Graphical abstract
Chemical compounds studied in this article: Cadmium dichloride (PubChem CID: 24947); Acetylthiocholine (PubChem CID: 74629); 5,5′-Dithiobis(2-nitrobenzoic acid) (PubChem CID: 6254); Thiobarbituric acid (PubChem CID: 2723628); Dihydronicotinamide-adenine dinucleotide phosphate (PubChem CID: 5884); Hydrogen peroxide (PubChem CID: 784); Cumene hydroperoxide (PubChem CID: 6629)
Keywords: Biomarker, Planktonic copepod, Cadmium, Centropages ponticus
Highlights
-
•
Ecotoxicological effects of cadmium chloride were tested in planktonic copepods Centropages ponticus.
-
•
Cadmium chloride toxicity influenced enzymatic activity and proteins synthesis in treated groups.
-
•
Synthesis of proteins, together with changes in antioxidant enzymes activity, could be used as biomarkers for further studies of copepods species.
Abstract
Pollution of the aquatic environment by heavy metals has become a worldwide problem. Most heavy metals exhibit toxic waste on aquatic organisms. Cadmium (Cd) is a highly toxic metal which affects aquatic organisms acutely and chronically. Planktonic calanoid copepods are the secondary dominant producers of pelagic ecosystems and play a considerable role in the transfer of energy and organic matter from primary producers to higher trophic levels. We investigated the effect of cadmium chloride on biochemical responses of the planktonic calanoid copepods Centropages ponticus which is a key species in the Mediterranean Sea. The response of copepods to cadmium chloride was examined under laboratory-controlled conditions during a 72-h exposure. Catalase (CAT), Glutathion Reductase (GR), Glutathione Peroxidase (GPx), Glutathione-S-Transferase (GST) and Acetylcholinesterase (AChE) were analyzed for cadmium chloride treatments (0, 0.2 and 0.4 μg/L) after 24, 48 and 72 h. Additionally, the thiobarbituric reactive species assay was used to evaluate lipid peroxidation (LPO) level of the copepod. In this study, it is observed that contents of protein increased gradually with an increase in concentrations of metals and exposure time. Our findings showed that cadmium chloride directly influenced malondialdehyde (MDA) levels in the treated copepods hinting that the copepods had suffered from oxidative damage. During exposure, the Cd treatments significantly influenced the biochemical markers (CAT, GR, GPx, GST and AChE). Thus, Centropages ponticus could be used as a suitable bioindicator of exposure to Cd using biochemicals markers.
1. Introduction
Over the last century, chemical pollution caused by human activities has become one of the most important stressors of aquatic ecosystems. Heavy metals are regarded as one of the most serious pollutants due to their environmental persistence and tendency to accumulate in aquatic environment [1]. Cadmium (Cd) is a biotoxic element and one of the major metals that are ubiquitously distributed in aquatic systems [2]. It’s also a widely-used heavy metal in the industry. In recent years; serious Cd pollution of the aquatic systems has become a dilemma [3]. Because of its extreme toxic properties to aquatic organisms, cadmium is known by the European Community as a priority hazardous substance in the field of water policy [4] as well as a metal of primary interest by USEPA [5]. It is a non-essential heavy metal that can lead to the disruption of cellular homeostasis [6], DNA damages [7], membrane depolarization and acidification of the cytoplasm [8]. Cd has also been reported to damage essential biochemical and physiological functions [9] and to stimulate the production of reactive oxygen species (ROS) such as superoxide anion and hydroxyl radicals [10]. These compounds are responsible for cell and tissue damage associated with the different pathological process [11]. Antioxidant compounds such as catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR) and glutathione peroxidase (GPx) are found to protect cells and tissues from oxidative damages and to neutralize the toxicity of ROS [12]. The use of molecular and enzymatic biomarkers is a sensitive tool in ecotoxicology because the effects on organisms and populations can be detected before ecosystem-damage are irreversible [13]. Cadmium can also lead to neuronal damage as neurotoxicity [14]. Acetylcholinesterase (AChE) is a principal component of cholinergic system which controls the nervous impulse transmission in cholinergic synapses. Over the last years, the acetylcholinesterase activities (AChE) have been widely used as environmental neurotoxicity biomarkers because they play important roles in the neurotransmission [15].
Zooplankton is an important component in aquatic ecosystems and a key organism within the food web and in the biogeochemical cycling of elements [16]. Copepods are one of the most important components of zooplankton and play major roles in the structure and functioning of marine planktonic food webs [17]. As zooplanktonic organisms, copepods are likely to be impacted by exposure to contaminants in the aqueous phase in the water column and through the ingestion of contaminated food. Therefore, it is crucial to address their responses when predicting the effects of any pollutants on pelagic ecosystems. Calanoid copepods are appropriate test organisms used internationally for toxicity screening of chemicals and for monitoring of effluents and contaminated waters due to their small size, short generation time and ease of culturing in the laboratory [18]. The planktonic copepod Centropages ponticus.is very interesting. This species has a large distribution area throughout the Mediterranean basin and a major ecological importance in the local food web [17]. Moreover, its ecology has been intensively studied. This species inhabits the water column and often dominates the zooplankton assemblage [17]. As a model for bioassays it’s suitable since it has a short life cycle and is easy to handle and maintain in the laboratory [19].
The present research survey therefore focused on Cd toxicity and antioxidant response of C. ponticus. We examined its biochemical response to Cd treatments via measurement of various biochemical parameters (CAT, GR, GPx, GST, AChE) and the MDA level. This is the first attempt to investigate Cd oxidative effects on the planktonic copepod C. ponticus, to explore the response mode of this copepod to Cd stress and to sieve out a potential biomarker to Cd pollution.
