Abstract
Context
Oral activated charcoal (AC) for toxin absorption should be applied as soon as possible. Extra-hospital AC-application on site by medical laypersons with pre-emptive obtained AC may save time, but may be inferior to AC-application by medical professionals.
Objective
1) Availability and incidence of pre-emptive stockpiling of AC on site in the German region Bavaria 2) time saved by AC-stockpiling and application on site, 3) quality of AC-application defined by completeness of the applied AC-dose, time needed, incidence of side-effects in lay-care and in professional-care, considering confounding variables: AC-formulation/powder/tablets, recommended AC-dose, patient’s age.
Method
telephone-interviews in cases with AC-recommendation by a Poison Information Centre (PIC). Lay-care was suggested according to risk-assessment by PIC. Ingestion sites were classified as either apt for AC-stockpiling or not apt.
Results
1) availability: In Bavaria only 20%–22% of eligible cases had AC on-hand, 2) time-saving was at least 14 min. 3) Lay-care/professional-care or patient’s age had no significant influence on the completeness of the applied AC-dose, which was higher with AC as powder but negatively correlated with the recommended AC-dose. No significant difference was seen with time needed for application and incidence of side-effects.
Conclusion
pre-emptive AC-stocking should be encouraged.
Keywords: Activated Charcoal, Poisons Information Centre, Layperson, Extra-hospital, Pre-hospital, pre-emptive stockpiling
1. Introduction
After ingestion of a potentially toxic substance, Single Dose Activated Charcoal (AC) is the most applied decontamination procedure [1]. It is easy to use, inexpensive and safe [2]. With increasing lag-time between toxin-ingestion and AC-application the efficacy of AC to decontaminate declines rapidly [3]. Thus, AC should be applied as soon as possible. Pre- and extra-hospital AC-application may shorten this lag time [4]. This implies AC application by non-medical persons (=laypersons). In cases where a consulting Poison Information Centre (PIC) sees an indication for AC and estimates toxin and circumstances to allow observation by laypersons instead of treatment by medical professionals, this could additionally save medical resources. In these cases prerequisite for an optimal scenario is provident stockpiling of AC at suitable sites prone for eventual toxin ingestion such as households, nurseries, schools, psychiatric wards. Application of AC by laypersons however may be inferior to application by medical professionals. We studied the local situation in Bavaria in terms of availability of AC, timesaving, feasibility and quality of AC-application by laypersons. With the results of this study the PIC Munich, wants to stimulate health authorities and – insurances to support and propagate precautionary stockpiling of AC at suitable places. Presently there is no policy of health care authorities and insurances policy concerning AC.
This study is not the first to address this subject; previous studies showed distinct regional differences: In Finland 42% of households had AC at home which shortened the lag-time till AC-application by 18 min compared with application in hospitals [5]. In another study in Kentucky, 10% of households had AC at home. Here, time saving there by AC-application at home was 36 min, including cases, in which AC had to be obtained from elsewhere [6].
Our study prospectively investigated cases in which the PIC recommended AC addressing three questions:
-
(1)
Availability of AC: In which percentage of cases AC was actually on site, where precautionary AC stockpiling would be reasonable and possible.
-
(2)Time saved by AC application in different scenarios:
-
a)AC stored on site, AC applied there,
-
b)AC-application in a pharmacy,
-
c)AC-application on site, however AC had to be obtained from elsewhere,
-
d)AC-application in a medical professional environment e.g. by paramedics or in doctor’s office to be compared with
-
e)AC-application in hospital or emergency-room.
-
a)
-
(3)In anticipation of eventual concerns of health care authorities against storage and application of AC by laypersons we studied the “quality” of the application of AC by laypersons and compared it with AC-application by professionals. Since there is no established definition for the quality of AC-application nor studies had yet addressed this subject, we investigated the “quality”
-
a)by the actually applied amount of AC (g/kg) in relation to the recommended dose (g/kg),
-
b)time needed for application
-
c)incidence of unwanted side-effects attributable to AC.
