Abstract
This study determined the presence and levels of Polycyclic Aromatic Hydrocarbons (PAHs) of groundwater in Moro, Edun-Abon, Yakoyo and Ipetumodu communities in Ife-North Local Government Area of Osun State. This was with a view to create public awareness about the safety of groundwater as a source for domestic purposes (e.g., drinking, cooking etc.) in non-industrial area. Water samples were collected on seasonal basis, comprising of three months (August–October) in the wet season and three months (December–February) in the dry season. The PAHs in the water samples were extracted with n-hexane using liquid–liquid extraction method, while their qualitative identifications and quantitative estimations were carried out with the use of gas chromatography. Levels of PAHs detected showed predominance of light PAHs (less than four fused rings) for both wet and the dry seasons. Higher concentrations of PAHs were recorded during the wet season than the dry season. The study concluded that the groundwater in the communities was contaminated with light PAHs and the total PAHs in this area exceeded the maximum permissible limit of 10 μg L−1 recommended by World Health Organization (WHO) for safety of groundwater.
Keywords: Polycyclic aromatic hydrocarbons, Groundwater, Water quality, Seasonal variation, Health impact
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are multi-ringed organic compounds which are ubiquitous in nature. They are derived from both natural processes of biogenic precursors [1]) and anthropogenic processes of incomplete combustion of organic matter and emissions of non-combustion-related petrogenic process [2]. They are a group of about 10,000 compounds, a few of which occur in considerable amounts in the environment, and in food [3]. PAHs comprise fused aromatic rings and do not contain hetero-atoms. Some PAHs have been demonstrated to be carcinogenic and mutagenic. However, those PAHs that have not been found to be carcinogenic may act as synergists. Exposure of humans to single PAHs does not occur because PAHs are always encountered as complex mixtures. PAHs containing up to four fused benzene rings are known as light PAHs and those containing more than four benzene rings are called heavy PAHs. Heavy PAHs are more stable and more toxic than light ones. PAHs are lipophilic in nature; nevertheless, some of them can dissolve quite well in water [3]. Water can become contaminated with PAHs from runoff in urban areas, waste water from certain industries such as those involved in creosote manufacturing and aluminium smelting), or from PAHs carried in the air. The level of PAHs in untreated drinking water is approximately five times that of treated municipal drinking water supplies [3].
Groundwater is often cheaper, more convenient and less vulnerable to pollution than surface water. Therefore, it is commonly used for public water supplies [4]. Groundwater makes up about twenty percent of the world's fresh water supplies, which is about 0.61% of the entire water, including oceans and permanent ice [4]. Many municipal water supplies are derived solely from groundwater. The volume of groundwater in an aquifer can be estimated by measuring water levels in local wells and by examining geologic records from well-drilling to determine the extent, depth and thickness of water-bearing sediments and rocks. Polluted groundwater is less visible, but more difficult to clean up than pollution in rivers and lakes. Groundwater pollution most often results from improper disposal of wastes on land. Major sources include industrial household chemicals and garbage landfills, industrial wastes, lagoons tailings, processed wastewater from mines, oil field brine pits, leaking underground oil storage tanks and pipelines, sewage sludge and septic systems. Polluted groundwater is mapped by sampling soils and groundwater near suspected or known sources of pollution, to determine the extent of the pollution and to aid in the design of groundwater remediation systems. Preventing groundwater pollution near potential sources such as landfills requires lining the bottom of landfills with watertight materials, collecting leachate withdrawal and keeping rain water off any potential contaminants along with regular monitoring of nearby groundwater to verify that contaminants have not leaked into the ground. The danger of pollution of municipal supplies is minimized by locating wells in areas of deep groundwater and impermeable soils and careful testing and monitoring of the aquifer and nearby potential pollution sources [4].
Several authors have studied pollution of water with PAHs in petrogenic and industrial environments. For example, Wakeham et al. [5] reported PAHs from sedimentary records of several lakes in Switzerland and Washington, indicative of high levels of PAHs pollution in modern input. Hostettler et al. [6] studied the trace of biomarker profiles in San Francisco Bay sediments, showing anthropogenic input of hydrocarbons in recent decade’s depositions. Anyakora and Coker [7] determined the PAHs in water bodies in the Niger Delta. Ogbuagu et al. [8] studied contamination of groundwater sources in Okrika Mainland with PAHs. However, there is no study on the determination of PAHs in water from non-industrial area hence this present study.
The motivation to this study was predicated on the increase of pollution in this century, which has led to the occurrence of many carcinogenic and mutagenic diseases. Because most of the foods consumed are produced from non-industrial areas, baseline index on the pollution level of PAHs needs to be established. Thus, this study investigated the level of PAHs pollution in groundwater of non-industrial area (Ife North Local Government Area, Osun State) so as to create public awareness about PAHs contamination in the area.