2. Materials and methods
2.1. Chemicals
All reagents were of analytical grade and all laboratory glassware was soaked in 10% (v/v) nitric acid (Merck) for at least 48 h and rinsed 3 times with distilled water prior to use in order to keep the testing solution to the nominal level of Cd. All chemical stock solutions were made volumetrically using deionised water. A cadmium stock of 100 mg Cd/L was prepared using cadmium chloride salt (Sigma aldrich). Working solutions were prepared by dilution of the stock solutions.
2.2. Biological material
The planktonic copepod Centopages ponticus was collected during low tides from the Bizerte Bay (37°17′09.23″N, 09°53′91,1″E) north east of Tunisia, by using plankton net with 200 μm mesh (0.57 m mouth diameter). The collected samples were immediately transported to the laboratory of Biodiversity and Functioning of Aquatic Systems (Faculty of Sciences of Bizerte, Tunisia). Copepods identification was based on morphological criteria following consultation of taxonomic keys and species descriptions [20] and using a dissecting microscope (Leica) at 40× magnification. Preliminary studies were performed in order to optimize the temperature of acclimatation. We have also demonstrated that slow temperature allows acclimatization which may result in an increase in the range of tolerance. Animals were then acclimatized to laboratory and test conditions in the temperature-controlled room at 18 °C for 48 h in 3 L beakers containing seawater with low continuous aeration with a 12:12-h light:dark cycle. Copepods were fed with natural food to avoid starvation and any negative effects resulting from the food. After the acclimation period only the healthy and active copepods which showed normal swimming ability were retained and used as test animals in the bioassay.
2.3. Cd exposure
In the bioassays, standard water quality parameters were as follows: salinity 34, dissolved oxygen 6 ± 0.1 mg L−1, pH 8.1 ± 0.1. We have chosen to maintain the temperature at 29 ± 1 °C during the toxicity tests to simulate the environmental temperature found in the Bizerte Bay where the copepods were collected. To perform the ecotoxicological test, adult copepods obtained as previously reported, were divided into 3 groups. Each group contained three replicates (90 individual/400 mL/replicate). The first group (control) was kept in filtered sea water, the second group treated with 0.2 μg L−1 CdCl2, the third group treated with 0.4 μg L−1 of CdCl2. The experimental time intervals were 24, 48 and 72 h. Water samples (50 mL) were taken from each beaker for cadmium analysis in order to assess the effective concentrations of the heavy metal by using atomic absorption spectrometry (Perkin Elmer PinAAcle 900T, USA).
2.4. Sample preparation
For the measurement of biomarkers, a total of 30 individuals were pooled in each sample in order to yield a sufficient sample volume for enzyme activity assays and for the determination of lipid peroxydation (LPO). The pools of Centropages ponticus were transferred manually with a pipette from tanks to a collection beakers containing water at the same temperature. To concentrate organisms, they were then quickly lifted out of the water on a special removable sieve. The specimens were subsequently transferred to an Eppendorf tube, and stored at −80 °C until further processing.
Before biochemical analysis, every pooled specimen of C. ponticus was homogenized in sodium phosphate buffer, 0.1 M, pH 7.5. The obtained homogenate was centrifuged at 10,000g for 30 min at 4 °C. The supernatant was removed and used to determine enzymes activities. Braford’s method [21] was used for quantitative determination of proteins with bovine serum albumin (BSA) as standard. All assays were performed in triplicate.
2.5. Determination of AChE activity
AChE activity was spectrophotometrically performed according to Ellman method [22] by measuring the absorbance increase of the sample at 412 nm in the presence of 3 mM acetylthiocholine as substrate and 0.1 mM 5,5,-dithiobis- 2-dinitrobenzoic acid (DTNB) at a controlled temperature of 20 °C. AChE activity measurement was performed in triplicate and was expressed as nmol of developed product per minute per mg of proteins.
2.6. Determination of MDA contents
Lipid peroxidation was measured using the thiobarbituric acid test for MDA according to Recknagel et al. [23] based on the reaction between malondialdeldehyde (MDA) and thiobarbituric acid (TBA) at 100 °C to form a complex that absorbs maximally at 532 nm.
2.7. Determination of antioxidant activities
Catalase activity (CAT) was assayed by measuring the rate of decomposition of H2O2 at 240 nm following the method of Clairborne [24]. Glutathione reductase activity (GR) was assayed by following at 37 °C and 340 nm the oxidation of NADPH by GSSG [25]. The enzymatic Glutathione S-transferase activity (GST) was measured at 340 nm according to Habig et al. [26] using CDNB as a substrate. Glutathione peroxidase (GPx) activity was measured by assaying glutathione recycling enzymes using cumene hydroperoxide as a substrate and monitoring NADPH oxidation at 340 nm [27]. Specific activity of enzymes is defined as nmol/min/mg protein.
2.8. Statistical analysis
GraphPad Prism 5.01 (GraphPad Software, Inc., San Diego, CA) was used to generate graphs. All statistical studies were performed with STATISTICA® 6.0 Software. The results were expressed as mean ± SD. Significant differences between groups were analyzed by one-way analysis of variance and Tukey HSD test where appropriate. A probability level of less than 0.05 was considered significant (95% confidence interval).