-
a)
Application by medical professionals was defined as AC-application in hospitals, by paramedics or in a doctor’s office; non-professional setting as application by laypersons at home, in pharmacies or other places.
2. Method
In this prospective, observational study from February 22, 2013 to July 27, 2014, calls where the Munich PIC recommended AC (over all 46.002 calls in this period, serving a population of approx. 12.000.000), were followed up by telephone after 1 or 2 days with a standardized questionnaire. As a further condition, one of the investigators had to be on duty in the PIC being involved in the first call. Indications for AC were given according to the position papers of EAPCCT and AACT [7]. Treatment in a hospital or emergency room was recommended a) in all cases where the alleged toxin was at worst estimated to be able to cause symptoms necessitating professional medical assistance [8], [9], b) in all cases where the toxin was ingested with abusive or self-harming intention; c) in all cases showing symptoms necessitating medical professional assistance. In cases where the alleged toxin was expected to produce only minor symptoms that were considered to be manageable with observation by medical non-professionals (laypersons), this was recommended in addition to single doses oral AC. In cases where AC was not on site, it was recommended to bring the patient to the next facility where AC is available and, if feasible, to apply AC there. In most cases this was a pharmacy. The recommended target dose of AC was 1 g/kg of bodyweight, but at least tenfold the estimated weight of the suspected toxin, the maximum being 50 g. AC doses less than 1 g/kg were advised when the estimated weight of an ingested substance with minor toxicity was smaller than 1 g. In such cases the recommendation was in accordance with the experimental results showing a sufficient binding with tenfold weight excess of AC over the toxin [10]. Formulation of AC and mode of application were at the discretion of the caller. We only suggested suspending AC in water. No suggestion was made concerning the kind of preparation of AC or a brand-name. However, when a caller asked for advice, the fastest opportunity to obtain AC was recommended regardless of the preparation. When asked for advice how to apply AC, we suggested to mix it with water, approximately 100 ml water per 5 g AC, but no more than 500 ml. When a layperson had called we made sure that the patient was alert and had no increased risk of aspiration and recommended to give the AC-suspension by mouth. For very small children we suggested the use of a baby-bottle. Medical professionals never asked for advice how to apply AC.
Case-relevant items were registered during the first consultation. Sites where the ingestion happened or the patient’s whereabouts at time of the call were categorized as sites in our opinion appropriate to store AC (e.g. household, nursery school, school, psychiatric ward, jail) or as not appropriate for AC-stockpiling (e.g. public space, recreational facilities, transport). Ingested substances and circumstances were categorized as a) minor hazardous and to be manageable with AC and observation by laypersons or b) as necessitating AC and evaluation by a physician, or c) as necessitating treatment in hospital or emergency-room. The caller’s consent to participate in the study was asked for at the end of the consultation. The quantity of AC applied and time-values were retrospectively estimated by the interview partner (=caller at first encounter). Statistical analysis was performed with SPSS-software Version 23 IBM Corp. Armonk, NY 2014. For parametric values we used the Mann-Whitney-test and for non-parametric data the Chi-square-test. For the AC-application quality parameters we used multiple linear, respectively a binary logistic regression models. A p < 0.05 was considered statistically significant. The study was approved by the local ethic committee.
