Abstract
A risk assessment of basil-based pesto sauces containing methyleugenol and related alkenylbenzenes was performed based on their levels detected in a series of pesto sauces available on the Dutch market. The estimated daily intake (EDI) values of alkenylbenzenes as a result of consumption of the different pesto sauces amounted to 1.2–44.3 μg/kg bw for individual alkenylbenzenes, 14.3–43.5 μg/kg bw when adding up the alkenylbenzene levels assuming equal potency, and 17.3–62.9 μg/kg bw when expressed in methyleugenol equivalents using alkenylbenzenes defined toxic equivalency factors (TEF). The margin of exposure approach (MOE), used to evaluate the potential risks, resulted in MOE values that were generally lower than 10000 indicating a priority for risk management when assuming daily consumption. The levels of methyleugenol detected in the pesto sauces would allow consumption of 1.1–29.8, 7.5–208, 15.1–416.5, and 32.4–892.5 g of pesto sauce on a daily basis, once a week, once every two weeks, and once a month, respectively, to achieve MOE values above the 10000 limit indicating low priority for risk management. It is concluded that consumption of pesto sauces would only be of concern if consumed on a daily basis over longer periods of time.
Abbreviations: EDI, estimated daily intake; BMDL10, lower confidence limit of the benchmark dose resulting in a 10% extra cancer incidence; EFSA, European Food Safety Authority; ESCO, scientific cooperation projects between EFSA and its counterparts in the Member States; MOE, margin of exposure; UPLC, Ultra Performance Liquid Chromatography; TEF, toxic equivalency factor; TEQ, toxic equivalency
Keywords: Margin of exposure (MOE), Basil-based pesto, Combined risk assessment, Toxic equivalency factors (TEFs)
1. Introduction
The genus ocimum, collectively called basil, belongs to the Lamiaceae family (the mint family), a family that contains 236 genera [34] and 7534 species [19]. The plants are aromatic and cultivated for culinary purposes. Basil adds a distinctive flavor to many foods, and is consumed fresh, dried, as essential oil, or processed. In its processed form, basil, especially Ocimum basilicum cv. Genovese gigante cultivar, is employed in the production of pesto, a typical Italian sauce. The worldwide consumption of pesto has increased due to recent interest in Mediterranean cuisine, which is considered to be healthy and valuable. The complex matrix of pesto (e.g. cheese, extra-virgin olive oil, pine nuts and/or walnuts, and garlic) significantly affects its taste and flavor [36]. Previously, the aromatic composition of basil was determined from different areas of north western Italy and showed methyleugenol (1,2-dimethoxy-4-prop-2-en-1-yl-benzene) and eugenol as main components [26], while estragole was shown to be present in pesto sauces made of basil cultivars different from the Genovese gigante cultivar [41], [23]. In 1999, the Committee of Experts on Flavoring Substances of the Council of Europe concluded that methyleugenol is a naturally-occurring genotoxic carcinogenic compound with a DNA-binding potency similar to that of safrole [7]. In 2001, the Scientific Committee on Food (SCF) of the European Union concluded that methyleugenol, and its structural analogues estragole and safrole (Fig. 1) are genotoxic and carcinogenic and that reductions in exposure and restrictions in use levels are indicated [37], [38], [39]. Rodent studies performed at high dose levels provided sufficient evidence of the hepatocarcinogenicity of methyleugenol, estragole and safrole, displaying similar characteristics regarding mode of action and tumor formation [27], [28], [29], [30], [32], [48], [20]. EC Regulation 1334/2008, which became effective in January 2011, prohibits the addition of methyleugenol, estragole and safrole to foods and restricts their concentration in compound foods of dairy products, meat preparations and meat products (including poultry and game), fish preparations and fish products, ready to eat savouries, non-alcoholic beverages, processed fruit and vegetables, and soups and sauces including pesto. For soups and sauces including pesto that have been prepared with flavorings or food ingredients with flavoring properties maximum allowed limits of 60 mg/kg for methyleugenol and 25 mg/kg for safrole have been defined while a limit for estragole has not been presented [17]. However, if the only food ingredients with flavoring properties that have been added are only the fresh, dried or frozen herbs, the maximum limit for methyleugenol does not apply. This implies that pesto sauce made with basil is permitted in food preparations, regardless of its genotoxic carcinogenic compounds content.
Fig. 1.
Structural formula of methyleugenol and its structural analogues.
A major issue in the safety assessment of alkenylbenzene-containing food items is how to provide guidance on the potential risks for human health resulting from the exposure to low levels of these genotoxic and carcinogenic compounds [9]. The European Food Safety Authority (EFSA) recommended the Margin of Exposure (MOE) approach [9] to evaluate the priority for risk management actions for compounds that are both genotoxic and carcinogenic and present in a botanical or botanical preparation of interest [10], [11]. The MOE compares the exposure levels causing malignant tumors in experimental animals with dietary intake estimates in humans (EDI), taking into account differences in consumption patterns. The Scientific Committee of the European Food Safety Authority recommends the use of the benchmark dose (the BMDL10; the lower confidence limit of the benchmark dose resulting in a 10% extra cancer incidence) to obtain the MOE [9]. The benchmark dose is a standardized reference point derived from the animal data by mathematical modelling within the observed range of experimental data [11]. Pesto eaters could be exposed to some of the highest levels of methyleugenol, because fresh pesto is prepared from a large quantity of fresh basil, reflected by basil amounts in pesto sauce products on the market that amounted on average to 36% (an average from different products in the market). It has been estimated that the theoretical dietary exposure to methyleugenol could reach ∼250 μg/kg bw per meal for adults and 500 μg/kg bw per meal for children, based on the assumptions that the percentage of methyleugenol in the essential oil is generally >40%, the amount of essential oil in O. basilicum cv. Genovese gigante corresponds 0.5% and that one portion of pesto contains ∼10 g of basil [26]. The French food control administration analysed methyleugenol in a limited number of well-defined food products and reported that the methyleugenol concentration for one sample of pesto sauce was 6.02 μg/g and for one sample of pasta with pesto was 1.53 μg/g [42]. Ávila et al. reported methyleugenol concentrations in a pesto sample amounting to 48 μg/g [5], and Siano et al. reported methyleugenol concentrations of 0.01–0.52 μg/g and estragole concentrations that amounted to 0.05–19.30 μg/g [41]. It appears that the current values reported on levels of the alkenylbenzenes in sauces of pesto are limited with values that vary orders of magnitude. The aim of the present study was to make a more detailed analysis of the presence of methyleugenol and possible other alkenylbenzenes in basil-containing sauce of pesto and perform an associated risk assessment based on the MOE approach, taking into consideration the possible combined exposure to different alkenylbenzenes. Given that the alkenylbenzenes are known to act by a similar mode of action, at a similar target organ causing the same adverse effect, being liver carcinogenicity caused by a genotoxic mode of action, combined exposure can be evaluated by dose addition. This implies that the response to the mixture can be predicted by summing the doses of the components if needed after adjusting for the differences in potencies. Dose addition is considered most appropriate for mixtures with components that affect the same endpoint by the same mode of action [13], [14], [15]. Dose addition is the underlying assumption of the toxic equivalency factor (TEF) approach [4].