3. Results
3.1. Protein content
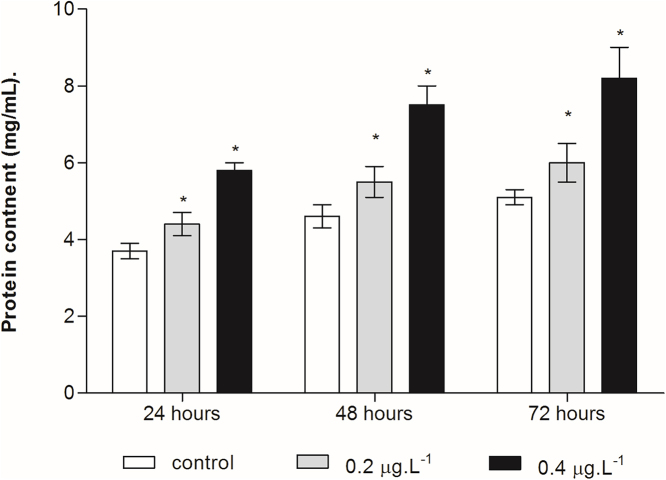
Fig. 1 shows the effect of Cd duration and exposure concentration on protein content in copepod. According to the two-way ANOVA test, both duration and concentration affected copepod’s protein content (p < 0.05). The protein content in all treated groups tended to increase with an increase of Cd concentration and time of exposure. The protein values in the 0.2 and 0.4 μg/L treatments increased 1.2, 1.5, 1.6 and 1.2, 2.0, 2.2 times, respectively, with respect to the control.
Fig. 1.
Effect of cadmium chloride on protein content in the planktonic copepod Centropages ponticus. Data are described as mean of three replicate ± standard deviation. Asterix indicate a significant difference among control group at p < 0.05.
3.2. The effect of cadmium on AChE activity
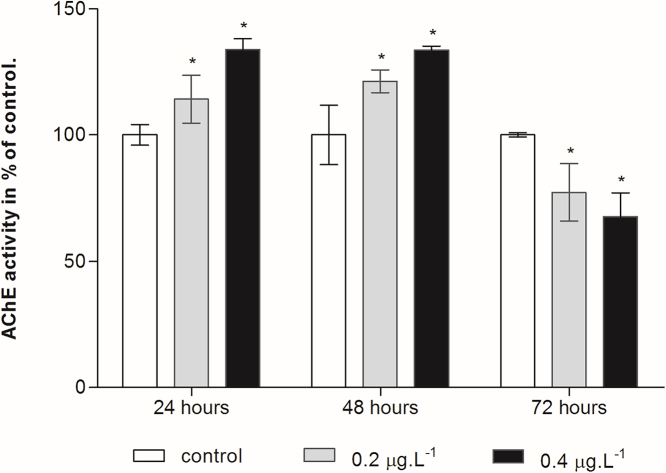
The response of AChE activity after exposure to Cd was reported in Fig. 2. AChE activity exhibited a significant increasing trend from control during the early exposure time (24 and 48 h). Subsequently, Cd treatment depressed the copepod’s AChE activity after 72 h of exposure and being significantly different from control group.
Fig. 2.
Effect of cadmium chloride on AChE activity in the planktonic copepod Centropages ponticus. Data are described as mean (in% of control) ± standard deviation. Asterix indicate a significant difference among control group at p < 0.05.
3.3. The effect of cadmium on MDA levels
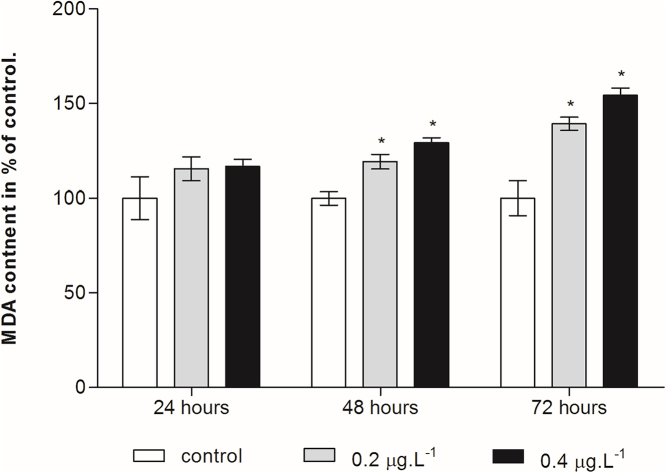
The influence of Cd on LPO content was measured as MDA level. Fig. 3 showed that there was no significant difference in MDA level between control and treated group after 24 h of exposure. While after 48 and 72 h of exposure, the copepod’s MDA level significantly intensified with the increase of Cd concentration (p < 0.05).
Fig. 3.
Effect of cadmium chloride on MDA levels in the planktonic copepod Centropages ponticus. Data are described as mean (in% of control) ± standard deviation. Asterix indicate a significant difference among control group at p < 0.05.
3.4. The effect of cadmium on antioxidant enzymes
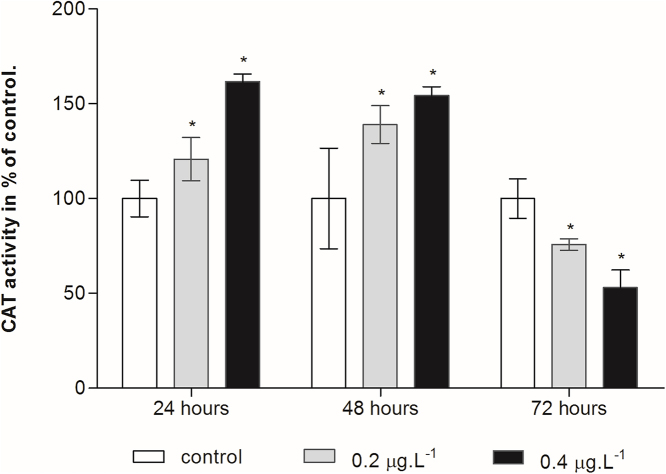
The activities of the antioxidant enzymes were evaluated in copepod exposed to 0.2 and 0.4 μg/L of cadmium chloride for 24, 48 and 72 h. Our results showed that exposure to CdCl2 for 24 and 48 h caused significant increase in CAT activity (Fig. 4). However, a significant decrease of CAT activity was detected during the 72-h exposure and it concerned both concentrations of tested cadmium.
