Abstract
Patients with heart failure (HF) have structural and functional changes of the gut as a result of microcirculatory disturbances. A disrupted gut epithelial barrier may lead to translocation of microbial products into systemic circulation, possibly aggravating HF by inducing inflammatory responses. Gut microbiota play an essential role in the maintenance of host homeostasis because large quantities of their gene products complement host physiological processes. Emerging evidence has suggested the potential clinical significance of gut microbiota in the pathophysiology of HF. Imbalances of gut microbe-derived metabolites can contribute to cardiac dysfunction and other morbidities in patients with HF. Therapeutic research for HF through targeting microbiota is under way. Thus, the novel concept of a heart-gut axis may lead to breakthroughs in the development of innovative diagnostics and therapeutic approaches for HF.
Keywords: Gut epithelial barrier, Gastrointestinal microbiome, Dysbiosis, Microbe-derived metabolites
INTRODUCTION
The potential role of the gut in the pathophysiology of heart failure (HF) has recently begun to attract increased attention. Patients with HF develop reduced cardiac output, increased tissue congestion, and develop peripheral vasoconstriction. These disturbances may impair gut epithelial function, contributing to malnutrition and cachexia complicating advanced HF, and may also lead to translocation of bacteria-derived endotoxins across the gut epithelial barrier, thereby inducing systemic inflammatory responses.1),2),3),4) There are more microbial cells residing in the human gut than human cells in the body, and their combined genomes contain millions of genes, at least one hundred-fold that of the number of human genes.
There is growing evidence that imbalances in the composition and function of gut microbiota, known as dysbiosis, are associated with a wide spectrum of host disorders ranging from gastrointestinal diseases to inflammatory, metabolic, and neurologic diseases. For example, we recently used 16S ribosomal RNA gene sequencing of fecal samples from HF patients to determine that gut microbial dysbiosis is associated with HF.5) Importantly, this result is suggestive of the potential impact that the gut microbiota may have on the pathophysiological processes involved in HF. In addition, a wide variety of metabolites derived from gut microbes may also influence HF. In this review, we present the novel concept of a heart-gut axis, and discuss how gut microbiota and microbe-derived metabolites may contribute to the pathophysiology of HF.
GUT EPITHELIAL DYSFUNCTION IN HF
The structure and function of the gut are fundamentally altered in patients with HF. Specifically, patients with HF have diminished intestinal blood flow, which correlates with the severity of HF. Cachectic patients in particular have notably decreased blood flow in the celiac trunk and the superior and inferior mesenteric arteries as measured by ultrasonography.6) HF patients also have a thickened bowel wall of terminal ileum and colon, suggesting bowel edema,7) and increased collagen accumulation in mucosal biopsies of the small intestine.8)
In the intestinal villi, arterioles and venules form a countercurrent microcirculatory system. Therefore, arteriolar oxygen shunts to venules before reaching the villus tip, resulting in the lowest oxygen concentration occurring at the villus tip. In patients with HF, there are microcirculatory disturbances in the gut due to reduced perfusion, increased congestion, and sympathetically mediated vasoconstriction. These hemodynamic alterations lead to exaggerated hypoxia, especially at the villus tip.1) Indeed, the intestinal epithelial cells can become functionally damaged as a result of bowel ischemia in HF.
