Abstract
Pristane and other adjuvants based on mineral oil saturated hydrocarbons (MOSH) may induce autoimmunity in rodents after intradermal injection; however there is a lack of information on immune effects after oral MOSH exposure. The aim of our study was to determine the impact of dietary exposure to pristane and other MOSH on the development of autoimmune arthritis.
Dark Agouti (DA) rats were given feed containing 4000 mg/kg pristane or a broad MOSH mixture in various concentrations (0–4000 mg/kg) for 90 days, or a single intradermal injection of 200 μl pristane (positive control). Arthritis scores, and serum and splenocyte markers previously associated with arthritis development, were determined.
All rats injected with pristane displayed arthritis symptoms and higher levels of certain serum markers. None of the rats fed pristane or MOSH developed arthritis symptoms or demonstrated clear changes in any measured arthritis-associated biological markers in serum or splenocytes.
The absence of clinical arthritis symptoms or any increase in common arthritis-associated biological markers in sera and spleen following dietary exposure to pristane or a broad MOSH mixture in a sub-chronic rat model of arthritis suggest that dietary MOSH have low capacity to promote development of autoimmunity.
Abbreviations: MOH, mineral oil hydrocarbons; MOSH, mineral oil saturated hydrocarbons; DA, Dark Agouti; i.d., intradermal; RF, rheumatoid factor; TLR, toll like receptor
Keywords: Mineral oil saturated hydrocarbons (MOSH), Autoimmune arthritis, Dark Agouti rat
1. Introduction
Mineral oils are commonly used as adjuvants to boost the immune system response to an antigen in human and veterinary vaccines [1], [2], [3]. Apart from therapeutic use, humans are exposed to mineral oils mainly via the food (see below), While oral administration of MOSH generally has low acute toxicity, MOSH has been reported to accumulate in tissues in both humans [4], [5] and animals [6]. In Fischer-344 rats, the formation of liver microgranulomas associated with inflammatory responses was observed after feeding with white mineral oils [7], [6], [8]. While there is generally little information about the potential of long term dietary MOSH to affect immune functions, single intradermal and intraperitoneal injections of certain mineral oils induce autoimmune responses in rodent models sharing both clinical and pathological features with human rheumatoid arthritis (RA) [9], [10], [11]. A few epidemiological studies suggest an association between exposure to high doses of MOH and an increased risk of developing autoimmune diseases. In a case control study in Sweden, self-reported occupational exposures to MOH primarily via skin and inhalation were associated with an increased relative risk of developing rheumatoid arthritis (RA) in men [12]. Furthermore, a significantly higher prevalence of RA and Systemic lupus erythematosus was observed in a population living close to an oil field waste site compared to another community with no known exposures of this type [13]. In the latest scientific opinion of mineral oils in food, The European Food Safety Authority (EFSA) identified a knowledge gap on potential effects on systemic autoimmune diseases or altered immune function after dietary exposure [14].
Mineral oil hydrocarbons (MOH) may unintentionally contaminate the food chain at various stages of food production or migrate from food packaging materials [15], while some MOH are intentionally used as food additives and in pesticides [14]. Oral and dermal exposure to MOH may also occur through use of cosmetics and pharmaceuticals and medicinal use [16], [17]. Some saturated hydrocarbons occur naturally in marine and terrestrial biota [14]. MOH consist of complex mixtures of mineral oil aromatic hydrocarbons (MOAH) and mineral oil saturated hydrocarbons (MOSH). Due to carcinogenic properties of MOAH, food grade MOH-products are treated in such a way that the MOAH content is minimized, while technical grades of MOH typically contain 15–35% MOAH. MOSH are present at different levels in nearly all foods, with the highest concentrations detected in bread, rolls, grains (mainly rice) confectionary (non-chocolate), vegetable oil, canned fish and oilseeds [14]. The estimated dietary MOSH exposure in Europe ranged from 0.03 to 0.3 mg/kg body weight (b.w.) per day, and higher in younger consumers and children, probably due to a higher intake of food per kg b.w. as well as age-related differences in dietary habits [14].
One model used for studies on arthritis is the arthritis-susceptible Dark agouti (DA) rats. In this model a single intradermal injection of medicinal white oil (commonly used for food, pharmaceutical and cosmetic use) as well as common commercial cosmetic products containing up to 80% mineral oils (like body lotion and baby oil), induced arthritis symptoms (joint synovitis). Percutaneous application of these products on abraded skin resulted in similar, but milder and transient, clinical arthritis symptoms in 5 out of 10 animals being exposed to a certain baby oil [10]. Although short term (five subsequent) per oral doses of medicinal white oil did not have any apparent effect in this arthritis model, the authors speculate that oral administration of adjuvants in conjunction with inflammation of the gut by other agents could parallel the arthritic effects of percutaneous exposure on abraded skin.
As described above, several MOSH have the ability to induce autoimmune responses in rodents after dermal/intradermal exposures, but so far, no studies have investigated whether long term (sub-chronic or chronic) dietary exposure to MOSH can promote autoimmunity [14], [18]. A single intradermal pre-administration of Incomplete Freund’s adjuvant or hexadecane was able to prevent development of disease induction by the intradermal injection with complete Freund’s adjuvant (including bacterial components and mineral oils) [19]. While incomplete Freund’s adjuvant has been reported not to demonstrate any significant immune responses after oral administration Silin et al., 2007, neither of the above studies investigated the potency to induce or prevent autoimmunity after oral exposure. Another aspect of concern is that previous safety evaluations regarding MOSH have been based on chemical and physical properties (such as viscosity) instead of sub-classes of mineral oils. The present study provides novel experimental data on the potential effects on autoimmunity of the whole MOSH range to which humans are exposed to via the diet. More precisely, we conducted a sub chronic study to determine whether 90 days with dietary exposures to a broad MOSH mixture can induce clinical signs and/or biological markers for autoimmune arthritis in the arthritis prone DA rat model.
