Abstract
The in vitro arsenite (AsIII) cytotoxicity dose-response (DR) of human keratinocytes (HEK001) was examined at greater statistical resolution than ever previously reported using the MTT assay to determine cell viability. Fifty-four 96-well plates were treated with AsIII concentrations of 0.25, 0.5, 1, 2, 3, 4, 5, 7, 10, 15, 20, 25, or 30 μM. Because of unexpected variation in viability response patterns, a two-stage DR analysis was used in which data on plate-specific viability (%), estimated as 100% times the ratio of measured viability in exposed to unexposed cells, were fit initially to a generalized lognormal response function positing that HEK001 cells studied consisted of: a proportion P of relatively highly sensitive (HS) cells, a proportion Po of relatively resistant cells, and a remaining (1–P–Po) fraction of typical-sensitivity (TS) cells exhibiting the intermediate level of AsIII sensitivity characteristic of most cells in each assay. The estimated fractions P and Po were used to adjust data from all 54 plates (and from the 28 plates yielding the best fits) to reflect the condition that P = Po = 0 to provide detailed DR analysis specifically for TS cells. Four DR models fit to the combined adjusted data were each very predictive (R2 > 0.97) overall but were inconsistent with at least one of the data set examined (p < 10−5). Adjusted mean responses at ≤3 μM were approximately equal (p > 0.30) and exceeded 100% significance (p ≤ 10−6). A low-dose hormetic model provided the best fit to the combined adjusted data for TS cells (R2 = 0.995). Marked variability in estimates of P (the proportion of apparent HS cells) was unexpected, not readily explained, and warrants further study using additional cell lines and assay methods, and in vivo.
Keywords: Arsenic, Arsenate, Cell culture, Cell death, Cytotoxicity, HEK001 cells
1. Introduction
Inorganic arsenic (iAs) at high doses is a known human carcinogen to urinary bladder, lung and skin [1]. In addition to carcinogenic effects, recent data suggest that chronic high exposure to iAs may also contribute to other non-carcinogenic health effects such as cardiovascular diseases, obesity, diabetes, developmental defects, and respiratory diseases [2], [3], [4].
iAs has been proposed as a threshold carcinogen for at least two decades [5], [6], [7], [8]. However, methods used have been inadequate to address this in a statistically rigorous fashion [7], although data from epidemiology studies, in animals, and in vitro as well as mechanistic research clearly support the likelihood that iAs toxicity has a threshold dose below which health effects are not expected [8]. Although the threshold question for iAs remains highly controversial [9], [10], recent evidence supports the possibility of a threshold for iAs-induced cancer and toxic effects, including in vitro studies [11], [12], [13], animal studies [14], [15], and epidemiologic investigations [16], [17], [18], [19], [20]. Strikingly consistent no-effect levels have been reported in animal models (approximately 1 ppm in water or diet) [8] and in vitro (>0.1 μM trivalent arsenicals) [8], [11], [12]. A general lack of increased (e.g., bladder) cancer risk in human populations exposed to arsenic in drinking water at concentrations below 100–150 ppb [8], [16], [19], [20] is consistent with the associated non-cytotoxic internal concentrations of trivalent arsenic metabolites (e.g., <0.1 μM) in urine. Recent evidence is also consistent with thresholds for non-cancer endpoints of iAs exposure [8], [15], [17], [18]. A practical threshold for cancer induced by exposure to iAs is thus possible in view of evidence that regenerative cell proliferation associated only with cytotoxic levels of iAs exposure consistently precedes iAs-exposure-induced epithelial tumors, such as urinary bladder, skin, and lung carcinomas [8], [12], [13]. Chemically induced regenerative cell proliferation, in turn, is a response to induced cell killing that for many chemicals is expected to have a threshold-like, S-shaped dose-response [21], as is the case for a wide array of metals (including iAs) and oxidative chemicals that stimulate the cytoprotective Nrf2-mediated antioxidant response element (ARE) pathway that generally is cytoprotective at relatively low levels of increased expression [22], [23], [24].
Low-dose nonlinearity for arsenic-induced cytotoxicity in vitro is suggested by data reported in previous studies done using various cell lines [13], [21], [25], [26], [27]. However, relatively small replicate numbers used in such studies limited their statistical power to resolve details of cytotoxic dose-response observed low concentrations, including those associated with relatively small departures from background response levels. The present study aimed to develop more detailed data adequate to assess whether the low-dose dose-response pattern exhibited by reductions in cell viability in vitro following exposure to trivalent arsenic is consistent with a linear or nonlinear (e.g., threshold-like) pattern. This study used more than 50 assay replicates conducted at 14 iAs concentrations specially selected to focus specifically on the initial portion of the low-dose dose-response curve for reduced cell viability. For reasons of practical feasibility in a first study conducted at this level of detail, a key limitation of the study design was that concentration effects were investigated using only a single cell line and single (MTT) assay method, with the recognition that broader generalizations concerning observed response patterns can only be based on results of similarly detailed studies using additional cell types and cytotoxicity assay methods.
2. Methods
2.1. Materials
Sodium arsenite (NaAsO2) (NaAsIII) (99% purity as certified by the vendor) purchased from Sigma (St. Louis, MO) was used without additional analysis. A 10 mM stock solution was prepared in Dulbecco’s PBS 1X (Gibco, Grand Island, NY). Treatment solutions of AsIII at concentrations (Ci) of 0.25, 0.5, 1, 2, 3, 4, 5, 7, 10, 15, 20, 25, and 30 μM, were prepared in cell culture medium fresh on the day of treatment. Levels of iAs, monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) were determined for Dulbecco’s PBS 1X and cell culture medium using hydride generation-atomic absorption spectrometry coupled with cryotrap (HG-CT-AAS). For Dulbecco’s PBS 1X, the concentration of iAs was 0.187 ng/mL, and concentrations of MMA and DMA were below the detection limit (0.05 ng arsenic/mL for both). In the cell culture medium, concentrations were below the detection limit for iAs (0.1 ng arsenic/mL), MMA and DMA. The 13 test concentrations were selected, based on preliminary experiments and analysis of data from a previous study of AsIII-induced cytotoxicity in HEK001 cells [13], to yield a relatively detailed expected characterization of dose-response for AsIII-induced cytotoxicity, including at relatively low levels of this response in the range of 85% to 100% viability relative to unexposed cells.