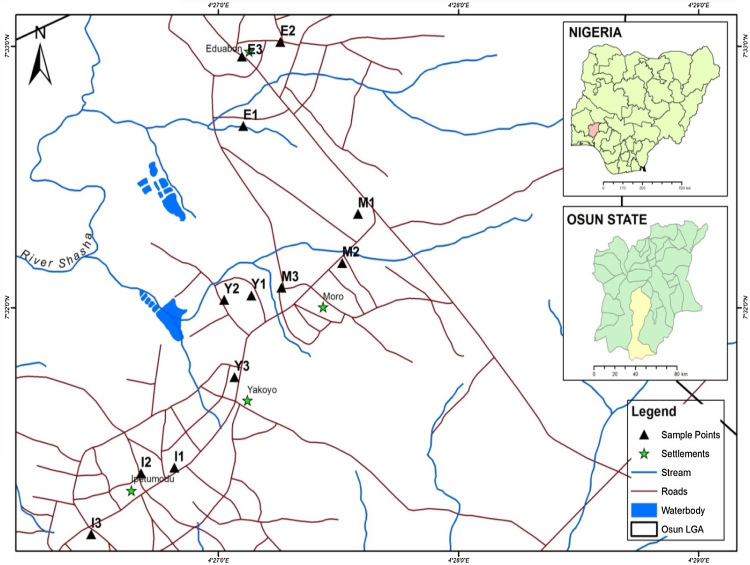
The study area includes Moro, Edun-Abon,Yakoyo and Ipetumodu in Ife North Local Government Area, Osun State. It has an area of 49 sq mi and a population of 153,694 as of 2006 census [9]. The study area lies within 7° 31.133′N, 004° 26.470′E and 7°32.359′N, 004° 27.581′E (Fig. 1). It is bounded by Ife Central, Ife East, Ife South and Ayedaade Local Governments Areas. The towns are suburbs to Ile-Ife and people in these towns are primarily Yoruba speaking ethnic groups and are known for trading and agricultural practices. Agricultural raw materials and foods consumed in the cities are transported to Ile − Ife, Osogbo, cities within and beyond the state. These people live essentially on ground water for domestic purposes and surface water for agricultural uses. It is, therefore, pertinent to evaluate the potability status of their water sources. The levels of PAHs in the groundwater have not been reported in the literature, hence the seasonal evaluation of the PAHs. Previous studies in our group conducted in the same site for the physico-chemical parameters of the underground water indicated that seasonal changes impact on the results obtained for both the dry and the wet season [10]. Thus, we speculate that seasonal changes may also lead to variation in the levels of PAHs in the ground water. This was confirmed from our experimental results where higher concentration of the PAHs was recorded for the wet season compared with the dry season. This study will also provide useful baseline data and information on the seasonal levels of PAHs in the groundwater of major towns in Ife North Local Government Area, Osun State Nigeria.
Fig. 1.
A Map of Ife North Local Government Showing the Sampling Sites.
2. Materials and methods
2.1. Pretreatment of sampling and storage vessels
Prior to sampling, sample bottles and storage glass wares were washed with detergent, rinsed with distilled water and pure acetone (99.9%) and then dried in an oven overnight at 100 °C. Winchester bottles were used in collecting water samples for determination of PAHs. Analytical grade chemical reagents and materials were used in the study.
2.2. Chemicals and reagents used
N-Hexane-BDH analytical grade, acetone-BDH analytical grade, silica gel 60–120 mesh-Oxford laboratory reagent, anhydrous sodium sulphate-M&B laboratory chemical, MnSO4-Kermel, HCl-BDH analytical grade.
2.3. Water sampling
Groundwater samples were collected on seasonal basis comprising of three months (August, September, October) during the wet season and three months (December, January, February) during dry season of the year 2014 from Moro (M), Edun-Abon (E), Yakoyo (Y) and Ipetumodu (I) communities of Ife North Local Government Area of Osun State. M1, M2, M3 = Moro roads 1, 2 and 3; E1, E2, E3 = Edunabon roads 1, 2 and 3; Y1, Y2, Y3 = Yakoyo roads 1, 2 and 3; I1, I2, I3 = Ipetumodu road 1, 2 and 3. Each town was divided into three zones (Table 1) and a composite sample (representing five wells of equal volume) were taken from the groundwater from each of the zones on a monthly basis to give a total of 3 composite samples for each town, and 12 composite samples for the four towns per month. A total of seventy-two (72) groundwater samples were collected for six months (including the wet and dry seasons) and the samples were collected in 2.5 L pre-treated Winchester bottles. Samples were stored at 4 °C in the refrigerator prior to extraction.
Table 1.
Description and Co-ordinates of Sampling Sites.
| Town | Location | N-coordination | E-coordination |
|---|---|---|---|
| Moro | |||
| M1 | Moro 1 | 07° 32.359′ | 004° 27.581′ |
| M2 | Moro 2 | 07° 32.171′ | 004° 27.515′ |
| M3 | Moro 3 | 07° 32.077′ | 004° 27.262′ |
| Edun-Abon | |||
| E1 | Edun-Abon 1 | 07° 32.695′ | 004° 27.103′ |
| E2 | Edun-Abon 2 | 07°32.017′ | 004° 27.257′ |
| E3 | Edun-Abon 3 | 07° 32.961′ | 004° 27.097′ |
| Yakoyo | |||
| Y1 | Yakoyo 1 | 07° 32.047′ | 004° 27.137′ |
| Y2 | Yakoyo 2 | 07° 32.030′ | 004° 27.023′ |
| Y3 | Yakoyo 3 | 07° 31.734′ | 004° 27.066′ |
| Ipetumodu | |||
| I1 | Ipetumodu 1 | 07° 31.388′ | 004° 26.816′ |
| I2 | Ipetumodu 2 | 07° 31.366′ | 004° 26.677′ |
| I3 | Ipetumodu 3 | 07° 31.133′ | 004° 26.470′ |
2.4. Extraction and clean-up
Extraction was carried out in a liquid–liquid extraction mode by thoroughly shaking 500 mL of water sample in a separating funnel with 20 mL of n-hexane using the method for organic chemical analysis of municipal and industrial wastewater, US EPA Method 610 [11]. The solution was transferred to a separating funnel and samples were extracted by shaking the funnel for 10 min with periodic venting to release excess pressure. The organic layer was allowed to separate from the water phase for a minimum of 15 min and the n-hexane extract was collected in a 120 mL Amber coloured bottle. Extraction was repeated three times and the extracts were combined in an Erlenmeyer flask. The concentrated extract was obtained by evaporating the organic layer under a gentle stream of nitrogen and stored in the refrigerator at 4 °C. The isolation of PAHs was done by solid-phase extraction in a normal phase mode. Activated silica gel was loaded into a glass-chromatographic column; an additional 1 g of Na2SO4 was added to the column and conditioned with n-hexane. The concentrated extract were dissolved in 5 mL n-hexane, loaded into the column and then eluted with 50 mL n hexane. The eluents were concentrated using a rotary evaporator and then reconstituted in 0.5 mL n-hexane for gas chromatography (GC) analysis.