Epithelial dysfunction impairs absorption of sugars, protein, and fat, which may have an adverse effect on nutritional status and contribute to cachexia, further complicating cases of advanced HF.7),8) In addition, impaired barrier function of gut epithelial cells also allows the translocation of bacteria residing in the gut along with their products into the circulatory system. In particular, lipopolysaccharide and endotoxin produced by gram-negative bacteria can, upon entering the systemic circulation, trigger inflammatory responses that further exacerbate HF.1),2),3),4) Consistently, circulating levels of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) have been reported to increase in patients with acute decompensated HF and remain unchanged even after clinical improvement.9),10) The increase in circulating levels of these cytokines is associated with an increased risk of mortality in patients with HF.11),12) However, TNF-α antagonists offer no benefit on the risk of death or hospitalization in patients with HF according to 2 large randomized placebo-controlled trials.13),14) On the other hand, plasma endotoxin levels are elevated during acute decompensation in patients with HF, but normalize after treatment of HF.15),16) Endotoxin levels are also significantly higher in hepatic venous blood than in the left ventricle during acute decompensation, suggesting that endotoxin can indeed enter systemic circulation from the gut.16)
GUT MICROBIOTA IN HF
Humans and gut microbiota have co-evolved for millions of years, developing a close and interactive relationship, and gut microbiota play a critical role in maintaining host homeostasis. There are more than 1014 microbial cells in the human gut, more than the number of human cells in the body. Until recently, our view of the microbial world has depended largely on culture-based approaches; however, recent advances in culture-independent approaches have provided a comprehensive view of the human gut microbiota. Next-generation metagenomic sequencing of 1,267 fecal samples was used to construct an integrated catalog of human gut microbiome, which comprises a collection of approximately 10 million microbial genes, at least 100-fold greater than the number of human genes.17) A large quantity and wide variety of these gene products complement host physiological processes. For example, gut microbiota facilitate maturation of the host immune system, metabolize dietary components to provide the host with energy sources, and biosynthesize multiple compounds that influence host cell function.18),19) Metagenomic analysis technology has also revealed that an altered composition of gut microbiota, known as dysbiosis, is associated with a wide spectrum of host illnesses ranging from gastrointestinal diseases to inflammatory, metabolic, hepatic, neurologic, and cardiovascular diseases (CVDs).20) Experimental studies with fecal microbiota transplantation from human donors to germ-free animals have suggested that dysbiotic gut microbiota may reproduce disease phenotypes in recipients.21)
Only a few studies have addressed the issue of gut microbiota in HF. The colonic microbiota profile was shown to be altered early after intestinal ischemia-reperfusion in rats as assessed by denaturing gradient gel electrophoresis.22) The composition of gut microbiota is also altered in guinea pigs with HF induced by aortic constriction, as determined by 16S ribosomal RNA gene sequencing.23) In patients with HF, higher levels of adherent bacteria are detected in the sigmoid colon mucus as evaluated by fluorescence in situ hybridization.7) Likewise, larger quantities of pathogenic bacteria are present in the feces of patients with HF, including Campylobacter, Shigella, and Salmonella, as assessed by microbial culture methods.24) In addition, we recently showed that HF is associated with gut microbial dysbiosis through 16S ribosomal RNA gene sequencing of fecal samples from HF patients.5) Furthermore, gut microbiota profiles of HF patients vary substantially between individuals, especially according to age.
GUT MICROBE-DERIVED METABOLITES IN HF
The blood metabolite profiles of germ-free and conventionally-raised mice differ significantly,25) and it is well recognized that gut microbiota have a substantial effect on the composition of blood metabolites in the host. Gut microbe-derived metabolites have also been shown to contribute to disease processes.26) For example, indoxyl sulfate and p-cresyl sulfate, known as uremic toxins, are derived from gut microbiotic fermentation products of dietary tryptophan and tyrosine, respectively. Indoxyl sulfate has prohypertrophic and profibrotic effects on the heart and kidney, and administration of indoxyl sulfate induces cardiac hypertrophy and renal injury in rodents.27),28)
Recent studies have revealed a role of trimethylamine N-oxide (TMAO) as a promising biomarker for predicting the risk of CVD.29),30) Gut microbiota, but not mammals, have trimethylamine (TMA) lyase enzymes, which can produce TMA from dietary phosphatidylcholine, choline, and carnitine. TMA is subsequently transported via portal circulation to the liver, where it is oxidized via hepatic flavin monooxygenases to form TMAO.31) According to a large cohort study, elevated plasma TMAO levels are associated with an increased risk of death, myocardial infarction, and stroke in patients undergoing elective coronary angiography.30) Moreover, recent studies have demonstrated that patients with HF have significantly higher plasma levels of TMAO than healthy control subjects, and elevated plasma TMAO levels are predictive of higher mortality risk in patients with HF.32),33),34) Increased TMAO levels are also associated with more advanced stages of left ventricular diastolic dysfunction, but not systolic dysfunction.33) In patients admitted to the hospital with acute decompensated HF, elevated plasma TMAO levels correlate with decreased renal function and can predict an increased risk of death or rehospitalization due to HF.35) Furthermore, increased dietary intake of choline or TMAO aggravates adverse cardiac remodeling following transverse aortic constriction in mice.36) Taken together, these observations suggest potential contributions of gut microbe-derived metabolites in the development of HF.