2.2. Cell culture
The HEK001 human keratinocyte cell line, derived from normal human skin and immortalized by transfection of HPV-16 E6 and E7 genes (expressing keratin 14 but not keratin 10, characteristic of basal keratinocytes) [28], was obtained from the American Type Culture Collection (ATCC) (Manassas, VA). Cells were cultured in Keratinocyte-SFM (1X) containing 5 ng/mL human recombinant EGF without bovine pituitary extract, and supplemented with 5 mL Glutamax™ (all from Gibco) and 100 units/ml penicillin and 100 μg/mL streptomycin (Hyclone, Logan, UT) at 37 °C in 5% CO2. AsIII was undetectable in the medium. Visual examination of the cultured cells under a phase contrast light microscope showed cells with rounded to columnar morphology. The karyotype for the cell line is 71‐86,XXY,+1,‐2,+3,+5,‐6,+7,+8,+9,‐10,+11,‐12,+13,+14,+16,add(17)(p11.2),+18,+19,+20,+20,+2‐9mar[cp20]. Cytogenetic analysis revealed the presence of an abnormal hypertriploid clone characterized by loss of chromosomes 2, 6, 10, and 12; gain of chromosomes 1, 3, 5, 7, 8, 9, 11, 13, 14, 16, 18, 19, 20, and several marker chromosomes; and additional material of unknown origin on the short arm of chromosome 17. The cp abbreviation denotes that the karyotype is a composite, due to karyotypic heterogeneity and chromosome complexity and morphology within the cells analyzed. Cells used for the study were passage 3 through passage 9.
2.3. Determination of cell viability
Ninety-six well plates were seeded with approximately 6000 cells/well. Each plate contained eight control wells (C = 0 μM AsIII + growth medium), eight wells at each of the eight lowest concentrations (0.25 μM ≤ Ci ≤ 7 μM), four wells at each remaining concentration (Ci ≥ 10 μM), and four wells containing growth medium but no cells for use as assay blanks. Twenty-four hours after seeding, treatment with AsIII at the concentrations listed above was begun and continued for 72 h, a treatment period selected based on previous similar studies including from our own laboratory showing that period to be adequate to reliably elicit an approximate maximal magnitude of measured cytotoxicity using the MTT assay [13], [26]. At the end of the treatment period, cell viability was determined by the MTT assay [29]. Absorbance (optical density, S) of each well was determined at 570 nm using a microplate reader (Bio-Tek Elx800, Winooski, VT). Estimated viability (Si) in the ith concentration group of treated cells on each plate was re-expressed as a corresponding percentage viability (PSi = 100% Si/So); in other words as a ratio of exposed cell viability (Si) relative to the control (unexposed) cell viability (So) measured on that plate. A total of 54 plates from eight experiments conducted over a 4-month period were analyzed in this way (Table 1). Preliminary experiments using unexposed and exposed HEK001 cells cultured as described demonstrated no significant effect of plate-specific well position on measured viability (data not shown).
Table 1.
| Plate (j) | Nx | Np | ij | P | Po | b (%/μM) | GM (μM) | GSD | R2 | pfit,adj |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 14 | 7 | 0.027 | 0.094 | 89.2 | 18.3 | 1.15 | 0.958 | 0.995 |
| 2 | 1 | 14 | 1 | 0 | 0 | 118. | 17.9 | 1.53 | 0.950 | 0.995 |
| 3 | 1 | 14 | 2 | 0.095 | 0.085 | 7.46 | 19.5 | 1.24 | 0.972 | 0.995 |
| 4 | 1 | 14 | 2 | 0.101 | 0.092 | 8.76 | 19.8 | 1.28 | 0.976 | 0.995 |
| 5 | 2 | 16 | 1 | 0 | 0 | 113. | 17.2 | 1.53 | 0.881 | 0.995 |
| 6 | 2 | 16 | 4 | 0.019 | 0.051 | 93.4 | 16.6 | 1.28 | 0.968 | 0.995 |
| 7 | 2 | 16 | 1 | 0 | 0.095 | 102. | 21.4 | 1.20 | 0.867 | 0.995 |
| 8 | 2 | 16 | 1 | 0 | 0.056 | 61.7 | 18.8 | 1.33 | 0.791 | 0.995 |
| 9 | 2 | 16 | 1 | 0 | 0.070 | 101. | 16.2 | 1.34 | 0.891 | 0.995 |
| 10 | 3 | 18 | 3 | 0.106 | 0.024 | 85.8 | 13.4 | 1.40 | 0.983 | 0.995 |
| 11 | 3 | 18 | 1 | 0.034 | 0.021 | 74.3 | 11.2 | 1.45 | 0.970 | 0.995 |
| 12 | 3 | 18 | 1 | 0.142 | 0.015 | 98.7 | 12.6 | 1.47 | 0.975 | <10−7 |
| 13 | 4 | 18 | 2 | 0.277 | 0.050 | 8.93 | 15.1 | 1.46 | 0.937 | ∼0 |
| 14 | 4 | 18 | 4 | 0.359 | 0.045 | 4.26 | 15.9 | 1.34 | 0.965 | 0.995 |
| 15 | 4 | 18 | 9 | 0.418 | 0 | 0.419 | 17.6 | 1.53 | 0.940 | 0.061 |
| 16 | 4 | 18 | 3 | 0.284 | 0 | 4.54 | 17.5 | 1.50 | 0.931 | 0.665 |
| 17 | 4 | 18 | 4 | 0.250 | 0.028 | 3.14 | 14.9 | 1.58 | 0.959 | 0.995 |
