IV | 3.9a | 0.3a | 4.6b | 0.2b | 4.0a | 0.2b | 4.7b | 0.3a | 3.6a | 0.2b | 3.9a | 0.2b | 3.4c | 0.2b |
| V | 4.4a | 0.3a | 5.7b | 0.3a | 5.1b | 0.2b | 5.5b | 0.3a | 4.4a | 0.3a | 4.5a | 0.3a | 4.1a | 0.2b |
| VI | 5.5a | 0.3a | 6.2b | 0.3a | 6.4b | 0.3a | 6.0b | 0.3a | 5.5a | 0.3a | 5.7a | 0.3a | 5.3a | 0.3a |
| VII | 5.8a | 0.4a | 6.4b | 0.3b | 4.7c | 0.3b | 6.8b | 0.3b | 6.4b | 0.3b | 6.2b | 0.3b | 6.1b | 0.3b |
| VIII | 4.8a | 0.3a | 5.3b | 0.3a | 5.3b | 0.3a | 5.3b | 0.3a | 5.4b | 0.3a | 4.6a | 0.3a | 4.3c | 0.3a |
| IX | 3.6a | 0.3a | 3.9a | 0.3a | 3.9a | 0.3a | 4.7b | 0.3a | 3.9a | 0.3a | 3.6a | 0.3a | 3.4a | 0.3a |
| X | 2.7a | 0.3a | 2.9a | 0.3a | 2.9a | 0.3a | 1.1b | 0.3a | 3.0c | 0.3a | 2.9a | 0.3a | 1.9b | 0.3a |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
Control devoid of additional nitrogen source
The results of the present study are in accordance with those reported by others (Hoq et al. 1994; Li et al. 2006; Murthy and Naidu 2012; Nikhil et al. 2012) who observed that YE is the most effective organic nitrogen source for xylanase production. Similarly, optimum xylanase production (1417.6 IU/gds/min) by T. lanuginosus RT9 and thermophilic Paecilomyces themophila was obtained when grown on a medium with yeast extract. In contrast, several reports (Haltrich et al. 1993; Hoq et al. 1994; Kuhad et al. 1998; Abdel-Sater and El-Said 2001; Kalogeris et al. 2003; Qinnghe et al. 2004; Bakri et al. 2008; Goyal et al. 2008) revealed that different nitrogen sources such as NH4NO3, (NH4)2HPO4, wheat bran and peptone, NaNO3, (NH4)2SO4, peptone and NaNO3 supported optimum xylanase production by Schizophyllum commune, T. lanuginosus RT9, T. harzianum, Thermoascus aurantiacus, Pleurotus ostreatus, C. sativus CS5 strain and Trichoderma viride in SmF and SSF, respectively.
Effect of different concentrations of yeast extract on xylanase production
In view of higher titers of xylanase production by the fungus in medium amended with yeast extract at only one and fixed concentration tested in the previous experiment, a repeat experiment with different concentrations (0.5, 1.0, 1.5 and 2.0%) of yeast extract was performed to find out the optimal concentration. Among all the concentrations tested in the present study, 1.5% yielded maximum (1400 U/mL) xylanase against 1000 U/mL at 0.5% YE on 7th day of incubation (Fig. 5). Likewise enzyme, highest amounts of extracellular protein was released in culture broth at 1.5% on 7th day (Table 5). Biomass production was higher with 1.5% of YE on all days of incubation when compared to that of lower and higher concentrations of YE (Table 5). This may be attributed to adequacy of 1.5% YE to support the production of biomass under growth conditions. There is no noticeable difference in pH recorded in culture broth at different concentrations of YE during the course of incubation (ST 4). Similarly, peptone and yeast extract served as the best nitrogen sources for xylanase production by the novel strain A. candidus (Garai and Kumar 2013). In the present study, maximal production of xylanase was obtained when the culture was grown at 1.5% yeast extract.
Fig. 5.
Secretion of xylanase by Fusarium sp. BVKT R2 grown on xylan in the presence of yeast extract. The values are the means of triplicates with standard deviation (SD). The line graphs bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Table 5.
Secretion of extracellular protein and biomass production by Fusarium sp. BVKT R2grown on xylan in the presence of yeast extract
| Incubation period (days) | Yeast extract (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | |||||
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 2.3a | 0.15a | 2.1a | 0.1a | 2.6b | 0.2b | 2.4a | 0.14a |
| II | 2.7a | 0.2a | 3.2b | 0.1b | 3.3b | 0.2a | 3.3b | 0.18a |
| III | 3.5a | 0.2a | 4.1b | 0.2a | 4.6c | 0.2a | 4.3b | 0.21a |
| IV | 4.5a | 0.2a | 4.9b | 0.2a | 5.5c | 0.3b | 5.0c | 0.26b |
| V | 5.3a | 0.3a | 5.8b | 0.3a | 6.0b | 0.3a | 6.5c | 0.29a |
| VI | 6.5a | 0.3a | 8.4b | 0.3a | 7.0c | 0.3a | 8.6b | 0.33a |
| VII | 6.9a | 0.3a | 10b | 0.3a | 12.3c | 0.4b | 13.2d | 0.35a |
| VIII | 5.3a | 0.3a | 4.5b | 0.3a | 9.2c | 0.3a | 9.0c | 0.31a |
| IX | 3.9a | 0.3a | 2.2b | 0.3a | 3.5a | 0.3a | 6.1c | 0.3a |
| X | 3.0a | 0.3a | 0.5b | 0.3a | 0.2b | 0.3a | 3.7a | 0.28a |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
Effect of inoculum size on xylanase production
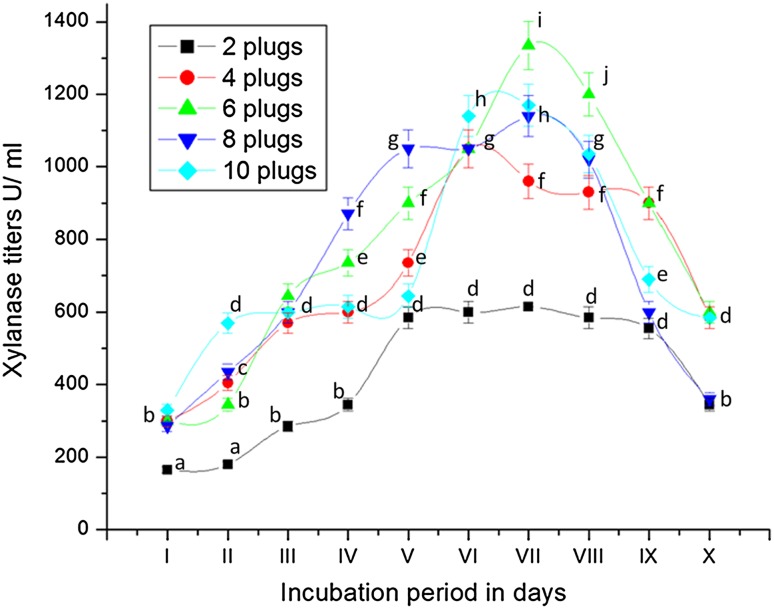
Inoculum size is one of the critical parameters for optimal production of xylanase by the culture. In the present study, a wide range of inoculum size (2, 4, 6, 8 and 10 agar plugs of 0.5 mm size) was tested. Increase in the inoculum size up to 6 plugs increased the production of xylanase in the present study (Fig. 6). Inoculum size higher than 6 agar plugs of 0.5 mm size did not improve xylanase yields which may be attributed to nutrient limitation. Biomass density generated at lower inoculum size is too low to utilize available nutrients for supporting the production of enzymes. Secretion of extracellular protein and biomass also followed the same pattern of xylanase enzyme and touched peak with use of inoculum of 6 plugs (Table 6). There is no significant difference in pH recorded in culture broth with different inoculum size (ST 5). Similarly, lower levels of inoculum may not be sufficient in initiating growth and enzyme synthesis (Petchluan et al. 2014).
