Abstract
Potential developmental toxicities of three different cigarette butt leachates were evaluated using the frog embryo teratogenesis assay–Xenopus (FETAX). Xenopus laevis embryos were exposed to regular cigarette butt (RCB), menthol (MCB) and electronic (ECB) in concentrations ranging from 0 to 4 butts/l for RCB and MCB and 0–10 butts/l for ECB. The embryos were from stage 8 to 11 and were exposed for a 96-h period in static renewal test conditions. Median lethal concentration (LC50), malformation (EC50), non-observed adverse effect concentration (NOAEC), and lowest observed adverse effect concentration (LOAEC) were calculated. Results from these studies suggest that each tested leachate is teratogenic for X. laevis embryos. The lowest LC50 was determined for ECB exposure at 17.9 cigarette butts/L. The LC50 value was the highest with RCB and MCB having LC50 s of approximately 1 cigarette butt/L. There were notable EC50 differences with RCB having the highest and ECB the lowest. The NOAEC and LOAEC levels for RCB and MCB were below 1 cigarette butt/L for both mortality and malformations; over 8 butts/L for ECB mortality and over 4 butts/L for malformations. From these results, we conclude that RCB leachate is the most toxic compound, while MCB leachate has the higher teratogenicity. ECB leachate has the lowest toxic and teratogenic effects on embryos but there were still noticeable effects. The results confirmed that the FETAX assay can be useful in an integrated biological hazard assessment for the preliminary screening for ecological risks of cigarette butts, and electronic cigarettes, in aquatic environment.
Keywords: Cigarette butt leachate, Xenopus laevis, Development
Cigarette butts are, collectively, the most common form of litter in the world. Approximately 5.6 trillion cigarettes are smoked every year worldwide. In the United States of America, cigarette waste constitutes an estimated 30% of the total litter (by count) on U.S. shorelines, in waterways, and on land [1]. The current method for measuring of how many cigarette butts are finding their way into streams, rivers, and coastal environments is the International Coastal Cleanup Day, which is organized annually by the Ocean Conservancy. The event involves more than 500,000 volunteers picking up debris from beaches, rivers, and streams around the world. The volunteers complete Marine Debris Data Cards that indicate the quantity and type of litter they pick up. During the 2013 international cleanup, 2,043,470 cigarette butts were collected, making this the most common debris item. Cigarette butts have topped the list in all International Coastal Cleanup Days since that category was added to the data cards as a separate item in 1990 [2].
The filters in one pack of 20 cigarettes weigh 0.12 ounces (with no tobacco attached) and displace a volume of 10 mL. Based on these figures, the cigarette butts collected in 2013 weighed approximately 766 lbs and displaced a volume of 1022 l. Owing to the ubiquitous nature and magnitude of cigarette butts discharged into the environment, studies have been undertaken to determine whether cigarette butt waste can exert ecotoxic effects in aquatic environments [3], [4]. The environmental impact of cigarette butt waste is related to both their persistence in the environment and potential toxic effects [5] and their chemical composition − containing over 4000 different chemicals [4]. Most of these chemicals are toxic and often leach into aquatic ecosystems, thereby threatening water supply sources and aquatic animals [6], [7] such as water fleas [3], which inhabit freshwater environments, and mosquito larvae [8].
Electronic cigarettes (EC) have, since their market introduction in 2004, gained a wide audience − as of 2013, there are several million e-cigarette users globally Michael Felberbaum (11 June 2013). Various reasons or factors have contributed to increased EC use; including the perception that vaping is a safer or healthier alternative to smoking conventional (tobacco) cigarettes. But the impact of EC use on the environment is unknown. EC deliver aerosol by heating a liquid solution known as e-liquid that usually contains nicotine, propylene glycol, glycerin, and flavorings. The EC combine the fluid chamber with a heating element, vaporizing the e-liquid. Since EC do not release secondhand smoke, they are touted by some as being a safe alternative, for users and bystanders, to traditional (analog) cigarette. But researchers have analyzed the impurities in both e-liquid [9], [10], [11], [12], and the composition of the emitted vapor [13], [14], [15], [16]. Goniewicz et al. [13] measured 0.03–0.57 μg of lead per e-cigarette in emitted vapor. Williams et al. [16] found metals (e.g., lead, nickel, and silver), silicate beads, and nanoparticles in e-cigarette aerosol. There is far less data regarding the environmental impact of EC disposal. While one EC is typically equivalent to 40 traditional cigarettes, greatly reducing related waste byproducts, some research has been conducted to determine whether or not EC waste should be classified as hazardous [17]. Krause and Townsend [17] found that some e-cigarettes may be classified as hazardous waste for lead leaching, though they did observe a large degree of variability among brands and products. They did stress that the rate of consumption and disposal of these products is assumed to be higher than many other electronic products.