2.5. GC analytical conditions
The samples were analyzed at the Department of Chemistry, University of Johannesburg, South Africa using Gas chromatograph model 7890A series (Agilent Technologies, Inc., Wilmington, DE, USA) equipped with a LECO Pegasus 4D Time of Flight mass spectrometry detection and an Agilent on-column injection system. Columns: Rxi-5 SilMS (30 m x 0.25 mm x 0.25 um) as a primary column and Rxi 17 Sil MS (1 m x 0.25 mm x 0.25 um) as the secondary column. The helium carrier gas was maintained at constant flow of 2.0 mL per min. The injection volume was set at 1 μL Splitless and the injection temperature was set at 250 °C. The oven temperature was programmed as follows: 40 °C held for 0.50 min; ramped from 40 °C − 280 °C at 10 °C/min and then held for 0.50 min at 280 °C. The mass spectrometry conditions were set up as follows: solvent delay of 180 s; transfer line temperature at 250 °C; Electron ionization at −70 eV; source temperature at 250 °C; stored mass range: 45–600 u; acquisition rate: 10 spectra/s for GC TOMS; detector offset voltage was set at 300 V.
3. Results and discussion
3.1. Mean concentration of PAHs at various locations in wet and dry seasons
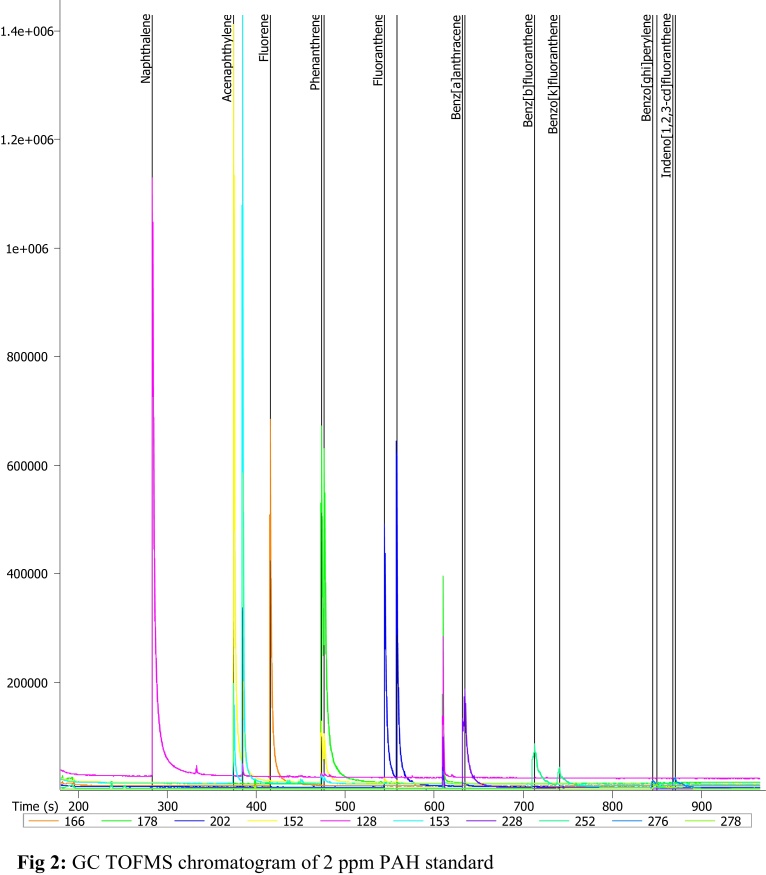
Fig. 2 shows the chromatogram of the certified reference standard used, while the supplementary (S) information on the calibration curves of individual PAHs standard used for the quantification of the PAHs determined in this study are presented in Figs. S1 and S2 in Supplementary material presents the spectra used for qualitative identification of individual PAHs. All these are the quality control measures taken to obtain reliable results. Tables S1 and S2 in Supplementary material present all PAHs identified in the calibration standards, their calibration equations and relative peak areas used for the quantification of PAHs identified in the samples. From this study, seven PAHs were identified to be present in the groundwater of the studied area (Table 2, Table 3). Fluorene has the highest mean concentration (119 ± 204 μg L−1) while acenaphthene has the lowest mean concentration (0.042 ± 0.342 μg L−1). It can be deduced that the PAHs detected in groundwater samples in wet and the dry seasons in the study area were light PAHs. Heavy PAHs are rarely present in water; this is due to the low water solubility of these compounds [12] (Fig. 3).
Fig. 2.
GC TOFMS chromatogram of 2 ppm PAH standard.
Table 2.
