Abstract
Background and Objectives
The effectiveness of adjunct balloon dilation after drug-eluting stent (DES) deployment has not been sufficiently evaluated. We evaluated whether adjunct balloon dilation was associated with a reduction in major adverse cardiac events (MACEs) after long everolimus-eluting stents (EESs) implantation.
Subjects and Methods
Drawing from 2 randomized trials, a total of 1,672 patients treated with long EES were analyzed. Of 1,672 patients, 1,061 patients (64%) received post-stent adjunct balloon dilation. MACE, defined as a composite of cardiac death, myocardial infarction, and target-lesion revascularization (TLR), was compared between patients who received post-stent adjunct balloon dilation and patients who did not in 595 propensity score-matched pairs.
Results
For the matched population, MACE occurred in 29 patients (4.9%) who received adjunct balloon dilation and in 29 patients (4.9%) who did not (hazard ratio [HR], 1.01; 95% confidence interval [CI], 0.60–1.69; p=0.972). However, significant interactions were observed among the subgroups for clinical presentation and vessel size. Adjunct balloon dilation was more favored within the subset of patients with stable angina vs. the subset of patients with acute coronary syndrome (p for interaction=0.037), and within the subset of lesions with small vessel diameter (reference vessel diameter [RVD] <3 mm) vs. the subset of lesions with larger vessel diameter (RVD ≥3 mm; p for interaction=0.027).
Conclusion
Adjunct balloon dilation was not associated with MACE reduction at 1 year among patients requiring long EES implantation. However, post-stent adjunct balloon dilation may be necessary for patients requiring long EES implantation who present with stable angina or for lesions with small vessel diameters.
Keywords: Coronary artery disease, Drug-eluting stents, Treatment outcome
INTRODUCTION
Post-stent adjunct balloon dilation is often used to achieve optimal stent expansion and complete apposition of stent struts against the vessel wall.1),2),3) Conversely, aggressive stent inflation with higher pressure may also be associated with increased neointimal hyperplasia due to the inflammatory response to vessel wall injury, and can lead to increased occurrence of peri-procedural myocardial infarction by thrombus or plaque debris embolization.4),5),6) In this context, post-stent adjunct balloon dilation is inconsistently practiced and is highly dependent on the individual operators' decisions. Adjunct balloon dilation has not been routinely performed in most randomized trials on the use of drug-eluting stents (DESs). Additionally, the clinical efficacy of post-stent adjunct balloon dilation has not been well evaluated in patients treated with second-generation DES, although 2 recent studies evaluated its efficacy for patients treated with bare-metal stents and first-generation DESs.7),8)
This study evaluated whether adjunct balloon dilation was associated with a reduction in major adverse cardiac events (MACEs) after long second-generation DES implantation, particularly everolimus-eluting stent (EES), using patient data from 2 randomized trials; Impact of intraVascular UltraSound guidance on outcomes of Xience Prime stents in Long lesions (IVUS-XPL) and REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation (RESET).9),10)
SUBJECTS AND METHODS
Study population
We identified patients treated with long EES (implanted stent ≥28 mm in length, Xience; Abbott Vascular, Santa Clara, CA, USA) from 2 recently conducted randomized trials. In the IVUS-XPL trial, which examined the superiority of intravascular ultrasound (IVUS)-guidance DES implantation for long coronary lesions, a total of 1,400 patients were randomized to receive either IVUS- or angiography-guided EES implantation.9) The RESET trial was a noninferiority trial comparing patients who had received 3 months of dual antiplatelet therapy following implantation of the Endeavor sprint zotarolimus-eluting stent (Medtronic, Inc., Santa Rosa, CA, USA) and 12 months of dual antiplatelet therapy following implantation of another DES.10) In the prespecified long lesion subset of this study,10),11) 543 patients were randomly allocated to receive either IVUS- or angiography-guided DES implantation. In this subset, the Endeavor sprint zotarolimus-eluting stent (n=271) or the EES (n=272) were also randomized. Finally, a total of 1,672 patients were included in this study, with 1,400 patients from the IVUS-XPL trial and 272 patients from the pre-specified long lesion subset of the RESET trial with EES implantation. Detailed protocols of these trials have been previously described.9),10),11) The study protocol was approved by the Institutional Review Boards at each participating center, and all participants gave written informed consent.