In order to derive water quality guidelines or conduct hazard assessments, toxicity data for a number of aquatic species is the minimum needed, so it is important to determine not only the toxicity of traditional cigarette butt leachate but also the toxicity of electronic cigarette related waste. In this study, we examined acute toxicity, teratogenic development, chronic and sublethal effects of cigarette butt leachate and EC leachate using Frog Embryo Teratogenesis Assay − Xenopus (FETAX). FETAX is an inexpensive alternative toxicity test system that can be used to evaluate ecological hazards from both complex environmental mixtures and pure chemical products [18]. This bioassay is a four-day, whole embryo-larval developmental toxicity screening assay that used embryos of the South African clawed frog, Xenopus laevis. The FETAX system is capable of monitoring acute, chronic, developmental, and behavioral toxicity for ecological and human health hazard assessment. With over 300 validation test compounds, the predictive accuracy of the FETAX model with conventional mammalian test systems is approximately 85% [19]. This allows for the examination of not only acute toxicity, but teratogenic development and chronic, sublethal effects on growth.
The specific aim of this study was to determine how different electronic cigarette butt leachate (ECBL) were compared to traditional cigarette butt leachate in terms of environmental (aquatic) toxicity, teratogenicity and impact on growth to embryos of Xenopus laevis. We hypothesized that the ECBL would be less toxic than the traditional cigarette butt leachate.
The objectives of this study were to determine the acute tocity (96-h LC50 and 96-h EC50) and lowest observed effect concentration (including the minimum concentration to inhibit growth) of regular cigarette butt (RCBL), menthol (MCBL), and electronic cigarette butt leachate (ECBL) to Xenopus laevis embryos.
1. Materials and methods
l-cysteine, human chorionic gonadotropin (HCG), NaCl, NaHCO3, KCl, CaCl2, CaSO4, and MgSO4 and other laboratory supplies were obtained from the Sigma Chemical Co., St. Louis, MO. Other supplies including 60 × 15 plastic Petri dishes, 10 mL disposable pipettes, transferable pipettes, and a 1 mL syringe were obtained from Fisher Scientific Supplies, location. Xenopus laevis frogs were obtained from Xenopus I, Inc. The Regular and menthol cigarettes contained 2.8% and 2.7% nicotine respectively, and the electronic cigarette cartridges contained 2.4% nicotine.
1.1. Cigarette butt collection, electronic cigarette purchase, and leachate preparation
Cigarette butts (CB) were collected from naturally smoked cigarettes, defined as cigarettes that were smoked by people, extinguished in cigarette disposal units and collected within 24 h. The electronic cigarettes were purchased, punctured, and then used discarded cigarette butts. We only tested one type of e-cigarette, as well as one brand of regular and menthol cigarette. The CB were selected randomly and placed in to clean plastic bags for transport to the laboratory. The collected CB were immediately processed as described below for leachate production.
Cigarette butt leachates (CBL) were made by soaking 3 butts in 300 mL FETAX solution for 1 h to produce the leachate stock solution of 10 CB/L. FETAX solution was prepared by adding 625 mg NaCl, 96 mg NaHCO3, 30 mg KCl, 15 mg CaCl2 60 mg CaSO4*2H2O, and 75 mg MgSO4 per liter distilled water [19], [20]. CBLs were made fresh for RCBL, MCBL, ECBL for each replicate experiment.