2. Materials and methods
2.1. Animals
Inbred DA male and female rats (DA/OlaHsd), 7–8 weeks of age, was obtained from Harland Laboratories (Indianapolis, USA). In the pilot experiment, the female rats were randomly assigned to two groups (pristane injection and control, n = 6) and in the main experiment 5 female and 5 male rats were randomly assigned to one of six experimental units (see experimental overview in Section 3.2.1; Table 2). The rats were housed two or three animals in each cage, and acclimatized for a minimum of 9 days. The makrolon cages, containing cage enrichments, were randomly placed in ventilated Scantainer filter cabinets, 12 h light/dark cycle, temperature 21 °C ± 2, relative humidity 35–75% ± 10. The cage position in the rack was changed at least twice a week. Feed and tap water were given ad libitum. The rats were given a standard diet until start of the experiment (Teklad 2018S, Envigo, Cambridgeshire, UK.) and standard rodent bedding (NESTPAKS, Datesand Ltd). All rats were marked by ear puncture before entering the experiments. The experiments were performed in conformity with Norwegian laws and regulations for live animals, and approval was given by the Norwegian Animal Research Authority under the Ministry of Agriculture (FOTS 7084).
Table 2.
Experimental overview and dietary mineral oil exposures in male and female Dark Agouti rats, calculated from the MOSH-concentrations in the feed and feed consumption at week 2 and 10.
| Exposure | Route | n (females + males) |
Dietary mineral oil exposure mg/kg/b.w./day (median) (females + males) |
Duration (days) | |
|---|---|---|---|---|---|
| Week 2 | Week 10 | ||||
| 200 μl Pristane day 0 | i.d. | 5 + 5 | 0 + 0 | 0 + 0 | 40a |
| 4000 mg Pristane/kg feed | p.o. | 5 + 5 | 283.3 + 355.1 | 260.0 + 244.5 | 90 |
| 0 mg MOSH/kg feed | p.o. | 5 + 5 | 0 + 0 | 0 + 0 | 90 |
| 40 mg MOSH/kg feed | p.o. | 5 + 5 | 3.8 + 3.3 | 2.9 + 2.3 | 90 |
| 400 mg MOSH/kg feed | p.o. | 5 + 5 | 30.3 + 30.8 | 29.4 + 25.7 | 90 |
| 4000 mg MOSH/kg feed | p.o. | 5 + 5 | 317.6 + 309.3 | 280.1 + 238.8 | 90 |
i.d., intradermal; p.o., per oral.
Pristane-injected rats were included in the experiment for a maximum of 40 days. Rats with arthritic score >10 and/or weight reduction >10% per week were sacrificed for animal welfare reasons.
2.2. Chemicals and reagents
Pristane (tetramethylpentadecane, purity ≥97%), Concanavalin A (ConA), Lipopolysaccharide (LPS), and 10% buffered formalin were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). All ELISA kits were purchased from eBioscience (San Diego, CA, USA), with the exception of the RF kit (from MyBioSource, San Diego, CA USA). All CBA kits were purchased from BD Bioscience (San Jose, CA, USA). Antibodies for detection of Toll-like receptor (TLR)2 and TLR 3 were purchased from Santa Cruz Biotechnology Inc. (Dallas, Texas 75220 USA); anti-TLR2 (H-175, rabbit polyclonal IgG), anti-TLR3 (N-14, goat polyclonal IgG), goat anti-rabbit IgG-FITC and donkey anti-goat IgG-APC. Hank’s Balanced Salt Solution (HBSS), fetal calf serum (FCS) and the cell growth medium RPMI 1640 were purchased from Gibco (Thermo Fisher Scientific, Waltham, MA USA), and penicillin/streptomycin mix was acquired from PAA The Cell Culture Company (GE Healthcare Little Chalfont, Buckinghamshire, UK).
2.3. Preparation of the MOSH −mixture
A MOSH mixture ranging from about C14 to C50 was prepared by combining the following products: 295 g/kg paraffin wax Ph Eur, low viscosity, Fluka 76233 (Buchs, Switzerland), 295 g/kg paraffin wax Ph Eur, high viscosity, Fluka 76234, 147 g/kg Catenex Ph 941 FU (Shell) and 263 g/kg distillate from paraffin highly liquid Ph Eur, BP NF, Merck JP K43210074 209 1.17174.1000 (Darmstadt, Germany). This distillate was obtained by discarding the first 25 ml from 700 ml and consecutive fractions of 25 ml. Fractions 2 and 3 were mixed at a ratio of 1:2.
2.4. Preparation of MOSH- and pristane containing feed
A standard pelleted diet for rats (AIN–93 M), as described by Reeves et al. Reeves et al. (1993), was selected. Prior to the preparation of the feed, the major ingredients were analysed by on-line HPLC-GC-FID for MOSH or polyolefin oligomeric saturated hydrocarbons (POSH) to rule out disturbing interferences. For all ingredients, the contamination was below 15 mg/kg and was considered acceptable. Before being incorporated into the diet, the MOSH mixture and pristane was dissolved in soybean oil and stirred for 4 h at 40 °C. MOSH were incorporated into the diet at 40, 400 and 4000 mg/kg and pristane at 4000 mg/kg, and in each case an equivalent mass of soybean oil was replaced by the MOSH solution. Diet concentrations were verified in March 2014 and again in September 2014. The measured doses were within 88–95% of the nominal concentrations.
2.5. Anaesthetics
Animals received anaesthetics prior to and during intradermal injections and at termination (3% Isofluran gas anaesthetics (Isoba vet; Intervet/Schering-Plough Animal Health, Lysaker, Norway), administered in surgical O2).
2.6. Arthritis score and ethical considerations
All four limbs, toes, foot and ankle, were assessed for arthritis symptoms (redness, swelling etc.) and given a score from 0 to 4 for each limb, were 0 is no symptoms and 4 are severe symptoms. Maximum total score was 16. If the rats showed signs of severe distress, the animals received pain relief, 0.3 mg/ml Temgesic (Buprenorfin, RB Pharmaceuticals, Berkshire, UK), a subcutaneously injection of 0.1 ml every eight to twelve hours. If the animals got a score over ten they were euthanized due to animal welfare reasons. If the weight reduction was >10% per week, the animals were also euthanized. The investigator was blinded with respect to dietary treatments. The pristane injected rats had visible shaved areas at the base of the tail, and consequently the injection treatment was not blinded to the investigator.