Stent implantation
EES implantation was performed according to standard techniques. If a lesion could not be covered with a single stent, overlapping stents were used. For patients allocated to the angiography-guided group, stent size, and length were chosen by visual estimation. Adjunct high-pressure dilation was performed if an optimal result was not achieved, which was defined as angiographic residual diameter stenosis <30% and absence of angiographically detected dissection. For patients allocated to the IVUS-guided group, stent size, and length were selected by online IVUS measurements, and adjunct high-pressure dilation was performed according to the discretion of operators based on IVUS findings. In the IVUS-XPL trial, the following IVUS criterion for stent optimization was used: a minimal lumen cross-sectional area greater than the lumen cross sectional area at the distal reference segments.9) After EES implantation, aspirin (at a dose of 100 mg/day) was prescribed indefinitely. The administration of clopidogrel (at a dose of 75 mg/day) depended on the randomized assignments in the previous trials.9),10),11)
Angiographic analyses
Angiographic measurements were performed by analysts who were blinded to patient and treatment assignments in an independent core laboratory at the Cardiovascular Research Center, Seoul, Korea. Before and after EES implantation, an off-line quantitative coronary angiographic system (CASS system; Pie Medical Instruments, Maastricht, The Netherlands) was used to perform quantitative coronary angiography analyses. Using a guiding catheter for magnification-calibration, the diameters of the reference vessel (the average of the proximal and distal reference lumen diameters) and the minimal luminal diameter were measured before and after EES implantation. These measurements were made from diastolic frames in a single matched view, revealing the smallest minimal luminal diameter. Pre-intervention lesion length was measured as the distance in millimeters from the proximal to the distal shoulder of the lesion when projected with the least foreshortening.12)
Follow-up and study endpoints
After stent implantation, clinical assessment was performed in the hospital and at 1, 3, 6, and 12 months after discharge. Follow-up assessments were performed during a clinic visit or by telephone interview. MACE was defined as cardiac death, target lesion-related myocardial infarction, or ischemia-driven target-lesion revascularization (TLR).
Clinical events were defined according to the Academic Research Consortium and an expert consensus document that defined the third universal definition for myocardial infarction.13),14) All deaths were considered cardiac-related unless an unequivocal non-cardiac cause could be established.13) At 1 year follow-up, a target lesion-related myocardial infarction was defined by the following parameters: the presence of clinical symptoms, electrocardiographic changes, or abnormal imaging findings indicative of myocardial infarction, and an increase in the creatine kinase myocardial band fraction above the upper normal limits or an increase in troponin-T/troponin-I above the 99th percentile of the upper normal limit. The territory of the myocardial infarction was supplied by the coronary artery containing the target lesions.9),14) Ischemia-driven TLR was defined as repeat percutaneous coronary intervention to or bypass surgery of the target lesion with; either 1) angiographic diameter stenosis ≥50% according to quantitative coronary angiographic analysis with documentation of a positive stress test, or 2) angiographic diameter stenosis ≥70% irrespective of the stress test results.13)
Statistical analyses
Categorical variables were reported as numbers and percentages and were compared using the χ2 test or Fisher's exact test. Continuous variables were reported as mean±standard deviation (SD) and were compared using the Student's t-test. Propensity score matching was performed to reduce treatment selection bias and potential confounding factors and to adjust for significant differences in patient or lesion characteristics. Propensity scores were estimated using a non-parsimonious multiple logistic regression model for treatment with adjunct balloon dilation vs. without adjunct balloon dilation. The following variables were selected to calculate the propensity score: age, gender, body mass index (BMI), hypertension, diabetes mellitus (DM), dyslipidemia, current smoker, clinical presentation (stable angina, unstable angina, and acute myocardial infarction), number of diseased vessels, number of treated lesions per patient, coronary arteries (left anterior descending artery, left circumflex artery, and right coronary artery), pre-intervention reference vessel diameter (RVD), pre-intervention lesion length, use of IVUS, and number of stents per lesion. New propensity scores were incorporated to assess the efficacy of adjunct balloon dilation. A local optimal algorithm with the caliper method was used to develop propensity score-matched pairs without replacement (a 1:1 match). The Hosmer-Lemeshow goodness-of-fit test was used to assess the model fit to the data (p=0.534). The C statistic for the model was 0.66. A matching caliper of 0.2 SDs of the logit of the estimated propensity score was enforced in order to ensure that matches of poor fit were excluded. The matching procedure was performed using R packages (R Project, Vienna, Austria), including MatchIt, RItools, and CEM. After propensity score matching, covariates were compared with the paired t-test for continuous variables and the McNemar test for categorical variables.
Cumulative incidences of MACE at 1 year were calculated using Kaplan-Meier estimates and compared using the log-rank test. Although patients could experience more than 1 MACE component, each patient was assessed until the occurrence of their first event and only once during analysis. Subgroup analyses were performed according to the clinical and angiographic subgroups. Statistical analyses were performed using IBM SPSS, version 19.0 (IBM Corporation, New York, NY, USA). All tests were 2-sided and a p value <0.050 was considered statistically significant.