1.2. Animal care and breeding
Animal care and breeding was performed according to the specifications set forth in the ASTM's Standard Guide for Conducting the Frog Embryo Teratogenesis Assay-Xenopus [19]. Animal care and use was performed in accordance with the requirements of the Jacksonville State University's Institution of Animal Care and Use Committee. Adult frogs were kept in glass aquariums in re-circulated water at room temperature (24 ± 2 °C). A 12-h day/12-h night cycle was maintained. Males and females were bred as single pairs. Breeding was induced by injecting each frog with human chorionic gonadotropin and placed in false bottom breeding chambers [21]. Males received 250–500 IU of HCG, and females received 500–1000 IU. The hormone concentration was 1000 IU/mL in sterile 0.9% NaCl and injected with a 1-mL tuberculin syringe fitted with a ½ inch long 26-gauge needle [19].
FETAX procedures were performed according to ASTM's Standard Guide for Conducting the Frog Embryo Teratogenesis Assay-Xenopus [19]. Eggs were collected and immediately inspected to determine fertility and quality. The jelly coat was removed by swirling the eggs for 1–3 min in 2% w/v l-cysteine solution prepared in FETAX solution, and pH was adjusted to 8.1 with 1 N NaOH. Immediately after the jelly coat was removed, l-cysteine was rinsed off with FETAX solution. Healthy, normally dividing blastulae were placed in 60 × 15 plastic Petri dishes, each dish containing 20 embryos. For each test, stock solutions of CBL were made fresh daily. Dead embryos were removed daily and a live count was taken. At the end of a four-day period, a final live/dead count was taken and the number and type of malformed was recorded. Embryos were anesthetized with MS222 and, then fixed in 3.0% (w/v) formalin. Malformation types were noted on the Scoresheet of Malformations at 96-h as described in the FETAX Atlas of Abnormalities [22]. Embryos were photographed and measured for length using Image Pro plus software. Two definitive dose-response tests were performed. The same clutch was used for each of the definitive 1 and a second clutch was used for definitive 2 for all leachate types.
1.3. Statistical analysis
Means and standard error were calculated. The 96-h LC50, 96-h EC50 for individual and combined experiments were determined using probit analysis by Systat 13. The Teratogenic Index (TI) was calculated by dividing the 96-h LC50/96-h EC50. Embryo length, malformation, and mortality data were analyzed with ANOVA followed by Bonferroni's t-test for multiple comparisons to determine the Minimum Concentration to Inhibit Growth (MCIG), The No and Lowest Observable Effects Concentration (NOEC,LOEC) for mortality and malformation.
2. Results
The overall average control lengths ranged from 0.96 to1.03 cm. The typical malformations seen in the controls included abnormal gut coiling, slight edema, and some tail and notochord malformations. For the overall experiment, 56 of the 480 control tadpoles exposed died, yielding a 11.6% mortality rate. Of the 424 surviving tadpoles, 17 were malformed for an overall malformation rate of 4.0%.
2.1. Regular cigarette butt leachate (RCBL)
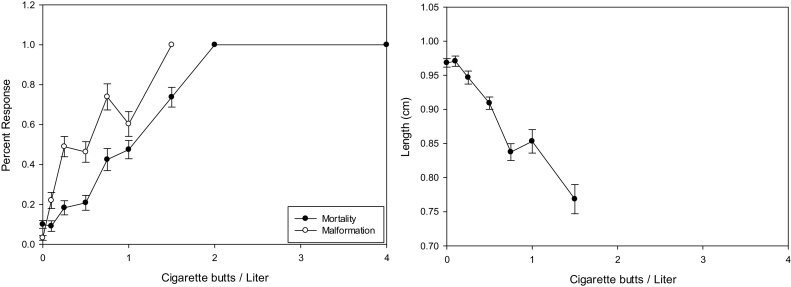
The Mortality and Malformation showed a sharp increase concentration response with Mortality and Malformation curves following each other. (Fig. 1. Left Panel). The 96-h LC50 for the two clutches of embryos exposed to RCBL was 0.68–1.65 CBs/L. The 96-h EC50 for the two clutches of embryos exposed to RCBL was 0.34–1.21 CBs/L. The Teratogenic Index (TI) for the two clutches was 1.36–1.95 CBs/L. The Minimum Concentration to Inhibit Growth (MCIG) for RCBL was calculated to be 0.25 and 0.5 CBs/L (Table 1). There was a sharp direct negative correlation between embryo length and regular cigarette butt leachate concentration (Fig. 1 Right Panel). The reduction in length of controls compared to the highest surviving concentration was 0.26 cm. The Lowest and No Observable Effects Concentrations (LOEC, NOEC) for Mortality were consistent with 0.5 CBs/L for the LOEC and 0.25 CBs/L for the NOEC. For Malformation, the NOEC was more variable due to clutch differences. Dominant malformations for RCB were loose gut coiling, facial malformations, notochord malformations, and stunting (Photo Plate 1, Top Right Panel).