2.7. Establishing the arthritis model and the corresponding immune markers (pilot)
Two groups of 6 female rats, one group serving as a negative control, were given a single intradermal injection, of 200 μl physiological buffer or 200 μl pristane. The rats were observed twice a week, for up to 40 days, for symptoms of arthritis. At termination, serum was collected as well as mesenteric lymph nodes and spleens.
2.8. Main experiment with MOSH in feed, pristane in feed and pristane injected
The rats were randomly assigned to one of 6 groups, 5 male and 5 female in each treatment group. The positive control group for arthritis was injected intradermally once with 200 μl pristane and given the control feed containing no MOSH. The other groups were given the different diets according to Table 2. The rats were observed twice a week, for 40 (the positive control group) or 90 days, for symptoms of arthritis. All animals were weighed once a week for the whole experimental period, i.e. a total of 40 (the positive control group) or 90 days. If the score were above 10 or the bodyweight was reduced by 10% or more in a week, the animals were euthanized and blood was collected from the heart into tubes without anticoagulant. A blood sample (100 μl), also without anticoagulant, was collected from femoral vein of all animals on day 10, 30 and 60. At the end of the experiment the animals were anesthetized and blood was collected from the heart (without anticoagulant), and the spleen and mesenteric lymph nodes were harvested. From all blood samples, serum was prepared and stored in aliquots at −80 °C for later analyses of serum rheumatoid factor and cytokines.
2.9. Preparation of a single cell suspension of splenocytes
The splenocytes were retrieved using a 70 μm cell strainer and rinsed with ice cold HBSS containing 2% fetal calf serum and 1% penicillin/streptomycin to produce single cell suspension. After centrifugation for 5 min at 10–15 °C and 420g the supernatant was discarded and the cells resuspended in 1 ml RPMI 1640 with 10% fetal calf serum and 1% penicillin/streptomycin. Twenty μl of the cell suspension was mixed with 10 ml Isoton II solution. The number of cells was counted in a Cell counter (both the Coulter Isoton II diluent and the cell counter, Coulter Z series, came from Beckman Coulter, Inc. Life Science Division Headquarters Indianapolis IN, USA).
2.10. Splenocyte analyses
2.10.1. Toll like receptor 2 and 3 expression
Additional biological markers of arthritis, i.e. expression of toll like receptor (TLR)2 and TLR3 on splenocytes, were determined in the main experiment. Spleen single cell suspensions were prepared and counted as previously described and resuspended in a wash buffer containing PBS, 0.1% sodium azide and 1% FCS, for a cell concentration of 2 × 107 cells/ml. One million cells from each cell suspension were first incubated with BD Fc blocker (0.5 μl/well; BD Biosciences, CA) at 4 °C for five minutes. Thereafter, cells from each animal was stained with a mixture of antibodies against TLR2 (H-175) and TLR3 (N-14) for 30 min at 4 °C, followed by a wash step and secondary staining with goat anti-rabbit IgG-FITC and donkey anti-goat IgG-APC (all from Santa Cruz Biotechnology Inc., Dallas, USA). A negative control, one sample per spleen was stained either only with secondary antibodies or with no antibodies. Cells were washed and resuspended in wash buffer before analyses on a LSRII flow cytometer (BD Bioscience).
2.10.2. Splenocyte cytokine secretion
The splenocytes were resuspended in RPMI 1640 with 10% fetal calf serum and 1% penicillin/streptomycin, 10 × 106cells/ml. In a 24 well plate, 1 ml of the suspension was transferred to each well in duplicates, and 100 μl ConA, LPS (5 g/ml) or no stimulant were added. The cells were cultivated for 48 h and the supernatant were collected after centrifugation for 10 min at 870g, and stored at −80 °C until detection of cytokines by cytometric bead array (CBA) or ELISA (see below).
2.11. ELISA
Serum were prepared according to protocol, as recommended by the manufacturers, for ELISA analysis of serum concentrations of rheumatoid factor (RF; IgG and IgM) and the cytokines IL-17, TNFα and IL-1b. Supernatant from splenocytes incubated in culture medium and stimulated with LPS or ConA or not stimulated were analyzed for the secretion of IL-17 by ELISA as suggested by the manufacturer. The ELISA plates were read on a EL808 ELISA plate reader (BIOTEK, Highland Park, Winoonski, Vermont, USA).
2.12. Cytometric bead array (CBA)
Splenocytes stimulated with LPS were analyzed for the cytokines IL-2 (E5), IFNγ (A6) and TNFα (C8). Splenocytes stimulated with ConA were analyzed for the cytokines IL-2(E5), IFNγ (A6), IL-4 (B9) and IL-10 (A8), using BD Cytometric bead array (CBA) Rat soluble protein flex set, acquired on a LSRII flow cytometer, and analyzed by the FCAP Array software (all from BD Biosciences).
2.13. Statistical analysis and data presentation
The statistical analysis was performed (SigmaPlot 13.0) using Mann Whitney test (pilot study) or two-way ANOVA, with ‘treatment’ and ‘sex’ as the two factors. If significant for the factor ‘treatment’, subsequent pairwise comparisons between the treatment groups were conducted using the Tukey post hoc test. It should be emphasized that the serum and splenocyte analyses were carried out in material collected at termination. Thus, since the pristane injected rats were terminated early (≤40 days) due to the severity of their symptoms and the groups exposed via feed were terminated at day 90, the levels cannot be directly compared. Therefore, the group intradermally treated with pristane was not included in the statistical analyses.