RESULTS
Of 1,672 patients, 1,061 (63.5%) received post-stent adjunct balloon dilation using a non-compliant balloon with an average diameter of 3.09±0.43 mm up to 16.3±4.1 atm. There were 595 matched pairs after propensity score matching. Baseline clinical characteristics are shown in Table 1. Clinical characteristics were not statistically different in patients who received adjunct balloon dilation vs. those who did not for the matched population. Angiographic and procedural characteristics according to the practice of post-stent adjunct ballooning of the target long lesions are shown in Table 2. Adjunct balloon dilation was more frequently undertaken for long target lesions of the left anterior descending artery and for use with IVUS for the total population. However, for the matched population, angiographic and procedural characteristics were not different between the 2 groups except for use with IVUS.
Table 1. Baseline clinical characteristics.
| Characteristics | Total population | Matched population | |||||
|---|---|---|---|---|---|---|---|
| ABD (+) | ABD (−) | p value | ABD (+) | ABD (−) | p value | ||
| No. of patients | 1,061 | 611 | - | 595 | 595 | - | |
| Age (years) | 64±10 | 64±9 | 0.589 | 63±10 | 64±9 | 0.250 | |
| Male sex | 721 (68) | 406 (66) | 0.527 | 406 (68) | 398 (67) | 0.667 | |
| BMI (kg/m2) | 24.7±3.0 | 24.7±2.9 | 0.919 | 24.8±3.0 | 24.7±3.0 | 0.671 | |
| Hypertension | 692 (65) | 381 (62) | 0.239 | 380 (64) | 369 (62) | 0.530 | |
| DM | 376 (35) | 213 (35) | 0.812 | 205 (35) | 207 (35) | 0.951 | |
| Dyslipidemia | 713 (67) | 389 (64) | 0.142 | 372 (63) | 379 (64) | 0.722 | |
| Current smoker | 244 (23) | 142 (23) | 0.909 | 140 (24) | 139 (23) | 1.000 | |
| Clinical presentation | - | - | 0.371 | - | - | 0.533 | |
| Stable angina | 559 (53) | 302 (49) | - | 313 (53) | 293 (49) | - | |
| Unstable angina | 354 (33) | 212 (35) | - | 200 (34) | 209 (35) | - | |
| Acute myocardial infarction | 148 (14) | 97 (16) | - | 82 (14) | 93 (16) | - | |
| No. of diseased vessels | - | - | 0.291 | - | - | 0.564 | |
| 1 | 331 (31) | 206 (34) | - | 199 (33) | 203 (34) | - | |
| 2 | 392 (37) | 232 (38) | - | 215 (36) | 224 (38) | - | |
| 3 | 338 (32) | 173 (28) | - | 181 (30) | 168 (28) | - | |
| No. of treated lesions per patient | 1.36±0.57 | 1.42±0.65 | 0.052 | 1.40±0.61 | 1.41±0.64 | 0.734 | |
Data are expressed as No. of patients (%) or mean±SD.
ABD = adjunct balloon dilation; BMI = body mass index; DM = diabetes mellitus; SD = standard deviation.
Table 2. Angiographic and procedural characteristics according to the presence of post-stent adjunct balloon dilation of target long lesions.
| Characteristics | Total population | Matched population | |||||
|---|---|---|---|---|---|---|---|
| ABD (+) | ABD (−) | p value | ABD (+) | ABD (−) | p value | ||
| No. of target long lesions | 1,061 | 611 | - | 595 | 595 | - | |
| Coronary arteries | - | - | 0.075 | - | - | 0.629 | |
| Left anterior descending artery | 684 (65) | 366 (60) | - | 363 (61) | 358 (60) | - | |
| Left circumflex artery | 155 (15) | 88 (14) | - | 91 (15) | 84 (14) | - | |
| Right coronary artery | 222 (21) | 157 (26) | - | 141 (24) | 153 (26) | - | |
| Pre-intervention QCA data | - | - | - | - | - | - | |
| RVD (mm) | 2.89±0.44 | 2.86±0.46 | 0.196 | 2.87±0.43 | 2.86±0.46 | 0.767 | |
| Minimum lumen diameter (mm) | 0.85±0.42 | 0.82±0.44 | 0.199 | 0.86±0.43 | 0.82±0.45 | 0.124 | |
| Diameter stenosis (%) | 70.4±14.0 | 71.2±14.7 | 0.234 | 70.0±14.4 | 71.2±14.8 | 0.092 | |
| Lesion length (mm) | 35.1±11.2 | 34.2±10.9 | 0.138 | 34.4±10.2 | 34.3±10.9 | 0.762 | |
| Use of IVUS (%) | 613 (58) | 234 (40) | <0.001 | 272 (46) | 242 (41) | 0.019 | |
| No. of stents per lesion | 1.28±0.51 | 1.38±0.56 | <0.001 | 1.32±0.53 | 1.36±0.54 | 0.155 | |
| Coronary perforation | 0 | 0 | - | 0 | 0 | - | |
| Edge dissection | 21 (2) | 11 (2) | 0.797 | 11 (2) | 11 (2) | 0.999 | |
| No reflow phenomenon | 2 (0.2) | 4 (0.7) | 0.125 | 1 (0.2) | 4 (0.7) | 0.250 | |
| Post-intervention QCA data | - | - | - | - | - | - | |
| RVD (mm) | 3.00±0.43 | 2.97±0.45 | 0.064 | 2.99±0.41 | 2.97±0.44 | 0.325 | |
| Minimum lumen diameter (mm) | 2.60±0.41 | 2.55±0.39 | 0.007 | 2.58±0.40 | 2.56±0.39 | 0.196 | |
| Diameter stenosis (%) | 13.2±8.2 | 13.8±8.5 | 0.187 | 13.4±7.8 | 13.7±8.4 | 0.759 | |
Data are expressed as No. of patients (%) or mean±SD.