Fig. 6.
Secretion of xylanase by Fusarium sp. BVKT R2 grown on xylan in MSM with different inoculum densities. The values are the means of triplicates with standard deviation (SD). The line graphs bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Table 6.
Secretion of extracellular protein and biomass production by Fusarium sp. BVKT R2 grown on xylan in MSM with different inoculum densities
| Incubation period (days) | Inoculum at a density of | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 Plugs | 4 Plugs | 6 Plugs | 8 Plugs | 10 Plugs | ||||||
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 1.7a | 0.2a | 1.6a | 0.1b | 2.3b | 0.2a | 3.2c | 0.1b | 4.5d | 0.1b |
| II | 2.2a | 0.2a | 2.1a | 0.2a | 2.6b | 0.2a | 4.5c | 0.2a | 6.7d | 0.2a |
| III | 2.7a | 0.2a | 2.9a | 0.2a | 3.4b | 0.3b | 7.6c | 0.2a | 8.1d | 0.2a |
| IV | 3.2a | 0.3a | 3.6a | 0.5b | 4.2b | 0.3a | 8.5c | 0.3a | 9.6d | 0.2c |
| V | 3.8a | 0.3a | 4.2b | 0.3a | 4.7b | 0.3a | 9.5c | 0.3a | 11.0d | 0.3a |
| VI | 4.5a | 0.3a | 4.9a | 0.3a | 6.2b | 0.3a | 10.1c | 0.3a | 13.1d | 0.3a |
| VII | 4.8a | 0.3a | 6.0b | 0.4b | 6.9b | 0.4b | 11.1c | 0.4b | 16.0d | 0.3a |
| VIII | 4.2a | 0.3a | 5.8b | 0.3a | 6.5c | 0.4a | 11.0d | 0.3a | 13.2e | 0.3a |
| IX | 3.5a | 0.3a | 3.8b | 0.3a | 5.4c | 0.3a | 8.4d | 0.3a | 10.2d | 0.3a |
| X | 2.4a | 0.3a | 2.5a | 0.3a | 3.8b | 0.3a | 6.1c | 0.3a | 8.5d | 0.3a |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
Qinnghe et al. (2004) reported that the production of xylanase by P. ostreatus was optimal with the inoculum level of four disks with size of 0.5 cm each for in 50 mL liquid culture (corn cob 2.5% + wheat bran 2.5%). The inoculum level of four disks with 0.5 cm size each for 4 g substrate culture was found to be the optimum for xylanase and cellulase production by Lentinus polychrous Lev. LPPT-1 (Petchluan et al. 2014). However, the production of xylanase increased when the inoculum size was increased from 1 to 2 disks but no increment in production of xylanase by further increase in inoculum size by T. lanuginosus (Kumar et al. 2009).
Effect of initial pH of the medium on xylanase production
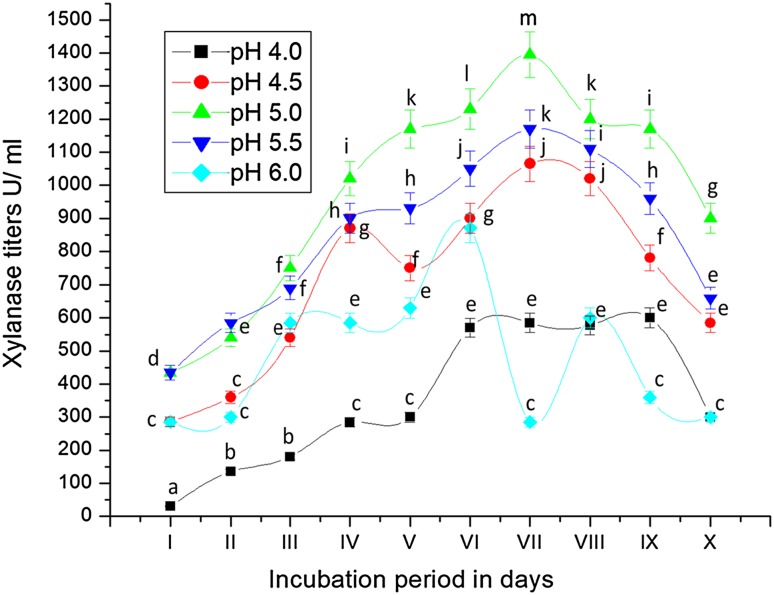
Optimal hydrogen ion concentration of the medium is essential for growth of organisms to produce metabolic enzymes and metabolic products. Hence, an experiment was carried out on growth of the potential fungus in the formulated medium set to different initial pH (4–6) and xylanase production. Xylanase production increased with increase in the initial pH of the medium from 4.0 to 5.5 with maximum yield at 5.0 (Fig. 7). Further increase in initial pH (6.0) significantly reduced the production of xylanase. Xylanase yield of 1400 U/mL was obtained in the medium set to an initial pH of 5.0 as against 550 U/mL yield by the same culture in medium set to pH of 4.0 on 7th day of incubation. Similar observations were made by Gupta et al. (2009) and Bakri et al. (2008) with a newly isolated C. sativus Cs5 strain in submerged fermentation. It might be due to the fact that the variation in external pH directly affects cytoplasmic pH of microbial cells which decreases the microbial growth or enzyme production by disrupting the plasma membrane or inhibiting the activity of different metabolic enzymes. Alteration of pH may also change the ionization state of nutrient molecules and reduce their availability to organism. pH of 4.1 and 4 was found optimum for the production of endoxylanase by Aspergillus awamori under submerged fermentation (Li et al. 2006).
Fig. 7.
Production of xylanase by Fusarium sp. BVKT R2 grown on xylan-amended MSM set to different pHs. The values are the means of triplicates with standard deviation (SD). The line graphs bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Growth of the culture in the medium set to initial pH ranging from 4.0 to 6.0 resulted in the maximum production of biomass (3–4 mg) on 7th day of incubation (Table 7). Secretion of extracellular protein into the broth increased up to 7th day of incubation and declined later (Table 7). The medium set to different initial pH underwent changes in pH during the course of growth of the culture fluctuating between 3.2 and 7.1 (ST 6).
Table 7.