Concentrations of PAHs in the Wet Season Groundwater Samples (μg L−1).
| Wet Season | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site | Naphthalene | Acenaphthene | Acenaphthylene | Anthracene | Fluoranthene | Fluorene | Phenanthene | Mean ± SD |
| M1 | 0.80 ± 0.14 | BDL | BDL | BDL | 0.94 ± 0.00 | 2.84 ± 0.00 | 4.29 ± 2.04 | 2.22 ± 1.67 |
| M2 | 4.57 ± 0.81 | 0.06 ± 0.00 | 0.09 ± 0.00 | 0.96 ±0.02 | BDL | 37.49 ± 0.48 | 1.26 ± 0.50 | 7.41 ± 14.83 |
| M3 | 1.69 ± 0.29 | BDL | 0.06 ± 0.00 | 0.23 ±0.00 | BDL | 45.62 ± 29.32 | 2.08 ± 0.49 | 9.94 ± 19.97 |
| E1 | 1.51 ± 0.17 | BDL | BDL | 0.00 ± 0.00 | BDL | 98.29 ± 67.26 | 2.10 ± 0.25 | 25.49 ± 48.55 |
| E2 | 0.67 ± 0.06 | 0.02 ± 0.00 | 0.08 ± 0.00 | 0.02 ± 0.00 | BDL | 11.89 ± 1.09 | 0.29 ± 0.02 | 2.16 ± 4.77 |
| E3 | 14.31 ± 1.25 | 0.11 ± 0.00 | BDL | 0.03 ±0 0.01 | BDL | BDL | BDL | 4.82 ± 8.22 |
| Y1 | 2.74 ± 0.31 | 0.05 ± 0.00 | 0.22 ± 0.00 | 2.91 ±2.03 | BDL | 165.19 ± 30.69 | 7.06 ± 3.28 | 9.69 ± 66.43 |
| Y2 | 11.25 ± 0.91 | BDL | BDL | BDL | BDL | 43.36 ± 0.00 | 0.55 ± 0.25 | 18.39 ± 22.28 |
| Y3 | 0.18 ± 0.03 | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.42 ±0.26 | BDL | 0.93 ± 0.00 | 0.76 ± 0.28 | 0.39 ± 0.39 |
| I1 | 90.01 ± 3.92 | 0.01 ± 0.00 | BDL | 0.01± 0.00 | BDL | 698.99 ± 0.00 | 12.65 ± 5.22 | 160.34 ± 303.45 |
| I2 | 4.61 ± 0.39 | 0.06 ± 0.00 | BDL | BDL | BDL | 199.63 ± 50.25 | 14.80 ±4.10 | 54.78 ± 96.77 |
| I3 | 1.16 ± 0.10 | 0.02 ± 0.00 | 0.08± 0.00 | BDL | BDL | 4.31 ± 0.00 | 9.09 ± 0.92 | 2.93 ± 3.86 |
| Range | 0.67–90.01 | 0.01–0.11 | 0.04–0.22 | 0.01–2.91 | – | 0.93–698.93 | 0.29–14.80 | 0.39–160.34 |
| Mean ± SD | 11.13 ± 25.24 | 0.04 ± 0.34 | 0.10 ± 0.06 | 0.51 ± 0.95 | 0.94 ±0.00 | 118.96 ± 203.78 | 5.00 ± 5.17 | 24.88 ± 45.29 |
BDL = Below Detection Limit; SD = Standard deviation.
Table 3.
Concentrations of PAHs in the Dry Season Groundwater Samples (μg L−1).
| Dry Season | |||||
|---|---|---|---|---|---|
| Site | Naphthalene | Anthracene | Fluorene | Phenanthrene | Mean ± SD |
| M1 | 0.64 ± 0.11 | 0.19 ± 0.08 | 15.57 ± 0.00 | 1.24 ± 0.21 | 4.41 ± 7.45 |
| M2 | 4.18 ± 0.26 | BDL | BDL | 6.72 ± 0.22 | 5.45 ± 1.79 |
| M3 | 0.14 ± 0.02 | BDL | BDL | 0.09 ± 0.02 | 0.12 ± 0.04 |
| E1 | 0.72 ± 0.18 | BDL | 7.19 ± 1.04 | 3.29 ± 1.71 | 3.73 ± 3.26 |
| E2 | 5.40 ± 0.47 | 0.01 ± 0.00 | 57.57 ± 38.61 | 7.99 ± 1.90 | 17.74 ± 26.76 |
| E3 | 11.24 ± 0.62 | 0.10 ± 0.00 | 358.52 ± 201.58 | 0.70 ± 0.22 | 92.64 ± 177.33 |
| Y1 | BDL | BDL | BDL | 0.09 ± 0.00 | 0.09 ±00 |
| Y2 | 0.02 ± 0.01 | 0.17 ± 0.00 | BDL | 0.43 ± 0.20 | 0.21 ± 0.20 |
| Y3 | 0.09 ± 0.03 | BDL | BDL | 0.52 ± 0.20 | 0.30 ± 0.30 |
| I1 | 0.15 ± 0.02 | BDL | BDL | 0.09 ± 0.00 | 0.12 ± 0.04 |
| I2 | 0.58 ± 0.09 | BDL | 0.78 ± 0.00 | 1.94 ± | 0.68 ± 0.14 |
| I3 | 0.17 ± 0.05 | 0.04 ± 0.01 | 3.45 ± 1.52 | 1.19 ± 0.38 | 1.21 ± 1.58 |
| Range | 0.02–11.24 | 0.01–0.19 | 0.78–358.52 | 0.09–7.99 | 0.09–92.64 |
| Mean ± SD | 2.12 ± 3.53 | 0.10 ± 0.08 | 73.85 ± 141.03 | 2.03 ± 2.80 | 10.56 ± 26.33 |
BDL = Below Detection Limit; SD = Standard deviation.
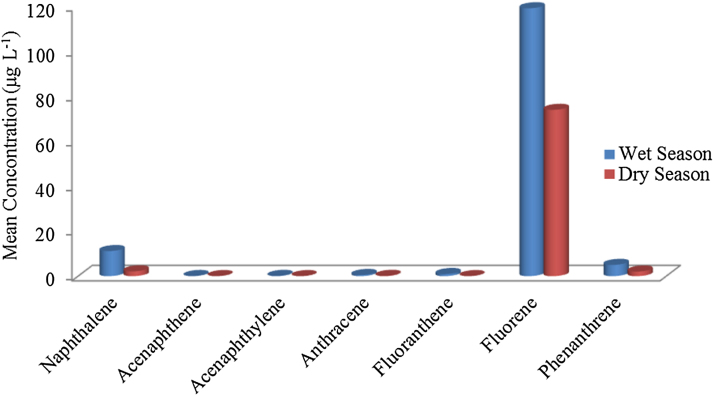
Fig. 3.