| 18 | 4 | 18 | 6 | 0.330 | 0.058 | 1.44 | 17.4 | 1.27 | 0.935 | 0.684 |
| 19 | 5 | 19 | 2 | 0.192 | 0 | 8.54 | 13.6 | 1.53 | 0.963 | 0.995 |
| 20 | 5 | 19 | 2 | 0.200 | 0.023 | 7.95 | 12.8 | 1.53 | 0.940 | 0.435 |
| 21 | 5 | 19 | 1 | 0.105 | 0.035 | 108. | 13.0 | 1.40 | 0.951 | <10−4 |
| 22 | 5 | 19 | 3 | 0.214 | 0.032 | 7.53 | 16.1 | 1.29 | 0.829 | <10−4 |
| 23 | 5 | 19 | 2 | 0.215 | 0.026 | 9.06 | 14.2 | 1.39 | 0.909 | 10−8 |
| 24 | 5 | 19 | 2 | 0.225 | 0.024 | 9.73 | 14.6 | 1.45 | 0.853 | 0.995 |
| 25 | 6 | 6 | 3 | 0.103 | 0.041 | 104. | 14.5 | 1.43 | 0.953 | 10−5 |
| 26 | 6 | 6 | 3 | 0.162 | 0.092 | 4.85 | 18.5 | 1.46 | 0.907 | 0.995 |
| 27 | 6 | 6 | 1 | 0.011 | 0 | 106. | 18.0 | 1.42 | 0.904 | 0.995 |
| 28 | 6 | 6 | 3 | 0.085 | 0.085 | 5.51 | 17.7 | 1.37 | 0.914 | 0.995 |
| 29 | 6 | 6 | 2 | 0.179 | 0 | 8.55 | 17.8 | 1.46 | 0.957 | 0.995 |
| 30 | 6 | 6 | 7 | 0.293 | 0.119 | 9.69 | 19.9 | 1.04 | 0.793 | 0.115 |
| 31 | 7 | 8 | 4 | 0.275 | 0.013 | 3.28 | 20.7 | 1.52 | 0.851 | 0.995 |
| 32 | 7 | 8 | 8 | 0.318 | 0.114 | 0.883 | 22.2 | 1.12 | 0.953 | 0.995 |
| 33 | 7 | 8 | 2 | 0.269 | 0.056 | 16.7 | 20.1 | 1.35 | 0.926 | 0.995 |
| 34 | 7 | 8 | 2 | 0.258 | 0 | 8.79 | 15.1 | 1.72 | 0.818 | 0.995 |
| 35 | 7 | 8 | 9 | 0.209 | 0 | 0.434 | 22.6 | 1.44 | 0.895 | 0.995 |
| 36 | 7 | 8 | 10 | 0.291 | 0.123 | 0.416 | 23.5 | 1.01 | 0.949 | 0.995 |
| 37 | 7 | 8 | 4 | 0.269 | 0 | 101. | 22.1 | 1.42 | 0.807 | 0.995 |
| 38 | 7 | 8 | 2 | 0.372 | 0 | 17.1 | 18.8 | 1.47 | 0.849 | 0.995 |
| 39 | 7 | 8 | 2 | 0.067 | 0.113 | 8.45 | 13.7 | 1.77 | 0.933 | 0.995 |
| 40 | 7 | 8 | 3 | 0.317 | 0.085 | 5.84 | 21.5 | 1.17 | 0.790 | 0.995 |
| 41 | 7 | 8 | 1 | 0 | 0 | 99.8 | 17.4 | 1.69 | 0.827 | 0.995 |
| 42 | 7 | 8 | 1 | 0.037 | 0 | 8.97 | 16.5 | 1.85 | 0.847 | 0.995 |
| 43 | 8 | 9 | 2 | 0.174 | 0 | 13.0 | 21.7 | 1.47 | 0.880 | 0.995 |
| 44 | 8 | 9 | 2 | 0.165 | 0 | 7.36 | 17.6 | 1.56 | 0.946 | 0.995 |
| 45 | 8 | 9 | 3 | 0.135 | 0.160 | 4.72 | 20.6 | 1.21 | 0.909 | 0.995 |
| 46 | 8 | 9 | 5 | 0.291 | 0 | 1.55 | 19.8 | 1.53 | 0.966 | 0.995 |
| 47 | 8 | 9 | 2 | 0.107 | 0.126 | 7.43 | 20.8 | 1.22 | 0.937 | 0.995 |
| 48 | 8 | 9 | 1 | 0.215 | 0.073 | 18.21 | 19.6 | 1.35 | 0.929 | 0.995 |
| 49 | 8 | 9 | 1 | 0.239 | 0.092 | 16.7 | 19.6 | 1.37 | 0.946 | 0.995 |
| 50 | 8 | 9 | 7 | 0.178 | 0.117 | 18.8 | 20.4 | 1.27 | 0.966 | 0.995 |
| 51 | 8 | 9 | 7 | 0.261 | 0.112 | 28.7 | 21.2 | 1.13 | 0.896 | 0.995 |
| 52 | 8 | 9 | 4 | 0.142 | 0 | 83.7 | 20.5 | 1.54 | 0.949 | 0.995 |
| 53 | 8 | 9 | 3 | 0.207 | 0.123 | 7.76 | 20.7 | 1.17 | 0.941 | 0.995 |
| 54 | 8 | 9 | 3 | 0.079 | 0.040 | 43.0 | 18.8 | 1.43 | 0.963 | 0.995 |
| AMa | All | – | – | 0.172 | 0.0483 | 35.0 | 17.9 | 1.40 | 0.915 | 0.829 |
| SDa | All | – | – | 0.113 | 0.0462 | 41.4 | 2.97 | 0.173 | 0.0546 | 0.353 |
| SEMa | All | – | – | 0.015 | 0.0063 | 5.64 | 0.404 | 0.0236 | 0.0074 | 0.048 |
| AMa, b | –b | – | – | 0.151 | 0.0511 | 35.1 | 16.9 | 1.41 | 0.952 | 0.821 |
| SDa, b | –b | – | – | 0.091 | 0.0419 | 41.2 | 2.94 | 0.138 | 0.0172 | 0.362 |
| SEMa, b | –b | – | – | 0.017 | 0.0079 | 7.76 | 0.555 | 0.0261 | 0.0033 | 0.068 |
LNS model variables (see Methods); those labeled without units are unitless; rational-valued entries listed are shown rounded to three significant digits. Nx = experiment #; Np = # cell-culture passages for cells used in this experiment; ij = initial index of concentrations Ci (1 ≤ ij ≤ 13) modeled for experiment j when data were subsequently combined over multiple experiments using Eq. (2) (see Methods, Results); R2 = squared coefficient of correlation (fraction of variance explained by regression); pfit,adj = p-value of chi-square goodness of fit test adjusted for n = 54 independent tests (values ≤10−10 are listed as ∼0); AM = arithmetic mean; SD = standard deviation; SEM = standard error of the mean; − = not applicable.
Summary statistics for the subset of 28 “best-fit” plates (Plates 1, 2, 3, 4, 6, 10, 11, 12, 13, 14, 16, 17, 19, 20, 21, 25, 28, 29, 33, 39, 44, 47, 48, 49, 50, 52, 53, and 54) for which R2 ≥ 0.91 and B ≥ 2 (see Methods).
Cell viability in a total of 4965 [=54(8×9 + 4×5) − 3] well measures were obtained in this study, excluding three defective measures involving Ci on plate j, specifically for: {i,j} = {1,30}, {5,16}, and {8,15}. For the “best-fit” subset of 28 plates, the corresponding total number of well measures is 2351.