3. Results
3.1. Availability of AC for application in non-medical setting
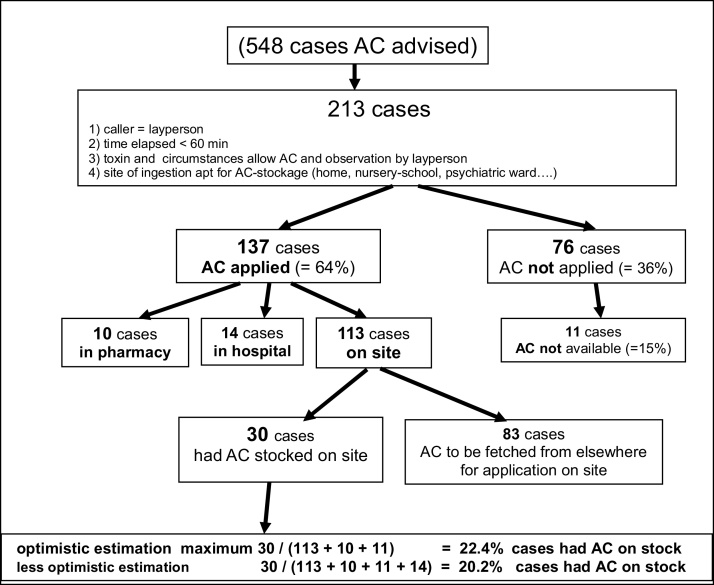
In all, there were 548 cases of AC recommendation. In 361 (=68.5%) cases, the PIC’s advice to apply AC was followed. To evaluate the availability of AC on site and time saving by application on site by having AC on-hand, we only evaluated cases, which might have profited from pre-emptive AC-stockpiling. Thus, the evaluation was restricted to cases, which met all following conditions: a) a layperson had called, b) latency ingestion-call was <60 min, c) the alleged toxin and accompanying circumstances allowed an observation by laypersons d) the site of ingestion was considered apt for AC storage. 213 cases met all criteria – see Fig. 1. Among those, 76 received no AC. In 11 cases (=14.5%) the cause for no AC application was non-availability of AC. 137 cases received AC: 14 in hospitals, 10 in pharmacies, 113 at site of ingestion (=“on site”- group), this was the caller’s home in 111 cases. Among those 113 “on site” – cases, 30 had AC already stored at site (=“onsite, AC on stock” −group), but in 83 cases AC had to be obtained from elsewhere prior to application on site (=“on site no AC” −group), in 80 cases, this was a pharmacy. As shown in Fig. 1 these 30 cases with AC on site show with an optimistic calculation 22.4% of the sites where toxin ingestions may happen and where pre-emptive AC stockpiling would be advisable, AC was actually on stock. A less optimistic calculation assumes that the 14 cases of AC applications in hospitals were due to non-availability of AC. That would mean that only 20.2% of sites where pre-emptive AC stockpiling would be advisable (i.e. mostly households), AC actually was on site (Table 1).
Fig. 1.
availability of AC.
Table 1.
adherence to AC advice, causes for non-application of AC.
| Caller |
||||
|---|---|---|---|---|
| layperson | medical professional | hospital | sum | |
| AC applied | 182 = 62.3% | 21 = 42.0% | 158 = 76.6% | 361 = 65.9% |
| AC not applied | 110 = 37.7% | 29 = 58.0% | 48 = 23.3% | 187 = 34.1% |
| causes for no AC | ||||
| revision of indication by new facts | 22 = 20.0% | 0 | 8 = 16.7% | 30 = 16.0% |
| caller or second opinion see no indication | 61 = 55.5% | 5 = 17.2% | 20 = 41.7% | 86 = 46.0% |
| contraindication against AC | 3 = 2.7% | 1 = 3.4% | 11 = 22.9% | 15 = 8.0% |
| refusal by patient | 3 = 2.7% | 1 = 3.4% | 6 = 12.5% | 10 = 5.3% |
| AC not available | 16 = 14.5% | 18 = 62.1% | 0 | 34 = 18.2% |
| other, unknown | 5 = 4.5% | 4 = 13.8% | 3 = 6.3% | 12 = 6.4% |
3.2. Time saving by stockpiling of AC
To prove the hypothesis that extra hospital AC application and stocking of AC on site shorten the time delay between toxin ingestion and AC application we examined the 137 cases of AC application mentioned above. Results are shown in Table 2. Median time delays between ingestion and call did not differ significantly. Median time lag between call and AC- application and consequently between ingestion and AC-application was significantly shortest when AC had been stored at site: 5 min, for on-site application compared to 24 min if AC was fetched from elsewhere, 19 min for AC-application in pharmacy and 34 min for AC-application in hospital (34 min). Pre-emptive stockpiling of AC on site saved at least 14 min compared with all other modes of AC-application. However, the differences among the other modalities did not reach statistical significance.