2. Materials and methods
2.1. Pesto sauce samples and chemicals
Basil containing pesto sauce samples were purchased from local Dutch markets. Table 1 presents an overview of the collected samples and their major characteristics as indicated on the label of each product. Methyleugenol, myristicin, estragole and apiol (purity, >97%), methanol (HPLC gradient) and acetonitrile (LC/MS gradient) were supplied by Sigma Aldrich (Steinheim, Germany). Nano pure water was obtained from a Barnstead Nanopure Type I ultrapure water system. Dimethyl sulfoxide (DMSO) was obtained from Acros organics (Geel, Belgium). Acetonitrile (ACN) (LC/MS grade) was purchased from Biosolve BV (Valkenswaard, The Netherlands). Trifluoroacetic acid TFA was obtained from Merck (Darmstadt, Germany).
Table 1.
Basil-containing pesto sauce samples used in the present study and their major characteristics. Information provided was derived from the product labels unless stated otherwise.
| Sample no. | Description | Ingredients |
|---|---|---|
| 1 | Pesto, Italian basil sauce, 85 g | Cold-pressed olive oil, fresh basil, fresh garlic, pine nuts, sea salt |
| 2 | Genovese pesto, biological, totally vegetarian, cheese free, gluten free, 130 g | Extra virgin olive oil 36%, Genovese basil in extra virgin olive oil 27.6%, salt, cashew nuts, walnuts, pine kernels, apple fibre, garlic, acidity |
| 3 | Combined with dried tomatoes and a hint of spice routes, biological, 135 g | Sunflower oil, breadcrumbs, cashew, basil, honey, garlic, olive oil, dried tomatoes (22.5%), vinegar, sea salt, natural flavour, pepper, thyme, paprika powder, cumin, chili pepper |
| 4 | Traditional terrasa pesto, bio, organic, 180 g | Extra virgin olive oil, fresh basil (35%), pecorino sheep cheese (6%), pine kernels (5%), garlic (3%), sea salt |
| 5 | Genovese pesto, costa ligure, 135 g | Sunflower oil, fresh basil 73%, salt, cashew, pine nuts, parmesan cheese, garlic, wine vinegar, starch, acidity regulators of critic acid, lactic acid |
| 6 | Green pesto, 190 g | Sunflower oil, basil 36%, cashew nut, whey powder, cheese, salt, potato, sugar, acid regulator, dried garlic |
| 7 | Italian Genovese pesto, 90 g | Sunflower oil, basil 35%, glucose, potato flake, cashew nut, milk, salt, rennet, preservatives |
| 8 | Excellent Genovese pesto, 135 g | Extra virgin olive oil, sunflower oil, fresh basil 69%, pine nut 13%, parmesan. |
| 9 | Tartufo pesto, costa ligure, 135 g | Sunflower oil, basil 42%, salt, cashew, pine nuts, parmesan cheese, garlic, wine vinegar, starch, acidity regulators of critic acid, lactic acid, 1% truffle |
| 10 | Limon pesto, 135 g | Oiled sunflower, fresh basil 59%, salt, cashew, pine nuts, parmesan cheese, garlic, wine vinegar, starch, acidity regulators of critic acid, lactic acid, 1.5 citron |
| 11 | Genovese pesto, 185 g | Sunflower oil (34%), basil 29%, potatoes, corn syrup, cashew nuts, grana padano cheese (4.5%), pecorino cheese (4.5%), salt, extra virgin olive oil, pine nuts (1%), natural flavours, garlic, concentrated lemon juice |
| 12 | Italian arugula pesto, 190 g | Sunflower oil, arugula 30%, basil 8%, pecorino romano cheese, sea salt, garlic, vegetable fibres, pine nuts, extra virgin olive oil, acidity regulator: lactic acid |
| 13 | Biological green pesto, 135g | Sunflower oil, fresh basil 79%, salt 1%, pine nuts, garlic, white wine vinegar, acid regulator (lactic acid, citric acid) |
| 14 | The real Italian seasoning, green pesto, 190 g | Basil 42%, sunflower oil, cashew nut 6.5%, water, parmigiano reggiano cheese 2.5%, salt, garlic, yogurt powder, olive oil 0.5%, pine 0.5%, acidity regulator: lactic acid, natural basil flavor, natural white pepper flavor, whey protein, antioxidant: E300 |
| 15 | Green pesto, classic basil pesto, garlic and parmesan cheese, 125 g | Basil 42%, sunflower oil 32.2%, cashew nuts, parmesan 4% (milk, salt, rennet), salt, pecorino cheese 2%, garlic 1.5%, glucose syrup, milk protein, acidity regulator: lactic acid, extra virgin olive oil, pine 0.5%, natural flavor |
| 16 | Green pesto, with more basil, 185 g | Basil 46%, sunflower oil, Italian cheese 7% (grana padano, pecorino romano), cashews, salt, extra virgin olive oil with virgin olive oil 2%, glucose syrup, sugar, potato flakes, flavor, garlic 0.5%, pine nut 0.5%, lactic acid |
| 17 | Green vegan pesto with extra virgin oil, bio, organic, 180 g | Extra virgin oil, basil 30%, walnuts, cashew nuts, pine kernels, sea salt |
| 18 | Italian green pesto, 90g | Basil 39%, 27% sunflower oil, water, lactose, 4% cashews, 4% parmigiano reggiano, 2.8% olive oil, 2% pine nuts, salt, spices extract, 1% pecorino cheese, garlic, acid: E270, natural flavoring |
| 19 | Fresh green pesto, in-house made (supermarket), 100 g | Sunflower oil, basil 23.7%, 9.5% cheese powder, walnuts, parsley, salt, vinegar, garlic 2%, 1.9%, pine nuts, sugar water, white pepper, food acids: lactic acid and citric acid, thickening agent, E415 and E415, preservative E202 |