2.4. Data analysis
The value and variance of PSi were estimated as E(PSi) ∼100%{[E(S)/E(So)] (1 +)} (reflecting a 1st-order correction for ratio-estimation bias) and Var(PSi) ∼[E(Si)/E(So)]2 ( + ), respectively, where γX = SD(X)/E(X) = the coefficient of X-variation for X = Si or So, and E(X), Var(X), and SD(X) = [Var(X)]1/2 denote the expected value, variance, and standard deviation of X, respectively [30]. Because preliminary data analyses indicated substantial, unanticipated variability in observed patterns of viability dose-response, viability data for each plate were analyzed in two stages. First, plate viability data were fit as a function of AsIII concentration C to the following generalized lognormal cell-viability model (LNS)
| F(C) = (PS/100%) = Po + P exp(−b C) + (1–P)Φ(ln(C/GM)/ln(GSD)) | (1) |
In this model, Po is the fraction of HEK001 cells that appeared to be relatively resistant (RR) to AsIII-induced viability reduction in each assay conducted, P is the assumed fraction of cells appeared to be relatively highly susceptible or hypersusceptible (HS) to AsIII-induced viability reduction, and b is a rate constant governing assumed 1st-order loss of HS cells exposed to AsIII. In Eq. (1), ln denotes the natural logarithm, Φ(z) denotes the cumulative standard normal distribution function evaluated at argument z, and z here involves functions of the geometric mean (GM) and geometric standard deviation (GSD) governing assumed lognormal loss of the remaining fraction, 1– Po −P, of AsIII-exposed cells. This remaining fraction represents those HEK001 cells in each assay that appeared to exhibit an intermediate or typical-sensitivity (TS) response to AsIII cytotoxicity—the level of sensitivity that was observed to pertain to most of the HEK001 cells examined in each assay. Eq. (1) implies that as C increases, F(C) increases from F(0) = Po to a limiting value of F(∞) = 1–P.
In stage 1, the LNS model was fit to plate-specific estimates of Ci-specific viability (PSi) by nonlinear weighted least-squares regression (NLWLSR), using inverse variances estimated as described above as weights [31], [32], and corresponding regression-explained fraction of total plate-specific PSi variance (R2) and chi-square goodness of fit statistics were also calculated.
In stage 2, data were combined over all plates and modeled as follows to characterize the dose-response relationship for AsIII cytotoxicity specifically in TS cells defined above. Estimated PSi values for each plate j were adjusted as follows
| (2) |
to reflect conditioning on LNS-model parameter values of Po = P = 0—that is, to represent a purely lognormal (LN) model of viability of TS cells. The initial concentration index ij ≥ 1 used for each jth plate was calculated as Min(i) over indices i {1,…,13} conditional on
| Ci ≥ FLNS−1(100.5{1–P}) | (3) |
where FLNS is the LNS model (Eq. (1)) fit to PSi estimates obtained for plate j, and FLNS−1(p) denotes the concentration predicted by this model at viability-response level PS = 100p% (0 ≤ p ≤ 1). Condition 3 thus excludes from all those points in PSi for plate j at which the response predicted by the LNS model for this plate is likely to be due only to HS cells. Eq. (2) and Condition 3 thus jointly imply that models fit to each jth set of data characterize a response that pertains only to TS cells, and not to either HS or RR cells.
Mean values of combined plate-specific data at each concentration Ci, weighted by the inverse-square of its respective standard error of the mean (SEM), were then fit numerically by NLWLSR as described above to LN, Cubic (C), Linear-Cubic (LC), and Hormetic-Cubic (HC) dose-response models defined by Eqs. (4)–(7), respectively:
| PSLN(C) = 100%{FLNS(C) | (Po = P = 0)} | (4) |
| PSC(C) = 100%{exp(−b C3)} | (5) |
| PSLC(C) = 100%{exp(a C − b C3)} | (6) |
| PSHN(C) = {30/d}[exp(−a C) − exp(−{a + d}C)] + 100%{exp(−b C3)} | (7) |
These fits were obtained for the entire set of 54 plates examined, and for a subset of (28) of “best-fit” plates for each of which a relatively predictive fit was obtained (R2 ≥ 0.91), together with an estimate of the LNS initial-slope parameter b that was not relatively small (b ≥ 2). The latter condition served to reduce ambiguity concerning the estimated LNS parameter P during stage 2 of data analysis.
Assessments of PS and data normality were done by Shapiro-Wilk tests [33], corresponding variance homogeneity was assessed by Bartlett’s tests [34], and p-value adjustments to corresponding padj values that account for multiple independent tests performed were done by Hommel’s modified Bonferroni procedure [35]. Nested F-tests, yielding statistics Fdf,df2 with df1 and df2° of freedom, were used to assess the significance of improved fits by PSLC(C) and by PSHN(C) each compared to PSC(C) [36]. To compare means of data, parametric one-way analysis of variance or (where indicated in case of unequal variances) nonparametric Kruskal-Wallis (KW) tests were used [34], [37]. All calculations were performed using Mathematica 10.2® [32].
3. Results
MTT measures obtained for 216 blank wells had an average (±1 SD) value of 0.000037 (±0.0014). The eight batches of plate-specific control measures (So) were each approximately normally distributed with means of 0.592–0.842 and had unequal variances (p < 10−7, by Shapiro-Wilk test); the batch-specific means differed significantly (p < 10−7, by Kruskal-Wallis test). The combined 54 sets of plate-specific sets of So measures obtained had an average (±1 SD) value of 0.731 (±0.107), each of these sets was approximately normally distributed (padj > 0.13), and all sets combined had significantly unequal variances (p = 0.000052) and means (p = ∼0, by KW test). The latter observation justified characterizing viability data on a plate-specific basis. Summaries of unadjusted viability (PS) data and corresponding LNS-model fit statistics obtained for each plate are listed in Table 1, based on MTT cell-viability measures obtained for nearly 5000 control and treated wells (see Table 1, note c). Of the 54 LNS-model fits summarized in Table 1, only four are clearly inconsistent (pfit,adj ≤ 0.01) with the modeled data conditional on the estimated errors in estimated mean response, but all fits nevertheless predict those mean-response patterns reasonably well (0.790 ≤ R2 ≤ 0.983). Six of the variables (Np, P, Po, B, R2, pfit,adj) defined in Table 1 exhibited no significant Pearson product-moment correlations (padj > 0.20), except for a negative association between estimates for LNS model parameters b and P (r = −0.66, padj = 10−7). A similar correlation pattern and similar summary statistics for estimated parameters and LNS-model fits were obtained for a subset of 28 “best-fit” plates (Table 1). Individual LNS-model fits to these 28 data sets appear in Supplemental Materials.