Mean Concentrations (μg L−1) Distribution of PAHs in the Groundwater for Wet and Dry Seasons.
Fig. 3 shows the distribution graph of mean concentration of PAHs identified to be present in the groundwater samples of the study area during the wet and dry seasons while Fig. 4 presents the frequency distribution of the total PAHs in the ground water samples. From the graph, the mean concentration of detected PAHs are in the order of fluorene > naphthalene > phenanthrene > fluoranthene > anthracene > acenaphthylene > acenaphthene. During the wet season the mean concentration of PAHs in the sites are in the order of I1> I2 > E1 > Y2 > M3 >YI > M2 > E3 > I3 >M1 >E2 > Y3, while in the dry season mean concentration of PAHs in the sites are in the order of E3 >E2 > M2 > M1 > E1 > I3 > I2 > Y3 >Y2 > I1 > M3 > Y1.
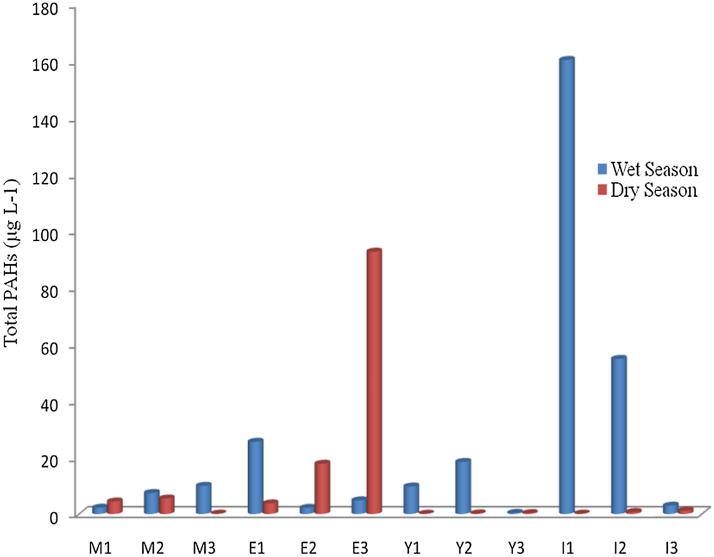
Fig. 4.
Frequency (Distribution of Total PAHs in the Groundwater Samples from the 12 Sampling Sites).
Naphthalene is the most occurring PAHs in the studied sites during the wet season (as indicated in Fig. 4), while phenanthrene is the most predominant in the groundwater samples in the studied sites during the dry season. Light PAHs detected in the groundwater of the studied area are less toxic and less carcinogenic compared to heavy PAHs [13]. Higher concentration of PAHs in wet season compared to dry season could be attributed to the ability of more of the lower molecular weight PAHs to leach easily through the soil to the groundwater as a result of rainfall in the wet season. Similarly, run-off from different places around the sampling site carries along some dissolved PAHs and pollutes the underground water more with this class of trace organics. In the same vein, an increase in volume of water in the ground during the wet season may enhance the dissolution of more of the PAHs, especially the low molecular weight PAHs, resulting to their higher concentration in the wet season. The presence of PAHs in towns of the studied local government could be attributed to anthropogenic activities such as burning of forest and firewood for cooking, which is a common practice in the area. Another source could be from the wearing away of the asphalt layer of the major roads in the area, vehicular emission of some of the PAHs due to high traffic movement along major roads around the local government area, burning of carbon containing waste in mechanic workshops in towns in the area, and other human activities. The released PAHs are deposited in soils and surface water and later leached to the ground water during heavy down pour or through runoff.
Generally, the PAHs detected in this study were above the set standard for drinking water which is 0.005 μg L−1 [14], and in most cases exceeded the maximum permissible limit of 10 μg L−1 recommended by WHO for safety groundwater [15]. Thus, their accumulative effect over time could be detrimental to human health and may cause some of the life-threatening diseases such as cancer associated with the presence of PAHs. Also, as stipulated by WHO (1984) the maximum permissible limits for total PAHs in drinking water is 0.2 μg/L [16]. Groundwater samples from this study area have total PAHs higher than the standard set by WHO, indicating a public health concern because PAHs have been confirmed to be carcinogenic and man stand the greatest risk of bioaccumulation.
Results obtained in this study were also lower compared to the values of 11.2–341.5 μg L−1 reported by [17], who worked on water samples in Agbadu bitumen field Southwest Nigeria (Table 4). Compared with Ogunfowokan et al. [18], the results in this study were also lower than the values of 0.1–15.8 mgL−1 (or 100–15810 μgL−1) reported for Osogbo and Ile-Ife, and 73.72 mgL−1 (or 73720 μg L−1) reported for industrial area of Lagos. Similarly, the results in this study were lower than those of Kalsson et al., 2008 who reported concentrations between 0.09–183 μg L−1 for water samples in Gully pots Northern Sweden. The inability to detect some of the PAHs during the dry season could probably be because they existed at concentrations below the detection limit of the machine caused by their volatile nature which is favoured by high temperature usually experienced during the dry season of the year in tropical regions like Nigeria.
Table 4.