3. Results
3.1. Pilot experiment
3.1.1. Arthritis incidence, severity and day of onset
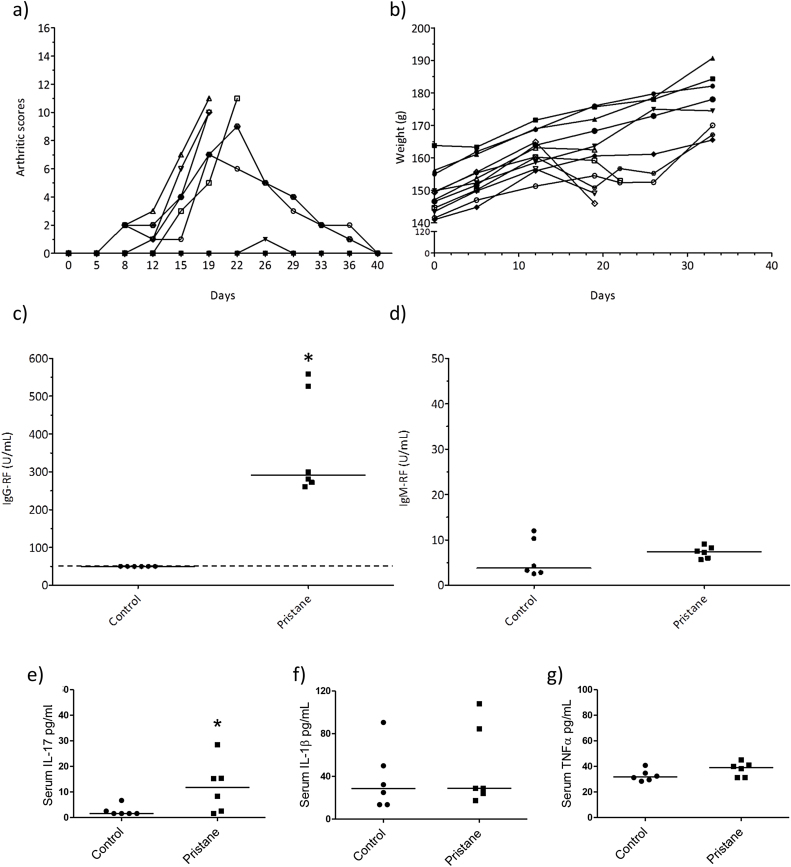
A single intradermal injection of 200 μl pristane on day 0 induced arthritic symptoms in all six female DA-rats (Table 1). Four of the pristane-injected rats were euthanized during the experiment (on days 19 and 22) due to severe symptoms, while the two remaining pristane-injected rats displayed clear but milder symptoms (maximum score 7 and 9) and showed remission of symptoms from day 19 and 22, respectively. These rats were monitored for the full 40 day period. None of the control rats intradermally injected with physiological saline displayed arthritic symptoms (Fig. 1a). All saline-injected rats showed a steady body weight increase during the experiment, while the body weight of pristane-injected rats declined and/or flattened out during the symptomatic phase (Fig. 1b).
Table 1.
Arthritis incidence, maximum score and day of onset in female Dark Agouti rats intradermally (i.d) injected day 0 with 200 μl pristane (n = 6) or physiological saline buffer (n = 6) and monitored twice a week for 40 days.
| Treatment | Arthritis incidence | Maximum scorea | Day of onset |
|---|---|---|---|
| Pristane i.d. | 6/6 | 7, 11, 11, 10, 10, 9 | 12, 12, 8, 8, 12, 8 |
| Saline i.d. | 0/6 | 0 | 0 |
Rats with arthritic score >10 and/or weight reduction >10% per week were sacrificed for animal welfare reasons.
Fig. 1.
Pilot study. (a) Arthritic scores, (b) body weight (gram; g), and serum levels at termination of (c) IgG-RF, (d) IgM-RF, (e) IL-17 (f), IL-1β and (g) TNFα in female DA-rats intradermally injected on day 0 with 200 μl pristane (n = 6) or physiological buffer (controls, n = 6) and monitored twice a week for up to 40 days. The dots represent the value for the individual animals while the lines represent the group median value. The dotted line indicates the lower detection limit for the ELISA-assay. Asterisk (*) denote statistically significant higher levels compared to the control group (p < 0.05, Mann Whitney test).
3.1.2. Serum analyses
At termination, the serum levels of IgG-RF (Fig. 1c) was significantly higher in rats injected with pristane compared to rats injected with saline (Mann Whitney test, p = 0.002), while the presence of IgM-RF in serum (Fig. 1d) was low and did not differ between the groups. The pristane-injected rats had significantly higher serum levels of IL-17 (Fig. 1e) than the control rats (Mann Whitney test, p < 0.05), while serum levels of IL-1β (Fig. 1f) and TNFα (Fig. 1g) did not differ between the groups. IL-10 and IL-6 were below the lower assay limits of detection for all rats irrespective of treatment, and only one out of six pristane-injected rats displayed a low, but detectable level of IL-22 (data not shown).
3.1.3. Mesenteric lymph nodes
In the pilot study, also wet weights of the mesenteric lymph nodes were measured, but no differences were observed between those rats receiving i.d. injection of pristane and the controls (data not shown). Mesenteric lymph nodes were therefore not collected from rats in the main experiment.
3.2. Main experiment
3.2.1. Feed consumption and body weight gain
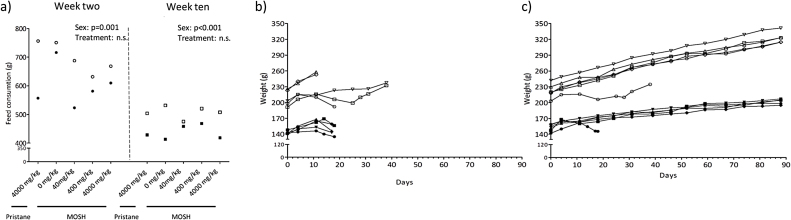
Feed consumption was monitored at two time points, week two and week ten of the experiment. In week 2 and 10, there were no significant differences in food consumption between treatment groups. The feed consumption was significantly higher in males than females at both time points (two way ANOVA, p = 0.011 and p = < 0.001, respectively; Fig. 2a). The MOSH intake via feed are presented in Table 2; the highest exposure group consumed 317 and 309 mg/kg b.w./MOSH per day (group medians, week two) for the female and male rats, respectively. Both male and female rats displayed a steady increase in body weight that did not differ between groups treated orally (Fig. 2c). The rats injected with pristane showed a decreased bodyweight along with disease development (Fig. 2b). The two males in the positive control group having remission of arthritis symptoms after peaking at score 6 or 7 regained weight increase, although not at the same rate as the animals in the other treatments groups.
Fig. 2.