| 20 | Artisan and full of flavor green pesto, 100 g | Sunflower oil, basil 30%, 8.5% grana padano cheese, cashews, olive oil, salt, pecorino cheese, fructose, pine, bamboo fiber, garlic, antioxidant E300 |
| 21 | Creamy green pesto with basil, cheese and pine nuts, 125 g | Vegetable oil (27% olive, rapeseed), basil 22%, 11% cheese powder, 7% pine nuts, parsley, 2.5% garlic puree, white wine vinegar, salt, sugar, vinegar, natural flavoring, preservative (E200, E210), black pepper, thickening agent (guar gum, xanthan gum) |
| 22 | Creamy red pesto with tomato, cheese and pine nuts, 125 g | Vegetable oil (13% olive, rapeseed), 16 tomatoes, basil 14%, 12% cheese powder, 8% pine nuts, parsley, 3% garlic puree, white wine vinegar, salt, sugar, vinegar, natural flavoring, preservative (E200, E210), thickener (guar gum, xanthan gum), 1.5% sundried tomatoes, red peppers |
| 23 | Pesto with Sicilian pistachio nuts, 130 g | Olive oil, Genovese basil 22%, pistachio nut (18%), cashew nuts, sea salt |
| 24 | Pesto with lemon zest, 135g | Olive oil, fresh basil 59%, cashew nuts, salt, cheese mix (2%), limon rinds (1.5%), fructose, pine nuts, wine vinegar, garlic, natural lemon flavoring, acidity regulator citric acid, lactic acid |
| 25 | Pesto with basil & truffle, 130g | Olive oil, Genovese basil 22%, cashew nuts, sea salt, pine nuts, white truffle |
| 26 | Rosso pesto with sun dried tomato and basil, 130g | Olive oil, sun dried tomato 25%, Genovese basil 25%, cashew nuts, sea salt, pine nuts, acidity regulator citric acid, lactic acid |
| 27 | Freshly made pesto, from Italian food products shop | Private recipe |
| 28 | Freshly made pesto, from cheese shop | Private recipe |
| 29 | Pesto spread, 90g | Sunflower oil, 15% pine nuts, low-fat cream cheese, basil 9%, spinach, white wine vinegar, 2.5 pecorino, garlic, salt, emulsifier (E472), bovine gelatin, preservative (E202, E224) |
| 30 | Special basil pesto, 100g | Sunflower oil, pine nut 15%, 12% old cheese, basil 11%, 10% spinach, white wine vinegar, dried garlic, sea salt, preservative (E202, E224) |
| 31 | Bio basil pesto,100g | Basil 32%, sunflower oil, cheese, olive oil 55, 4% pine nuts, vinegar, garlic, lemon juice, sea salt, spices, coconut blossom sugar |
Bold values emphasizes the variation of source and content of botanical under study, the basil.
2.2. Methanol extracts
All basil-containing pesto sauces were extracted using methanol based on the method described by Ávila et al. [5], with minor modifications. In short, 5 g of each sauce sample were sonicated in an ultrasonic bath for 10 min and macerated for 12 h at 50 °C with 80 ml of methanol in a closed glass vessel. Finally, an aliquot of the extract solution was centrifuged at 13000g for 5 min, and stored at −20 °C until Ultra Performance Liquid Chromatography (UPLC) analysis. Samples were extracted and then analyzed on UPLC in triplicate. The accuracy of the developed method was assessed by means of a recovery study and standard deviations of the replicates. For recovery, pure standards of commercially available alkenylbenzenes were added in two different quantities (final concentration of 5 and 10 μM) to 5 g of two pesto sauce samples (sample 16 and 21) which covers the whole range of analysis in a final volume of methanol of 80 ml. Samples were prepared and analyzed as described above to quantify the alkenylbenzene levels enabling determination of the recovery. The extraction capacity linearity was demonstrated to applying a range of weight of sample to final volume of the solution ratio of 1.5% up to 10%.
2.3. UPLC analysis
For quantification of alkenylbenzenes in the extracts, 3.5 μl of each sample was subjected to UPLC analysis. The UPLC system consisted of a Waters (Waters, Milford, MA) Acquity solvent manager, sample manager, and photodiode array detector. Chromatographic separation was achieved using an ACQUITY UPLC BEH C18 1.7 μm column, 2.1 × 50 mm. The column was thermostated at 35 °C and the sample manager was set at 10 °C. An isocratic gradient was made for the separation using a mixture of acetonitrile (ACN) and ultrapure water containing 0.1% (v/v) trifluoroacetic acid (TFA). The mobile phase consisted of 40% acetonitrile for 4 min, which was the time needed to separate alkenylbenzenes of interest. During the whole run, the flow rate was 0.6 ml/min. Under these conditions, the retention times for methyleugenol, estragole, myristicin, and apiol were 1.5, 2.2, 2.4, and 2.8 min, respectively. Identification of the alkenylbenzenes was achieved by comparing the UV spectrum and retention time of the peak to the UV spectrum and retention time of the peaks of commercially available reference compounds. Quantification was done by comparing the area of the alkenylbenzene peak to that of the calibration curve of the reference compounds determined using UPLC with photodiode array detection (UPLC/PDA) at a wavelength 201 for methyleugenol and estragole, and a wavelength 210 for myristicin and apiol.