Table 2.
median lag-times in cases of AC-application with respect to site of application: site, where toxin ingestion happened „on site“ with or without pre-emptive stock of AC, in pharmacy, in hospital including emergency-rooms.
| Time | ingestion call | call- start AC | ingestion-start AC | ||||
|---|---|---|---|---|---|---|---|
| n | median | IQR* | median | IQR | median | IQR | |
| AC-application on site, AC stockpiled | 31 | 10 min | 8 | 5 min** | 6 | 17 min** | 14 |
| AC-application in pharmacy | 10 | 15 min | 6 | 19 min | 27 | 33 min | 27 |
| AC-application on site, no AC on stock | 83 | 10 min | 14 | 24 min | 23 | 40 min | 33 |
| AC-application in hospital | 14 | 10 min | 5 | 34 min | 33 | 52.5 min | 21 |
IQR = interquartile-range.
p < 0,005 compared with all other modalities; other comparisons: p > 0,05.
3.3. “Quality” of AC application: quantity of AC applied, time needed for application, incidence of side effects from AC
We compared the “quality” of AC-application by laypersons with application by medical professionals. As indicators for the “quality” we used the actual applied dose in g/kg bodyweight as percentage of the recommended dose in g/kg, the time needed for AC-application and the rate of AC-related side-effects. As shown in Table 3, at first glance laypersons seem to have performed worse than medical professionals: Laypersons only applied the recommended dose AC completely in 29.7% of the cases vs. 78.5% with medical professionals. The average percentage of the AC dose applied in relation to the recommended dose was 60.1% vs. 91.6%. Laypersons needed more time for AC application: 20.9 vs. 16.0 min. However, there are confounding and interrelated factors influencing these indicators. Laypersons used AC-tablets (75.6%) more often, whereas professionals preferred powder (92.6%); the laypersons’ patients were younger than those treated by professionals. As another confounding factor we analysed the recommended dose (g/kg) AC. On account of these multiple factors we used a logistic regression model for statistical analysis (see Table 4). Regarding the applied AC dose we found statistically significant effects with the recommended AC-dose and the AC-formulation. The effect of the applying person (lay/professional) just missed statistical significance, probably due to the small sample size. Regarding the time needed for AC-application we found no significant influence of any of the factors.
Table 3.
„quality“ of AC-application.
| layperson |
professional |
AC dose recommended g/kg |
AC applied given as% of recommended dose AC |
Recommended dose AC applied completely |
time needed for AC application |
patients age |
number of reported side effects |
|
|---|---|---|---|---|---|---|---|---|
| n, (%) | n, (%) | mean, (95% CI) | mean, (95% CI) | n, (%) | mean, (95% CI) | mean, (95% CI) | n, (%) | |
| layperson | 172 | 0.76 (0.0.71…0.82) |
60.89 (53.60…68.17) |
51 (29.7%) |
20.90 (15.89…25.97) |
11.76 (8.76…14.63) |
7 (4.0%) |
|
| professional | 189 | 0.74 (0.71…0.77) |
91.60 (87.07…96.12) |
148 (78.5%) |
15.97 (13.07…18.87) |
30.92 (28.0633.67) |
16 (8.5%) |
|
| AC − tablets | 130 (75.6%) |
14 (7.4%) |
55.75 (47.72…63.79) |
38 (26.4%) |
20.67 (14.74…26.60) |
13.42 (10.03…16.82) |
6 (4.2%) |
|
| AC − powder | 42 (24.4%) |
175 (92.6%) |
90.90 (86.57…95.23) |
161 (74.2%) |
16.78 (14.08…19.48) |
27.44 (24.48…30.01) |
17 (7.8%) |
Table 4.
logistic regressions.