Secretion of extracellular protein and biomass production by Fusarium sp. BVKT R2 grown on xylan set to different initial pHs
| Incubation period (days) | Initial pH of the medium set to | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | ||||||
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 0.7a | 0.1a | 0.6a | 0.1a | 0.9b | 0.2b | 1.4b | 0. 1a | 1.2b | 0.1a |
| II | 1.0a | 0.2a | 0.9a | 0.2a | 1.4b | 0.2a | 1.9b | 0.2a | 1.9b | 0.1b |
| III | 1.6a | 0.2a | 1.6a | 0.2a | 2.0b | 0.2a | 2.5b | 0.2a | 2.5b | 0.2a |
| IV | 2.2a | 0.2a | 2.4a | 0.2a | 3.0b | 0.3b | 3.9c | 0.2a | 3.6b | 0.2a |
| V | 2.9a | 0.3a | 2.6a | 0.3a | 3.9b | 0.3a | 4.2b | 0.3a | 4.2b | 0.3a |
| VI | 4.0a | 0.3a | 4.5a | 0.3a | 4.8b | 0.3a | 4.7b | 0.3a | 5.3c | 0.3a |
| VII | 4.6a | 0.3a | 5.5b | 0.3a | 5.5b | 0.4b | 5.7b | 0.3a | 6.6c | 0.3a |
| VIII | 4.2a | 0.3a | 5.1b | 0.3a | 4.4a | 0.3a | 4.3a | 0.3a | 6.5c | 0.3a |
| IX | 3.5a | 0.3a | 3.8a | 0.3a | 3.7a | 0.3a | 3.7a | 0.3a | 2.7b | 0.3a |
| X | 2.5a | 0.3a | 2.9a | 0.3a | 2.9a | 0.3a | 2.9a | 0.3a | 3.4b | 0.3a |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
Different investigations on xylanase production revealed that the best initial pH of medium for xylanase production by different fungi by fermentation is 4.5 (Fadel 2001), 6.0 (Qinnghe et al. 2004) and 6.5 (Carmona et al. 2005). Most of the fungi prefer acidic environment for their growth (Collins et al. 2005). According to Loveleen et al. (2010), production of cellulase and xylanase by Scopulariopsis acremonium and protease by Rhizopus microsporus var. oligosporus increased with increase in pH value, reaching the maximum at pH 5.5, followed by a gradual decrease thereafter in SmF. Nikhil et al. (2012) observed the highest xylanase production (1274.5 IU/gds/min) at pH 6 while Abdelrahim and Bayoumi (2011) also noticed maximum xylanase production at pH of 6 by Sporotrichum thermophile and Chaetomium thermophile.
Effect of incubation temperature on xylanase production
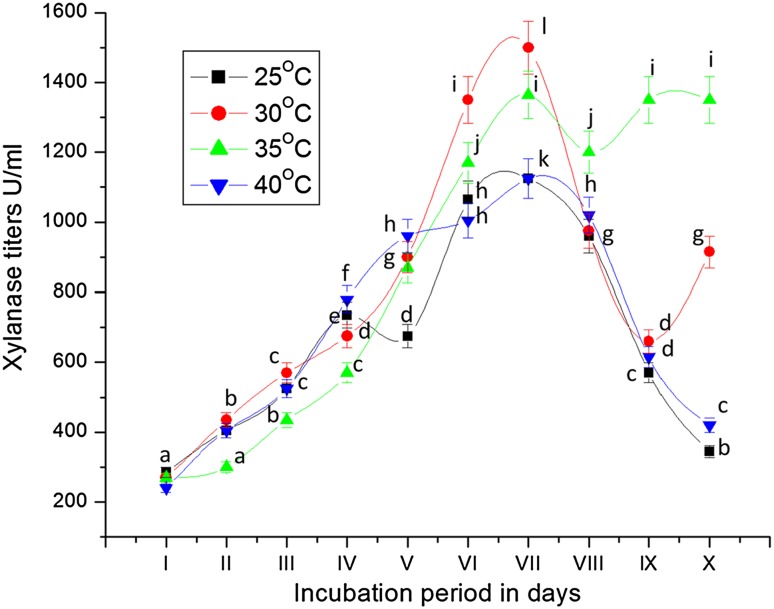
Incubation temperature is also a critical factor for growth of organisms. To study the influence of temperature on the production of xylanase, the culture was grown at different temperatures (25, 30, 35 and 40 °C). In the present study, the highest production of xylanase with titers of 1500 U/mL was observed on 7th day of incubation indicating mesophilic nature of the fungus (Fig. 8). There is no significant difference in biomass and extracellular protein yield by the fungus grown at 25, 30, 35 and 40°C (Table 8). The culture broth used for growth of the fungus exhibited change in pH (ST 7). Production of maximum xylanase titers at 30°C by the fungus in the present study is in agreement with many reports on optimum temperature requirement of 30 °C for xylanase production by P. janthinellum FM-5, Fusarium oxysporum, S. acremonium, Streptomyces rameus, Penicillium citrinum xym2 and Aspergillus fumigatus RSP-8 (Milagres et al. 1993; Kuhad et al. 1998; Loveleen et al. 2010; Bhosale et al. 2011; Saha and Ghosh 2014; Ravichandra et al. 2015). Similarly, Wejse et al. (2005) and Laxmi et al. (2008) also observed xylanase production by A. niger with optimum incubation temperature of around 30 °C. T. harzianum showed maximum xylanase activity when incubated in xylan containing medium at 34 °C (Pathak et al. 2014). Haltrich et al. (1996) and Nikhil et al. (2012) reported xylanase production of 2.64 U/mL at 28 °C and 966.8 IU/gds/min at 25 °C by A. niger and Aspergillus flavus FPDN1, respectively. The incubation temperature of 25 °C was best for xylanase production by T. viride (Goyal et al. 2008).
Fig. 8.
Secretion of xylanase by Fusarium sp. BVKT R2 grown on xylan in MSM at different temperatures. The values are the means of triplicates with standard deviation (SD). The line graphs bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Table 8.
Secretion of extracellular protein and biomass production by Fusarium sp. BVKT R2 grown on xylan at different temperatures
| Incubation period (days) | Temperature at (°C) | |||||||
|---|---|---|---|---|---|---|---|---|
| 25 | 30 | 35 | 40 | |||||
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 1.5a | 0.1a | 2.0a | 0.2b | 1.3a | 0.1a | 0.6b | 0.1a |
| II | 2.0a | 0.2a | 2.4a | 0.2a | 1.7b | 0.2a | 1.0b | 0.2a |
| III | 2.4a | 0.2a | 3.0a | 0.2a | 2.2a | 0.2a | 1.6b | 0.2a |
| IV | 2.9a | 0.2a | 3.7b | 0.3b | 3.0a | 0.2a | 2.4a | 0.2a |
| V | 4.0a | 0.3a | 4.2a | 0.3a | 3.7a | 0.3a | 3.3b | 0.3a |
| VI | 4.7a | 0.3a | 4.9a | 0.3a | 4.4a | 0.3a | 4.3a | 0.3a |
| VII | 5.9a | 0.3a | 6.0a | 0.3a | 5.7a | 0.3a | 5.5a | 0.3a |
| VIII | 4.7a | 0.3a | 5.6b | 0.3a | 4.3a | 0.3a | 4.6a | 0.3a |
| IX | 3.4a | 0.3a | 4.0b | 0.3a | 3.4a | 0.3a | 3.4a | 0.3a |
| X | 2.5a | 0.3a | 3.1b | 0.3a | 2.4a | 0.3a | 2.3a | 0.2b |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
The optimum temperature for xylanase production by Aspergillus japonicum, Penicilium oxalicum and T. aurantiacus was 25, 45 and 50 °C, respectively (Simoes and Tauk-Torniseielo 2006; Muthezhilan et al. 2007; Dhillon et al. 2000). Haas et al. (1992) obtained highest xylanase activity by P. chrysogenum at 28 °C. Likewise, Kheng and Omar (2005) reported the ambient temperature for xylanase production to be 28 ± 3 °C using the fungal isolate Aspergillus niger USM AI 1 with palm kernel cake (PKC) as substrate. According to Anand et al. (2013), a thermostable and alkali-tolerant bacterium Geobacillus thermodenitrificans produced xylanase at 60 °C but with very low activity (2.75 U/mL). The xylanases produced by A. niger and A. flavus remained totally stable at 45 °C (de Alencar Guimaraes et al. 2013). Bacillus species and P. citrinum xym2 were observed to synthesize high titer of xylanase at 50 and 30 °C, respectively (Nagar et al. 2010; Saha and Ghosh 2014). Thus, different bacterial and fungal species exhibited diverse ideal temperature optima for their growth and secretory products. It might be due to that at higher or lower temperature than optimum, the growth of the fungus was inhibited and, hence, the xylanase production was also decreased (Yaun and Rugyu 1999; Rahman et al. 2003).