2.4. Determination of estimated daily intake (EDI) of alkenylbenzenes resulting from the use of pesto sauce
The estimate daily intake (EDI) of alkenylbenzenes resulting from the use of pesto sauce was determined based on the alkenylbenzene content in the pesto sauce as determined in the present study (see results) and a body weight of 70 kg, the default value for adult body weight proposed by EFSA [12]. Daily consumption of pesto sauce was based on the reported use of about 10 g basil for a meal [26], and on the basil content of the samples as mentioned on the label (Table 1). If there was no information on the basil content in the pesto sauce on the label the average content of basil in pesto sauce of 36.0 ± 19.6% (n = 27) (Table 1) was used. Firstly, the EDI was calculated for individual compounds for all of the samples. And since several alkenylbenzenes were found in some of the pesto samples, and the different alkenylbenzenes show similarity in structure, target organ, type of adverse effects and mode of action through formation of a DNA reactive 1′-sulfoxymetabolite contributing to formation of liver tumors, a combined exposure assessment and subsequent risk assessment were also performed for those samples. To this end two approaches were used; summing up the EDIs of the alkenylbenzenes assuming equal potency, and a so-called toxic equivalency (TEQ) approach calculating the estimated daily intake (EDI) values of the alkenylbenzenes using their toxic equivalency factor (TEF) relative to methyleugenol. Methyleugenol was taken as the reference compound (TEF = 1.0), expressing the concentrations of the other alkenylbenzenes in methyleugenol equivalents. TEF values for the different alkenylbenzenes for the TEQ approach need to be defined, which was done by calculating an average of three endpoints. The first one was the slope of the dose-response curve for DNA-adduct formation in female CD-1 mice liver upon exposure to different alkenylbenzenes [32]. The second one, was the in vivo level of formation of the hepatocarcinogenic 1′-sulfoxymetabolites of the different alkenylbenzenes predicted using human physiologically based kinetic (PBK) models at a dose level of 0.01 mg/kg bw [2], [1], [31], [3]. And finally, the BMDL10 values derived from in vivo tumor data for safrole, estragole, and methyleugenol [27], or by read-across from safrole for myristicin and apiol [2], [1] (Table 4). The TEF values for the different alkenylbenzenes defined by the three approaches relative to methyleugenol were subsequently averaged to define the final TEF values. Using these TEF values the EDI of alkenylbenzenes in the pesto sauces expressed in (μg methyleugenol equivalents/kg bw per day) was calculated using the formula:
Table 4.
Data used for estimation of the TEF values for the different alkenylbenzenes as derived from data reported for (i) the dose-dependent level of DNA adduct formation in the liver of alkenylbenzene exposed female CD-1 mice [32], (ii) the formation of the proximate carcinogenic 1′-sulfoxy metabolites in liver in μmol/kg bw as predicted by available PBK models at a dose level of 0.01 mg/kg bw [2], [1], [31], [3], and (iii) BMDL10 values reported in the literature [2], [1], [46]. The TEF values were calculated relative to methyleugenol as the reference compound, expressing the concentrations of the other alkenylbenzenes in methyleugenol equivalents.
| Compound | Formation of DNA adduct in 107 nucleotides versus dose in mg/kg bw |
Formation of the proximate carcinogenic 1′-sulfoxy metabolite |
In vivo tumor data |
Average TEF value ± SD | |||
|---|---|---|---|---|---|---|---|
| slope of the curve | TEF | formation level (μmol/kg bw) at 0.01 mg/kg bw dose | TEF | BMDL10 (mg/kg bw per day) | TEF | ||
| Safrole | 1.68 | 1.05 | 3.28E-05 | 1.08 | 1.9 | 8,05 | 3.39 ± 4.04 |
| myristicin | 0.39 | 0.24 | 2.44E-05 | 0.8 | 1.9 | 8,05 | 3.03 ± 4.36 |
| apiol | 0.11 | 0.07 | 1.62E-05 | 0.53 | 5.7 | 2,68 | 1.09 ± 1.40 |
| estragole | 2.48 | 1.55 | 1.41E-04 | 4.62 | 3.3 | 4,64 | 3.60 ± 1.78 |
| methyleugenol | 1.60 | 1.00 | 3.05E-05 | 1.00 | 15.3 | 1,00 | 1.00 |
2.5. Calculation of the margin of exposure
Risk assessment of consumption of the pesto sauce samples was performed using the Margin of Exposure (MOE) approach [9]. For the individual alkenylbenzene approach, the BMDL10 values of each compound were used [2], [1], [46] and then compared with the EDIs of the alkenylbenzenes resulting from the use of pesto sauce. For the equal potency assumption approach, the BMDL10 value of the predominating alkenylbenzene in the samples was used and compared to the sum of the EDIs. And finally, for the TEQ approach, the BMDL10 of the reference compound methyleugenol was compared to the EDI expressed in methyleugenol equivalents.
3. Results
3.1. Determination of methyleugenol content in the basil-containing pesto sauce samples, EDI, and resulting MOEs
Table 2 presents the levels of methyleugenol detected in the various pesto sauces. The results obtained revealed that methyleugenol was detected in all the samples analyzed. The level of methyleugenol in the different samples varied about 28-fold ranging between 3.6 and 99.3 μg/g pesto sauce. Estimated daily intakes (EDIs) of methyleugenol from basil containing pesto sauce were calculated using the methyleugenol content in the samples, a daily consumption of pesto calculated based on the reported use of about 10 g basil for a meal [26], and the basil content of the samples as mentioned on the label (Table 1) or the assumption that the basil content in pesto sauce was on average 36.0 ± 19.6% (n = 27) (Table 1) in case this% of basil in the pesto sauce was not mentioned on the label, and an adult body weight of 70 kg. The EDI values thus obtained for methyleugenol vary in the range of 3.2–44.3 μg/kg bw per day. The MOE values were calculated using the BMDL10 value reported in the literature for methyleugenol of 15.3 mg/kg bw per day [46] (Table 2). The MOE values obtained vary from 345 to 4781 and were all lower than 10000, the value suggested by EFSA to judge if there is a priority for risk management [9].
Table 2.
Level of methyleugenol detected in the pesto sauce samples, EDI for methyleugenol upon consumption of these pesto samples, and the resulting MOE.