Comparison of results of this study (mean concentrations of PAHs) with similar studies in other parts of the world.
| Study | Location | Mean concentrations of PAHs | Comment |
|---|---|---|---|
| Present study | Groundwater, Ife North Local Government | BDL–118.958 μg L−1 | – |
| [18] | Osogbo and Ife Lagos |
0.1–15.81 mg L−1 0.1–73.72 mg L−1 |
Conc in present study lower. |
| [17] | Water sample Agbabu bitumen field South west Nigeria | 11.2–341.5 μg L−1 | Conc in present study lower. |
| [19] | Water samples-Gully pots nortern Sweeden | 0.09–183 μg L−1 | Conc in present study lower. |
| WHO, 2011 [20] | 0–0.005 μg L−1 | Conc in present study higher. |
3.2. Analysis of variance of PAHs in wet and dry seasons of the groundwater samples
ANOVA results presented in Table 5a, showed that at p ≤ 0.05 significant level, fcal > fcrit, which implies that there is a significant difference in concentration of naphthalene (Nap), acenaphthalene (Ace), acenaphthylene (Aceyl), anthracene (Ant), fluranthrene (Flura) and fluorene (Fluo) for the period of wet season, while fcal < fcrit for phenathrene (Phe) indicates that there is no significant difference in the concentration of phenathrene in the groundwater for the periods of wet season investigated. Table 5b showed that at p ≤ 0.05 significant level, fcal > fcrit for napthalene, anthracene, fluorene and phenathrene, implying that there is a significant difference in the concentration of PAHs in the water for the different months of the dry season studied.
Table 5a.
Analysis of Variance for PAHs in Wet Season (ANOVA).
| Sum of Squares | df | Mean Square | F | Sig. | ||
|---|---|---|---|---|---|---|
| Naphthalene | Between Groups | 1747.067 | 3 | 582.356 | 0.886 | 0.488 |
| Within Groups | 5258.431 | 8 | 657.304 | |||
| Total | 7005.497 | 11 | ||||
| Acenaphthene | Between Groups | 0.001 | 3 | 0 | 0.291 | 0.831 |
| Within Groups | 0.012 | 8 | 0.001 | |||
| Total | 0.013 | 11 | ||||
| Acenaphthylene | Between Groups | 0.007 | 3 | 0.002 | 0.484 | 0.703 |
| Within Groups | 0.041 | 8 | 0.005 | |||
| Total | 0.048 | 11 | ||||
| Anthracene | Between Groups | 2.42 | 3 | 0.807 | 1.181 | 0.376 |
| Within Groups | 5.465 | 8 | 0.683 | |||
| Total | 7.884 | 11 | ||||
| Fluorathrene | Between Groups | 0.221 | 3 | 0.074 | 1 | 0.441 |
| Within Groups | 0.59 | 8 | 0.074 | |||
| Total | 0.812 | 11 | ||||
| Fluorene | Between Groups | 150210.4 | 3 | 50070.14 | 1.441 | 0.301 |
| Within Groups | 278025.5 | 8 | 34753.19 | |||
| Total | 428235.9 | 11 | ||||
| Phenathrene | Between Groups | 254.432 | 3 | 84.811 | 13.87 | 0.002 |
| Within Groups | 48.918 | 8 | 6.115 | |||
| Total | 303.35 | 11 | ||||
At P ≤ 0.05, F > Fcrit for Napthalene, Acephthalene, Acenaphthylene, Anthracene, Flurathrene, Fluorene while at P ≤ 0.005, F < Fcrit for Phenathrene.
Table 5b.
Analysis of Variance for PAHs in Dry Season (ANOVA).
| Sum of Squares | df | Mean Square | F | Sig. | ||
|---|---|---|---|---|---|---|
| Naphthalene | Between Groups | 63.55 | 3 | 21.183 | 2.587 | 0.126 |
| Within Groups | 65.519 | 8 | 8.19 | |||
| Total | 129.069 | 11 | ||||
| Anthracene | Between Groups | 0.005 | 3 | 0.002 | 0.251 | 0.859 |
| Within Groups | 0.051 | 8 | 0.006 | |||
| Total | 0.056 | 11 | ||||
| Fluorene | Between Groups | 43448.04 | 3 | 14482.68 | 0.264 | |
| Within Groups | 72349.8 | 8 | 9043.725 | |||
| Total | 115797.8 | 11 | ||||
| Phenanthrene | Between Groups | 28.688 | 3 | 9.563 | 1.434 | 0.303 |
| Within Groups | 53.367 | 8 | 6.671 | |||
| Total | 82.055 | 11 | ||||
At P ≤ 0.05, F > Fcrit Naphthalene, Anthracene, Fluorene, Phenathrene.