ABD = adjunct balloon dilation; IVUS = intravascular ultrasound; RVD = reference vessel diameter; SD = standard deviation; QCA = quantitative coronary angiographic.
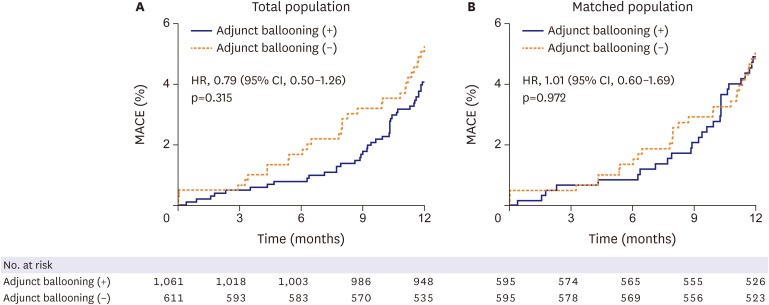
Clinical outcomes at 1 year are shown in Table 3. For the total population, MACE occurred in 42 patients (4.1%) who received adjunct balloon dilation and in 31 patients (5.1%) who did not, and the difference was not statistically significant (hazard ratio [HR], 0.79; 95% confidence interval [CI], 0.50–1.26; p=0.315) (Figure 1A). Similarly, for the matched population, MACE occurred in 29 patients (4.9%) who received adjunct balloon dilation and in 29 patients (4.9%) who did not, and the difference was also not statistically significant (HR, 1.01; 95% CI, 0.60–1.69; p=0.972) (Figure 1B). There were no significant differences between the 2 groups for any of the measured clinical events including cardiac death, target-lesion related myocardial infarction, ischemia-driven TLR, and definite or probable stent thrombosis for total population and matched population (Table 3).
Table 3. Clinical outcomes at 1 year post-procedure.
| Clinical outcomes | Total population | Matched population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients, No (%)* | HR (95% CI) | p value | Patients, No (%)* | HR (95% CI) | p value | ||||
| ABD (+) | ABD (−) | ABD (+) | ABD (−) | ||||||
| No. | 1,061 | 611 | - | - | 595 | 595 | - | - | |
| MACE† | 42 (4.1) | 31 (5.1) | 0.79 (0.50–1.26) | 0.315 | 29 (4.9) | 29 (4.9) | 1.01 (0.60–1.69) | 0.972 | |
| Cardiac death | 4 (0.4) | 5 (0.8) | 0.47 (0.13–1.74) | 0.246 | 3 (0.5) | 4 (0.7) | 0.76 (0.17–3.39) | 0.718 | |
| Target lesion related MI | 1 (0.1) | 1 (0.2) | 0.59 (0.04–9.36) | 0.701 | 1 (0.2) | 1 (0.2) | 1.01 (0.06–16.16) | 0.994 | |
| Ischemia-driven TLR | 39 (3.8) | 25 (3.9) | 0.91 (0.55–1.50) | 0.700 | 26 (4.4) | 24 (4.0) | 1.10 (0.63–1.91) | 0.749 | |
| Definite or probable stent thrombosis | 2 (0.2) | 3 (0.5) | 0.39 (0.07–2.31) | 0.279 | 1 (0.2) | 3 (0.5) | 0.33 (0.04–3.21) | 0.319 | |
| Acute | 0 | 2 | - | - | 0 | 2 | - | - | |
| Sub-acute | 1 | 0 | - | - | 0 | 0 | - | - | |
| Late | 1 | 1 | - | - | 1 | 1 | - | - | |
ABD = adjunct balloon dilation; CI = confidence interval; HR = hazard ratio; MACE = major adverse cardiac event; MI = myocardial infarction; TLR = target-lesion revascularization.