Adjusted viability data (defined by Eq. (2)) that were combined for dose-response analysis are summarized in Table 2. Fits of LN, Cubic, LC, and HC models obtained to the complete set of adjusted data, and to the “best-fit” data subset, are shown in Fig. 1, Fig. 2, respectively. Corresponding fit details and statistics are listed in Table 3. Using data from all n = 54 plates as well as only from the 28 “best-fit” plates, approximately equal levels of adjusted mean viability (p > 0.30) occurred at the five concentrations ≤3 μM AsIII examined. When combined (using either n = 54 or n = 28 plates), the overall mean level of relative viability at concentrations ≤3 μM AsIII significantly exceeded that exhibited by unexposed (control) cells (p ≤ 10−6). Although the LN, Cubic, LC, and HC models were each very predictive (R2 > 0.97), none were statistically consistent with the entire corresponding data set examined (p < 10−5). The LC model fit to each data set contained a linear coefficient that is positive (i.e., hormetic, which in the present context, indicates increased viability with increasing concentration at low concentrations), but not significantly so (Table 3). LC model fits were not significantly better than corresponding Cubic-model fits (F12,11 = 0.53 and p = 0.85 with n = 54, F12,11 = 0.18 and p = 0.997 with n = 28). The HC model, which also exhibits low-dose hormetic behavior, provided the best fit to the combined data (R2 = 0.995) (Table 3), and provided a significantly better fit than the Cubic model (F12,10 = 5.62 and p = 0.0051 with n = 54, F12,10 = 30.14 and p = 0.00081 with n = 28).
Table 2.
Summary of adjusted viability data ().a
| i | Ci (μM) | All plates (n = 54)b |
Plates with R2 ≥ 0.91 and b ≥ 2 (n = 28)b |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (%) | SEM (%) | Nj | padj | Mean (%) | SEM (%) | Nj | padj | ||
| 1 | 0.25 | 107.2 | 1.85 | 13 | 0.935 | 108.7 | 1.92 | 6 | 0.910 |
| 2 | 0.50 | 108.2 | 2.29 | 28 | 0.930 | 108.6 | 1.47 | 16 | 0.910 |
| 3 | 1 | 104.2 | 1.61 | 38 | 0.376 | 104.4 | 1.67 | 22 | 0.061 |
| 4 | 2 | 107.4 | 1.69 | 44 | 0.935 | 105.8 | 1.72 | 26 | 0.910 |
| 5 | 3 | 101.7 | 1.74 | 45 | 0.935 | 99.6 | 1.62 | 26 | 0.910 |
| 6 | 4 | 103.1 | 1.65 | 46 | 0.890 | 100.7 | 1.41 | 26 | 0.910 |
| 7 | 5 | 96.4 | 1.44 | 50 | 0.935 | 97.2 | 1.28 | 28 | 0.910 |
| 8 | 7 | 94.5 | 1.46 | 51 | 0.041 | 92.7 | 1.56 | 28 | 0.027 |
| 9 | 10 | 88.9 | 1.82 | 53 | 0.935 | 86.2 | 1.99 | 28 | 0.910 |
| 10 | 15 | 55.2 | 2.66 | 54 | 0.935 | 63.4 | 3.67 | 28 | 0.051 |
| 11 | 20 | 36.6 | 2.54 | 54 | 0.935 | 28.3 | 3.06 | 28 | 0.910 |
| 12 | 25 | 5.21 | 1.50 | 54 | 0.547 | 3.50 | 1.63 | 28 | 0.910 |
| 13 | 30 | 3.40 | 0.79 | 54 | 0.001 | 3.47 | 0.79 | 28 | 0.062 |
Mean = weighted arithmetic mean of adjusted percent-viability data using inverse estimated variances as weights; Ci = ith test concentration of AsIII; SEM = standard error of the mean; Nj = # plates from which data at concentration Ci were averaged (only concentrations i = ij through i = 13 were included from each jth plate—see Methods and Table 1); padj = p-value of Shapiro-Wilk test of approximate normality of estimated weighted means, adjusted for 13 independent tests. Mean values are shown to ≥0.1% accuracy or 3 significant digits, and SEM values to ≥0.01% accuracy.
See Table 1.
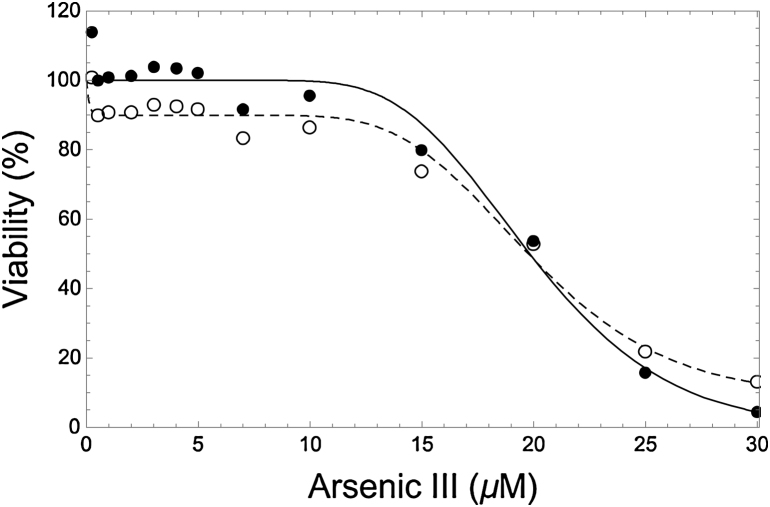
Fig. 1.
Observed viability data (open points) and corresponding LNS-model fit (dashed curve) for plate #4, compared to data values (solid points) that are all adjusted to reflect this fitted model with assumed parameter values of P = Po = 0 (solid curve).