| regression coefficient | standard error | 95% Confidence interval |
|||
|---|---|---|---|---|---|
| lower limit | upper limit | Sig. p | |||
| intercept | 117.171 | 8.018 | 101.399 | 132.944 | 0.000 |
| recommended dose AC_ (g_kg) | −31.093 | 7.428 | −45.703 | −16.482 | 0.000 |
| AC_formula_tablet/powderpowder = 0. tablet = 1 | −28.422 | 5.627 | −39.491 | −17.352 | 0.000 |
| layperson/professional professional = 0; lay = 1 |
−10.915 | 5.805 | −22.334 | 0.505 | 0.061 |
| age (patient) | −0.003 | 0.111 | −0.222 | 0.216 | 0.980 |
| time needed for AC application | |||||
| regression coefficient | standard error | 95% Confidence interval | |||
| lower limit | upper limit | Sig. p | |||
| intercept | 16.200 | 5.793 | 4.804 | 27.596 | 0.005 |
| recommended dose AC_ (g_kg) | 4.296 | 5.353 | −6.234 | 14.827 | 0.423 |
| AC_formula_tablet/powderpowder = 0. tablets = 1 | 1.180 | 4.117 | −6.918 | 9.278 | 0.775 |
| layperson/professional professional = 0; lay = 1 |
1.828 | 4.252 | −6.536 | 10.192 | 0.668 |
| Patient age | −0.116 | 0.081 | −0.275 | 0.042 | 0.150 |
AC- dose applied as% of recommended dose.
3.4. Side effects
Side effects attributable to AC were reported in 23 out of 361 AC-applications (=6.4%): Symptoms were (multiple entries per case possible): Vomiting: 16 times; constipation, diarrhoea, nausea, abdominal pain, sore throat: each of these 3 times. Serious side effects like aspiration or ileus were not observed. The difference of the incidence of side-effects did not reach statistical significance (Table 5).
Table 5.
binary logistic regression: rate of side −effects.
| regression coefficient | standard error | 95% confidence −interval |
sig. p | OR | ||
|---|---|---|---|---|---|---|
| lower limit | upper limit. | |||||
| Intercept | −3.042 | 1.675 | −5.625 | −1.558 | 0.048 | 0.001 |
| AC_given_g/kg | 0.608 | 0.960 | −0.879 | 3.316 | 1.836 | 0.325 |
| AC_formula_tablet/powder powder = 0. tablets = 1 |
−0.126 | 1.635 | −1.890 | 1.490 | 0.882 | 0.875 |
| layperson/professional professional = 0; lay = 1 |
−0.522 | 1.005 | −2.570 | 0.888 | 0.593 | 0.556 |
| patient age | 0.006 | 0.014 | −0.022 | 0.031 | 1.006 | 0.603 |
4. Discussion
Counselling by PICs reduces health care costs since PICs can judge when treatment by a healthcare professional is required after poison ingestion and when treatment and observation by laypersons is sufficient [11], [12], [13], [14]. Many cases of ingestions happen in households, in nurseries, at school, psychiatric wards, or prisons. These are sites, where a pre-emptive stockpiling of AC and it’s application by laypersons would be possible. AC must be applied as soon as possible [10] and is quickest when AC is available at site of the ingestion. Pre-emptive AC-stockpiling at theses sites would be an easily realizable precautious measure, which may help not only optimizing the therapy after ingestion of toxic substances but also may help saving health expenditures.
Our data allow estimating that in the area covered by the Munich PIC this optimal prearrangement was present in 20.2%–22.4% of the investigated cases. Studies in literature found 41% of households storing AC in Finland, [5] and 9.5% in Kentucky, USA, [6]. In our study, we found that in 14 out of 137 cases, where circumstances would have allowed on-site AC-application by laypersons, AC was given in hospitals unnecessarily (10.2%). Assuming this was due to the lack of AC, these hospital-treatments could have been avoided by pre-emptive AC-stockpiling.