Fig. 4.
Effect of cadmium treatment on CAT activity in the planktonic copepod Centropages ponticus. Data are described as mean (in% of control) ± standard deviation. Asterix indicate a significant difference among control group at p < 0.05.
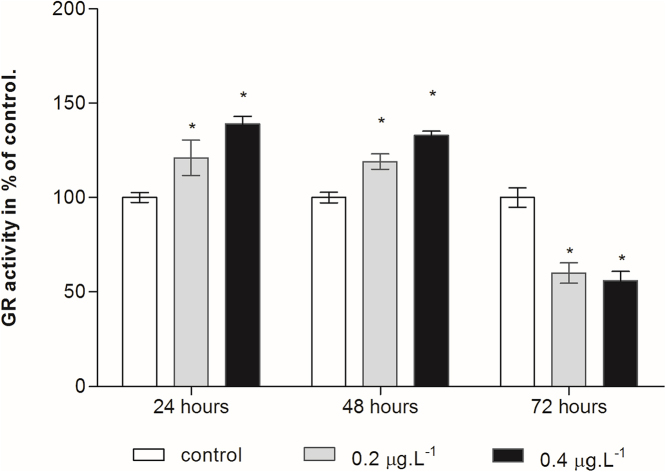
Fig. 5 showed the response of GR activity to Cd treatments. Until 24 h, both 0.2 μg L−1 and 0.4 μg L−1 Cd treatments significantly stimulated copepod’s GR activity. The GR activity reached its peak stimulation in 48 h in the two treated groups, and then significantly decreased at 72 h (p < 0.05).
Fig. 5.
Effect of cadmium treatment on GR activity in the planktonic copepod Centropages ponticus. Data are described as mean (in% of control) ± standard deviation. Asterix indicate a significant difference among control group at p < 0.05.
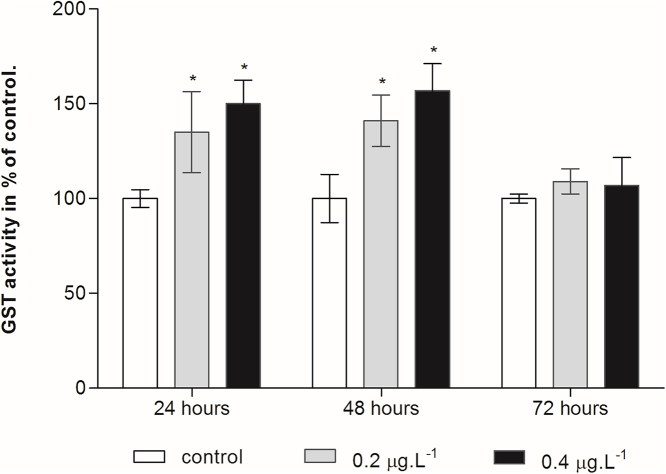
Fig. 6 showed that GST activity in copepods was significantly higher after 24-h (135% and 150%) and after 48-h exposure (140.8% and 156.9%) for the two exposed groups as compared with control groups. However, after 72-h exposure, GST activity remained unchangeable as compared with control groups showing values of 382.6 and 373.6 nmol/min mg protein for 0.2 and 0.4 μg/L Cd respectively.
Fig. 6.
Effect of cadmium treatment on GST activity in the planktonic copepod Centropages ponticus. Data are described as mean (in% of control) ± standard deviation. Asterix indicate a significant difference among control group at p < 0.05.
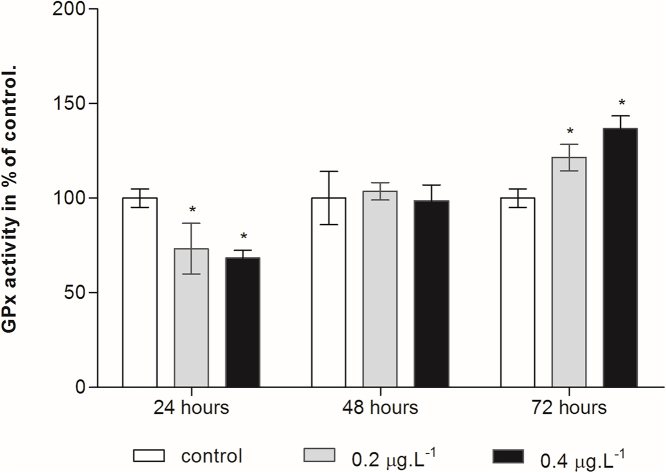
Fig. 7 showed the response of GPx activity to Cd treatments. In fact, the two Cd treatments (0.2 and 0.4 μg/L) exerted a significant inhibiting effect in a dose-dependent manner after 24 h of exposure (p < 0.05) as compared to the control groups. 48 h later, the GPx activity of the treated groups returned to the same level as the control and thereafter increased to 72 h of exposure in a dose dependant manner.
Fig. 7.
Effect of cadmium treatment on GPx activity in the planktonic copepod Centropages ponticus. Data are described as mean (in% of control) ± standard deviation. Asterix indicate a significant difference among control group at p < 0.05.
4. Discussion
Contamination of costal marine ecosystems with heavy metals has increased worldwide. They are gradually intensified in various aquatic organisms as they reach higher trophic levels of the food chain [28]. Health hazards created by heavy metals have become a great concern since they affected human’s health via the food chain [29]. In recent years massive efforts have been devoted to identify viable and ecologically relevant invertebrate toxicity testing models. The use of aquatic invertebrate as indicator organisms for biomonitoring the quality of the aquatic ecosystems is becoming increasingly important [30]. Since their position in aquatic food chains is very prominent, planktonic copepods play an important role in the transfer of aquatic pollutants across the marine food web [29]. The purpose of this study is to illustrate the potential of using the plonktonic copepod Centropages ponticus as an invertebrate marine model organism for ecotoxicology study. Therefore an attempt has been made to study the influence of cadmium chloride exposure, on the response pattern of some biochemical markers in this organism.