THERAPEUTIC PERSPECTIVES FOR HF
Fecal microbiota transplantation from healthy donors has proven to be quite effective for severe cases of Clostridium difficile infection.37) This finding has opened a new field of gut microbiota-targeting therapeutic strategies aimed against a broad range of diseases. However, fecal microbiota transplantation carries the potential risk of transferring subclinical disease phenotypes from apparently healthy donors. Therefore, other strategies based on gut microbiota are under investigation, including the administration of single species, cocktails of defined microbial species, or agents that target specific microbiota-derived molecules. For example, 3,3-dimethyl-1-butanol, a structural analog of choline, inhibits microbial TMA lyase activity, thus lowering plasma TMAO levels and preventing atherosclerotic lesion development in Apoe-deficient mice fed a high choline diet.38)
Therapeutic management of HF through targeting gut microbiota is under investigation. Germ-free mice have attenuated blood pressure elevation and cardiac fibrosis induced by angiotensin II as compared to conventionally-raised mice.39) Oral administration of probiotics or vancomycin to rats reduces myocardial infarct size in ischemia-reperfusion injuries and attenuates cardiac remodeling following myocardial infarction.40),41) Further, a high intake of dietary fiber modifies the composition of gut microbiota and ameliorates cardiac hypertrophy as well as hypertension in mineralocorticoid-excess mice. Oral supplementation of acetate, one of the short-chain fatty acids produced by gut microbiota fermentation from dietary fiber, has similar protective effects.42) In addition, oral treatment with charcoal adsorbents that lower circulating levels of indoxyl sulfate decreases cardiac fibrosis in addition to improving markers of renal injury in subtotal-nephrectomized rats.43) There have also been a few reports describing clinical improvement by gut microbiota-mediated therapy in patients with HF. A pilot study of 3-month probiotic therapy with Saccharomyces boulardii for 7 chronic HF patients demonstrated a significant improvement in left ventricular ejection fraction from 39.0% to 45.6% and a significant decrease in left atrial diameter from 4.49 to 4.20 cm.44) However, further investigation is required to determine whether gut microbiota-targeting therapeutic strategies will be clinically feasible for patients with HF.
CONCLUSION
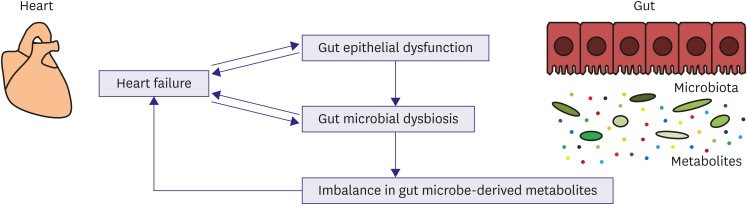
Emerging evidence supports a novel link between HF, gut epithelial dysfunction, and gut microbiota dysbiosis. Disrupted gut barrier function and altered gut microbiota composition may result in aberrant production and absorption of microbe-derived metabolites in HF patients. This imbalance in gut microbe-derived metabolites together with gut epithelial dysfunction may contribute to cardiac dysfunction, inflammation, malnutrition, and other morbidities in patients with HF (Figure 1). A better understanding of the heart-gut axis will promote the development of innovative diagnostic and therapeutic approaches for HF. Importantly, gut microbiota profiles can be determined noninvasively by the collection of fecal samples and may serve as a potential promising biomarker. Furthermore, in the era of precision medicine, personalized characterization of gut microbiome in HF patients may be useful for individualized risk stratification and treatment decisions.45)
Figure 1.
Novel concept of a heart-gut axis. In patients with HF, microcirculatory disturbances result in gut epithelial dysfunction. HF is also associated with gut microbiota dysbiosis and possibly aberrant production of gut microbe-derived metabolites. The imbalance in microbe-derived metabolites together with gut epithelial dysfunction could contribute to cardiac dysfunction, inflammation, malnutrition, and other morbidities in HF patients.