| Sample no. | Methyleugenol concentration in the pesto sample Average ± SD (μg/g) | EDI (μg/kg bw per day) |
MOE |
|---|---|---|---|
| 1 | 17.9 ± 2.4 | 7.1 | 2154 |
| 2 | 11.4 ± 1.5 | 5.9 | 2593 |
| 3 | 11.1 ± 1.5 | 4.4 | 3474 |
| 4 | 35.6 ± 4.8 | 14.5 | 1053 |
| 5 | 32.3 ± 4.3 | 6.3 | 2421 |
| 6 | 41.2 ± 5.5 | 16.4 | 936 |
| 7 | 29.6 ± 4 | 12.1 | 1266 |
| 8 | 27.6 ± 3.7 | 5.0 | 3066 |
| 9 | 25.3 ± 3.4 | 8.6 | 1778 |
| 10 | 38.1 ± 5.1 | 9.2 | 1659 |
| 11 | 20.5 ± 2.7 | 10.1 | 1515 |
| 12 | 3.8 ± 0.5 | 6.8 | 2255 |
| 13 | 34.1 ± 4.6 | 6.2 | 2481 |
| 14 | 60.5 ± 8.1 | 20.6 | 744 |
| 15 | 21.8 ± 2.9 | 7.4 | 2063 |
| 16 | 39.0 ± 5.2 | 12.1 | 1263 |
| 17 | 12.4 ± 1.7 | 5.9 | 2591 |
| 18 | 35.8 ± 4.8 | 13.1 | 1167 |
| 19 | 56.4 ± 7.5 | 34.0 | 450 |
| 20 | 15.2 ± 2.0 | 7.2 | 2114 |
| 21 | 22.9 ± 3.1 | 6.4 | 2404 |
| 22 | 15.1 ± 2.0 | 14.9 | 1029 |
| 23 | 8.4 ± 1.1 | 15.4 | 993 |
| 24 | 33.0 ± 4.4 | 5.5 | 2805 |
| 25 | 9.8 ± 1.3 | 8.0 | 1915 |
| 26 | 5.6 ± 0.7 | 3.2 | 4781 |
| 27 | 14.3 ± 1.9 | 5.7 | 2696 |
| 28 | 13.4 ± 1.8 | 5.3 | 2877 |
| 29 | 3.6 ± 0.5 | 5.7 | 2678 |
| 30 | 3.8 ± 0.5 | 4.9 | 3100 |
| 31 | 99.3 ± 13.3 | 44.3 | 345 |
3.2. Determination of other alkenylbenzenes in the pesto sauce samples, EDI for the combined exposure, and resulting MOEs
In four samples (16, 18, 19, and 21) other alkenylbenzenes were detected in addition to methyleugenol, including estragole, myristicin, and/or apiol (Table 3). The levels of methyleugenol, detected in these samples, were already presented in Table 2. Estragole that is known to be present in some basil cultivars in addition to methyleugenol, was detected in two samples (16,18), with one of these samples indeed containing another cultivar than the Genovese cultivar as mentioned on the label (Table 1). The estragole level varied 10-fold between the two samples in which it was detected. Myristicin and apiol were detected in two samples of green pesto (Table 3), which may be due to the fact that these samples contain other herbs in addition to basil (e.g. parsley) (Table 1). For samples 16, 18, 19 and 21 the EDI for the individual alkenylbenzenes varied between 6.4–34.0 μg/kg bw per day for methyleugenol, 1.2–10.6 μg/kg bw per day for estragole, 8.6–9.6 μg/kg bw per day for myristicin, and 2.2 μg/kg bw per day for apiol the latter being detected in only one sample (Table 3).
Table 3.
Level of alkenylbenzenes methyleugenol, estragole, myristicin, and apiol in the four pesto sauce samples that contained other alkenylbenzenes in addition to methyleugenol and EDI values for the individual alkenylbenzenes, as well as combined EDI values calculated either by assuming equal potency, or in methyleugenol equivalents using the TEF values presented in Table 4.
| Sample no. | Alkenylbenzenes level in the pesto sample average ± SD (μg/g) |
EDI (μg/kg bw per day) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| individual |
combined exposure |
|||||||||
| methyleugenol | estragole | myristicin | apiol | methyleugenol | estragole | myristicin | apiol | assuming equal potency | based on TEF methyleugenol equivalents | |
| 16 | 39.0 ± 5.2 | 34.1 ± 2.8 | 12.1 | 10.6 | 22.7 | 50.3 | ||||
| 18 | 35.8 ± 4.8 | 3.2 ± 1.5 | 13.1 | 1.2 | 14.3 | 17.3 | ||||
| 19 | 56.4 ± 7.5 | 15.8 ± 0.01 | 34 | 9.6 | 43.5 | 62.9 | ||||
| 21 | 22.9 ± 3.1 | 13.2 ± 1.2 | 3.4 ± 0.2 | 6.4 | 8.6 | 2.2 | 17.2 | 34.8 | ||
Since several alkenylbenzenes were found in some pesto samples, and because these alkenylbenzenes are known to display similarity in structure, target organ, type of adverse effects and mode of action through formation of a DNA reactive 1′-sulfoxymetabolite contributing to formation of liver tumors, a combined exposure and risk assessment was also performed. To this end the combined exposure was estimated in two ways, assuming either equal potency or using a so-called toxic equivalency (TEQ) approach. When assuming equal potency of the different alkenylbenzenes the combined EDI can be calculated by adding up the EDIs of the different alkenylbenzenes. Table 3 presents the combined EDI values obtained for the four pesto sauce samples in which more than one alkenylbenzene were detected. The values obtained varied from 14.3 to 43.5 mg/kg bw per day with methyleugenol being the major alkenylbenzene making up 53.3–91.6% of these values except for sample 22 where methyleugenol makes up 37.2% of the alkenylbenzenes and myristicin appeared to be the major one at 50.0%. In a second approach the combined exposure was calculated in methyleugenol equivalents taking the relative potency of the different alkenylbenzenes into account. For this TEQ approach the required toxic equivalency factor (TEF) relative to methyleugenol were estimated as summarized in Table 4. TEF values for the different alkenylbenzenes were defined by calculating the average of the relative potency as available for three end points, including (i) the slope of the dose-response curve for DNA-adduct formation in female CD-1 mice liver upon exposure to different alkenylbenzenes [32], (ii) the in vivo level of formation of the hepatocarcinogenic 1′-sulfoxymetabolites of the different alkenylbenzenes predicted using human physiologically based kinetic models at a dose level of 0.01 mg/kg bw [2], [1], [31], [3], and (iii) the BMDL10 values derived from in vivo tumor data for safrole, estragole, and methyleugenol [27]), or by read-across from safrole for myristicin and apiol [2], [1]. Methyleugenol was taken as the reference compound (TEF = 1.0), expressing the concentrations of the other alkenylbenzenes in methyleugenol equivalents (Table 4).
Using these TEF values and the toxic equivalency approach (TEQ) the combined EDI values obtained vary in the range of 17.3–62.9 μg methyleugenol equivalents/kg bw per day (Table 3).
Using the EDI values thus obtained MOE values were calculated. To this end different BMDL10 values were used for different approaches to obtain the MOE values. The BMDL10 values for the individual alkenylbenzenes were taken from literature [2], [1], [46] and were used to caluclate MOE values for intakes of each individual alkenylbenzene (Table 5). MOE values thus obtained were all below 10000. Assuming equal potency, the BMDL10 of methyleugenol of 15.3 mg/kg bw per day [46], being a dominant alkenylbenzenes in the samples was compared with the combined EDI. The MOE values thus obtained varies between 351 and 1071 (Table 5). For the TEQ approach, the BMDL10 of methyleugenol of 15.3 mg/kg bw per day [46] was compared to the EDI expressed in methyleugenol equivalents to calculate the MOE. When using the TEQ approach, MOE values ranged from 243 to 883.