Variation in protein levels in living organisms is one of the main signs of an applied stress. Siddiki and Suponglemla [31] were considered protein content change as a useful index of metal toxicity in the fresh water Indian cat fish Clarias batrachus. Our results illustrated in Fig. 1 indicated that contents of protein increased gradually with an increase in concentrations of cadmium chloride and exposure time. The increase in the amount of protein could be due to the de novo synthesis of stress proteins provoked by metal exposure. These stress proteins may constitute enzymes involved in metallothioneine biosynthesis and those required for antioxidants and some heat shock proteins synthesis [32]. This agrees with previous findings indicating that exposure of endemic amphipod to CdCl2 triggers a preferential increase in stress protein synthesis [33]. Ivanina et al.[34] showed that exposure to cadmium resulted in a dose-dependent increase in the rate of protein synthesis in oyster cells. Increase tissue proteins in fish exposed to various toxicants has also been reported by [35].
Acetylcholine is the primary neurotransmitter in the sensory and neuromuscular systems in most species. The activity of this system is vital to normal behaviour and represents a prime target on which some toxicants can exert a detrimental effect [15]. Measurement of AChE activity in aquatic organisms has already been used as a biomarker of effects of neurotoxic contaminants. Our study clearly shows that Cd exposure elicits a strong induction of AChE activity during the earliest time of exposure (24 and 48 h) and is inhibited after 72 h. The results are in agreement with Emadeldeen [36] who has observed an increase on the AChE activity in the copepod Apocyclops borneoensis exposed to 1000 μg/L of Ni, after 24 h, and then significantly decreases with the increase of exposure time. Thus, increase in AChE activity in response to metals seems to be an immediate response to acute exposure, while decreases in AChE activity would be expected after more extended exposure. Bainy et al. [37] have reported that the increase in AChE activity would be related to a de novo synthesis of the enzyme as a response to an initial inhibition and they suggest that cadmium interacts with the acetylcholine receptor and thereby affects its binding efficiency, leading to an increase in AChE synthesis which decomposes the higher levels of acetylcholine. After 72 h of treatment with cadmium the AChE inhibition agrees with several studies that indicate a classical inhibitory impact of metals on AChE activity [38]. According to Talesa [39], AChE activity in invertebrates displays less defined substrate specificity and a marked variability in the kinetic behaviour, depending therefore on the duration and the metal concentration used compared to vertebrates.
Lipid peroxidation is a main indicator of cellular membrane damage by heavy metals [40]. MDA levels following CdCl2 exposure are investigated in our study, and the results are shown in Fig. 3. Our results demonstrate that Cd treatment increases significantly the levels of MDA in copepods after 48 and 72 h. The increase in lipid peroxidation implied that excess ROS had been produced in copepods upon exposure to Cd. Our findings support the results of Wang and Wang [41] who reported similar results in Tigriopus japonicus copepod exposed to 100 μg/L of Cd. The increase in lipid peroxidation is also observed in Gammarus pulex after 32 μg/L of Cd exposure [42]. Li et al. [40] have demonstrated that the MDA contents increase with exposure time and dose and show time and dose-dependency in Crab Sinopotamon henanense exposed to Cd. The increased MDA concentration helps to conclude that the antioxidant defense system of copepods is damaged under Cd stress, which resultes in cell structural damages.
Recent studies have shown that heavy metals including cadmium, are pro-oxidants and cause alterations of antioxidant defense systems [43]. CAT is responsible for eliminating the intracellular H2O2 by transforming H2O2 into H2O and O2. First, our result reveales an activation of CAT activity after 24 and 48 h of Cd exposure and then an inhibition after 72 h (Fig. 3), indicating that when Cd enteres the organism, the activity of superoxide dismutase is subsequently stimulated to increase the concentration of H2O2. The higher concentration of H2O2 induces the activity of CAT [44]. With increasing time and concentration of Cd (72 h) the scavenging capability of CAT is reduced. The decrease in CAT activity could be due to its inhibition by the excess production of ROS as evidenced by MDA in the present study and triggeres a sequence of reactions leading to death. These findings are in accordance with Lei et al. [45] who notes that CAT activity is increased in freshwater crab Sinopotamon yangtsekiense exposes to Cd on day 5 and decreases with increasing Cd concentration and exposure time. Similarly, study conducted on Gammarus pulex, that are exposed to Cd and assessed for antioxidant stress activity shows that the activity of CAT increases for 48 h. However, a decrease is observed compared with the control group and the authors claimed that CAT is the first enzyme to respond to oxidative stress [42].
GR plays an important role in cellular antioxidant protection because it catalyzes the regeneration of GSH from GSSG. The GR activity is increased in copepod after 24 and 48 h of exposure to Cadmium and decreases significantly after 72 h. The increase of GR activity probably plays an important role in preventing the alteration of the GSH status on first days of exposure to cadmium. Thus, the significant rise at the earliest exposure time correlates with the elevation in CAT activity. However, a decrease in GR activity may result in GSH depletion if extra synthesis of GSH cannot occur to protect its redox status. Ensibi et al. [12] have indicated that the activities of antioxidant enzymes may be elevated or inhibited by chemical stress depending on the intensity and the duration of the stress applied as well as on susceptibility of the exposed species. Kanak et al. [46] have emphasized that little change in GR activity could be attributed to the significant changes in the other first line of defense enzymes or may indicate that the tolerance limit of organisms to metals is not exceeded.