Fig. 1.
Representative concentration response graphs for Mortality & Malformation (Right Panel) and Growth (Left Panel) from a single experiment with Regular Cigarette Butt Leachate (RCBL). The Mortality & Malformation indicated an increasing response with concentration of RCBL. The growth curve indicated decreasing length with increasing concentration of RCBL. Error bars represent standard error.
Table 1.
Individual analysis of each replicate experiment with 96-h LC50, EC50(malformation), Teratogenic index (TI), Minimum Concentration to Inhibit Growth (MCIG), Mortality and Malformation LOEC (Lowest Observable Effects Concentration) and NOEC (No Observable Effects Concentration).
| Clutch | 96-hLC50 CBs/L | 96-h EC50 CBs/L | TI | MCIG CBs/L | Mortality CBs/L |
Malformation CBs/L |
|||
|---|---|---|---|---|---|---|---|---|---|
| LOEC | NOEC | LOEC | NOEC | ||||||
| RCBL | 1a | 0.68 (0.56–0.82) | 0.34 (0.27–0.45) | 1.95 | 0.25 | 0.5 | 0.25 | ≤0.1 | N.C. |
| RCBL | 2 | 1.65 (1.35–2.06) | 1.21 (0.88–1.85) | 1.36 | 0.5 | 0.5 | 0.25 | 2 | 1 |
| MCBL | 1 | 1.078 (0.99–1.33) | 0.96 (0.71–1.60) | 1.43 | 0.5 | 2 | 1 | 0.25 | 0.1 |
| MCBL | 2 | 1.140 (1.02–1.29) | 0.30 (0.23–0.37) | 3.77 | 0.5 | 1.5 | 1 | <0.1 | N.C. |
| ECBL | 1 | 9.704 (7.54–14.03) | 20.34 (12.91–75.01) | 0.47 | 10 | 10 | 8 | >10 | N.C. |
| ECBL | 2 | 26.807 (N.C.) | 15.584 (10.98–34.91) | 1.72 | >10 | 8 | 4 | >10 | N.C. |
CBs = cigarette butts. Teratogenic Index (TI) = 96-h LC50/96-h EC50. Numbers in Parenthesis are 95% Fieller Bounds. RCBL = Regular Cigarette Butt Leachate, MCBL = Menthol Cigarette Butt Leachate, ECBL = Electronic Cigarette Butt Leachate.
All first experiments were conducted with the same clutch and all of the second experiments were conducted with the same clutch. N.C.= Not Calculable.
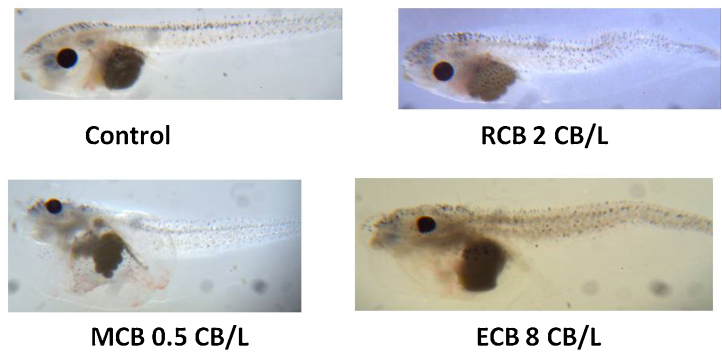
Plate 1.