Feed consumption and animal weights. DA-rats were exposed to a single intradermal pristane injection (200 μl), feed containing 4000 mg/kg pristane, or feed containing 0 (control), 40, 400 and 4000 mg/kg of a broad MOSH mixture. (a) Feed consumption (gram; g) week two and week 10 for the dietary exposed male (n = 5) and female (n = 5) rats. (b) Body weight curve (gram; g) for individual male (n = 5) and female (n = 5) DA-rats intradermally injected day 0 with 200 μl pristane and weighed weekly for a maximum of 40 days. (c) Group median body weight (g) for male (n = 5) and female (n = 5) DA-rats, weighed weekly for 90 days. Filled symbols = females, open symbols = males. Injection; circular symbols, pristane in feed; diamonds, 0 mg/kg MOSH; downward triangle, 40 mg/kg MOSH; hexagon, 400 mg/kg MOSH; square, 4000 mg/kg MOSH upward triangle. Two-way ANOVA results (treatment and sex as the two factors) are stated in the figures if statistically significant (p < 0.05).
3.2.2. Arthritis incidence, severity and day of onset
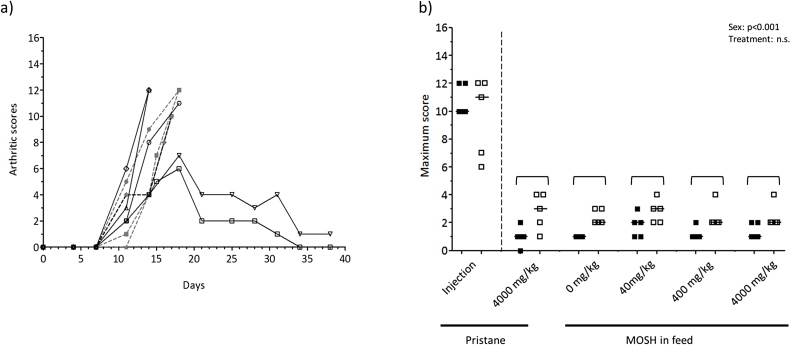
In the positive control group a single intradermal injection of 200 μl pristane on day 0 induced arthritic symptoms from day 7 in all 10 rats. All females and 3 males displayed significant and severe symptoms (maximum score 12) and were therefore euthanized during the experiment. Two males showed remission of symptoms after 18 days and were sacrificed on day 40 (Fig. 3a). Overall, in the main experiment the rats injected with 200 μl pristane, displayed arthritis symptoms within the same time span and degree of severity as those in the pilot experiment.
Fig. 3.
Arthritis scores. (a) Arthritic scores in individual DA-rats intradermally injected with 200 μl pristane on day 0 and monitored twice a week for up to 40 days. (b) maximum arthritic scores per animal during the 90 days, in female (closed symbols) and male (open symbols) DA-rats exposed to a single intradermal pristane injection (200 μl), feed containing 4000 mg/kg pristane, or feed containing 0 (control), 40, 400 and 4000 mg/kg of a broad MOSH mixture. The symbols represent the value for the individual animals while the lines represent the group median value. Two-way ANOVA results (treatment and sex as the two factors) are stated in the figures if statistically significant (p < 0.05), and statistically significant group differences (p < 0.05) from the post-hoc test are indicated with brackets.
The rats in the groups receiving pristane and MOSH in the feed as well as the group receiving no treatment had arthritis scores fluctuating between zero and four during the 90 days of observation. The maximum arthritis score per animal during the 90 days are presented in Fig. 3b. There were no significant differences between the treatment groups. However, at all doses of MOSH males had a significant higher maximum score than females (p = <0.001; 4000 mg/kg pristane, p = 0.007; 0 mg/kg MOSH, p = 0.019; 400 mg/kg MOSH, p = 0.048; 40 mg/kg MOSH and 4000 mg/kg MOSH).
3.2.3. Serum analyses
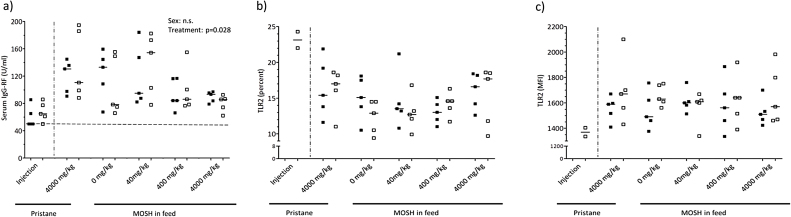
IgG rheumatoid factor (IgG-RF) were measured in serum from blood collected at day 90, or at the day of termination in the pristane injection group. Although there was an overall statistically significant difference due to treatment (two way ANOVA, p = 0.028), there were no significant differences in the post hoc test between the groups exposed via feed or between sexes within the different groups (Fig. 4a).
Fig. 4.
Rheumatoid factor and TLR expression. (a) IgG rheumatoid factor (IgG-RF) measured in serum collected at termination, (b) TLR 2 (percent) and (c) TLR 2 (mean fluorescence intensity (MFI)) measured in splenocytes, from female (closed symbols, n = 5) and male (open symbols, n = 5) DA-rats exposed to a single intradermal pristane injection (200 μl), feed containing 4000 mg/kg pristane, or feed containing 0 (control), 40, 400 and 4000 mg/kg of a broad MOSH mixture. The symbols represent the value for the individual animals while the lines represent the group median value. Two-way ANOVA results (treatment and sex as the two factors) are stated in the figures if statistically significant (p < 0.05).
3.2.4. Splenocytes
3.2.4.1. TLR2 and TLR3 expression
Neither% TLR2+ cells nor TLR2 expression per cell showed any significant differences between treatment groups or sexes (Fig. 4b and c). The same is applicable for TLR3 expression, which was low for all samples (data not shown).