As a GSH-dependent enzyme, GST catalyzes the conjugation reaction of xenobiotics with GSH [47]. GST also facilitates conjugation of electrophilic substances including ROS and the byproducts of LPO to glutathione in order to make the xenobiotics more hydrophilic for transportation or excretion [12]. In this study, significant changes in the GST activity are observed in early time-courses in both concentrations tested, but remain unchanged for 72 h in comparison to control group. Our results are in agreement with other studies. Wang and Wang [41] have found that heavy metals (Cd and Ni) significantly increase GST activity of the benthic copepod Tigriopus japonicus after 7 days exposure. Similarly Emadeldeen [36] has found that Ni treatments increase GST activity in day 7, and is significantly different from control under 100 μg/ L Ni treatment in copepod Apocyclops borneoensis. On the other hand, a decrease in the GST activity is reported in mussels Mytilus galloprovincialis exposed to benzo[a]pyrene [48]. It is also shown that Cu cause a reduction of marine gastropod Nucella lapillus GST activity [14]. However, Jemec et al. [49] have noticed no impact of Cd concentrations up to 40 μg/L on GST activity in Daphnia magna. Therefore, GST response might be dependent on species, intensity and duration of the chemical stress applied to the organism in addition to the susceptibility of the exposed species.
Our results show that copepod’s GPx activity significantly decreases after 24 h of exposure and increases after 72 h. Yet, after 48 h it remaines unchangeable. GPx detoxifies ROS by reducing lipid hydroperoxides to stable alcohols and by removing hydrogen peroxide formed in the cytosol [50]. Either stimulation or inhibition of GPx activities by Cd have been measured in copepods [41]. The decrease of GPx activity with Cd concentration at 24 h might be attributable to the interaction of Cd with selenium or with the seleno-prosthetic group precursor and therefore deactivates the enzyme activity, as reported in other studies [51]. However, during the later exposure time, GPx activity increases in a dose-dependent mode and becomes involved in the detoxification of H2O2 and organic hydroperoxide due to ROS accumulation. However, an increase in GPx activity can be attributed to a stress that copepods face. These results are consistent with previous studies [41].
5. Conclusion
The aim of the present study is to investigate the impact of cadmium chloride at slighter concentrations than environmental ones on planktonic copepods (Centropages ponticus) during 72 h of exposure. The toxic impact is evaluated on the basis of results of biochemical examinations. In this research, Cd exposure induces some changes in copepods antioxidant stress enzymes and causes significant changes in MDA levels. Our results support the notion that the mode of cadmium toxicity in copepods is evident through both lipid peroxidation and general cellular oxidative stress. These results indicate that antioxidant defense components in C. ponticus are significantly affected by cadmium contamination. The biochemical response to sublethal exposure in copepods could be a valuable tool, in particular using biomarkers like CAT, GR, GST, GPx activities and MDA levels. These biomarkers could be proposed as a battery to monitor cadmium pollution in aquatic environments.
Conflict of interest
The authors declare that there are no conflict of interest.
Acknowledgements
We would like to thank Dr Noura Hamdi for the English corrections. This study was supported by the Ministry of Higher Education and Scientific Research of Tunisia through the bilateral cooperation program between Tunisia and France (LMI COSYS-MED laboratory). Anonymous reviewers are acknowledged for their constructive remarks that resulted in significant improvement of this paper.
References
- 1.Benzer S., Arslan H., Uzel N., Gül A., Yılmaz M. Concentrations of metals in water, sediment and tissues of Cyprinus carpio L., 1758 from Mogan Lake (Turkey) Iran. J. Fish. Sci. 2013;12(1):45–55. [Google Scholar]
- 2.Ghiasi F., Mirzargar S.S., Bdakhshan H., Shamsi S. Effects of low concentrations of cadmium on the level of lysozyme in serum, leukocyte count and phagocytic index in Cyprinus carpio under the wintering conditions. J. Fish. Aquat. Sci. 2010;5:113. [Google Scholar]
- 3.Zamora L.M., Catherine K.K., Sarah J.P., Patti V. Sensitivity and response time of three common Antarctic marine copepods to metal exposure. Chemosphere. 2015;120:267–272. doi: 10.1016/j.chemosphere.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 4.European Commission Directive 2455/2001/EC of the European parliament and of the council of 20 November 2001 establishing improvements on directive /60/EC. List of priority hazardous substances in the field of water policy. Off. J. Eur. Communities. 2000 L331/331-L331/335. [Google Scholar]
- 5.U.S.EPA . U.S.EPA; Washington DC, USA: 2007. Framework for Metals Risk Assessment. Risk Assessment Forum EPA-120/R-07/001. [Google Scholar]