Time lags between call and AC application naturally were shortest when AC was stored at site, median 5 min. When no AC was at hand and the patient had to be brought to a pharmacy where AC was available and applied, median time lag was 19 min longer; when AC had to be purchased, brought and applied at site, median time lag lengthened to 24 min. AC-administration in a hospital prolonged the median time lag to 34 min. However, in our study, probably due to the small number of cases only the time saved by AC-application on sites with AC in stock was statistically significant. In the Finnish study, time lag PIC-call/start AC was 24.5 min when AC was stored at home, and 41.6 min when AC had to be provided from elsewhere [5]. In the Kentucky study, time lag ingestion/start AC was 73 min when AC was applied in an emergency room and 38 min when applied at home [6]. Other studies compared call/start-time lags between prehospital AC-administration by paramedics with AC- administration in hospitals. Mean time savings ranged from 46.6 min [15] to 90 min [16]. Our study confirms these results and suggests that AC can be given safely by laypersons without serious side effects [5], [15], [16], [17], [18]. The rate of minor side-effects found in our study is in agreement with those reported by other studies [6], [17].
The time advantage achieved with AC application on site by laypersons may be thwarted by smaller doses of AC being applied in these settings. In our study, only 29% received the advised doses AC completely. The Finnish study found a complete AC application in 79% [5]. Osterhoud [18] found the patients’ age to influence the completeness of AC-applications. We did not see a relevant effect of patients’ age. Mean time needed for AC-application in our study ranged from 16 to 20.5 min which is within the range known from other studies [17], [18], [19], [20].
In anticipation of eventual concerns we wanted demonstrate that the “quality” of AC-application by laypersons is not that much inferior or possibly dangerous, that health care authorities could refuse the idea of pre-emptive AC-storage and AC-application by laypersons after counselling by a PIC. Therefore, a secondary goal of our study was assessing the “quality” of AC-application to support the hypothesis that AC application by laypersons is possible and safe. To our knowledge this was the first time that the subject “quality” of AC-application was investigated and there are no established criteria to assess this. We are aware that laypersons and medical professionals applied AC to quite different patients: The mean age of patients treated by laypersons was 11,7 years, in the group treated by professionals the mean age was 30,9 years (p < 0,005). It can be assumed that other features as are intention of ingestion, type of toxins, severity of intoxication or mode of AC-application also differed, but were not investigated. Nevertheless, our study results do not show a difference in the “quality of AC-application” that would justify rejecting the recommendation of precautionary stockpiling of AC and eventual AC-application by laypersons.
Within this investigation we made more by incidence two remarkable observations: 1) that the recommended dose AC (g/kg) and the actually applied dose (g/kg) are negatively correlated, meaning, that the more AC (g/kg) is recommended the less is actually given. This observation may add a new aspect to the question about the optimum AC-dose, but needs confirmation prior to an elaborate interpretation. 2) We found more AC could be applied when it was given as powder compared with AC −tablets. In our protocol had we left the choice of AC formulation to the discretion of the caller. We only suggested to dispense AC in water. However, medical professionals preferred AC-powder whereas laypersons (and probably pharmacists) favoured tablets. Thus, both factors are interconnected and it was not our study’s primary goal to investigate this question. Thus, eventually more specifically designed future studies with larger sample sizes may modify our observation. In Bavaria AC is sold by pharmacies without prescription. We suppose pharmacists prefer selling AC-tablets.
In a debate about AC for gut decontamination after ingestion of potentially toxic substances, it must not be omitted to mention that there is no scientific evidence that AC reduces morbidity and mortality after toxin ingestion [20], [21], [22]. Because of ethical and practical reasons, in the near future no well-designed and large studies are expected to answer this elemental question. However, single dose AC is recommend with good reason for gut decontamination despite the lack of evidence [2].
Our study bears several important limitations: 1) It is a monocentric study with a relatively small sample. Regional effects might influence the result: for example, the good accessibility of pharmacies in urban areas. Socio-economic features of the callers were not investigated, thus our results may be not comparable with results from other, e.g. more rural regions. 2) Data about times of AC application and AC doses applied were only estimated by the caregivers and not measured exactly. 3) Calls with recommendation to apply AC were only backtracked, when one of the authors of the study was on duty in the PIC, thus letting an unknown number of AC- recommendations uninvestigated. However, since the investigators worked in equal parts in all types of shifts, the investigated AC recommendations can be expected to be representative.