*Event rates are cumulative 1-year Kaplan-Meier event rates. HRs are derived from the Cox proportional hazard regression models, †MACE from cardiac death, target lesion-related myocardial infarction, or ischemia-driven TLR at 1-year.
Figure 1.
Kaplan-Meier estimates of occurrence of MACEs for total population (A) and matched population (B). Cumulative incidence curves for MACEs of cardiac death, target lesion-related myocardial infarction, and TLR.
CI = confidence interval; HR = hazard ratio; MACE = major adverse cardiac event; TLR = target-lesion revascularization.
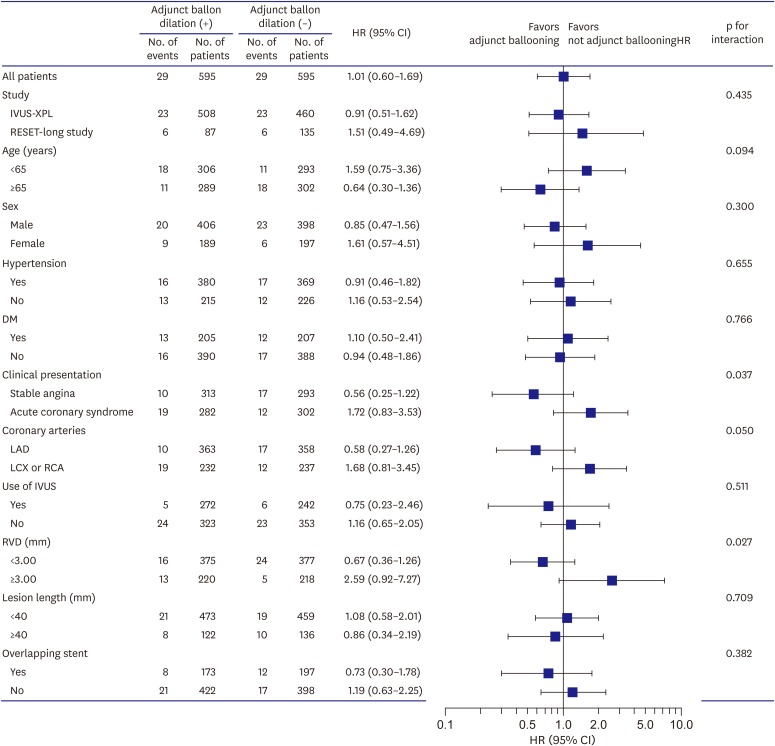
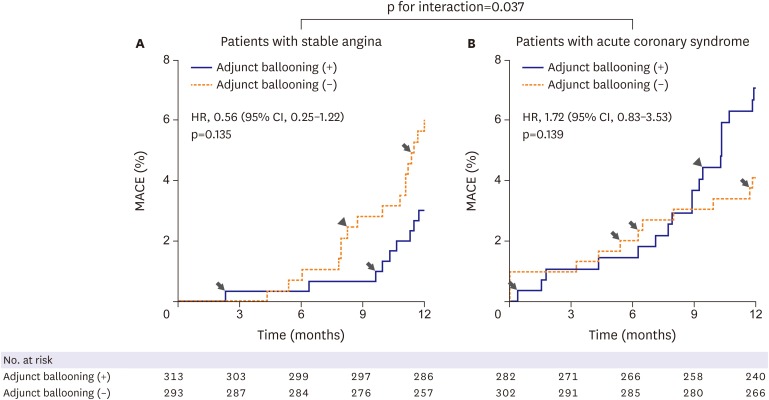
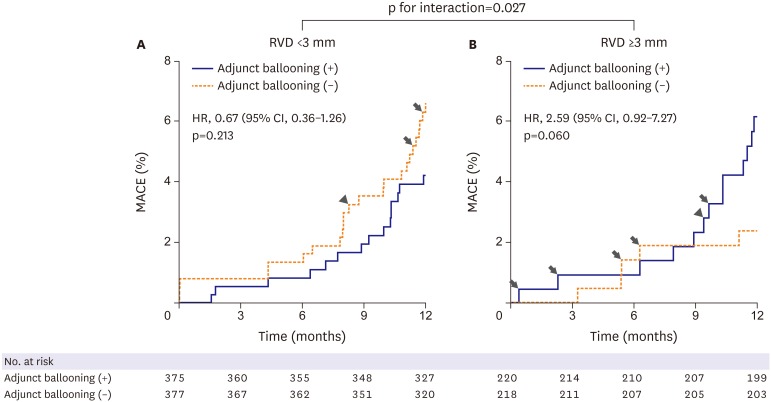
Subgroup analyses for the matched population are shown in Figure 2. There were no statistically significant interactions among the subgroups for clinical variables of age, sex, hypertension, DM, use of IVUS, and lesion length. However, significant interactions were observed among the subgroups for clinical presentation and reference vessel size. Adjunct balloon dilation was more favored within the subset of patients with stable angina compared with those with acute coronary syndrome (p for interaction=0.037) (Figure 3). Adjunct balloon dilation was also more favored within the subset of lesions with small vessel diameter (RVD <3 mm) compared with those with larger vessel diameter (RVD ≥3 mm; p for interaction=0.027) (Figure 4).