- 6.Macías-Mayorga D., Laizc I., Moreno-Garridoa I., Blasco J. Is oxidative stress related to cadmium accumulation in the Mollusc Crassostrea angulata? Aquat. Toxicol. 2015;161:231–241. doi: 10.1016/j.aquatox.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Wei-Na W.M.C., An-Li W., Ting-Ting T., Peng W., Ying Z., Yuan L. Effects of cadmium on respiratory burst, intracellular Ca2+ and DNA damage in the white shrimp Litopenaeus vannamei. Comp. Biochem. Physiol. Part C. 2009;149:581–586. doi: 10.1016/j.cbpc.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Van-Kerkhove E., Pennemans V., Swennen Q. Cadmium and transport of ions and substances across cell membranes and epithelia. Biometals. 2010;23:823–855. doi: 10.1007/s10534-010-9357-6. [DOI] [PubMed] [Google Scholar]
- 9.Cirillo T., Cocchieri R.A., Fasano E., Lucisano A., Tafuri S., Ferrante M.C., Carpene E., Andreani G., Isani G. Cadmium accumulation and antioxidant responses in Sparus aurata exposed to waterborne cadmium. Arch. Environ. Contam. Toxicol. 2012;62:118–126. doi: 10.1007/s00244-011-9676-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Qian W., Jiarui L., Qingqing S., Fei W., Lan W. Cadmium induces hydrogen peroxide production and initiates hydrogen peroxide-dependent apoptosis in the gill of freshwater crab, Sinopotamon henanense. Comp. Biochem. Physiol. Part C. 2012;156:195–201. doi: 10.1016/j.cbpc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Dabas A., Nagpure N.S., Kumar R., Kushwaha B., Kumar P., Lakra W.S. Assessment of tissue-specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrel, Channa punctatus. Fish Physiol. Biochem. 2012;38:469–482. doi: 10.1007/s10695-011-9527-7. [DOI] [PubMed] [Google Scholar]
- 12.Ensibi C., Pérez-López M., Soler-Rodríguez F., Míguez-Santiyán M.P., Daly-Yahia M.N., Hernández-Moreno D. Effects of deltamethrin on biometric parameters and liver biomarkers in common carp (Cyprinus carpio L.) Environ. Toxicol. Pharmacol. 2013;36:384–391. doi: 10.1016/j.etap.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Cao L., Huang W., Liu J.H., Yin X.B., Dou S.Z. Accumulation and oxidative stress biomarkers in Japanese flounder larvae and juveniles under chronic cadmium exposure. Comp. Biochem. Physiol. C: Pharmacol. Toxicol. 2010;151:386–392. doi: 10.1016/j.cbpc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Cunha I., Mangas-Ramirez E., Guilhermino L. Effects of copper and cadmium on cholinesterase and glutathione S-transferase activities of two marine gastropods (Monodonta lineata and Nucella lapillus) Comp. Biochem. Physiol. Part C. 2007;145:648–657. doi: 10.1016/j.cbpc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Ensibi C., Hernández-Moreno D., Míguez-Santiyán M.P., Daly-Yahia M.N., Rodríguez F.S., Pérez-López M. Effects of carbofuran and deltamethrin on acetylcholinesterase activity in brain and muscle of the common carp. Environ. Toxicol. 2014;29(4):386–393. doi: 10.1002/tox.21765. [DOI] [PubMed] [Google Scholar]
- 16.Swadling K.M., Gibson J.A.E., Ritz D.A., Nichols P.D., Hughes D.E. Grazing of phytoplankton by copepods in eastern Antarctic coastal waters. Mar. Biol. 1997;128:39–48. [Google Scholar]
- 17.Neffati N., Daly-Yahia-Kefi O., Bonnet D., Carlotti F., Daly-Yahia M.N. Reproductive traits of two calanoid copepods: Centropages ponticus and Temora stylifera, in autumn in Bizerte Channel. J. Plankton Res. 2012:1–17. [Google Scholar]
- 18.Michalec F.G., Holzner M., Menu D., Hwang J.-S., Souissi S. Behavioral responses of the estuarine calanoid copepod Eurytemora affinis to sub-lethal concentrations of waterborne pollutants. Aquat. Toxicol. 2013;138:129–138. doi: 10.1016/j.aquatox.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Ensibi C., Pringault O., Hannaoui W., Daly-Yahia M.N. Effects of cadmium exposure on reproduction and survival of the planktonic copepod centropages ponticus. J. Mar. Sci. Res. Dev. 2015;5(159) [Google Scholar]
- 20.Razouls C., Durand J. Inventaire des copépodes planctoniques méditerranéens. Vie Milieu. 1991;41:73–77. [Google Scholar]
- 21.Bradford M.M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Ellman G.L., Courtney K.O., Andrres V., Featherstone R.M. A new rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 23.Recknagel R.O., Glende E.A., Waller R.L., Lowrey K. Lipid peroxidation biochemistry, measurement and significance in liver cell injury. In: Plaa G.L., Hewitt W.R., editors. Toxicology of the Liver. Raven Press; New York: 1982. pp. 213–241. [Google Scholar]
- 24.Claiborne A. CRC-Handbook of Methods in Oxygen Radical Research. CRC; Florida, USA: 1985. Catalase activity. [Google Scholar]
- 25.Cribb A.E., Leeder J.S., Spielberg S.P. Use of a microplate reader in an assay of glutathione reductase using 5, 5’-dithiobis(2-nitrobenzoic acid) Anal. Biochem. 1989;183:195–196. doi: 10.1016/0003-2697(89)90188-7. [DOI] [PubMed] [Google Scholar]
- 26.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 27.Mohandas J., Marshall J.J., Duggin G.G., Horvath J.S., Tiller D.D. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Cancer Res. 1984;44:5086–5091. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- 28.Ruangsomboon S., Wongrat L. Bioaccumulation of cadmium in an experimental aquatic food chain involving phytoplankton (Chlorella vulgaris), zooplankton (Moina macrocopa), and the predatory catfish Clarias macrocephalus x C. gariepinus. Aquat. Toxicol. 2006;78:15–20. doi: 10.1016/j.aquatox.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q., Liuyan Y., Wen-Xiong W. Bioaccumulation and trophic transfer of dioxins in marine copepods and fish. Environ. Pollut. 2011;159:3390–3397. doi: 10.1016/j.envpol.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Defur P.L. Use of invertebrates in testing for endocrine disruptors. ILAR J. 2004;45:484–493. doi: 10.1093/ilar.45.4.484. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui A.A., Suponglemla C. Cadmium chloride intoxication and evaluation of protein changes in Clarias batrachus (Linn) Int. J. Curr. Microbiol. App. Sci. 2014;3(1):787–794. ISSN: 2319-7706. [Google Scholar]