Control embryo top left. Top right panel is RCBL (2CB/L) and the embryo exhibits minor malformations of head, gut and overall embryonic delayed development. The RCBL embryo also exhibited significant tail abnormalities. Bottom left is MCBL (0.5 CB/L) and the embryo exhibits severe edema in the heart, craniol-facial, and abdomial areas. There are also significant gut and facial abnormalities. Bottom Right panel is ECBL at 8 CB/L and the embryo exhibits some tail abnormalities. The embryo has moderate to severe edema, heart and gut abnormalities. The embryo also has facial abnormalities as well.
2.2. Menthol Cigarette Butt Leachate (MCBL)
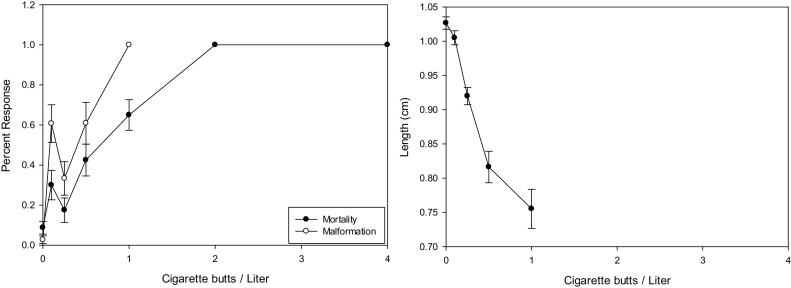
The Mortality and Malformation showed an increase concentration response with a distinct separation of the mortality and malformation curves (Fig. 2 left panel). The 96-h LC50 for the two clutches of embryos was consistent at just over 1.078 and 1.140 CBs/L indicating good agreement between the two replicated experiments. But the 96-h EC50 showed a little more variability between 0.3–0.96 CBs/L. It is the differences in the 96-h EC 50 that account for the TI being from 1.43 to 3.77. The MCIG was consistent at 0.5 CBs/L. There was a sharp dramatic direct negative correlation between embryo length and MCBL (Fig. 2 right panel). The reduction in length of controls compared to the highest surviving concentration was 0.21 cm. The growth reduction was 0.20 cm for the highest surviving concentrations. The malformation endpoint was the most sensitive with the LOEC being 0.25 to <0.1 CBs/L. The malformations seen with MCBL were most dramatic. There was significant consistent edema, gut, heart, and craniofacial abnormalities (Photo Plate 1 bottom left panel).
Fig. 2.
Representative concentration response graphs for Mortality & Malformation (Right Panel) and Growth (Left Panel) from a single experiment with Menthol Cigarette Butt Leachate (MCBL). The Mortality & Malformation indicated an increasing response with concentration of MCBL. The growth curve indicated decreasing length with increasing concentration of MCBL. Error bars represent standard error.