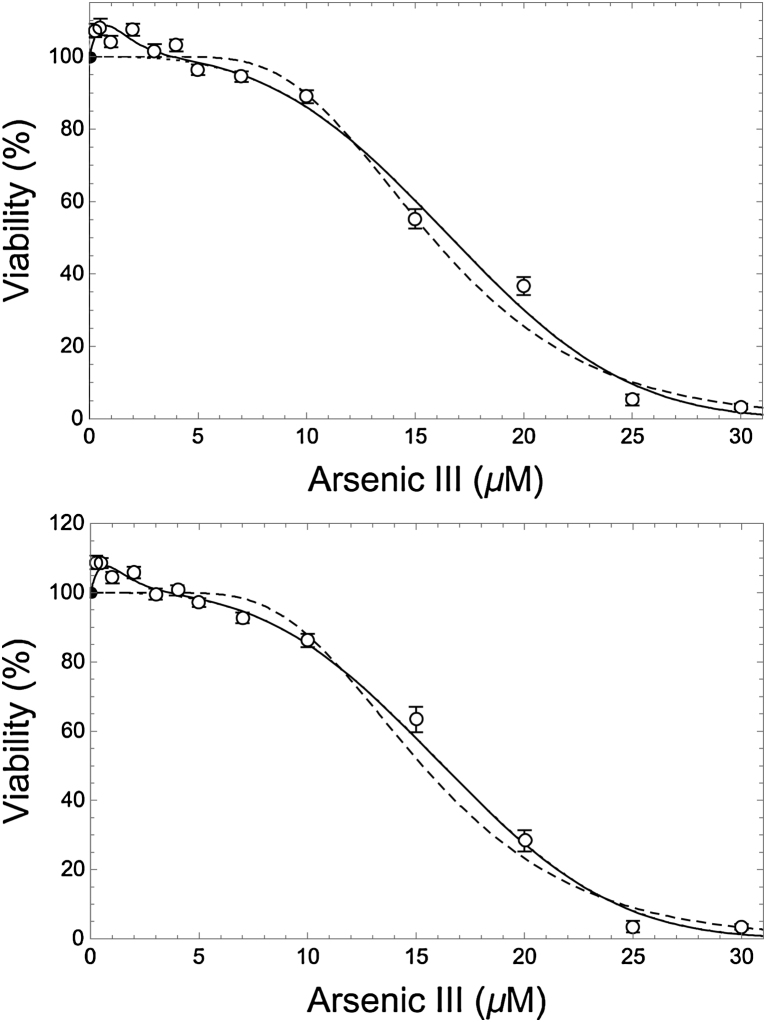
Fig. 2.
Model fits to adjusted viability data from all 54 plates (top plot), and from 28 plates for which the LNS model yielded fits (to corresponding unadjusted data sets) that are all relatively predictive (R2 ≥ 0.91) and have relatively steep initial slopes (b ≥ 2—see Table 1) (bottom plot). Error bars denote ±1 SEM. Fitted models shown in each plot are: lognormal (dashed curve), cubic-hormetic (solid curve), and cubic (dotted curve, which nearly coincides with the dashed and solid curve at C ≤ 3 μM and C ≥ 4 μM, respectively).
Table 3.
Model fits to adjusted viability data ().a
| Model | Plates includedb | Parameter | Estimate | SE | p=0 | R2 | pfit |
|---|---|---|---|---|---|---|---|
| LN | 54 | GM | 15.77 | 0.705 | <10−9 | 0.979 | ∼0 |
| GSD | 1.435 | 0.061 | ∼0 | ||||
| LN | 28 | GM | 15.30 | 0.860 | <10−8 | 0.977 | ∼0 |
| GSD | 1.442 | 0.070 | ∼0 | ||||
| Cubic | 54 | b (×104) | 1.500 | 0.154 | <10−8 | 0.983 | ∼0 |
| 28 | b (×104) | 1.612 | 0.208 | <10−5 | 0.986 | ∼0 | |
| LC | 54 | a (×103) | 2.889 | 3.93 | 0.48 | 0.983 | ∼0 |
| b (×104) | 1.603 | 0.218 | 10−5 | ||||
| 28 | a (×103) | 1.695 | 4.20 | 0.69 | 0.986 | ∼0 | |
| b (×104) | 1.681 | 0.280 | <10−4 | ||||
| HC | 54 | a | 1.207 | 8.99 | 0.90 | 0.992 | <10−5 |
| d | 0.1678 | 18.9 | 0.99 | ||||
| b (×104) | 1.502 | 0.116 | 10−7 | ||||
| 28 | a | 1.053 | 1.69 | 0.55 | 0.995 | 0.0008 | |
| d | 0.7963 | 4.24 | 0.19 | ||||
| b (×104) | 1.681 | 0.116 | <10−6 |
Models LN, Cubic, LC, and HC are defined by Equations 4–7, respectively (Methods). those labeled without units are unitless. SE = standard error; p0 = p-value from a t-test of the null hypotheses that the parameter value is zero (values ≤10−10 are listed as ∼0); R2 = squared coefficient of correlation (fraction of variance explained by regression); pfit = p-value of chi-square goodness of fit test. Parameter and SE estimates are rounded to 4 and at least 3 significant digits, respectively.
See Table 2.
4. Discussion and conclusions
The current study used a greater number of replicate plates than in any previously reported study of in vitro AsIII cytotoxicity and assessed cytotoxicity at many different concentrations. Markedly variable and unexplained dose-response patterns were observed concerning unadjusted data on HEK001 cell viability after AsIII exposure, The LNS model plausibly describes diverse dose-response patterns of AsIII cytotoxicity observed for an estimated fraction 1–P–Po of HEK001 cells in each test that appeared to exhibit a typical level of resistance to AsIII cytotoxicity. Each test-specific fraction Po of apparently highly resistant cells was estimated after adjusting each corresponding set of viability data to exclude an estimated proportion P of tested cells that appeared to be relatively highly susceptible to cytotoxicity induced at very low AsIII concentrations (as low as 0.25 μM). Such unexpected and unexplained phenotypic heterogeneity in apparent ultra-low-dose sensitivity to AsIII was characterized as inter-test variation in the fraction P, estimated to range from 0 to 42%, and to average approximately 17% (Table 1). This heterogeneity challenges the hypothesis that AsIII cytotoxic effects have a dose-response threshold (i.e., do not occur at relatively low doses), to the extent that the fractions of evidently highly AsIII-susceptible HEK001 cells that were estimated to occur in this study accurately model hypersensitive cell phenotypes that also occur in vivo. Only if such relatively highly susceptible subpopulations of cultured HEK001 cells are not relevant in vivo—for example, if they are an unrealistic artifact of this line of cultured cells, or of the in vitro cell culture conditions used—would the good fits obtained in this study by the LNS model to adjusted HEK001 cell-viability data provide evidence consistent with a threshold-like dose-response for AsIII cytotoxicity, conditional on the assumption that P = 0 under biologically relevant conditions in vivo. In contrast, patterns of AsIII-induced cell toxicity observed in this study indicate that AsIII is cytotoxic to a relatively small and relatively highly sensitive subset of HEK001 cells in approximate linear proportion to the AsIII concentration (consistent with slope parameter b in Eq. (1)). To the extent that “typical-sensitivity” HEK001 cells posited by the LNS model do exist, data obtained in this study do not provide evidence that low concentrations of AsIII are toxic to these cells in linear proportion to AsIII concentration. Consequently, results obtained in this study are novel and interesting, and merit further study to address the question of whether and to what extent subpopulations of cells that are relatively highly sensitive to AsIII (in contrast to cells that exhibit more typical levels of sensitivity to AsIII) exist in other cell lines and in vivo.