These variations in different incubation temperatures are due to the different nature of microorganisms and their environmental conditions. Less activity of fungal enzymes at low temperature (15–25 °C) and at high temperature (35–40 °C) as compared to 30 °C might be due to slow growth at low temperature and inactivation of the enzyme at high temperature (Loveleen et al. 2010). But our isolate Fusarium sp. BVKTR2 displayed maximum production of xylanase at 30 °C temperature.
Effect of agitation on xylanase production
Mechanical agitation is a crucial factor for production of xylanases since this aids in uniform distribution of nutrients and supply of oxygen to all individual cells of organisms. For investigating the influence of agitation on xylanase production, the culture was grown at 100, 150, 200 and 250 rpm along with estimation of biomass and xylanase production. Among all the agitation levels tested in the present study, 200 rpm was found optimal for xylanase production (Fig. 9). Growth of Fusarium sp. BVKTR2 at 200 rpm speed resulted in the production of xylanase to the extent of 1900 U/mL as against xylanase yields of 900 U/mL at 150 rpm by the same culture on 7th day of incubation. Growth of the culture reached peak on 5th day of incubation at all speeds as reflected by biomass which remained constant (Table 9). But secretion of extracellular protein touched peak on 5th day of incubation. The initial change in pH of the culture filtrate fluctuated between 3.2 and 7.3 (ST 8). According to different studies, optimal agitation varied based on the substrate and the organism used for production. For instance, the enzyme production by A. niger was deleteriously affected by agitation (Lejeune and Baron 1995; Sanghi et al. 2009; Sepahy et al. 2011). The highest xylanase production by B. pumilus VLK-1 at an agitation rate of 200 rpm after 48 h of incubation was reported (Battan et al. 2006). Agitation at 150 rpm was optimum for the production of thermostable and cellulase free xylanase by Streptomyces species AB106 (Techapu and Prosesor 2003).
Fig. 9.
Production of xylanase by Fusarium sp. BVKT R2 grown on xylan MSM with different agitation rates. The values are the means of triplicates with standard deviation (SD). The line graphs bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Table 9.
Secretion of extracellular protein and biomass production by Fusarium sp. BVKT R2 grown on xylan with different agitation rates
| Incubation period (days) | Agitation (rpm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 150 | 200 | 250 | |||||
| Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | Protein (mg/mL) | Biomass (mg/flask) | |
| I | 1.2a | 0.1a | 1.5a | 0.1a | 1.7b | 0.2b | 1.3a | 0.1a |
| II | 1.8a | 0.2a | 1.7a | 0.2a | 2.5b | 0.2a | 1.6a | 0.2a |
| III | 2.8a | 0.2a | 2.1a | 0.2a | 4.3b | 0.2a | 2.6a | 0.2a |
| IV | 3.5a | 0.2a | 2.7b | 0.2a | 6.3c | 0.3b | 3.6a | 0.2a |
| V | 4.1a | 0.3a | 3.6a | 0.3a | 6.8b | 0.3a | 4.3a | 0.3a |
| VI | 4.8a | 0.3a | 4.6a | 0.3a | 7.3b | 0.3a | 4.8a | 0.3a |
| VII | 5.9a | 0.3a | 5.9a | 0.3a | 6.8b | 0.3a | 5.4a | 0.3a |
| VIII | 6.8a | 0.3a | 4.9b | 0.3a | 5.0b | 0.3a | 4.8b | 0.3a |
| IX | 4.1a | 0.3a | 3.4b | 0.3a | 3.6b | 0.3a | 2.2c | 0.3a |
| X | 2.5a | 0.3a | 1.1b | 0.3a | 1.6b | 0.3a | 4.2c | 0.3a |
All data presented are the means of triplicates
Values in rows with respect to each parameter (protein/biomass) for each time interval bearing common letters in superscript are not significantly different from each other (P ≤ 0.05) according to DMR test
Higher production of xylanase in shake cultures may be due to the formation of pellets by hyphae. The maximum enzyme yield (77 U/mL) was achieved at 150 rpm and a variation of 30 rpm on either side of this resulted in reduction of xylanase productivity by A. fumigatus RSP-8 (Ravichandra et al. 2015).
Effect of incubation time
Time course plays a very critical role in fungal metabolic activity and growth for economical production of enzymes. It is obvious that production costs are directly proportional to the production time. Thus, an enzyme should be produced at the shortest possible time for overall economy of production. In the current and other experiments, xylanase production was studied for 10 days at 1-day interval. The production of xylanase increased with increase in incubation time up to 7th day and decreased thereafter with/without addition of different carbon and nitrogen sources. Our results are in agreement with Irfan et al. (2014) who reported maximum xylanase activity with T. viride IR05 in SSF after 7 days of incubation. In contrast, there are reports on maximum xylanase secretion by T. harzianum, T. viride and T. lanuginosus MC 134 at 8, 14–17 and 6 days of incubation period, respectively (Abdel-Sater and El-Said 2001; Goyal et al. 2008; Kumar et al. 2009).
Neves et al. (2011) reported higher xylanase production on 5th day of incubation by Lichthemia blakesleeana. Torres and Cruz (2013) observed maximum xylanase activity on 4th day of incubation by mangrove fungi and prolonged incubation periods decreased enzyme production. Okafor et al. (2007) isolated a strain of Penicillium chrysogenum PCL501 from wood wastes which exhibited the highest xylanase activity after 96 h of fermentation and the lowest after 120 h. Similarly, time course analyses from other studies showed that regardless of substrate and fermentation setup, the maximal xylanase production by filamentous fungi was generally obtained after 5 days of incubation (Bakir et al. 2001; Li et al. 2006; Murthy and Naidu 2012). It is not uncommon, however, for some fungi to produce high levels of xylanase towards the 6th day of incubation and beyond (Jiang et al. 2010). Increased fermentation time and decreased enzyme synthesis might be due to the depletion of macro- and micronutrients in the fermentation medium with the passage of time, which altered the fungal physiology resulting in the inactivation of secretory machinery of the enzymes (Nochure et al. 1993). At higher incubation time, production cost would increase due to the increase in maintenance. Besides, at higher incubation times contamination is also one of the major problems.