2.3. Electronic Cigarette Butt Leachate (ECBL)
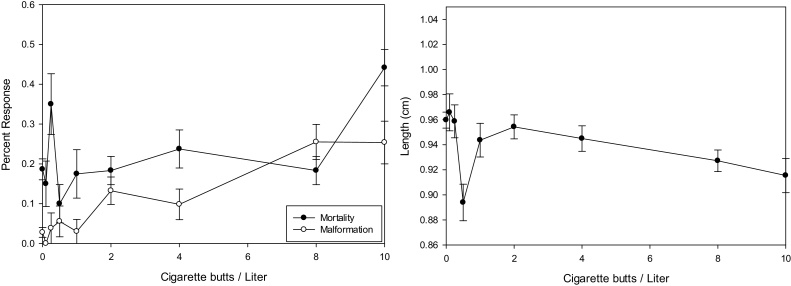
The Mortality and Malformation showed a unique concentration response with mortality and malformation curves overlapping for most of the concentration (Fig. 3 left panel). There was one point at 0.5 CBs/L that showed a dramatic drop in embryo length; however, it was not consistent with other concentrations tested. The 96-h LC50 indicated a low toxicity with 9.7 and 26.8 CBs/L. The two experiments were different with the second showing much lest toxicity than the first. Neither experiment yielded many malformations at all concentrations tested. The TI of the experiment indicated widely different numbers of 0.47–1.72 due to the change in the 96-h LC50 of Experiment 2. The MCIG was right at the highest concentration tested of 10 CBs/L or higher. Embryo growth was negatively impacted; however, the growth curve had a much more gradual slope (Fig. 3 right panel). The reduction in length of controls compared to the highest surviving concentration was only 0.05 cm. The malformations seen with ECBL were slight edema, loose gut coiling, and some tail abnormalities (Photo Plate 1 bottom right panel). These malformations were moderate to severe when they occurred.
Fig. 3.
Representative concentration response graphs for Mortality & Malformation (Right Panel) and Growth (Left Panel) from a single experiment with Electronic Cigarette Butt Leachate (ECBL). The Mortality & Malformation indicated an increasing response with concentration of ECBL. The growth curve indicated decreasing length with increasing concentration of ECBL. Error bars represent standard error.
3. Discussion
This research indicates the overall 96-h LC50 of RCBL and MCBL was 1.2 and 1.14 CBs/L respectively (Table 2). The overall 96-hEC50 (malformation) of RCBL and MCBL was 0.90 and 0.50 CBs/L (Table 2). It would be expected that embryos with malformations would not survive in the environment. These values are within the range of previously tested aquatic species (Table 3). This demonstrating that Xenopus laevis embryos are good indicators of toxicity associated with cigarette butt leachate. In addition, the FETAX assay showed much lower CBs/L for other endpoints measured such as MCIG of 0.01 and 0.25 CBs/L (Table 2).
Table 2.
Overall summary analysis of Regular Cigarette Butt Leachate (RCBL), Menthol Cigarette Butt Leachate (MCBL), and Electronic Cigarette Butt Leachate (ECBL) with the 96-h LC50, EC50 (malformation), Teratogenic index (TI), Minimum Concentration to Inhibit Growth (MCIG), Mortality and Malformation LOEC (Lowest Observable Effects Concentration) and NOEC (No Observable Effects Concentration).
| 96-h LC50 CBs/L | 96-h EC50 Malformation CBs/L | TI | MCIG CBs/L | Mortality CBs/L |
Malformation CBs/L |
|||
|---|---|---|---|---|---|---|---|---|
| LOEC | NOEC | LOEC | NOEC | |||||
| RCBL | 1.20 (1.04–1.38) | 0.90 (0.71–1.21) | 1.33 | 0.01 | 0.5 | 0.25 | 0.1 | 0.05 |
| MCBL | 1.14 (1.05–1.25) | 0.50 (0.43–0.59) | 2.28 | 0.25 | 1.5 | 1 | 0.1 | <0.1 |
| ECBL | 14.60 (11.10–22.66) | 16.31 (12.50–25.14) | 0.90 | 10 | 10 | 8 | 8 | 4 |
CBs = cigarette butts, TI = Teratogenic Index (96-h LC50/96-h EC50). Numbers in Parenthesis are 95% Fieller Bounds.
Table 3.
Combined analysis of Regular Cigarette Butt Leachate (RCBL) in Literature.
3.1. Impact of RCBL and MCBL
The RCBL and MCBL had an overall TI of 1.33–2.28 (Table 2) indicating that there is some teratogenic risk associated with the leachate with MCBL having more risk. This would be expected because nicotine is known to be highly teratogenic in the FETAX assay [25]. This indicates that the CBL are a potential teratogenic hazard to aquatic life in rivers, streams, and lakes and [agrees with] is consistent with data from Lee and Lee [26]. The MCBL indicates a higher risk due to either the menthol present or possibly interactions of the additives with the nicotine and other products from smoking cigarettes. Another possibility is that the additives somehow added the ability of nicotine and other products to leave the cigarettes. This does not support the statement by Slaughter et al. [4] that “There is no research to support that flavored cigarettes (eg, menthol) alter toxicity or impart additional toxicity.” Although the overall toxicity differences are not large the impact on malformations was significant.