Figure 2.
Subgroup analyses of the rates of MACEs at 1-year post-procedure.
CI = confidence interval; DM = diabetes mellitus; HR = hazard ratio; IVUS = intravascular ultrasound; IVUS-XPL = Impact of intraVascular UltraSound guidance on outcomes of Xience Prime stents in Long lesions; LAD = left anterior descending; LCX = left circumflex; MACE = major adverse cardiac event; RCA = right coronary artery; RESET = REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation; RVD = reference vessel diameter.
Figure 3.
MACEs at 1-year post-procedure according to clinical presentation. Arrow indicates cardiac death and arrow head indicates myocardial infarction, and other events are TLR.
CI = confidence interval; HR = hazard ratio; MACE = major adverse cardiac event; TLR = target-lesion revascularization.
Figure 4.
MACEs at 1-year post-procedure according to vessel size. Arrow indicates cardiac death and arrow head indicates myocardial infarction, and other events are TLR.
CI = confidence interval; HR = hazard ratio; MACE = major adverse cardiac event; RVD = reference vessel diameter; TLR = target-lesion revascularization.
DISCUSSION
Adjunct balloon dilation was not associated with MACE reduction at 1 year, even among patients requiring long EES implantation. However, significant interactions were observed among the subgroups for clinical presentation and vessel size. Adjunct balloon dilation after long EES implantation was more favored within the subset of patients with stable angina compared with those with acute coronary syndrome, and within the subset of lesions with small vessel compared with those with larger vessel.
Compared with angiography-guided EES implantation, IVUS-guided EES implantation resulted in a significantly lower rate of composite MACE at 1 year (5.8% vs. 2.9%, respectively; HR, 0.48; p=0.009) in the IVUS-XPL trial.9) This finding raised a question regarding the effectiveness of routine post-stent balloon dilation. However, it is unclear whether routine post-stent balloon dilation would have achieved better clinical outcomes.15) Additionally, the clinical efficacy of this strategy has not been well evaluated in patients with second-generation DESs, although recent studies evaluated this strategy mostly among patients with bare-metal stents and first-generation DESs.7),8) Frobert et al.8) reported that post-stent balloon dilation was not associated with a significantly lower risk of stent thrombosis, but it was associated with a higher rate of restenosis in an analysis of 93,697 stents (55,426 of bare-metal stents, 59%). Pasceri et al.7) compared clinical outcomes for DES-treated patients based on whether they received routine post-stent balloon dilatation (n=279) or selective post-stent balloon dilation after suboptimal results (n=262). The MACE incidence at 12 months was 19.5% in the selective post-stent balloon dilation group and 12.5% in the routine post-stent balloon dilation group (p=0.040). This was mainly driven by lower TLR rates. However, in this study, first-generation DESs were used up to 55% of the time. There is no consistency in the practice of post-stent adjunct balloon dilation and the procedure varies according to the operators' discretion. Furthermore, adjunct balloon dilation was not routinely performed or discussed in prior studies on the use of DESs. From the Swedish coronary angiography and angioplasty registry, 63,740 of 93,697 stents (68%) were post-dilated following stent implantation.8) In the DEScover Registry involving 6,509 patients treated with a first-generation DES in routine practice in the United States, post-dilatation was performed for 46% of patients.16)
Our finding that post-stent adjunct balloon dilation was not associated with a reduction of MACEs at 1 year—even among patients with long lesions—could be attributed to the use of second-generation DESs in our trials, particularly EESs, which offer improved stent performance with different vascular healing and re-endothelialization properties.17),18) An optical coherence tomographic study reported that EES offered more favorable strut coverage than the first-generation sirolimus-eluting stent.18) A meta-analysis reported that the lowest rate of stent thrombosis was observed in the EES implantation among different types of DESs.19)
However, we also identified significant interactions in patients with stable angina (vs. acute coronary syndrome) and in patients with small vessel disease (vs. large vessel disease). Consistent with our findings, previous studies have shown that post-stent balloon dilation, was associated with an increased risk of death and myocardial infarction in patients with acute myocardial infarction but not in patients with stable angina.20) Patients presenting with acute coronary syndrome had plaques that were more unstable and had a higher volume of necrotic core according to virtual histology-IVUS compared with those presenting with stable angina.21) Post-intervention elevation of cardiac troponin levels was more frequently observed in lesions with a large necrotic core by ultrasound,22) and in lipid-rich lesions detected by near-infrared spectroscopy.23) Distal embolization due to aggressive mechanical expansion from post-stent balloon dilation may be a possible underlying mechanism.