Xylanase production under optimum conditions
Under optimized submerged fermentation conditions (pH 5.0, temperature 30 °C, 5 days of incubation period, shaking at 200 rpm, 6 agar plugs of inoculum, 1.5% of sorbitol and 1.5% of yeast extract) Fusarium BVKT R2 produced 4200 U/mL of xylanase, which is 3.2-fold higher than un-optimized conditions (period of incubation 7 days, pH of 5.0, temperature of 30 °C, amount of inoculum 5 agar plugs and agitation of 150 rpm without addition of sorbitol as additional carbon source and yeast extract as additional nitrogen source). It is higher than the xylanase production of 180 IU/mL by Bacillus sp. GRE7 under submerged fermentation using oat spelt xylan as the substrate (Kiddinamoorthy et al. 2008).
Saccharification
Release of soluble sugars in saccharification process from local lignocellulose biomasses upon growth of Fusarium sp. BVKT R2 was assessed as percentage at different time intervals. Figure 10 illustrates the saccharification of sawdust, rice straw and cotton stocks. Our results clearly demonstrate that Fusarium sp. BVKT R2 strain has the ability to saccharify all the tested biomass feedstocks. The level of saccharification varied from substrate to substrate and with incubation time (Fig. 10). The maximum saccharification (%) was observed with sawdust after 120 h of incubation.
Fig. 10.
Release of soluble sugars from lignocellulosic substrates upon growth of Fusarium sp. BVKT R2. The values are the means of triplicates with standard deviation (SD). The bars bearing same letters for each time interval are significantly not different (P ≤ 0.05) from each other according to DMR test
Solubilization of sugars from lignocellulosic mass by BVKT R2 in the present study could be facilitated by action of xylanase enzyme secreted by the culture during growth. The ability of different organisms to saccharify lignocellulosic biomass was evaluated by different researchers (Harshvardhan et al. 2013; Santhi et al. 2014; Premalatha et al. 2015). Saccharification of pretreated sugarcane bagasse with crude cellulase enzyme of Fomitopsis sp. RCK 201 at loading of 20 U/g released 1.5 to 2.4-fold higher sugars than untreated sugar cane bagasse (Deswal et al. 2014). Among different plant biomasses tested with cellulolytic enzymes of Enhydrobacter sp. ACCA2, maximum saccharification (61.33%) of bamboo was observed on 3rd day of incubation (Premalatha et al. 2015). Saccharification of macro-alga Ulva lactuca biomass with cellulolytic enzyme from a marine Bacillus sp. increased recovery of glucose (450 mg/g) (Harshvardhan et al. 2013).
Conclusion
Process parameters, which play a critical role, were optimized for the maximum production of xylanase. Among different xylans used, birchwood xylan was found to be the best substrate for xylanase production by Fusarium sp. BVKT R2. Additional carbon and nitrogen sources, sorbitol (1.5%) and yeast extract (1.5%), pH (5.5), temperature (30 °C), agitation (200 rpm), incubation time (5 day) and volume of inoculum 6 agar plugs induced maximum xylanase production. Under optimal conditions, xylanase production is as high as 4200 U/mL and reduction in the incubation time (5 days) was achieved after optimization. Further, the fungal culture also saccharified different biomass materials indicating clearly its potential for biomass saccharification and applications in allied industries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the financial assistance provided by the University Grants Commission, New Delhi, in the form of fellowships to G. Ramanjaneyulu, A. Ramya and K. Dileep Kumar to carry out the above research.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0977-1) contains supplementary material, which is available to authorized users.
References
- Abdelrahim AA, Bayoumi A. Thermostable xylanases production by thermophilic fungi from some lignoce llulosic substrates. J Basic Appl Sci Res. 2011;1(12):2777–2785. [Google Scholar]
- Abdel-Sater MA, El-Said AHM. Xylan-decomposing fungi and xylanolytic activity in agricultural and industrial wastes. Int Biodeterior Biodegrad. 2001;47:15–21. doi: 10.1016/S0964-8305(00)00113-X. [DOI] [Google Scholar]
- Aditi K, Ray RR. Effect of salic in on induction and carbon catabolite repression of endoxylanase synthesis in MTCC 10889. Chem Pap. 2014;68(4):451–456. [Google Scholar]
- Altaf SA, Umar DM, Muhammad MS. Production of xylanase enzyme by Pleurotus eryngii and Flamulina velutipes grown on different carbon sources under submerged fermentation. World Appl Sci J. 2010;8:47–49. [Google Scholar]
- Altaf SA, Sughra MG, Nasreen K, Umar DM, Sher MM, Noor-e-Saba KM, Fariha RK, Lu C. Characterization of crude xylanase produced by edible mushroom Pleurotus eryngii. J Bioprocess Biotech. 2016;6(2):1–6. [Google Scholar]
- Anand A, Kumar V, Satyanarayana T. Characteristics of thermostable endoxylanase and b-xylosidase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1 and its applicability in generating xylooligosaccharides and xylose from agro-residues. Extremophiles. 2013;17:357–366. doi: 10.1007/s00792-013-0524-x. [DOI] [PubMed] [Google Scholar]
- Arabi MIE, Jawhar M, Bakri Y. Effect of additional carbon source and moisture level on xylanase production by Cochliobolus sativus in solid fermentation. Microbiology. 2011;80:150–153. doi: 10.1134/S0026261711010024. [DOI] [PubMed] [Google Scholar]
- Bailey M, Biely J, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23(3):257–270. doi: 10.1016/0168-1656(92)90074-J. [DOI] [Google Scholar]
- Bakir U, Yavascaoglu S, Guvenc F, Ersayin A. An endo-b 1,4- xylanase from Rhizopus oryzae: production, partial purification and biochemical characterization. Enzyme Microb Technol. 2001;29:328–334. doi: 10.1016/S0141-0229(01)00379-9. [DOI] [Google Scholar]
- Bakri Y, Mohammed J, Mohammed IEA. Improvement of xylanase production by Cochliobolus sativus in submerged culture. Food Technol Biotechnol. 2008;46(1):116–118. [Google Scholar]
- Battan B, Sharma JK, Dhiman SS. High level xylanase production by alkalophilic B. pumilus ASH under solid state fermentation. World J Microbiol Biotechnol. 2006;22:1281–1287. doi: 10.1007/s11274-006-9173-x. [DOI] [Google Scholar]
- Beck CI, Scoot D. Enzymes in foods for better or worse. Adv Chem Ser. 1974;138:1–17. doi: 10.1021/ba-1974-0138.ch001. [DOI] [Google Scholar]