One possible explanation for the substantial variability in estimated values of P obtained in this study is that variation in values of P, as well as our observation of statistically significantly increased adjusted viability in the (presumptive) remainder of “typical‐sensitivity” cells exposed to ≤3 vs. 0 μM AsIII, was due to karyotypic heterogeneity present in the cell line. The cell line was derived originally from cells from the scalp of a 65-year-old male [28] that were transfected with a plasmid p1321 containing human papillomavirus (HPV) 16 E6 and E7 genes, which may have resulted in the heterogeneity of the karyotype. Alternatively, the observed heterogeneity might be a result of cell passages, or possibly to artifacts of variable aspects of in vitro testing such as plating-induced damage to a fraction (P) of plated HEK001 cells. Additional experiments will be required to determine the cause of substantial variation in the apparent value of P that was observed in this study.
Ideally, a detailed study of this type would be repeated using multiple cell lines and multiple viability and viability assay methods in parallel, despite the fact that low-concentration AsIII-induced cytotoxicity is well known to involve induction of apoptotic pathways [38], [39], [40], [41], [42]. Even using a single cell line and a single assay method for reasons of practical feasibility for a first study conducted at this level of detail, the study design was quite expensive and very labor intensive. Nevertheless, results from this study represent a monumental effort that clearly yielded interesting and unexpected results. Future studies can extend this approach to additional cell lines and assay methods.
Acknowledgements
The research was funded (without input to study design, data analysis, or conclusions) by Electric Power Research Institute (EPRI) contracts MA10003705 (Exponent) and MA10003723 (UNMC). Our sincere thanks to Dr. Mirek Styblo at the University of North Carolina for the analysis of the arsenic concentrations in Dulbecco’s PBS and the cell culture medium and to Dr. Bhavana Dave and the Cytogenetics Laboratory at the University of Nebraska Medical Center for the karyotyping of the HEK001 cell line.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2016.12.003.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.IARC . Some Drinking-Water Disinfectants and Contaminants, Including Arsenic. International Agency for Research on Cancer (IARC), World Health Organization; France: 2004. IARC monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 2.Chiou J.M., Wang S.-L., Chen C.-J., Deng Lin C.-R.W., Tai T.-Y. Arsenic ingestion and increased microvascular disease risk: observations from the south-western arseniasis-endemic area in Taiwan. Int. J. Epidemiol. 2005;343:936–943. doi: 10.1093/ije/dyi108. [DOI] [PubMed] [Google Scholar]
- 3.Maull E.A., Ahsan H., Edwards J., Longnecker M.P., Navas-Acien A., Pi J., Silbergeld E.K., Styblo M., Tseng C.-H., Thayer K.A., Loomis D. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ. Health Perspect. 2012;120:1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naujokas M.F., Anderson B., Ahsan H., Aposhian H.V., Graziano J.H., Thompson C., Suk W.A. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ. Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abernathy C.O., Chappell W.R., Meek M.E., Gibb H. Guo H-R. Is ingested inorganic arsenic a threshold carcinogen. Fundam. Appl. Toxicol. 1996;29:168–175. doi: 10.1006/faat.1996.0018. [DOI] [PubMed] [Google Scholar]
- 6.Carlson-Lynch H., Beck B.D., Boardman P.D. Arsenic risk assessment. Environ. Health Perspect. 1994;102(4):354–355. doi: 10.1289/ehp.94102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clewell H.J., Gentry P.R., Barton H.A., Shipp A.M., Yager J.W., Andersen M.E. Requriements for a biologically realistic cancer risk assessment for inorganic arsenic. Int. J. Toxicol. 1999;18:131–147. [Google Scholar]
- 8.Cohen S.M., Arnold L.L., Beck B.D., Lewis A.S., Eldan M. Evaluation of the carcinogenicity of inorganic arsenic. Crit. Rev. Toxicol. 2013;43:711–752. doi: 10.3109/10408444.2013.827152. [DOI] [PubMed] [Google Scholar]
- 9.Moon K.A., Guallar E., Umans J.G., Devereux R.B., Best L.G., Francesconi K.A., Goessler W., Pollak J., Silbergeld E.K., Howard B.V., Navas-Acien A. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Ann. Intern. Med. 2013;159:649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez K.F., Ungewitter E.K., Crespo-Mejias Y., Liu C., Nicol B., Kissling G., Yao H. Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ. Health Perspect. 2016;124:336–343. doi: 10.1289/ehp.1509703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentry P.R., McDonald T.B., Sullivan D.E., Shipp A.M., Yager J.W., Clewell H.J., 3rd Analysis of genomic dose-response information on arsenic to inform key events in a mode of action for carcinogenicity. Environ. Mol. Mutagen. 2010;51:1–14. doi: 10.1002/em.20505. [DOI] [PubMed] [Google Scholar]
- 12.Yager J.W., Gentry P.R., Thomas R.S., Pluta L., Efremenko A., Black M., Arnold L.L., McKim J.M., Wilga P., Gill G., Choe K.Y., Clewell H.J. Evaluation of gene expression changes in human primary uroepithelial cells following 24-hr exposures to inorganic arsenic and its methylated metabolites. Environ. Mol. Mutagen. 2013;54:82–98. doi: 10.1002/em.21749. [DOI] [PubMed] [Google Scholar]
- 13.Dodmane P.R., Arnold L.L., Kakiuchi-Kiyota S., Qiu F., Liu X., Rennard S.I., Cohen S.M. Cytotoxicity and gene expression changes induced by inorganic and organic trivalent arsenicals in human cells. Toxicology. 2013;312:18–29. doi: 10.1016/j.tox.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S.M., Arnold L.L., Klaunig J.E., Goodman J.I. Response to the Waalkes et al., Letter to the editor concerning our “Letter to the editor, Re: lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human relevant doses, Waalkes et al., Arch Toxicol, 2014. Arch. Toxicol. 2015;89:2167–2168. doi: 10.1007/s00204-015-1614-6. [DOI] [PubMed] [Google Scholar]