HF = heart failure.
Footnotes
Funding: This work was supported in part by grants from the Japan Society for the Promotion of Science (KAKENHI 26670395) to H.A. H.A. has received research funding from Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Shionogi & Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Bristol-Myers Squibb K.K., MSD K.K., Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, and Sumitomo Dainippon Pharma Co., Ltd.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62:485–495. doi: 10.1016/j.jacc.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 2.Rogler G, Rosano G. The heart and the gut. Eur Heart J. 2014;35:426–430. doi: 10.1093/eurheartj/eht271. [DOI] [PubMed] [Google Scholar]
- 3.Nagatomo Y, Tang WH. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail. 2015;21:973–980. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundaram V, Fang JC. Gastrointestinal and liver issues in heart failure. Circulation. 2016;133:1696–1703. doi: 10.1161/CIRCULATIONAHA.115.020894. [DOI] [PubMed] [Google Scholar]
- 5.Kamo T, Akazawa H, Suda W, et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One. 2017;12:e0174099. doi: 10.1371/journal.pone.0174099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandek A, Swidsinski A, Schroedl W, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64:1092–1102. doi: 10.1016/j.jacc.2014.06.1179. [DOI] [PubMed] [Google Scholar]
- 7.Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Arutyunov GP, Kostyukevich OI, Serov RA, Rylova NV, Bylova NA. Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. Int J Cardiol. 2008;125:240–245. doi: 10.1016/j.ijcard.2007.11.103. [DOI] [PubMed] [Google Scholar]
- 9.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 10.Milani RV, Mehra MR, Endres S, et al. The clinical relevance of circulating tumor necrosis factor-α in acute decompensated chronic heart failure without cachexia. Chest. 1996;110:992–995. doi: 10.1378/chest.110.4.992. [DOI] [PubMed] [Google Scholar]
- 11.Rauchhaus M, Doehner W, Francis DP, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 12.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 13.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti-TNF Therapy Against Congestive Heart Failure Investigators Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 14.Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 15.Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 16.Peschel T, Schönauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5:609–614. doi: 10.1016/s1388-9842(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Jia H, Cai X, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischbach MA, Segre JA. Signaling in host-associated microbial communities. Cell. 2016;164:1288–1300. doi: 10.1016/j.cell.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 21.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Li Q, Wang C, Tang C, Li J. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PLoS One. 2012;7:e42027. doi: 10.1371/journal.pone.0042027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips Campbell RB, Duffourc MM, Schoborg RV, et al. Aberrant fecal flora observed in guinea pigs with pressure overload is mitigated in animals receiving vagus nerve stimulation therapy. Am J Physiol Gastrointest Liver Physiol. 2016;311:G754–G762. doi: 10.1152/ajpgi.00218.2016. [DOI] [PubMed] [Google Scholar]
- 24.Pasini E, Aquilani R, Testa C, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4:220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 27.Lekawanvijit S. Role of gut-derived protein-bound uremic toxins in cardiorenal syndrome and potential treatment modalities. Circ J. 2015;79:2088–2097. doi: 10.1253/circj.CJ-15-0749. [DOI] [PubMed] [Google Scholar]
- 28.Yang K, Wang C, Nie L, et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol. 2015;26:2434–2446. doi: 10.1681/ASN.2014060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–96. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trøseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016;102:841–848. doi: 10.1136/heartjnl-2015-308826. [DOI] [PubMed] [Google Scholar]
- 36.Organ CL, Otsuka H, Bhushan S, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9:e002314. doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile . N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karbach SH, Schönfelder T, Brandão I, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam V, Su J, Koprowski S, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gan XT, Ettinger G, Huang CX, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 42.Marques FZ, Nelson EM, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 43.Lekawanvijit S, Kompa AR, Manabe M, et al. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS One. 2012;7:e41281. doi: 10.1371/journal.pone.0041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015;179:348–350. doi: 10.1016/j.ijcard.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 45.Zmora N, Zeevi D, Korem T, Segal E, Elinav E. Taking it personally: personalized utilization of the human microbiome in health and disease. Cell Host Microbe. 2016;19:12–20. doi: 10.1016/j.chom.2015.12.016. [DOI] [PubMed] [Google Scholar]