- Beg QK, Kapoor M, Mahajan L, Hoondal GS. Microbial xylanases and their Industrial applications: a review. Appl Microbiol Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- Bhosale HJ, Sukalkar SR, Uzma SMZ, Kadam TA (2011) Production of xylanase by Streptomyces rameus grown on agricultural wastes. Biotechnol Bioinf Bioeng 1(4):505–512
- Biely P. Microbial xylanolytic systems. Trends Biotechnol. 1985;3:288–290. doi: 10.1016/0167-7799(85)90004-6. [DOI] [Google Scholar]
- Carmona EC, Fialho MB, Buchgnani EB, Coelho GD, Brocheto-Braga MR, Jorge JA. Production, purification and characterization of a minor form of xylanase from Aspergillus versicolor. Process Biochem. 2005;40:359–364. doi: 10.1016/j.procbio.2004.01.010. [DOI] [Google Scholar]
- Chakdar H, Kumar M, Pandiyan K, Singh A, Nanjappan K, Kashyap PL, Srivastava AK. Bacterial xylanases: biology to biotechnology. 3 Biotech. 2016;6(2):150. doi: 10.1007/s13205-016-0457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Gerday C, Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev. 2005;29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Damiano VB, Bocchini DA, Gomes E, Da Silva R. Application of crude xylanase from Bacillus licheniformis 77-2 to the bleaching of eucalyptus Kraft pulp. World J Microbiol Biotechnol. 2003;19:139–144. doi: 10.1023/A:1023244911314. [DOI] [Google Scholar]
- Despres J, Forano E, Lepercq P, Comtet-Marre S, Jubelin G, Chambon C, Yeoman CJ, Berg Miller ME, Fields CJ, Martens E, Terrapon N, Henrissat B, White BA, Moson P. Xylan degradation by the human gut Bacteroides xylanisolvens XB1AT involves two distinct gene clusters that are linked at the transcriptional level. BMC Genomics. 2016;17:326. doi: 10.1186/s12864-016-2680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deswal D, Gupta R, Nandal P, Kuhad RC. Fungal pretreatment improves amenability of lignocellulosic material for its saccharification to sugars. Carbohydr Polym. 2014;99:264–269. doi: 10.1016/j.carbpol.2013.08.045. [DOI] [PubMed] [Google Scholar]
- de Alencar Guimaraes NC, Sorgatto M, Peixoto-Nogueira S de C, Betini JHA, Zanoelo FF, Marques MR, Lourdes M de, Moraes T de, Polizeli, Giannesi GC (2013) Bioprocess and biotechnology: effect of xylanase from Aspergillus niger and Aspergillus flavus on pulp biobleaching and enzyme production using agro industrial residues as substract. Springer Plus 2:380 [DOI] [PMC free article] [PubMed]
- Dhillon A, Gupta JK, Jauhari BM, Khanna SA. A cellulase poor, thermostable, alkali-tolerant xylanase produced by Bacillus circulans AB16 grown on rice straw and its application in biobleaching of eucalyptus pulp. Bioresour Technol. 2000;73:273–277. doi: 10.1016/S0960-8524(99)00116-9. [DOI] [Google Scholar]
- Dodd D, Cann IKO. Enzymatic deconstruction of xylan for biofuel production. GCB Bioenergy. 2009;1:2–17. doi: 10.1111/j.1757-1707.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D, Mackie RI, Cann IKO. Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes. Mol Microbiol. 2011;79(2):292–304. doi: 10.1111/j.1365-2958.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel M. Production physiology of cellulase and β-glucosidase enzymes of Aspergillus niger grown under solid state fermentation conditions. J Biol Sci. 2001;1(5):401–411. doi: 10.3923/jbs.2001.401.411. [DOI] [Google Scholar]
- Garai D, Kumar V. Response surface optimization for xylanase with high volumetric productivity by indigenous alkali tolerant Aspergillus candidus under submerged cultivation. 3 Biotech. 2013;3(2):127–136. doi: 10.1007/s13205-012-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N, Mahatma KK, Kumar A. Xylanase: applications and biotechnological aspects. Saarbrucken: Lambert Academic Publishing AG & Co. KG; 2010. [Google Scholar]
- Gilbert HJ, Hazelwood GP. Bacterial cellulases and xylanases. J Gen Microbiol. 1999;139:187–194. doi: 10.1099/00221287-139-2-187. [DOI] [Google Scholar]
- Goyal M, Kalra KL, Sareen VK, Soni G. Xylanase production with xylan rich lignocellulosic wastes by a local soil isolate of Trichoderma viride. Braz J Microbiol. 2008;39:535–541. doi: 10.1590/S1517-83822008000300025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, Rajeeva G, Santosh KY, Nandan SD. Optimization of xylanase production from free and immobilized cells of Fusarium solani F7. BioResources. 2009;4(3):932–945. [Google Scholar]
- Haas H, Herfurth E, Stoffler G, Redl B. Purification, characterization and partial amino acid sequences of a xylanase produced by Penicillium chrysogenum. Biochem Biophys Acta. 1992;1117:279–286. doi: 10.1016/0304-4165(92)90025-P. [DOI] [PubMed] [Google Scholar]
- Haltrich D, Preiss M, Steiner W. Optimization of a culture medium for increased xylanase production by a wild strain of Schizophyllum commune. Enzyme Microb Technol. 1993;15:854–860. doi: 10.1016/0141-0229(93)90097-L. [DOI] [Google Scholar]
- Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupaneie S. Production of fungal xylanases. Bioresour Technol. 1996;58:137–161. doi: 10.1016/S0960-8524(96)00094-6. [DOI] [Google Scholar]
- Harshvardhan K, Mishra A, Jha B. Purification and characterization of cellulase from a marine Bacillus sp. H1666: a potential agent for single step saccharification of seaweed biomass. J Mol Catal B Enzym. 2013;93:51–56. doi: 10.1016/j.molcatb.2013.04.009. [DOI] [Google Scholar]
- Hoq MM, Carsten H, Wolf-Dieter D. Cellulase-free xylanase by Thermomyces lanuginosus RT9: effect of agitation, aeration, and medium components on production. J Biotechnol. 1994;37(1):4958. doi: 10.1016/0168-1656(94)90202-X. [DOI] [Google Scholar]
- Irfan M, Muhammad N, Quratulain S. One-factor-at-a-time (OFAT) optimization of xylanase production from Trichoderma viride-IR05 in solid-state fermentation. J Rad Res Appl Sci. 2014;7:317–326. [Google Scholar]
- Isil S, Nilufer A. Xylanase production from T. harzianum 1073 D3 with alternative carbon and nitrogen sources. Food Technol Biotechnol. 2005;43:37–40. [Google Scholar]
- Jiang Z, Cong Q, Yan Q, Kumar N, Du X. Characterisation of a thermostable xylanase from Chaetomium sp. and its application in Chinese steamed bread. Food Chem. 2010;120:457–462. doi: 10.1016/j.foodchem.2009.10.038. [DOI] [Google Scholar]
- Joshi C, Khare SK. Induction of xylanase in thermophilic fungi Scytalidium thermophilum and Sporotrichum thermophile. Braz Arch Biol Technol. 2012;55:21–27. doi: 10.1590/S1516-89132012000100003. [DOI] [Google Scholar]
- Kalogeris E, Iniotaki F, Topakas E, Christakopoulos P, Kekos D, Macris BJ. Performance of an intermittent agitation rotating drum type bioreactor for solid state fermentation of wheat straw. Bioresour Technol. 2003;86:207–213. doi: 10.1016/S0960-8524(02)00175-X. [DOI] [PubMed] [Google Scholar]