Table 5.
The MOE values obtained for samples containing several alkenylbezenes. MOE values were calculated for each individual alkenylbenzenes, and based on combined exposure estimates either assuming equal potency, or based on a TEQ approach.
| Sample no. | MOE individual alkenylbenzenes |
MOE based on equal potency and BMDL10 of the highest level alkenylbenzene in the sample | MOE based on TEF | |||
|---|---|---|---|---|---|---|
| methyleugenol | estragole | myristicin | apiol | |||
| 16 | 1263 | 3209 | 674 | 304 | ||
| 18 | 1166 | 355 | 1071 | 883 | ||
| 19 | 450 | 199 | 351 | 243 | ||
| 21 | 1028 | 221 | 2582 | 892 | 353 | |
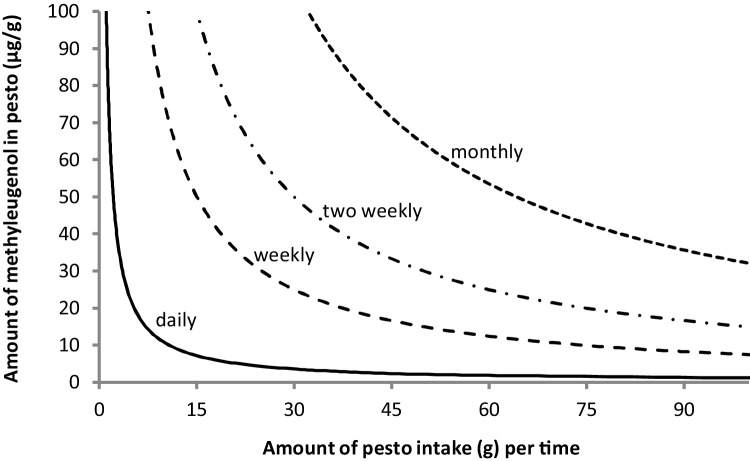
From the data thus obtained it is clear that the estimated daily consumption of the pesto sauces at 10 g basil per meal per day (based on wet weight) would result in MOE values that indicate a priority for risk management for all pesto sauces. It is of interest to evaluate what level of methyleugenol, amount of pesto sauce consumed and/or what exposure frequency would result in an MOE of 10000. To evaluate how these parameters could be modified to result in MOE values that would be of low priority for risk management, Fig. 2 shows the relation between the level of methyleugenol detected in a pesto sauce sample and the amount of pesto sauce consumed that would result in MOE values of 10000 at a certain frequency of intake. For these calculations, it was assumed that Haber’s rule would apply so the daily dose would be linearly dependent on the frequency of intake. The results presented indicate that daily intake of for example 30 g pesto sauce would be of low priority for risk management at a methyleugenol level of 3.6 μg/g, while weekly intake of 30 g would be of low concern at a methyleugenol value of 25.0 μg/g. The curves can also be used to derive that for the pesto sauce samples of the present study with methyleugenol levels that vary from 99.3 to 3.6 μg/g pesto sauce, the consumption of 1.1–29.8, 7.5–208, 15.1–416.5, and 32.4–892.5 g of pesto sauce on a daily basis, once a week, once every two weeks, and once a month, respectively, could be considered of low concern.
Fig. 2.
Relation between the methyleugenol content (μg/g) and daily pesto sauce consumption that would result in an MOE of 10000 upon daily (−), weekly (- −), two weekly (-.-.-.) or monthly (….) consumption. Combinations of methyleugenol levels and pesto sauce intake that result in values below the respective curves result in MOE values above 10000 and would be of low priority for risk management.
4. Discussion
The aim of the present study was to make a more detailed analysis of the presence of methyleugenol and possible other alkenylbenzenes in basil-containing sauce of pesto and perform an associated risk assessment based on the MOE approach, taking into consideration the combined exposure to different alkenylbenzenes.
It is important to consider the variance in aromatic composition of basil at different growth stages from different areas. The methyleugenol content of the volatile oil from commercial samples of sweet basil, and in the leaf, flower and stem for the same cultivar of sweet basil were in the range of 0–2.4% [21], [44], [40]. It was also reported that the content of methyleugenol in the essential oil of basil plants decreases from 100% to 10% as plant height and maturity increase [26]. Different studies revealed different levels of estragole and methyleugenol in basil for different genotypes and as a function of region and harvesting. The concentrations in fresh basil amount 10.2–2029 μg/g for estragole and 0.07–560 μg/g for methyleugenol [5], [41], [22]. For pesto sauce, studies showed different concentrations of estragole that varied between 0.05 and 19.3 μg/g, and of methyleugenol at values that varied between 0.01 and 52 μg/g [5], [41]. The levels of methyleugenol determined in the pesto sauces of the present study amounted to 3.6–99.3 μg/g pesto sauce, and of estragole, being present in only two out of the 32 pesto sauce samples analyzed, to 3.2 and 34.1 μg/g pesto sauces. Based on these levels and taking the basil content (%) as indicated on the label of the pesto sauces into account, the level of methyleugenol in basil used to prepare the pesto sauce was estimated to vary between 22.4–310.3 μg/g. The determination of the levels of alkenylbenzenes in basil-containing sauce of pesto revealed that all the samples analyzed contained methyleugenol. In addition to methyleugenol, two of the samples contained estragole, while two other samples contained myristicin, and one sample contained both myristicin and apiol in addition to methyleugenol. Based on chemical analysis the EDI values could be determined assuming a body weight of 70 kg and intake of 10 g of basil (based on wet weight) per meal [26], provided by an amount of pesto sauce that could be calculated taking into account the basil content (%) in the pesto sauce as indicated on the label (Table 1). For samples containing only methyleugenol, the EDI values ranged from 3.2–44.3 μg/kg bw per day. These EDI values resulted in MOE values of 345–4781. Since several alkenylbenzenes were found in four of the pesto samples, and these alkenylbenzenes show high similarity in structure, target organ, type of adverse effects and mode of action through formation of a DNA reactive 1′-sulfoxymetabolite contributing to formation of liver tumors, for these four samples a combined exposure assessment and subsequent risk assessment were performed. EDI values based on individual compounds amounted to 1.2–34.0 μg/kg bw per day. These EDI values resulted in MOE values of 199–3209 for the individual levels of estragole, myristicin and apiole, indicating that these alkenylbenzenes add to the possible health risks. Assuming equal potency of the alkenylbenzenes, combined EDI values obtained ranged from 14.3–43.5 μg/kg bw per day resulting in MOE values ranging from 351 to 1071. Estimation of the TEF values used in this study was based on in vitro and in vivo data. It is of interest to note that in an ideal situation TEF values would be derived from in vivo data. However in practice in vitro endpoints are also frequently used, for example in the case of TEF values derived for dioxins [45] and, more recently, in the case of interim TEF values defined for pyrrolizidine alkaloids [25]. Using a TEF concept combined EDI values ranged from 17.5–59.7 μg methyleugenol equivalents/kg bw leading to MOE values ranging from 243 to 883. MOE values resulting from this combined exposure assessment were 1.5–5-fold lower than the MOE values obtained when considering the presence of only methyleugenol in these four samples, and are all below 10000. Combined risk assessment based on equal potency did not vary much from results obtained using the TEQ approach. This can be ascribed to the minor variation in potencies of the different alkenylbenzenes detected in the pesto samples.