3.2.4.2. Spontaneous cytokine secretion from splenocytes
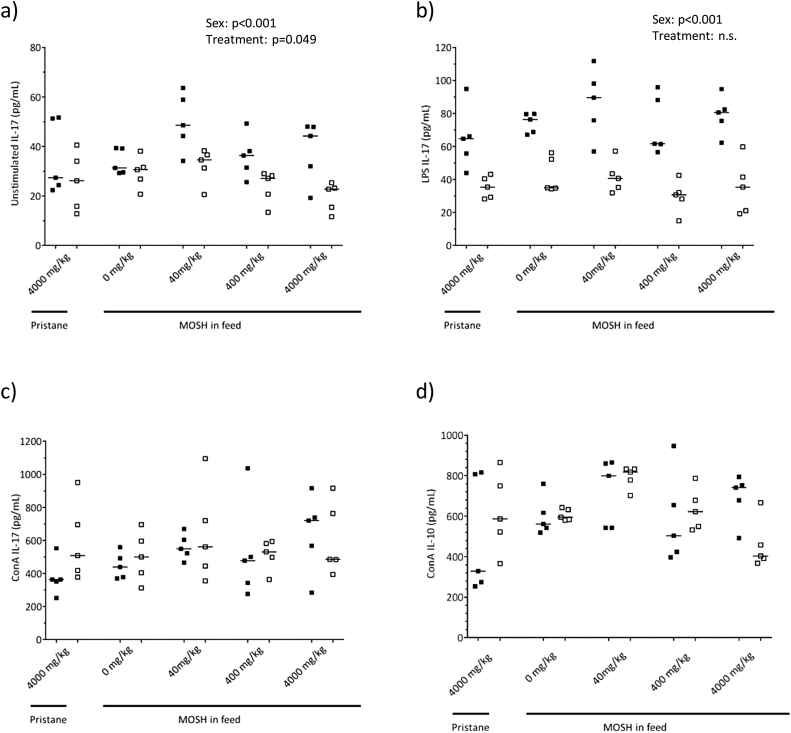
In unstimulated cells there was an overall effect of treatment (two way ANOVA, factor ‘treatment’ p = 0.049) on IL-17 secretion, but there were no statistical differences between the different treatment groups in the post hoc test. There was also an overall significant difference between males and females related to secretion of IL-17 (two ways ANOVA, factor ‘sex’ p < 0.001) (Fig. 5a). Levels of IL-2, TNFα or IFNγ measured in splenocytes were mostly below the limit of the assays (data not shown).
Fig. 5.
Cytokine secretion from splenocytes. Concentrations of secreted IL-17a in supernatants of (a) unstimulated (b) LPS and (c) ConA stimulated splenocytes, and (d) IL-10 in supernatants of ConA stimulated splenocytes, from female (closed symbols, n = 5) and male (open symbols, n = 5) DA-rats exposed to feed containing 4000 mg/kg pristane, or feed containing 0 (control), 40, 400 and 4000 mg/kg of a broad MOSH mixture. The symbols represent the value for the individual animals while the lines represent the group median value. Two-way ANOVA results (treatment and sex as the two factors) are stated in the figures if statistically significant (p > 0.05).
3.2.4.3. Cytokine release from LPS stimulated splenocytes
LPS stimulated cells showed no significant difference in IL-17 secretion between treatment groups. There was an overall lower IL-17 secretion in cells from males (two ways ANOVA p < 0.001) compared to females (Fig. 5b). There were no significant differences between treatments groups in levels of IL-2, TNFα or IFNγ measured in splenocytes (data not shown).
3.2.4.4. Cytokine release from ConA stimulated splenocytes
In splenocytes stimulated with ConA there was no difference in IL-17a levels between the different treatment groups (Fig. 5c).
No significant differences in IL-2 secretion from splenocytes stimulated with ConA were observed between treatment groups. There was an overall significant difference between male and female (two ways ANOVA p < 0.001). There were no significant differences between the different treatment groups in IFNγ (p = 0.196, data not shown) or IL-10 (p = 0.057) secretion from splenocytes (Fig. 5d). IL-4 secretion was below the detection limit (data not shown).
4. Discussion
Whereas some mineral hydrocarbons given intradermally may induce autoimmunity, experimental data on the potential of oral MOSH exposure to cause autoimmunity is currently lacking [18]. Our study demonstrates that dietary exposure to a broad MOSH mixture or pristane for 90 days neither causes clinical signs of autoimmune arthritis nor induce biological markers associated with immune responses, in the susceptible, arthritis-prone DA rats.
In accordance with previous murine arthritis studies [20], [10] and the pilot study performed to establish the positive arthritis control, all rats injected with pristane displayed severe arthritic symptoms in terms of red and swollen paws/ankles corresponding to high arthritis scores. Rats exposed to dietary pristane or three dose levels of MOSH, on the other hand, did not show signs of arthritis and elevated arthritis scores compared with rats fed the control feed (0 mg/kg MOSH). The arthritis score is a subjective measurement, based on a visual inspection and grading of the four limbs of each animal. Precautions were taken to reduce the subjective impact on the results, by allowing the same technician to perform the scoring throughout the experiment and blinding the technician with regard to exposure groups. In spite of such precautions, some subjectivity will influence the background level of scores. Notably, males in general had significantly higher maximum arthritis scores than females. We presume that this gender difference might be explained by an observation bias, as the male rats have larger paws than females. Since the score did not differ from the control group (0 mg/kg MOSH) and none of the other arthritis parameters were elevated for male rats, there is no evidence suggesting that the slightly higher scores reported for male rats was related to the higher intake of MOSH-containing feed compared to female rats.
In addition to the subjective arthritis score, we included measurements of a selection of objective biological markers associated with immune reactions. In murine models, parenteral exposures to certain mineral oils may induce autoimmune symptoms associated with rheumatoid arthritis and systemic lupus erythematous (SLE) [21], [10], [11] and these models have been employed to study the pathogenesis of autoimmune diseases. In addition to clinical signs in terms of affected/swollen limbs/joints, several biological markers suggested to be involved in both the development and the maintenance of autoimmune diseases, have been investigated. Increased systemic (serum) levels of rheumatoid factor (RF) [11], IL-17 [22], [23], IL-6, TNFα [23] and IL-1β [9], [23] have been reported in animal models of arthritis. Furthermore, it has been reported that toll like receptor 3 (TLR3) up-regulation in splenic macrophages participate in both the initiation and the maintenance of pristane-induced arthritis in DA rats [24], [25] and also the proportion of TLR2+ dendritic cells and macrophages were considerably increased in the spleen [26]. Moreover, murine arthritis models have frequently been applied to investigate therapeutic effects of a wide range of treatments. Decreased severity of arthritis symptoms in rats have been associated with reduced splenocyte secretion of cytokines IL-6 [27], TNFα [28], [29], [27], IFNγ [28], [27], IL-1β [28], [29] and augmented levels of IL-10 [29]. In general, major limitations of previous studies were the application of mineral oil in one high dose with the lack of dose-response studies, and furthermore that only injections and percutaneous routes have been employed [18]. It is therefore not known if the results from these studies have relevance for chronic exposure to mineral oils via the oral route. Consequently, the biological markers (as well as clinical symptoms/signs) were selected due to their previous association with arthritis development in murine models and included in the present study to investigate potential effects of dietary MOSH exposure in DA-rats.