Although the limitations of our study may reduce the impact of the quantitative evaluation, they do not devaluate its qualitative results: A) As already shown by other studies, after assessment and counselling by PIC in cases with ingestion of minor toxic substances laypersons can apply safely AC without serious side effects. B) Stockpiling of AC at sites where ingestion may occur will reduce the time lag until AC-application. C) Only in a minority of sites where storage of AC seems reasonable, AC is actually at hand. D) If there was more AC stored at sites prone for ingestion of toxic substances, unnecessary treatment in medical institutions could be avoided. This could help to save expenditure within the health service. E) AC-powder can be more efficiently applied compared with AC −tablets, albeit there may be important confounding variables such as the applying persons’ medical background.
5. Conclusion
Efforts should be made to promote AC stockpiling at sites where ingestion of toxic substances is foreseeable e.g. areas with children, nursery schools, psychiatric wards etc. AC as powder may be preferred. This should be accompanied with educational measures for caregivers concerning the application of AC. PICs have an important role in assessing cases after ingestion of toxic substances.
Acknowledgement
This work was supported by the German Research Foundation (DFG) and the Technische Universität München within the funding programme Open Access Publishing,
Contributor Information
Rudolf Pfab, Email: rudi.pfab@tum.de.
Sabrina Schmoll, Email: Sabrina.schmoll@tum.de.
Gabriele Dostal, Email: Gabriele.Dostal@tum.de.
Jochen Stenzel, Email: Jochen.Stenzel@tum.de.
Alexander Hapfelmeier, Email: alexander.hapfelmeier@tum.de.
Florian Eyer, Email: Florian.Eyer@tum.de.
References
- 1.Mowry J.B., Spyker D.A., Cantilena L.R., Jr, McMillan N., Ford M. Annual report of the american association of poison control centers' national poison data system (NPDS): 31 st annual report. Clin Toxicol (Phila). 2014;52(10):1032–1283. doi: 10.3109/15563650.2014.987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juurlink D.N. Activated charcoal for acute overdose: a reappraisal. Br. J. Clin. Pharmacol. 2016;81(3):482–487. doi: 10.1111/bcp.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isbister G.K., Kumar V.V. Indications for single-dose activated charcoal administration in acute overdose. Curr. Opin. Crit. Care. 2011;17(4):351–357. doi: 10.1097/MCC.0b013e328348bf59. [DOI] [PubMed] [Google Scholar]
- 4.Greene S.L., Kerins M., O'Connor N. Prehospital activated charcoal: the way forward. Emerg. Med. J. 2005;22(10):734–737. doi: 10.1136/emj.2005.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamminpää A., Vilska J., Hoppu K. Medical charcoal for a child's poisoning at home: availability and success of administration in Finland. Hum. Exp. Toxicol. 1993;12(1):29–32. doi: 10.1177/096032719301200106. [DOI] [PubMed] [Google Scholar]
- 6.Spiller H.A., Rodgers G.C., Jr. Evaluation of administration of activated charcoal in the home. Pediatrics. 2001;108(6):E100. doi: 10.1542/peds.108.6.e100. [DOI] [PubMed] [Google Scholar]
- 7.: Chyka P.A., Seger D., Krenzelok E.P., Vale J.A. Position paper: single-dose activated charcoal. Clin Toxicol (Phila). 2005;43(2):61–87. doi: 10.1081/clt-200051867. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Oz B., Levichek Z., Koren G. Medications that can be fatal for a toddler with one tablet or teaspoonful: a 2004 update. Paediatr. Drugs. 2004;6(2):123–126. doi: 10.2165/00148581-200406020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Matteucci M.J. One pill can kill: assessing the potential for fatal poisonings in children. Pediatr. Ann. 2005;34(12):964–968. doi: 10.3928/0090-4481-20051201-12. [DOI] [PubMed] [Google Scholar]