The ECBL was much less toxic with an overall 96-h LC50 of 14.6 CBs/L (Table 2) indicating that the ECBL was at least 10 fold less toxic than the regular cigarette butts. Surprisingly, the 96-h EC50 (malformation) was even higher at 16.3 CBs/L. The overall teratogenic index was 0.90 indicating there was also little teratogenic risk. Because the ECBL would be expected to contain nicotine, this might mean that ECBs might not allow the nicotine to be bioavailable or some other mechanisms are in place to prevent excessive nicotine exposure to the environment. There was much less effect on growth as indicated by the very high MCIG compared to the traditional cigarette butts.
Electronic cigarettes are approximately 10 fold less toxic Xenopus embryos than traditional cigarettes. This indicates that the impact of discarded ECB on aquatic environments would potentially be less than that of traditional cigarette butts. The impact is further minimized by the fact that 1 ECB is equivalent to approximately 40 traditional cigarettes [17]. The aquatic environmental impact of ECBs thus could be potentially 400 times less than that of traditional cigarettes. The methods that were used in this study, however, were only a 1 h soaking/mixing time with ECB that had not been physically damaged as they might be in a landfill. This data would also not include potential impacts by long-term metal erosion or batter degradation into the aquatic environment among other factors. The purpose of this paper was to examine what would leak out of easily discarded cigarette butts, and not on more elaborate electronic cigarettes with refillable cartridges. The pathways of this cigarette waste to aquatic environments are complex and varied, and that makes correlations to risk difficult to study and determine [4].
FETAX proved to be a useful testing assay for cigarette butt leachate and is viable for further testing. Because traditional menthol cigarettes resulted in different results than regular cigarettes, especially with regards to gross malformations, future studies should examine mentholated cigarettes in more detail. In addition, longer soaking/mixing times, as well as testing the liquid contents of refillable e-cigarettes (flavored and non-flavored), should be assessed.
Acknowledgement
We would like to thank Jacksonville State University Biology Department for support of this project.
References
- 1.Moriwaki H., Kitajima S., Katahira K. Waste on the roadside, ‘poi-sute' waste: its distribution and elution potential of pollutants into environment. Waste Manage. 2009;29(3):1192–1197. doi: 10.1016/j.wasman.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Ocean_Concervancy . Ocean Concervancy; Washington, D.C: 2014. Marine Debris: More than An Eye Sore. [Google Scholar]
- 3.Register Kathleen. Cigarette butts as litter-toxic as well as ugly? Underwater Naturalist. Bull. Am. Littoral Soc. 2000;25(2):23–29. [Google Scholar]
- 4.Slaughter E., Gersberg R.M., Watanabe K., Rudolph J., Stransky C., Novotny T.E. Toxicity of cigarette butts, and their chemical components, to marine and freshwater fish. Tob. Control. 2011;20(1):25–29. doi: 10.1136/tc.2010.040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee, Joyce, Get your butt off the ground! Consequences of cigarette waste and litter reducing methods. Pomona: Pomona Senior Theses, 2012, 44.