It should be emphasized that the serum analyses were carried out in sera collected at termination. Thus, since the positive control rats were terminated early (≤40 days) due to the severity of their symptoms and the feed treatments groups were terminated at day 90, the serum levels cannot be directly compared. Furthermore, as all of the objective markers except IL-17 (discussed below) were measured at termination, early and mild effects of the dietary MOSH may have been overlooked. [10] demonstrated that repeated percutaneous application of baby oil on abraded rat skin gave early and mild arthritis symptoms lasting for only 4-6 days; however these results were based solely on subjective arthritis scores, as no biological markers were reported. In the pilot experiment, pristane injected rats had, at termination, a significant higher level of serum IL-17 than the negative control animals, while other arthritis-associated cytokines were not affected (IL-1β, TNFα). Therefore, IL-17 was chosen as the marker to measure in the sera collected at several time points during the main experiment (day 10, 30, 60 and 90). Serum IL-17, however, was only detectable in the 90-day serum samples, showing no differences between the feed treatment groups. As the only serum marker measured during the whole time course of the study, the result supports the observation that MOSH and pristane in feed did not induce any arthritis symptoms in these rats at any time period during the 90 days. In agreement, also serum levels of IL-1β and TNFα at day 90 did not differ between the groups.
Patients with rheumatoid arthritis (RA) have increased titers of rheumatoid factor (RF) in sera. Overproduction of RF may be related to a pathogenic effect observed in arthritis, although the mechanisms have not been revealed [11]. RF is also speculated to be a predisposing factor for the development of RA as increased levels of RF in symptom free individuals have been associated with an increased risk of RA [30]. In accordance with the human data, increased titers of both RF-IgM and RF-IgG was observed during the development of pristane-induced arthritis in an murine model using DA rats, however only RF IgM was significantly elevated [11]. In the present study, an increased serum level of RF-IgG but not RF-IgM was detected in the positive control (at day 40 or earlier) in the pilot experiment. In the main experiment, in agreement with the other endpoints, the serum levels of RF-IgG were not affected by dietary MOSH or pristane exposures. In the present study, the RF-IgG levels in pristane injected rats did not differ from the levels in the rats exposed via feed for 90 days, but as discussed above, this may be a result of RF analysis performed in sera collected at different ages and stages of the disease.
To further investigate the possible influence of MOSH ingestion on immune modulation or function; splenocytes were investigated with regard to surface markers and cytokine production as indicated by the previously published literature. Splenocytes were cultivated in medium, or in medium with LPS or Con A to stimulate B or T cells, respectively. However, regardless of stimulation, we observed no significant differences in cytokine secretion between the feed treatment groups. Furthermore, no effects on TLR2 expression from splenocytes (expressed as% TLR2+ cells and TLR2 expression per positive cell) were observed after MOSH exposure in feed. Also TLR3 expression did not differ between groups, but the levels were low compared to what has previously been reported in DA rats. This may be due to lack of an amplifying step in our detection procedure as previously described [24]. The general lack of splenocyte effects in our study is in agreement with our recent observations that dietary MOSH exposures for 120 days did not induce significant immunosuppressive effects in Fisher-433 rats (Nygaard et al., in preparation). In that study, we used the same feed preparations and thus the same doses of the broad MOSH mixture as in the present study, and another arm of immune function was assessed by detection of KLH-specific IgM antibodies 5 days after a KLH injection.
Addition of MOSH or pristane to the feed apparently did not affect the feed intake as we observed no differences in consumption of feed between the treatment groups regardless of diet; thus it is presumed that amount of MOSH or pristane consumed is proportional to the concentrations in the feed. The present dose levels thus are about 10, 100 and 1000 times higher than the mean human exposure from food, as estimated by EFSA [14].
To our best knowledge, no comparative papers exist on the toxicokinetics of pristane administered via intradermal or oral routes; however we could speculate that intradermal injection of pristane would result in substantial differences compared with dietary exposure. By comparing radioactivity levels in tissue of rats dosed either intraperitoneally or per os with the same quantity of a tritiated MOSH mixture, Ebert et al. [31] found that 24 h after administration, the concentrations measured in liver and fat of injected animals were approximately 20 and 1000 higher, respectively, than those observed in rats receiving the mixture orally. Similar differences could occur between intradermally and dietary exposed animals, resulting in higher pristane concentrations in target tissues involved in the autoimmune arthritis process.
5. Conclusion
The present study is to our best knowledge the first sub-chronic oral study to investigate the potential of dietary mineral oils to induce autoimmunity, using a susceptible experimental rat model, and several dose levels of a broad MOSH mixture relevant for human dietary exposure. The absence of clinical arthritis symptoms as well as lack of changes in common arthritis-associated biological markers in sera and spleen is in support of the notion that MOSH exposure via the dietary route have a low capacity to promote development of autoimmunity, in this case arthritis.
Acknowledgements
This research was part of a grant project co-funded by the European Food Safety Authority (EFSA), grant agreement GP/EFSA/BIOCONTAM/2013/01 (“Bioaccumulation and toxicity of mineral oil hydrocarbons in rats − specificity of different subclasses of a broad mixture relevant for human dietary exposures”). The conclusions, findings, and opinions expressed in this scientific manuscript reflect only the view of the authors and not the official position of the European Food Safety Authority. We thank Florence Blas-Y-Estrada, Xavier Blanc (INRA), Astri Grestad, Tone Rasmussen, Trude K. Olsen and Kari G. Løken (NIPH) for excellent technical assistance.