- 10.Jürgens G., Hoegberg L.C., Graudal N.A. The effect of activated charcoal on drug exposure in healthy volunteers: a meta-analysis. Clin. Pharmacol. Ther. 2009;85(5):501–505. doi: 10.1038/clpt.2008.278. [DOI] [PubMed] [Google Scholar]
- 11.Galvao T.F., Silva E.N., Silva M.T., Bronstein A.C., Pereira M.G. Economic evaluation of poison centers: a systematic review. Int. J. Technol. Assess. Health Care. 2012;28(2):86–92. doi: 10.1017/S0266462312000116. [DOI] [PubMed] [Google Scholar]
- 12.Friedman L.S., Krajewski A., Vannoy E., Allegretti A., Wahl M. The association between U. S. Poison Center assistance and length of stay and hospital charges. Clin. Toxicol. (Phila). 2014;52(3):198–206. doi: 10.3109/15563650.2014.892125. [DOI] [PubMed] [Google Scholar]
- 13.LoVecchio F., Curry S., Waszolek K., Klemens J., Hovseth K., Glogan D. Poison control centers decrease emergency healthcare utilization costs. J. Med. Toxicol. 2008;4(4):221–224. doi: 10.1007/BF03161204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keyes C., De Tamble L. Prehospital activated charcoal: a prospective randomised trial. Clin. Toxicol. (Phila). 1999;37:610. (abstract 65). [Google Scholar]
- 15.Crockett R., Krishel S.J., Manoguerra A., Williams S.R., Clark R.F. Prehospital use of activated charcoal: a pilot study. J. Emerg. Med. 1996;14(3):335–338. doi: 10.1016/0736-4679(96)00030-3. [DOI] [PubMed] [Google Scholar]
- 16.Alaspää A.O., Kuisma M.J., Hoppu K., Neuvonen P.J. Out-of-hospital administration of activated charcoal by emergency medical services. Ann. Emerg. Med. 2005;45(2):207–212. doi: 10.1016/j.annemergmed.2004.07.448. [DOI] [PubMed] [Google Scholar]
- 17.Personne M. Use of activated charcoal in the pre-hospital situation. Clin. Toxicol. (Phila). 2004;42:402. (abstract 6). [Google Scholar]
- 18.Osterhoudt K.C., Alpern E.R., Durbin D., Nadel F., Henretig F.M. Activated charcoal administration in a pediatric emergency department. Pediatr. Emerg. Care. 2004;20(8):493–498. doi: 10.1097/01.pec.0000136064.14704.d1. [DOI] [PubMed] [Google Scholar]
- 19.Fischer T.F., Singer A.J. Comparison of the palatabilities of standard and superactivated charcoal in toxic ingestions: a randomized trial. Acad. Emerg. Med. 1999;6(9):895–899. doi: 10.1111/j.1553-2712.1999.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 20.Eddleston M., Juszczak E., Buckley N.A., Senarathna L., Mohamed F., Dissanayake W., Hittarage A., Azher S., Jeganathan K., Jayamanne S., Sheriff M.R. Warrell DA; Ox-Col Poisoning Study collaborators. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371(9612):579–587. doi: 10.1016/S0140-6736(08)60270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Silva H.A., Fonseka M.M., Pathmeswaran A., Alahakone D.G., Ratnatilake G.A., Gunatilake S.B., Ranasinha C.D., Lalloo D.G., Aronson J.K., de Silva H.J. Multiple-dose activated charcoal for treatment of yellow oleander poisoning: a single-blind, randomised, placebo-controlled trial. Lancet. 2003;361(9373):1935–1938. doi: 10.1016/s0140-6736(03)13581-7. [DOI] [PubMed] [Google Scholar]
- 22.Merigian K.S., Blaho K.E. Single-dose oral activated charcoal in the treatment of the self-poisoned patient: a prospective, randomized, controlled trial. Am. J. Ther. 2002;9(4):301–308. doi: 10.1097/00045391-200207000-00007. [DOI] [PubMed] [Google Scholar]