Small vessels are still at high risk of restenosis even after receiving new-generation DESs.24),25) Post-procedural minimal lumen diameter by angiography is a major factor in restenosis after DES implantation.26),27) However, it is expected that the post-procedural minimal lumen diamater will be subsequently smaller in lesions of small vessels compared to those in large vessels. It is generally more difficult to obtain optimal acute angiographic results with a sufficient minimal lumen diameter. Additionally, the relatively acute gain in post-stent adjunct balloon dilation will be greater in lesions of small vessels compared to those of large vessels. Thus, routine post-stent adjunct balloon dilation may be more beneficial for lesions of small vessels treated with DES implantation than for lesions of large vessels. Furthermore, all patients enrolled in this study had diffuse long lesions. Diffuse long lesions, compared with focal lesions, have a higher probability of underlying plaques and heavy calcification. Besides the presence of heavy calcium and greater amounts of plaques, diffuse lesions have a greater chance of leading to small vessel diameter. This corresponds to the distal margin of long DES implantation and location in the middle or distal segment of major epicardial arteries, as well as requiring 2 more overlapping stents. Therefore, these subsets of small vessels and long lesions with a high-risk of stent failure may require routine post-stent adjunct balloon dilation.
This study has several limitations. First, this study did not have a randomized comparison design. Second, our patient sample size and a 1 year clinical follow-up period may not be sufficient to assess clinical outcomes. Third, post-stent adjunct dilation performance after stent deployment was determined by corresponding physicians. However, all analyzed patients were drawn from 2 randomized trials, allowing us to minimize risk for any potential bias using an endpoint analysis with precisely defined criteria, using core laboratories, and blinding the event adjudication committee members to 1 another. Fourth, although we found some interaction in the subgroup analyses, our major findings need to be interpreted as negative results.
In conclusion, adjunct balloon dilation was not associated with MACE reduction at 1 year among patients requiring long coronary stent implantation. However, post-stent adjunct balloon dilation may be necessary for patients who present with stable angina or for lesions with small vessels requiring long EES implantation. Large randomized trials comparing the efficacy of routine post-stent adjunct balloon dilation are required.
Footnotes
Funding: This study was supported by a grant from the Korea Healthcare Technology Research and Development Project, Ministry of Health and Welfare, Korea (No. A085136 and HI15C1277), the Mid-career Researcher Program through National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST), Korea (No. 2015R1A2A2A01002731), and the Cardiovascular Research Center, Seoul, Korea.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Hong SJ, Hong MK.
- Data curation: Hong SJ, Ahn CM, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Her AY, Kim YH, Jang Y, Hong MK.
- Formal analysis: Hong SJ, Hong MK.
- Funding acquisition: Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK.
- Methodology: Hong SJ, Ahn CM, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Her AY, Kim YH, Jang Y, Hong MK.
- Supervision: Hong SJ, Ahn CM, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Her AY, Kim YH, Jang Y, Hong MK.
- Writing - original draft: Hong SJ, Hong MK.
- Writing - review & editing: Hong SJ, Ahn CM, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Her AY, Kim YH, Jang Y, Hong MK.