- 32.Goering P.L., Fisher B.R. Metals and stress proteins. In: Goyer R.A., Cherian M.G., editors. Handbook of Experimental Pharmacology, Vol. 115, Toxicology of Metals, Biochemical Aspects. Springer- Verlag; New York: 1995. pp. 229–266. [Google Scholar]
- 33.Timofeyev M.A., Shatilina Z.M., Bedulina D.S. Evaluation of biochemical responses in Palearctic and Lake Baikal endemic amphipod species exposed to CdCl2. Ecotoxicol. Environ. Safe. 2008;70:99–105. doi: 10.1016/j.ecoenv.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Ivanina A.V., Cherkasov A.S., Sokolova I.M. Effects of cadmium on cellular protein and glutathione synthesis and expression of stress proteins in eastern oysters, Crassostrea virginica Gmelin. J. Exp. Biol. 2008;211:577–586;. doi: 10.1242/jeb.011262. [DOI] [PubMed] [Google Scholar]
- 35.Choudhary L., Bhardwaj S. Effect of heavy metal pollution on survival and protein changes in liver and intestine of fresh water fish Tilapia mossambica. J. Adv. Lab. Res. Biol. 2015 ISSN 0976-7614. [Google Scholar]
- 36.Emadeldeen H.M. Biochemical response of the cyclopoida copepod Apocyclops borneoensis exposed to nickel. Jordan J. Biol. Sci. 2014;7(1):41–47. ISSN 1995-6673. [Google Scholar]
- 37.Bainy A.C.D., Gennari M.H.M., Paolo Di.M., de Almeida E.A. In vivo effects of metals on the acetylcholinesterase activity of the Perna perna mussel’s digestive gland. Biotemas. 2006;19(1):35–39. ISSN 0103-1643. [Google Scholar]
- 38.Tsangaris C., Evangelos P., Efthimia C. Assessment of the impact of heavy metal pollution from a ferro nickel smelting plant using biomarkers. Ecotoxicol. Environ. Saf. 2007;66:232–243. doi: 10.1016/j.ecoenv.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Talesa V., Principato G.B., Giovannini E., Norton S.J., Rosi G. Presence of a solubile tetrameric (blood) and membrane-bound dimeric forms of cholinesterase in the mollusk Murex brandaris (Gastropoda: neogastropoda) Exp. Zool. 1994;270:233–244. [Google Scholar]
- 40.Li Y., Chai X., Wu H., Jing W., Wang L. The response of metallothionein and malondialdehyde after exclusive and combined Cd/Zn exposure in the crab Sinopotamon henanense. PLoS One. 2013;8(11):e80475. doi: 10.1371/journal.pone.0080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M.H., Wang G.Z. Biochemical response of the copepod Tigriopus japonicus Mori experimentally exposed to cadmium. Arch. Environ. Contam. Toxicol. 2009;57:707–717. doi: 10.1007/s00244-009-9319-6. [DOI] [PubMed] [Google Scholar]
- 42.Duman F., Kar M. Evaluation of effects of exposure conditions on the biological responses of Gammarus pulex exposed to cadmium. Int. J. Environ. Sci. Technol. 2015;12:437–444. [Google Scholar]
- 43.Almeida J.A., Barreto R.E., Novelli E.L.B., Castro F.J., Moron S.E. Oxidative stress biomarkers and aggressive behavior in fish exposed to aquatic cadmium contamination. Neotrop. Ichthyol. 2009;7:103–108. [Google Scholar]
- 44.Atli G., Alptekin O., Tukel S., Canlin M. Response of catalase activity to Ag+, Cd+, Cr6+, Cu2+ and Zn2+ in five tissues of fresh water fish Oreochromis niloticus. Comp. Biochem. Physiol. C. 2006;143:218–224. doi: 10.1016/j.cbpc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Lei W., Wang L., Liu D., Xu T., Luo J. Histopathological and biochemical alternations of the heart induced by acute cadmium exposure in the freshwater crab Sinopotamon yangtsekiense. Chemosphere. 2011;84:689–694. doi: 10.1016/j.chemosphere.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 46.Kanak E.G., Dogan Z., Eroglu A., Atli G., Canli M. Effects of fish size on the response of antioxidant systems of Oreochromis niloticus following metal exposures. Fish. Physiol. Biochem. 2014;40(1083) doi: 10.1007/s10695-014-9907-x. [DOI] [PubMed] [Google Scholar]
- 47.Frova C. Glutathione transferases in the genomics era: new insights and perspectives. Biomol. Eng. 2006;23:149–169. doi: 10.1016/j.bioeng.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Akcha F., Izuel C., Venier P., Budzinski H., Burgeot T., Narbonne J.F. Enzymatic biomarker measurement and study of DNA adduct formation in benzo(a)pyrene contaminated mussels Mytilus galloprovincialis. Aquat. Toxicol. 2000;49:269–287. doi: 10.1016/s0166-445x(99)00082-x. [DOI] [PubMed] [Google Scholar]
- 49.Jemec A., Drobne D., Tisler T. The applicability of acetylcholinesterase and glutathione S-transferase in Daphnia magna toxicity test. Comp. Biochem. Physiol. Part C. 2007;144:303–309. doi: 10.1016/j.cbpc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Bus J.S., Gibson J.E.l. Lipid peroxidation and its role in toxicology. Rev. Biochem. Toxicol. 1979;1:125–149. [Google Scholar]
- 51.Giguère A., Campbell P.G.C., Hare L., Cossu-Leguille C. Metal bioaccumulation and oxidative stress in yellow perch (Perca flavescens) collected from eight lakes along a metal contamination gradient (Cd, Cu, Zn, Ni) Can. J. Fish. Aquat. Sci. 2005;62:563–577. [Google Scholar]