It is of interest to note that these MOE values refer to regular daily consumption of pesto which may be an overestimation of realistic human consumption. Levels of methyleugenol in pesto sauce samples and consumption frequency of pesto sauce directly impact the EDI and the resulting MOE values. The levels of methyleugenol detected in the pesto sauces of the present study vary between 99.3 and 3.6 μg/g and would allow consumption of 1.1–29.8, 7.5–208, 15.1–416.5, and 32.4–892.5 g of pesto sauce on a daily basis, once a week, once every two weeks, and once a month, respectively, to achieve MOE values above the 10000 safety limit for low priority for risk management. It is concluded that consumption of pesto sauces would only be of concern if consumed on a daily basis over longer periods of time. The results of the present paper reveal that pesto consumption does not always represent a cancer risk and provide insight into when this might be the case and under which level and frequency of consumption pesto consumption would not raise a concern.
Acknowledgment
AMA and AJAM acknowledge financial support from the SOIT foundation (The foundation for Stimulation of Innovation in Toxicology).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2016.11.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Alajlouni A.M., Al Malahmeh A.J., Kiwamoto R., Wesseling S., Soffers A.E., Al-Subeihi A.A., Vervoort J., Rietjens I.M. Mode of action based risk assessment of the botanical food-borne alkenylbenzene apiol from parsley using physiologically based kinetic (PBK) modelling and read-across from safrole. Food Chem. Toxicol. 2016;89:138–150. doi: 10.1016/j.fct.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Al-Malahmeh A.J., Al-Ajlouni A.M., Wesseling S., Soffers A.E., Al-Subeihi A.A., Kiwamoto R., Vervoort J., Rietjens I.M. Physiologically based kinetic modeling of the bioactivation of myristicin. Arch. Toxicol. 2016 doi: 10.1007/s00204-016-1752-5. http://link.springer.com/journal/204/onlineFirst/page/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlSubeihi A.A., Spenkelink B., Punt A., Boersma M.G., van Bladeren P.J., Rietjens I.M.C.M. Physiologically based kinetic modeling of bioactivation and detoxification of the alkenylbenzene methyleugenol in human as compared with rat. Toxicol. Appl. Pharmacol. 2012;260:271–284. doi: 10.1016/j.taap.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 4.ATSDR (Agency for Toxic Substances and Disease Registry) U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2004. Guidance Manual for the Assessment of Joint Toxic Actions of Chemical Mixtures. [Google Scholar]
- 5.Ávila M., Zougagh M., Escarpa A., Ríos Á. Determination of alkenylbenzenes and related flavour compounds in food samples by on-column preconcentration-capillary liquid chromatography. J. Chromatogr. A. 2009;1216:7179–7185. doi: 10.1016/j.chroma.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Council of Europe – Committee of Experts on Flavouring Substances . 2001. Opinion of the Scientific Committee on Food on Methyleugenol (4-Allyl-1,2-dimethoxybenzene)http://ec.europa.eu/food/fs/sc/scf/out102_en.pdf [Google Scholar]
- 9.EFSA (European Food Safety Authority) European Food Safety Authority. Opinion of the scientific committee on a request from EFSA related to a harmonized approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005;282:1–31. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2005.282/epdf [Google Scholar]
- 10.EFSA, (European Food Safety Authority) Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, on request of EFSA. EFSA J. 2009;7:1249. https://www.pharmamedtechbi.com/∼/media/Images/Publications/Archive/The%20Tan%20Sheet/17/037/05170370014_b/090914_efsa_botanicals_guidance.pdf [Google Scholar]
- 11.EFSA (European Food Safety Authority) Use of the benchmark dose approach in risk assessment, Guidance of the Scientific Committee. EFSA J. 2009;1150:1–72. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2009.1150/epdf [Google Scholar]
- 12.EFSA (European Food Safety Authority) Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012;10(3):2579. http://www.efsa.europa.eu/en/efsajournal/doc/2579. pdf [Google Scholar]
- 13.EPA (United states Environmental Protection Agency) Guidelines for the health risk assessment of chemical mixtures. Fed. Reg. 1986;51:34014–34025. [Google Scholar]
- 14.EPA (United states Environmental Protection Agency) U.S. Environmental Protection Agency, Office of Research and Development; 1990. Technical Support Document on Health Risk Assessment of Chemical Mixtures. EPA/600/8-90/064. [Google Scholar]
- 15.EPA (United states Environmental Protection Agency) U.S. Environmental Protection Agency, Risk Assessment Forum; Washington, DC: 2000. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. EPA/630R-00/002. [Google Scholar]
- 17.European Commission, (2008) Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Off J Eur. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R1334&from=EN
- 19.Kew, Royal Botanic Gardens. World Checklist of Selected Plant Families; http://apps.kew.org/wcsp/incfamilies.do.