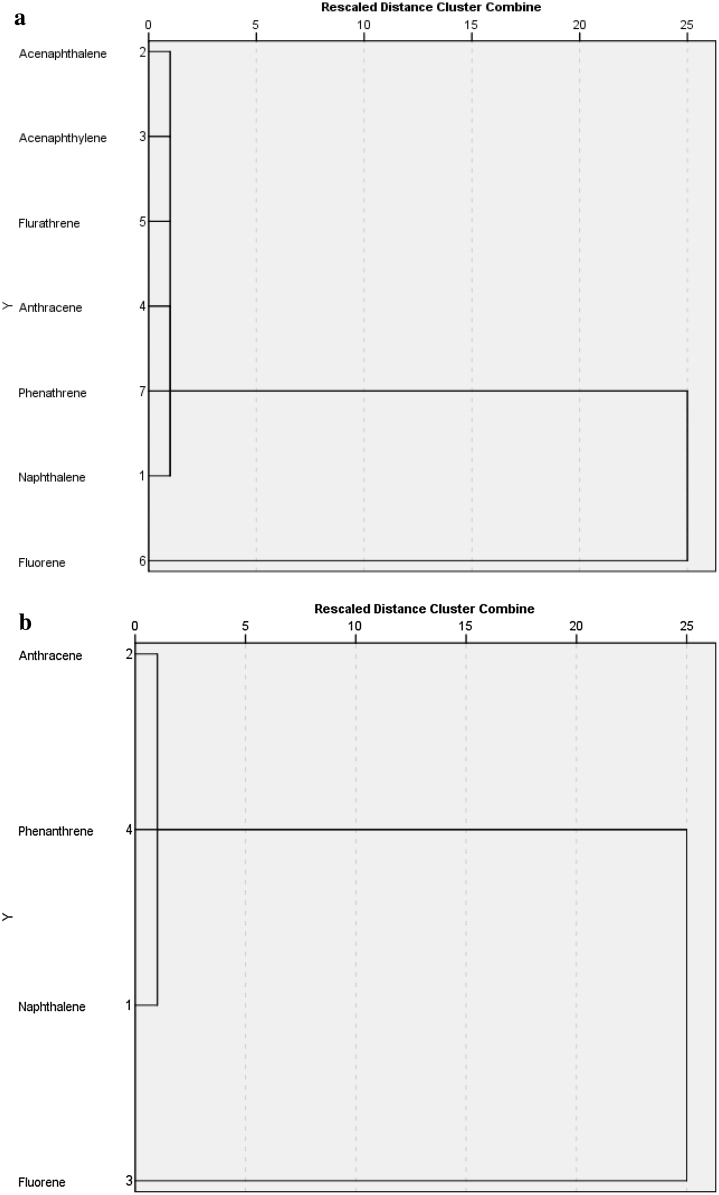
3.3. Cluster analysis of PAHs in wet and dry seasons
The cluster analysis of PAHs for wet season is presented in Fig. 5a. This shows that there is close relationship among acenaphthene, acenaphthylene, anthracene, and fluoranthrene. There is an extended relationship between acenaphthene, acenaphthylene, anthracene, fluranthrene and fluorene, phenanhrene. This implies that their presence in the study area groundwater might probably be from the same source such as; burning activities of carbon containing substances or wastes in the area, oil spill from mechanic workshops, emission from vehicular movement and other human activities. that leached from the surface ground and seepage to the groundwater. The presence of naphthalene on its own without clustering implies that naphthalene might be from another source different from that of other PAHs. Due to the volatile nature of naphthalene, its presence might be movements from another area in air, runoff water to these communities and leaching into the groundwater. The cluster analysis of PAHs for the dry season is presented in Fig. 5b. This can be grouped into three, the first cluster consists of naphthalene and anthracene; this implies that they might be from the same source while the other two groups include fluorene and phenanthrene, which are separate. This implies that fluorene and phenanthrene are from different sources. Different factors are responsible for their presence in the dry season, because temperature increases during this period, their presence might be from anthropogenic activities from another area that moves with air to these study areas.
Fig. 5.
(a) Caluster Diagram Showing the Relationship among PAHs Present in Wet Season. (b) Cluster Diagram Showing the Relationship among PAHs Present in Dry Season.
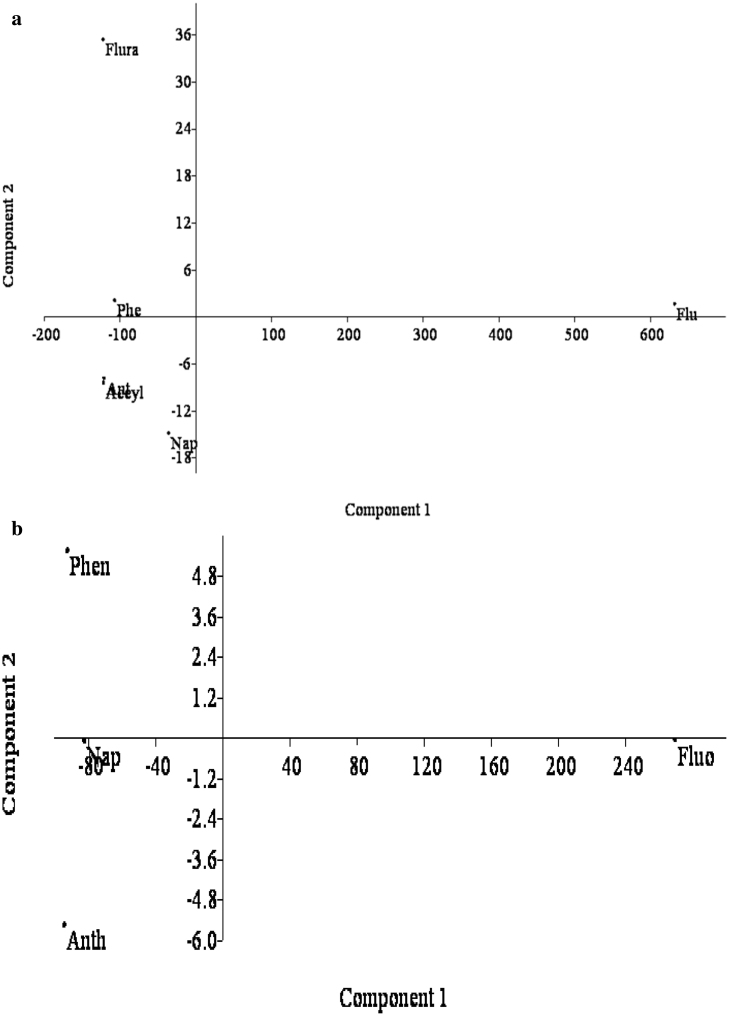
3.4. Principal component analysis of PAHs
The principal component analyses (PCA) in wet and dry seasons are presented in Fig. 6a and b. The wet season PCA showed that there is a relationship among phenanthrene, anthracene, acenaphthene, acenaphthylene and fluoranthene. The dry season shows a relationship between naphthalene and anthracene. The PCA of wet and dry seasons further confirmed the clustering relationship shown by the cluster analysis.