- 6.Novotny T.E., Lum K., Smith E., Wang V., Barnes R. Cigarettes Butts and the Case for an Environmental Policy on Hazardous Cigarette Waste. Int. J. Environ. Res. Public Health. 2009;5:1691–1705. doi: 10.3390/ijerph6051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novotny, Thomas E., Sarah N. Hardin, Lynn R. Hovda, Dale J. Novotny, Mary Kay McLean, Safdar Khan. Tobacco and cigarette butt consumption in humand and animals. Tobacco Control, 20 (2011) 17–20. [DOI] [PMC free article] [PubMed]
- 8.Dieng H. Indirect effects of cigarette butt waste on the dengue vector Ades aegypti (Diptera: Culicidae) Acta Trop. 2013;130:123–130. doi: 10.1016/j.actatropica.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Bahl V., Lin S., Xu N., Davis B., Wang Y., Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Etter J.F., Zäther E., Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108:1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- 11.Trehy M.L. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J. Liq. Chromatogr. Relat. Technol. 2011;32:1442–1446. [Google Scholar]
- 12.Drummond M. Bradley, Upson Dona. Electronic cigarettes potential harms and benefits. Ann. Am. Thorac. Soc. 2014;11(2):236–242. doi: 10.1513/AnnalsATS.201311-391FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goniewicz M.L. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingebrethsen B.J., Cole S.K., Alderman S.L. Electronic cigarette aerosol particle size distribution measurements. Inhal. Toxicol. 2012;24:976–984. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- 15.McAuley T.R., Hopke P.K., Zhao J., Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal. Toxicol. 2012;24:850–857. doi: 10.3109/08958378.2012.724728. [DOI] [PubMed] [Google Scholar]
- 16.Williams Monique, Villarreal Amanda, Bozhilov Krassimir, Lin Sabrina, Talbot Prue. Prue talbot metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause Max J., Townsend Timothy G. vol. 39. Elsevier; 2015. Hazardous waste status of discarded electronic cigarettes; pp. 57–62. (Waste Management). [DOI] [PubMed] [Google Scholar]
- 18.Bantle John A., Fort Douglas J., Rayburn James R., DeYoung D.J., Bush Shirley J. Futher validation of FETAX: evaluation of the developmental toxicity of five known mammalian teratogens and non-teratogens. Drug Chem. Toxicol. 1990;13(4):267–282. doi: 10.3109/01480549009032286. [DOI] [PubMed] [Google Scholar]
- 19.Bantle, John A., T. D. Sabourin, Standard Guide for Conducting the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). American Society for Testing and Materials (ASTM) E1439-91 (ASTM International) 11.06 (1991), 1–11.
- 20.Dawson D.A., Bantle J.A. Development of a reconstituted water medium and initial validation of FETAX. J. Appl. Toxicol. 1987;7:237–244. doi: 10.1002/jat.2550070403. [DOI] [PubMed] [Google Scholar]
- 21.McCallum Malcolm, Rayburn James. A simple method for housing Xenopus during oviposition and obtianing eggs for use in FETAX. Herpetolog. Rev. 2006;37(3):332. [Google Scholar]
- 22.Bantle John A., James James N., Finch Robert A., Linder Gregory. Oklahoma State University Press; Stillwater, OK: 1991. Atlas of Abnormalities: A Guide for The Performance of FETAX. [Google Scholar]
- 23.Warne M.St.J., Patra R.W., Cole B. The Australasian Society of Ecotoxicology and The International Chemometrics Society; 2002. Toxicity and a Hazard Assessment of Cigarette Butts to Aquatic Organisms. Interact 2002-Programme and Abstract Book. Sydney: The Royal Australian Society Chemical Institute; p. 192. [Google Scholar]
- 24.Micevska T., Warne M.S., Pablo F., Patra R. Variation in, and causes of, toxicity of cigarette butts to a cladoceran and microtox. Arch. Environ. Contam. Toxicol. 2006;50(2):205–212. doi: 10.1007/s00244-004-0132-y. [DOI] [PubMed] [Google Scholar]
- 25.Dawson Douglas A., Fort Douglas J., Newel John A. Evaluation of the developmental toxicity of nicotine and cotinine with frog embryo teratogenesis assay: xenopus. Teratog. Carcinog. Mutagen. 1988:329–338. doi: 10.1002/tcm.1770080603. [DOI] [PubMed] [Google Scholar]
- 26.Lee Wenjau, Lee Chih Chun. Developmental toxicity of cigarette butts – An underdeveloped issue. Ecotoxicol. Environ. Saf. (Elsevier) 2015;113:362–368. doi: 10.1016/j.ecoenv.2014.12.018. [DOI] [PubMed] [Google Scholar]