- Kheng PP, Omar IC. Xylanase production by a local fungal isolate, Aspergillus niger USM AI 1 via solid state fermentation using palm kernel cake (PKC) as substrate. J Sci Technol. 2005;27(2):325–336. [Google Scholar]
- Kiddinamoorthy J, Anceno JA, Haki DG, Rakshit SK. Production, purification and characterization of Bacillus sp. GRE7 xylanase and its application in eucalyptus Kraft pulp biobleaching. World J Microbiol Biotechnol. 2008;24:605–612. doi: 10.1007/s11274-007-9516-2. [DOI] [Google Scholar]
- Knob A, Carmona EC. Xylanase production by Penicillium sclerotiorum and its characterization. World Appl Sci J. 2008;4(2):277–283. [Google Scholar]
- Kuhad RC, Manchanda M, Singh A. Optimization of xylanase production by a hyperxylanolytic mutant strain of F. oxysporum. Process Biochem. 1998;33:641–647. doi: 10.1016/S0032-9592(98)00025-9. [DOI] [Google Scholar]
- Kumar KS, Ayyachamy M, Kugen P, Suren S. Production of β-xylanase by a Thermomyces lanuginosus MC 134 mutant on corn cobs and its application in biobleaching of bagasse pulp. J Biosci Bioeng. 2009;107(5):494–498. doi: 10.1016/j.jbiosc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Latif F, Asgher M, Saleem R, Akram A, Legge R. Purification and characterization of xylanase produced by C. thermophile NIBGE. World J Microbiol Biotechnol. 2006;22:45–50. doi: 10.1007/s11274-005-5745-4. [DOI] [Google Scholar]
- Laxmi GS, Sathish T, Subba Rao C, Brahmaiah P, Hymavathi M, Prakasham RS. Palm fiber as novel substrate for enhanced xylanase production by isolated Aspergillus sp. RSP-6. Curr Trends Biotechnol Pharm. 2008;2(3):447–455. [Google Scholar]
- Lejeune R, Baron GV. Effect of agitation on growth and enzyme production of Trichoderma reesei in batch fermentation. Appl Microbiol Biotechnol. 1995;43:249–258. doi: 10.1007/BF00172820. [DOI] [Google Scholar]
- Li Y, Lin J, Meng D, Lu J, Gu G, Mao Z. Effect of pH, cultivation time and substrate concentration on the endoxylanase production by Aspergillus awamori ZH-26 under submerged fermentation using central composite rotary design. Food Technol Biotechnol. 2006;44:473–477. [Google Scholar]
- Lopes F, Motta F, Andrade CCP, Rodrigues MI, Maugeri-Filho F. Thermo-stable xylanases from non conventional yeasts. Microb Biochem Technol. 2011;3(3):36–42. [Google Scholar]
- Loveleen KS, Maninder A, Sehgal VK. Use of Scopulariopsis acremonium for the production of cellulase and xylanase through submerged fermentation. Afr J Microbiol Res. 2010;4(14):1506–1510. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol regent. J Gen Microbiol. 1951;131:3017–3027. [PubMed] [Google Scholar]
- Mc Cleary BV. Enzymatic modification of plant polysaccharides. Int J Macromole. 1986;8:349–354. doi: 10.1016/0141-8130(86)90054-1. [DOI] [Google Scholar]
- Milagres AMF, Lacis LS, Prade RA. Characterization of xylanase production by a local isolate of Penicillium janthinellum. Enzyme Microbiol Technol. 1993;15:248–253. doi: 10.1016/0141-0229(93)90145-R. [DOI] [Google Scholar]
- Miller GL. Use of 3,5-dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Murthy PS, Naidu MM. Production and application of xylanase from Penicillium sp. utilizing coffee by-products. Food Bioprocess Technol. 2012;5(2):657–664. doi: 10.1007/s11947-010-0331-7. [DOI] [Google Scholar]
- Muthezhilan R, Ashok R, Jayalakshmi S (2007) Production and optimization of thermostable alkaline xylanase by Penicillium oxalicum in solid state fermentation. Afr J Microbiol Res 1(2):20–28
- Nagar S, Gupta VK, Kumar D, Kumar L, Kuhad RC. Production and optimization of cellulase-free, alkali-stable xylanase by Bacillus pumilus SV-85S in submerged fermentation. J Ind Microbiol Biotechnol. 2010;37:71–83. doi: 10.1007/s10295-009-0650-8. [DOI] [PubMed] [Google Scholar]
- Nair SG, Sindhu R, Shashidhar S. Fungal xylanase production under solid state and submerged fermentation conditions. Afr J Microbiol Res. 2008;2:82–86. [Google Scholar]
- Nakamura S, Ishiguro Y, Nakai R, Wakabayashi K, Aono R, Horikoshi K. Purification of a thermophilic alkaline xylanase from thermophilic Bacillus sp. strain TAR-1. J Mol Catal B Enzym. 1995;1:7–15. doi: 10.1016/1381-1177(95)00003-8. [DOI] [Google Scholar]
- Neves ML, da Silva MF, Souza-Motta CM, Spier MR, Soccol CR, Porto TS, Moreira KA, Porto AL. Lichtheimia blakesleeana as a new potential procedure of phytase and xylanase. Artic Mol. 2011;16:4807–4817. doi: 10.3390/molecules16064807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikhil B, Adhyaru D, Thakor P. Production of xylanase by Aspergillus flavus FPDN1 on Pearl millet bran: optimization of culture conditions and application in bioethanol production. Int J Res Chem Environ. 2012;2(3):204–210. [Google Scholar]
- Ninawe S, Lal R, Kuhad RC. Isolation of three xylanase-producing strains of actinomycetes and their identification using molecular methods. Curr Microbiol. 2006;53(3):178–182. doi: 10.1007/s00284-005-0285-6. [DOI] [PubMed] [Google Scholar]
- Nochure SV, Roberts MF, Demain AI. True cellulase production by Clostridium thermocellum grown on different carbon sources. Biotechnol Lett. 1993;15:641–646. doi: 10.1007/BF00138556. [DOI] [Google Scholar]
- Oakley AJ, Heinrich T, Thompson CA, Wilce MCJ. Characterization of a family 11 from Bacillus subtilis B230 used for paper bleaching. Acta Cryst D. 2003;59:627–636. doi: 10.1107/S0907444903001227. [DOI] [PubMed] [Google Scholar]
- Okafor UA, Emezue TN, Okochi VI, Onyegeme-Okerenta BN, Nwodo-Chinedu S. Xylanase production by Penicillium chrysogenum (PCL501) fermented on cellulosic wastes. Afr J Biochem Res. 2007;1:48–53. [Google Scholar]
- Otero DM, Cadaval CL, Teixeira LM, Rosa CA, Sanzo AVL, Kalil SJ. Screening of yeasts capable of producing cellulase-free xylanase. Afr J Biotechnol. 2015;14(23):1961–1969. doi: 10.5897/AJB2015.14476. [DOI] [Google Scholar]
- Pathak P, Bhardwaj NK, Singh AK. Production of crude cellulase and xylanase from Trichoderma harzianum PPDDN10 NFCCI-2925 and its application in photocopier waste paper recycling. Appl Biochem Biotechnol. 2014;172:3776–3797. doi: 10.1007/s12010-014-0758-9. [DOI] [PubMed] [Google Scholar]
- Paul J, Varma AK. Influence of sugars on endoxylanase and bxylosidase activities of Bacillus strain. Biotechnol Lett. 1990;12:19–27. doi: 10.1007/BF01028494. [DOI] [Google Scholar]