References
- 1.Brodie BR, Cooper C, Jones M, Fitzgerald P, Cummins F, Postdilatation Clinical Compartative Study (POSTIT) Investigators Is adjunctive balloon postdilatation necessary after coronary stent deployment? Final results from the POSTIT trial. Catheter Cardiovasc Interv. 2003;59:184–192. doi: 10.1002/ccd.10474. [DOI] [PubMed] [Google Scholar]
- 2.Russo RJ, Silva PD, Teirstein PS, et al. A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial) Circ Cardiovasc Interv. 2009;2:113–123. doi: 10.1161/CIRCINTERVENTIONS.108.778647. [DOI] [PubMed] [Google Scholar]
- 3.Hur SH, Kitamura K, Morino Y, et al. Efficacy of postdeployment balloon dilatation for current generation stents as assessed by intravascular ultrasound. Am J Cardiol. 2001;88:1114–1119. doi: 10.1016/s0002-9149(01)02044-6. [DOI] [PubMed] [Google Scholar]
- 4.Romagnoli E, Sangiorgi GM, Cosgrave J, Guillet E, Colombo A. Drug-eluting stenting: the case for post-dilation. JACC Cardiovasc Interv. 2008;1:22–31. doi: 10.1016/j.jcin.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann R, Guagliumi G, Musumeci G, et al. Vascular response to sirolimus-eluting stents delivered with a nonaggressive implantation technique: comparison of intravascular ultrasound results from the multicenter, randomized E-SIRIUS, and SIRIUS trials. Catheter Cardiovasc Interv. 2005;66:499–506. doi: 10.1002/ccd.20542. [DOI] [PubMed] [Google Scholar]
- 6.Iakovou I, Mintz GS, Dangas G, et al. Increased CK-MB release is a “trade-off” for optimal stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2003;42:1900–1905. doi: 10.1016/j.jacc.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Pasceri V, Pelliccia F, Pristipino C, et al. Clinical effects of routine postdilatation of drug-eluting stents. Catheter Cardiovasc Interv. 2014;83:898–904. doi: 10.1002/ccd.24999. [DOI] [PubMed] [Google Scholar]
- 8.Fröbert O, Sarno G, James SK, Saleh N, Lagerqvist B. Effect of stent inflation pressure and post-dilatation on the outcome of coronary artery intervention. A report of more than 90,000 stent implantations. PLoS One. 2013;8:e56348. doi: 10.1371/journal.pone.0056348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015;314:2155–2163. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 10.Kim BK, Hong MK, Shin DH, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation) J Am Coll Cardiol. 2012;60:1340–1348. doi: 10.1016/j.jacc.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Kang TS, Mintz GS, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6:369–376. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 15.Steg PG, Serruys PW, Abdelghani M, Wijns W. The year in cardiology 2015: coronary intervention. Eur Heart J. 2016;37:335–343. doi: 10.1093/eurheartj/ehv708. [DOI] [PubMed] [Google Scholar]
- 16.Srour JF, Abbott JD. Routine postdilation of drug-eluting stents: worth the gain. Catheter Cardiovasc Interv. 2014;83:905–906. doi: 10.1002/ccd.25481. [DOI] [PubMed] [Google Scholar]
- 17.Dangas GD, Serruys PW, Kereiakes DJ, et al. Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions) JACC Cardiovasc Interv. 2013;6:914–922. doi: 10.1016/j.jcin.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Kim JH, Shin DH, et al. Serial randomized comparison of strut coverage of everolimus- and first-generation sirolimus-eluting stents. Can J Cardiol. 2015;31:723–730. doi: 10.1016/j.cjca.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang ZJ, Marroquin OC, Stone RA, et al. Differential effects of post-dilation after stent deployment in patients presenting with and without acute myocardial infarction. Am Heart J. 2010;160:979–986.e1. doi: 10.1016/j.ahj.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong MK, Mintz GS, Lee CW, et al. Comparison of virtual histology to intravascular ultrasound of culprit coronary lesions in acute coronary syndrome and target coronary lesions in stable angina pectoris. Am J Cardiol. 2007;100:953–959. doi: 10.1016/j.amjcard.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Hong YJ, Mintz GS, Kim SW, et al. Impact of plaque composition on cardiac troponin elevation after percutaneous coronary intervention: an ultrasound analysis. JACC Cardiovasc Imaging. 2009;2:458–468. doi: 10.1016/j.jcmg.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Maehara A, Muller JE, et al. Plaque characterization to inform the prediction and prevention of periprocedural myocardial infarction during percutaneous coronary intervention: the CANARY Trial (Coronary Assessment by Near-infrared of Atherosclerotic Rupture-prone Yellow) JACC Cardiovasc Interv. 2015;8:927–936. doi: 10.1016/j.jcin.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv. 2012;5:626–635. doi: 10.1016/j.jcin.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Claessen BE, Smits PC, Kereiakes DJ, et al. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv. 2011;4:1209–1215. doi: 10.1016/j.jcin.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Kastrati A, Dibra A, Mehilli J, et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.601823. [DOI] [PubMed] [Google Scholar]
- 27.Hong SJ, Kim MH, Ahn TH, et al. Multiple predictors of coronary restenosis after drug-eluting stent implantation in patients with diabetes. Heart. 2006;92:1119–1124. doi: 10.1136/hrt.2005.075960. [DOI] [PMC free article] [PubMed] [Google Scholar]