Fig. 6.
(a) Parincipal Component Loadings of PAHs in Wet Season. (b) Principal Component Loadings of PAHs in Dry Season.
4. Conclusion
The PAHs detected are light PAHs (2–3 rings), which includes; naphthalene, acenaphthene, acenaphthylene, anthracene, fluoranthene, fluorene, and phenanthrene, for the wet season, and naphthalene, anthracene, fluorene, and phenanthrene, for the dry season. Heavy PAHs were not detected in any of the samples. The total amount of PAHs in this area exceed the maximum permissible limit of 10 μg L−1 recommended by WHO for safety of groundwater [15]. The drinking of the groundwater by the people over a period of time may cause disorderliness in the proper functioning of the body system. It could also lead to risk of cancer diseases and low intelligence quotient in children.
Acknowledgment
The authors acknowledge Obafemi Awolowo University (OAU) Ile-Ife, Nigeria, for providing the research platform for this study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2016.10.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Young L.Y., Cerniglia C.E. Wiley; New York: 1995. Microbial Transformation and Degradation of Toxic Organic Chemicals. [Google Scholar]
- 2.Harvey D. 1st edition. McGraw-hill Companies; 2000. Modern Analytical Chemistry. [Google Scholar]
- 3.ATSDR . ATSDR; Atlanta, Georgia, USA: 1995. Chemical and Physical Information, in Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) pp. 209–221. [Google Scholar]
- 4.USGS . United States Geological Survey; 2013. What Is Hydrology and What Do Hydrologists Do? The USGS Water Science School. (23 May 2013. Retrived 21 Jan. 2014) [Google Scholar]
- 5.Wakeham S.G., Schaffner C., Giger W. Polycyclic aromatic hydrocarbons in recent lake sediments − I: compounds having anthropogenic origin. Geochim. Cosmochim. Acta. 1980;44:403–413. [Google Scholar]
- 6.Hostettler F.D., Pereira W.E., Kvenvolden K.A., Geen A., Luoma S.N., Fuller C.C., Anima R. A Record of Hydrocarbon Input to San Francisco Bay as Traced by Biomarker Profiles in Surface Sediment and Sediment Cores. Mar. Chem. 1999;64:115–127. [Google Scholar]
- 7.Anyakora Chimezie, Coker Herbert. Determination of polynuclear aromatic hydrocarbons (PAHs) in selected water bodies in the Niger Delta. Afr. J. Biotechnol. 2006;5(21):2024–2031. [Google Scholar]
- 8.Ogbuagu D.H., Okoli C.G., Gilbert C.L., Madu S. Determination of the contamination of groundwater sources in okrika mainland with polynuclear aromatic hydrocarbons (PAHs) Br. J. Environ. Clim. Change. 2011;1(3):90–102. [Google Scholar]
- 9.NIPOST . Nigeria postal sevices; 2009. Post Offices with Map of Local Government Area. [Google Scholar]
- 10.Oluyemi E.A., Adekunle A.S., Adenuga A.A., Makinde W.O. Physico-chemical properties and heavy metal content of water sources in Ife North Local Government Area of Osun State, Nigeria. Afr. J. Environ. Sci. Technol. 2010;4:691–697. [Google Scholar]
- 11.EPA . National Technical Information Service, PB84-211614; Springfield, Virgina 22161: 1984. EPA Method Study 20, Method 610 (PNA’s) EPA 600/4-84-063. [Google Scholar]
- 12.Anyakora C.A., Ogbeche K.A., Palmer P., Coker H., Ukpo G., Ogah C. A screen for Benzo(a)pyrene, a carcinogen: in the water samples from the Niger Delta region. Nig. J. Hosp. Med. 2004;14:288–293. [Google Scholar]
- 13.Brown J., Peake B. Sources of heavy metals and polycyclic aromatic hydrocarbons in urban storm water runoff. Sci. Total Environ. 2006;359:145–155. doi: 10.1016/j.scitotenv.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 14.WHO . 3rd ed. vol. 1. Recommendations; Geneva, Switzerland: 2006. (Guidelines for Drinking Water Quality: Incorporation First Addendum). [Google Scholar]
- 15.Environment Canada . Supply and Services Canada; Ottawa, Ontario: 1994. Canadian Environmental Protection Act Priority Substances List Assessment Report: Polycyclic Aromatic Hydrocarbons. (61 pp.) [Google Scholar]
- 16.World Health Organization (WHO), Guidelines for Drinking Water Quality, Geneva, 1984.
- 17.Olajire A., Alade A.O., Adeniyi A.A., Abemiwo O.M. Distribution of polycyclic aromatic hydrocarbons in surface soils and water from the vicinity of Agbabu bitumen field of southwestern Nigeria. J. Environ. Sci. Health. 2007;42(8):1043–1049. doi: 10.1080/10934520701418474. [DOI] [PubMed] [Google Scholar]
- 18.Ogunfowokan A.O., Asubiojo O.J., Fatoki O.S. Isolation and determination of polycyclic aromatic hydrocarbons in runoff and sediments. Water Air Soil Pollut. 2003;147(4):245–261. [Google Scholar]
- 19.Karlsson K., Maria V. Polycyclic aromatic hydrocarbons (PAH) in water and sediment from gully pots. Water Air Soil Pollut. 2008;188:271–282. [Google Scholar]
- 20.World Health Organization (WHO) 2011. Guidelines for Drinking-water Quality-4th edition. WHO Library Cataloguing-in-Publication Data. ISBN 978 92 4 154815 1. 564 pp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.