- 20.Kobets T., Duan J.D., Brunnemann K.D., Etter S., Smith B., Williams G.M. Structure activity relationships for DNA damage by alkenylbenzenes in turkey egg fetal liver. Toxicol. Sci.: Off. J. Soc. Toxicol. 2016;150:301–311. doi: 10.1093/toxsci/kfv322. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence M., Shu C.K. Essential oils as components of mixtures: their method of analysis and differentiation. In: Ho C.T., Manley C.M., editors. Flavor Measurement. Marcel Dekker; New York: 1993. [Google Scholar]
- 22.Lee S.-J., Umano K., Shibamoto T., Lee K.- Identification of volatile components in basil (Ocimum basilicum L.). and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91:131–137. [Google Scholar]
- 23.Marotti M., Piccaglia R., Giovanelli E. Differences in essential oil composition of basil (Ocimum basilicum L.) italian cultivars related to morphological characteristics. J. Agric. Food Chem. 1996;44:3926–3929. [Google Scholar]
- 25.Merz K.H., Schrenk D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016;263(May (6)):44–57. doi: 10.1016/j.toxlet.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Miele M., Dondero R., Ciarallo G., Mazzei M.J. Methyleugenol in ocimum basilicum L. cv. genovese gigante. Agric. Food Chem. 2001;49(January (1)):517–521. doi: 10.1021/jf000865w. [DOI] [PubMed] [Google Scholar]
- 27.Miller E.C., Swanson A.B., Phillips D.H., Fletcher T.L., Liem A., Miller J.A. Structure–activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983;43:1124–1134. [PubMed] [Google Scholar]
- 28.NTP Toxicology and carcinogenesis studies of methyleugenol (CAS No. 93-15-2) in F344/N rats and B6C3F1 mice. Natl. Toxicol. Program Tech. Rep. Ser. 2000;491:1–412. PMID:12563349. [PubMed] [Google Scholar]
- 29.Phillips D.H., Miller J.A., Miller E.C., Adams B. Structures of the DNA adducts formed in mouse liver after administration of the proximate hepatocarcinogen 1’-hydroxyestragole. Cancer Res. 1981;41:176–186. [PubMed] [Google Scholar]
- 30.Phillips D.H., Reddy M.V., Randerath K. 32P-Post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally occurring alkenylbenzenes. II. Newborn male B6C3F1 mice. Carcinogenesis. 1984;5(12):1623–1628. doi: 10.1093/carcin/5.12.1623. [DOI] [PubMed] [Google Scholar]
- 31.Punt A., Paini A., Boersma M.G., Freidig A.P., Delatour T., Scholz G., Schilter B., van Bladeren P.J., Rietjens I.M.C.M. Use of physiologically based biokinetic (PBBK) modeling to study estragole bioactivation and detoxification in humans as compared with male rats. Toxicol. Sci. 2009;110:255–269. doi: 10.1093/toxsci/kfp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randerath K., Haglund R.E., Phillips D.H., Reddy M.V. 32P-Post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. I. Adult female CD-1 mice. Carcinogenesis. 1984;5(12):1613–1622. doi: 10.1093/carcin/5.12.1613. [DOI] [PubMed] [Google Scholar]
- 34.Raymond M. Harley, Sandy Atkins, Andrey L. Budantsev, Philip D. Cantino, Barry J. Conn, Renée J. Grayer, Madeline M. Harley, Rogier P.J. de Kok, Tatyana V. Krestovskaja, Ramón Morales, Alan J. Paton, P. Olof Ryding, (2004) "Labiatae" pages 167275, In: Klaus Kubitzki, (ed.), Joachim W. Kadereit, (volume ed.), The Families and Genera of Vascular Plants volume VII, SpringerVerlag: Berlin; Heidelberg, Germany. ISBN 9783540405931, 2275–2283.
- 36.Salvadeo P., Boggia R., Evangelisti F., Zunin P. Analysis of the volatile fraction of Pesto Genovese by headspace sorptive extraction (HSSE) Food Chem. J. 2007;105(3):1228–1235. [Google Scholar]
- 37.SCF (Scientific Committee on Food) Health and Consumer Protection Directorate-General. Directorate C, Scientific Opinions; Brussels, Belgium: 2001. Opinion of the Scientific Committee on Food on Estragole (1-Allyl-4-methoxybenzene)http://ec.europa.eu/food/fs/sc/scf/out104_en.pdf Available at. [Google Scholar]
- 38.SCF (Scientific Committee on Food) Health and Consumer Protection Directorate-General. Directorate C, Scientific Opinions; Brussels, Belgium: 2001. Opinion of the Scientific Committee on Food on Methyleugenol (4-Allyl-1, 2-dimethoxybenzene)http://ec.europa.eu/food/fs/sc/scf/out102_en.pdf [Google Scholar]
- 39.SCF (Scientific Committee on Food) Health and Consumer Protection Directorate General. Directorate C, Scientific Opinions; Brussels, Belgium: 2001. Opinion of the Scientific Committee on Food on the Safety of the Presence of Safrole (1-Allyl-3, 4-methylene Dioxy Benzene) in Flavourings and Other Food Ingredients with Flavouring Properties.http://ec.europa.eu/food/fs/sc/scf/out116_en.pdf [Google Scholar]
- 40.Sheen L.Y., Tsai Ou Y.H., Tsai S.J. Flavor characteristic compounds found in the essential oil of Ocimum basilicum L: with sensory evaluation and statistical analysis. J. Agric. Food Chem. 1991;39:939–943. [Google Scholar]
- 41.Siano F., Ghizzoni C., Gionfriddo F., Colombo E., Servillo L., Castaldo D. Determination of estragole, safrole and eugenol methyl ether in food products. Food Chem. 2003;81:469–475. [Google Scholar]
- 42.Smith B., Cadby P., Leblanc J.-C., Setzer R.W. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic Example: methyleugenol, CASRN: 93-15-2. Food Chem. Toxicol. 2010;48:S89–S97. doi: 10.1016/j.fct.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 44.Tsai S.J., Sheen L.Y. Essential Oil of Ocimum Basilicum L. Cultivated in Taiwan. In: Sze W., Woo F.C., editors. Trends in food science. Proceedings of the 7th World Congress of Food Science and Technology. Singapore Institute of Food Science and Technology; Singapore: 1987. pp. 66–70. [Google Scholar]
- 45.Van den Berg M., Birnbaum L.S., Denison M., De Vito M., Farland W., Feeley M., Fiedler H., Hakansson H., Hanberg A., Haws L., Rose M., Safe S., Schrenk D., Tohyama C., Tritscher A., Tuomisto J., Tysklind M., Walker N., Peterson R.E. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006;93(October (2)):223–241. doi: 10.1093/toxsci/kfl055. Epub 2006 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van den Berg S.J., Restani P., Boersma M.G., Delmulle L., Rietjens I.M.C.M. Levels of genotoxic and carcinogenic compounds in plant food supplements and associated risk assessment. Food Nutr. Sci. 2011;2:989–1010. [Google Scholar]
- 48.Zhou G.-D., Moorthy B., Bim J., Donnelly K., Randerath K. DNA adducts from alkoxyallylbenzene herb and spice constituents in cultured human (HepG2) cells. Environ. Mol. Mutagen. 2007;48:715–721. doi: 10.1002/em.20348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.