- 15.Sidhu M.S., Desai K.P., Lynch H.N., Rhomberg L.R., Beck B.D., Venditti F.J. Mechanisms of action for arsenic in cardiovascular toxicity and implications for risk assessment. Toxicology. 2015;331:78–99. doi: 10.1016/j.tox.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji J.S., Perez V., Garry M.R., Alexander D.D. Association of low-level arsenic exposure in drinking water with cardiovascular disease: a systematic review and risk assessment. Toxicology. 2014;323:78–94. doi: 10.1016/j.tox.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji J.S., Perez V., Garry M.R., Alexander D.D. Association of low-level arsenic exposure in drinking water with cardiovascular disease: a systematic review and risk assessment. Toxicology. 2014;323:78–94. doi: 10.1016/j.tox.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji J.S., Garry M.R., Perez V., Chang E.T. Low-level arsenic exposure and developmental neurotoxicity in children: a systematic review and risk assessment. Toxicology. 2015;337:91–107. doi: 10.1016/j.tox.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Lamm S.H., Robbins S., Chen R., Lu J., Goodrich B., Feinleib M. Discontinuity in the cancer slope factor as it passes from high to low exposure levels – arsenic in the BFD-endemic area. Toxicology. 2014;326:25–35. doi: 10.1016/j.tox.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Lamm S., Ferdosi H., Dissen E., Li J., Ahn J. A systematic review and meta-regression analysis of lung cancer risk and inorganic arsenic in drinking water. Int. J. Environ. Res. Public Health. 2015;12:14990. doi: 10.3390/ijerph121214990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogen K.T. Linear-no-threshold default assumptions for noncancer and nongenotoxic-cancer risks: a mathematical and biological critique. Risk Anal. 2016;36(3):589–604. doi: 10.1111/risa.12460. http://onlinelibrary.wiley.com/doi/10.1111/risa.12460/epdf [DOI] [PubMed] [Google Scholar]
- 22.Simmon S.O., Fan C.-Y., Yeoman K., Wakefield J., Ramabhadran R. NRF2 oxidative stress induced by heavy metals is cell type dependent. Curr. Chem. Genomics. 2011;5:1–12. doi: 10.2174/1875397301105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla S.J., Huang R., Simmons S.O., Tice R.R., Witt K.L., VanLeer D., Ramabhadran R., Austin C.P., Xia M. Profiling environmental chemicals for activity in the antioxidant response element signaling pathway using a high throughput screening approach. Environ. Health Perspect. 2012;120(8):1150–1156. doi: 10.1289/ehp.1104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Wu K.C., Lu Y.F., Ekuase E., Klaassen C.D. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid. Med. Cell Longev. 2013;2013:305861. doi: 10.1155/2013/305861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham-Evans B., Tchounwou P.B., Cohly H.H.P. Cytotoxicity and proliferation studies with arsenic in established human cell Lines: keratinocytes, melanocytes, dendritic cells, dermal fibroblasts, microvascular endothelial cells, monocytes and T-cells. Int. J. Mol. Sci. 2003;4:13–21. [Google Scholar]
- 26.Komissarova E.V., Saha S.K., Rossman T.G. Dead or dying: the importance of time in cytotoxicity assays using arsenite as an example. Toxicol. Appl. Pharmacol. 2005;202:99–107. doi: 10.1016/j.taap.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Komissarova E.V., Rossman T.G. Arsenite induced poly(ADPribosyl)ation of tumor suppressor P53 in human skin keratinocytes as a possible mechanism for carcinogenesis associated with arsenic exposure. Toxicol. Appl. Pharmacol. 2010;243(3):399–404. doi: 10.1016/j.taap.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugerman P.B., Bigby M. Preliminary functional analysis of human epidermal T cells. Arch. Dermatol. Res. 2000;292:9–15. doi: 10.1007/pl00007461. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Kendall M., Stuart A. 4th ed. vol. 247. MacMillan Publishing Co.; NY: 1977. The advanced theory of statistics; p. 260. (Distribution Theory). [Google Scholar]
- 31.Carrol R.J., Ruppert D. Chapman and Hall; New York, NY: 1988. Transformation and Weighting in Regression; pp. 9–62. [Google Scholar]
- 32.Wolfram Research . Wolfram Research, Inc.; Champaign, IL: 2015. Wolfram Mathematica 9 Documentation Center. ( www.wolfram.com, http://reference.wolfram.com/mathematica/guide/Mathematica.html) [Google Scholar]
- 33.Royston P. Approximating the Shapiro-Wilk W-test for non-normality. Stat. Comput. 1992;2(3):117–119. [Google Scholar]
- 34.Snedecor G.W., Cochran W.G. 8th ed. Iowa State University Press; Ames, IA: 1989. Statistical Methods; pp. 217–236. [Google Scholar]
- 35.Wright S.P. Adjusted p-values for simultaneous inference. Biometrics. 1992;48:1005–1013. [Google Scholar]
- 36.Selvin S. Duxbury Press, Wadsworth Publishing Co.; Belmont, CA: 1995. Practical Biostatistical Methods; pp. 1–137. [Google Scholar]
- 37.Lehman E.L., D’Abrera H.J.M. Holden-Day; San Francisco, CA: 1975. Nonparametrics: Statistical Methods Based on Ranks; pp. 204–210. [Google Scholar]
- 38.Yedjou C.G., Moore P., Tchounwou P.B. Dose- and time-dependent response of human leukemia (HL-60) cells to arsenic trioxide treatment. Int. J. Environ. Res. Public Health. 2006;3(2):136–140. doi: 10.3390/ijerph2006030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H., He J., Ju P., Zhong X., Liu J. Studies on the mechanism of arsenic trioxide-induced apoptosis in HepG2 human hepatocellular carcinoma cells. Chin. J. Clin. Oncol. 2008;5:22–25. [Google Scholar]
- 40.Stevens J.J., Graham-Evans B., Walker A.M., Armstead B., Tchounwou P.B. Cytotoxic effect of arsenic trioxide in adenocarcinoma colorectal cancer (HT-29) cells. Met. Ions Biol. Med. 2008;10:458–462. [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Xu Y., Wang H., Xue P., Li X., Li B., Zheng Q., Sun G. Arsenic induces mitochondria-dependent apoptosis by reactive oxygen species generation rather than glutathione depletion in Chang human hepatocytes. Arch. Toxicol. 2009;83(10):899–908. doi: 10.1007/s00204-009-0451-x. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S., Yedjou C.G., Tchounwou P.B. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J. Clin. Exp. Cancer Res. 2014;33:42. doi: 10.1186/1756-9966-33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.