- Petchluan P, Charida P, Nareerat C. Characterization of xylanase and cellulase from Lentinus polychrous Lev. LP-PT-1. Chiang Mai J Sci. 2014;41(5.1):1007–1019. [Google Scholar]
- Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol. 2005;67:577–591. doi: 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- Premalatha N, Gopal NO, Jose PA, Anandham R, Kwon SW. Optimization of cellulase production by Enhydrobacter sp. ACCA2 and its application in biomass saccharification. Front Microbiol. 2015;6:1046. doi: 10.3389/fmicb.2015.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qinnghe C, Xiaoyu Y, Tiangui N, Cheng J, Qiugang M. The screening of culture condition and properties of xylanase by white-rot fungus Pleurotus ostreatus. Process Biochem. 2004;39:1561–1566. doi: 10.1016/S0032-9592(03)00290-5. [DOI] [Google Scholar]
- Raghukumar C, Muraleedharan U, Gaud VR, Mishra R. Xylanases of marine fungi of potential use for biobleaching of paper pulp. J Ind Microbiol Biotechnol. 2004;31:433–441. doi: 10.1007/s10295-004-0165-2. [DOI] [PubMed] [Google Scholar]
- Rahman AK, Sugitani N, Hatsu M, Takamizawa K. A role of xylanase, α-l-arabinofuranosidase, and xylosidase in xylan degradation. Can J Microbiol. 2003;49:58–64. doi: 10.1139/w02-114. [DOI] [PubMed] [Google Scholar]
- Ramanjaneyulu G, Rajasekhar Reddy B. Optimization of xylanase production through response surface methodology by Fusarium sp. BVKT R2 isolated from forest soil and its application in saccharification. Front Microbiol. 2016;7:1450. doi: 10.3389/fmicb.2016.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanjaneyulu G, Praveen Kumar Reddy G, Dileep Kumar K, Rajasekhar Reddy B. Isolation and screening of xylanase producing fungi from forest soils. Int J Curr Microbiol Appl Sci. 2015;4:586–591. [Google Scholar]
- Ramanjaneyulu G, Ramya A, Shanthi Kumari B, Dileep Kumar K, Rajasekhar Reddy B. Xylanase producing microflora in Eastern Ghats of Andhra Pradesh, India. J For Res. 2016 [Google Scholar]
- Ravichandra K, Yaswanth VVN, Nikhila B, Jamal A, Srinivasa Rao P, Uma A, Ravindrababu V, Prakasham RS. Xylanase production by isolated fungal strain, Aspergillus fumigatus RSP-8 (MTCC 12039): impact of agroindustrial material as substrate. Sugar Tech. 2015 [Google Scholar]
- Saha BC, Bothast RJ. Enzymology of xylan degradation. ACS Symp Ser. 1999;723:167–194. doi: 10.1021/bk-1999-0723.ch011. [DOI] [Google Scholar]
- Saha SP, Ghosh S. Optimization of xylanase production by Penicillium citrinum xym2 and application in saccharification of agro-residues. Biocatal Agric Biotechnol. 2014;3:188–196. [Google Scholar]
- Sanghi A, Garg N, Kuhar K, Kuhad RC, Gupta VK. Enhanced production of cellulase-free xylanase by alkalophilic Bacillus subtilis ASH and its application in biobleaching of kraft pulp. BioResources. 2009;4:1109–1129. [Google Scholar]
- Santhi VS, Bhagat AK, Saranya S, Govindarajan G, Jebakumar SRD. Seaweed (Eucheuma cottonii) associated microorganisms, a versatile enzyme source for the lignocellulosic biomass processing. Int Biodeterior Biodegrad. 2014;96:144–151. doi: 10.1016/j.ibiod.2014.08.007. [DOI] [Google Scholar]
- Sepahy AA, Ghazi S, Sepahy MA. Cost-effective production and optimization of alkaline xylanase by indigenous Bacillus mojavensis AG13 fermented on agricultural waste. Enzyme Res. 2011 doi: 10.4061/2011/593624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes MLG, Tauk-Torniseielo SM. Optimization of xylanase biosynthesis by Aspergillus japonicus isolated from a ‘Caatinga’ area in the Brazilian state of Bahia. Afr J Biotechnol. 2006;5:1135–1141. [Google Scholar]
- Sridevi A, Sandhya A, Ramanjaneyulu G, Narasimha G, Devi PS. Biocatalytic activity of Aspergillus niger xylanase in paper pulp biobleaching. 3 Biotech. 2016;6(165):1–7. doi: 10.1007/s13205-016-0480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Wen F, Si T, Xu JH, Zhao H. Direct conversion of xylan to ethanol by recombinant Saccharomyces cerevisiae strains displaying an engineered minihemicellulosome. Appl Environ Microbiol. 2012;78(11):3837–3845. doi: 10.1128/AEM.07679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunna A, Antranikian G. Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol. 1997;17:39–67. doi: 10.3109/07388559709146606. [DOI] [PubMed] [Google Scholar]
- Techapu C, Prosesor R. Thermostable and alkaline tolerant microbial cellulose free xylanase produced from agriculture waste. Proc Biochem. 2003;38(1):1327–1340. doi: 10.1016/S0032-9592(02)00331-X. [DOI] [Google Scholar]
- Tony C, Charles G, Georges F. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev. 2005;29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Torres JM, Cruz DTE. Production of xylanases by mangrove fungi from the Philippines and their application in enzymatic pretreatment of recycled paper pulps. World J Microbiol Biotechnol. 2013;29:645–655. doi: 10.1007/s11274-012-1220-1. [DOI] [PubMed] [Google Scholar]
- Uday USP, Choudhury P, Bandyopadhyay TK, Bhunia B. Classification, mode of action and production strategy of xylanase and its application for biofuel production from water hyacinth. Int J Biol Macromol. 2016;82:1041–1054. doi: 10.1016/j.ijbiomac.2015.10.086. [DOI] [PubMed] [Google Scholar]
- Wahyuntari B, Mubarik NR, Setyahadi S. Effect of pH, temperature and medium composition on xylanase production by Bacillus sp. AQ-1 and partial characterization of the crude enzyme. Microbiol Indones. 2009;3(1):17–22. doi: 10.5454/mi.3.1.3. [DOI] [Google Scholar]
- Walsh GA, Power RF, Headon DR. Enzymes in animal feed industry. Trends Biotechnol. 1993;11:424–430. doi: 10.1016/0167-7799(93)90006-U. [DOI] [PubMed] [Google Scholar]
- Wejse PL, Ingvorsen K, Mortensen KK. Salinity and temperature effects on accessibility of soluble and cross-linked insoluble xylans to endoxylanases. IUBMB Life. 2005;57(11):761–763. doi: 10.1080/15216540500364271. [DOI] [PubMed] [Google Scholar]
- Wong KKY, Saddler JN. Trichoderma xylanases: their properties and application. In: Visser J, Beldman G, Someren MAK, Voragen AGJ, editors. Xylans and xylanases. Amsterdam: Elsevier; 1992. pp. 171–186. [Google Scholar]
- Yan Q, Hao S, Jiang Z, Zhai Q, Chen W. Properties of a xylanase from Streptomyces matensis being suitable for xylooligosaccharides production. J Mol Catal B Enzym. 2009;58:72–77. doi: 10.1016/j.molcatb.2008.11.010. [DOI] [Google Scholar]
- Yaun Q, Rugyu M. Study on temperature oscillation in production of xylanase by Aspergillus niger. Beijing Hugagong Daxue Xuebao. 1999;26:11–16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.