References
- 1.Aucouturier J., Dupuis L., Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001;19(17–19):2666–2672. doi: 10.1016/s0264-410x(00)00498-9. [DOI] [PubMed] [Google Scholar]
- 2.Cox J.C., Coulter A.R. Adjuvants–a classification and review of their modes of action. Vaccine. 1997;15(3):248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 3.Del Giudice G., Rappuoli R. Inactivated and adjuvanted influenza vaccines. Curr. Top. Microbiol. Immunol. 2015;386:151–180. doi: 10.1007/82_2014_406. [DOI] [PubMed] [Google Scholar]
- 4.Barp L., Kornauth C., Wuerger T., Rudas M., Biedermann M., Reiner A., Grob K. Mineral oil in human tissues, part I: concentrations and molecular mass distributions. Food Chem. Toxicol. 2014;72:312–321. doi: 10.1016/j.fct.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Biedermann M., Barp L., Kornauth C., Wurger T., Rudas M., Reiner A., Grob K. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci. Total Environ. 2015;506–507:644–655. doi: 10.1016/j.scitotenv.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 6.Firriolo J.M., Morris C.F., Trimmer G.W., Twitty L.D., Smith J.H., Freeman J.J. Comparative 90-day feeding study with low-viscosity white mineral oil in Fischer-344 and Sprague-Dawley-derived CRL:CD rats. Toxicol. Pathol. 1995;23(1):26–33. doi: 10.1177/019262339502300104. [DOI] [PubMed] [Google Scholar]
- 7.Barp L., Biedermann M., Grob K., Blas Y.E.F., Nygaard U.C., Alexander J., Cravedi J.P. Accumulation of mineral oil saturated hydrocarbons (MOSH) in female Fischer 344 rats: comparison with human data and consequences for risk assessment. Sci. Total Environ. 2017;575:1263–1278. doi: 10.1016/j.scitotenv.2016.09.203. [DOI] [PubMed] [Google Scholar]
- 8.Trimmer G.W., Freeman J.J., Priston R.A., Urbanus J. Results of chronic dietary toxicity studies of high viscosity (P70H and P100H) white mineral oils in Fischer 344 rats. Toxicol. Pathol. 2004;32(4):439–447. doi: 10.1080/01926230490465865. [DOI] [PubMed] [Google Scholar]
- 9.Herman S., Kny A., Schorn C., Pfatschbacher J., Niederreiter B., Herrmann M., Hoffmann M.H. Cell death and cytokine production induced by autoimmunogenic hydrocarbon oils. Autoimmunity. 2012;45(8):602–611. doi: 10.3109/08916934.2012.719948. [DOI] [PubMed] [Google Scholar]
- 10.Sverdrup B., Klareskog L., Kleinau S. Common commercial cosmetic products induce arthritis in the DA rat. Environ. Health Perspect. 1998;106(1):27–32. doi: 10.1289/ehp.9810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wernhoff P., Olofsson P., Holmdahl R. The genetic control of rheumatoid factor production in a rat model of rheumatoid arthritis. Arthritis Rheum. 2003;48(12):3584–3596. doi: 10.1002/art.11342. [DOI] [PubMed] [Google Scholar]
- 12.Sverdrup B., Kallberg H., Bengtsson C., Lundberg I., Padyukov L., Alfredsson L., Klareskog L. Association between occupational exposure to mineral oil and rheumatoid arthritis: results from the Swedish EIRA case-control study. Arthritis Res. Ther. 2005;7(6):R1296–R1303. doi: 10.1186/ar1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlgren J., Takhar H., Anderson-Mahoney P., Kotlerman J., Tarr J., Warshaw R. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site: a cross sectional study. Environ. Health. 2007;6:8. doi: 10.1186/1476-069X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EFSA (2012). Scientific Opinion on Mineral Oil Hydrocarbons in Food. Retrieved from.
- 15.Grob K., Biedermann M., Artho A., Egli J. Food contamination by hydrocarbons from packaging materials determined by coupled LC-GC. Z. Lebensm. Unters. Forsch. 1991;193(3):213–219. doi: 10.1007/BF01199968. [DOI] [PubMed] [Google Scholar]
- 16.Niederer M., Stebler T., Grob K. Mineral oil and synthetic hydrocarbons in cosmetic lip products. Int. J. Cosmet. Sci. 2016;38(2):194–200. doi: 10.1111/ics.12276. [DOI] [PubMed] [Google Scholar]
- 17.Noti A., Grob K., Biedermann M., Deiss U., Bruschweiler B.J. Exposure of babies to C15-C45 mineral paraffins from human milk and breast salves. Regul. Toxicol. Pharmacol. 2003;38(3):317–325. doi: 10.1016/s0273-2300(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 18.Kimber I., Carrillo J.C. Oral exposure to mineral oils: is there an association with immune perturbation and autoimmunity? Toxicology. 2016;344–346:19–25. doi: 10.1016/j.tox.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Mia M.Y., Zheng C.L., Hossain M.A., Yamasaki F., Tokunaga O., Kohashi O. The preventive effects of incomplete Freund's adjuvant and other vehicles on the development of adjuvant-induced arthritis in Lewis rats. Immunology. 1999;98(2):267–272. doi: 10.1046/j.1365-2567.1999.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt-Weber C.B., Pohlers D., Siegling A., Schadlich H., Buchner E., Volk H.D., Kinne R.W. Cytokine gene activation in synovial membrane, regional lymph nodes, and spleen during the course of rat adjuvant arthritis. Cell. Immunol. 1999;195(1):53–65. doi: 10.1006/cimm.1999.1509. [DOI] [PubMed] [Google Scholar]
- 21.Reeves W.H., Lee P.Y., Weinstein J.S., Satoh M., Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30(9):455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astry B., Harberts E., Moudgil K.D. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J. Interferon Cytokine Res. 2011;31(12):927–940. doi: 10.1089/jir.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolina M., Bolon B., Middleton S., Dwyer D., Brown H., Duryea D., Zack D. The evolving systemic and local biomarker milieu at different stages of disease progression in rat adjuvant-induced arthritis. J. Clin. Immunol. 2009;29(2):158–174. doi: 10.1007/s10875-008-9238-8. [DOI] [PubMed] [Google Scholar]
- 24.Meng L., Zhu W., Jiang C., He X., Hou W., Zheng F., Lu Toll-like receptor 3 upregulation in macrophages participates in the initiation and maintenance of pristane-induced arthritis in rats. Arthritis Res. Ther. 2010;12(3):R103. doi: 10.1186/ar3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W., Meng L., Jiang C., Xu J., Wang B., Han Y., Lu S. Overexpression of toll-like receptor 3 in spleen is associated with experimental arthritis in rats. Scand. J. Immunol. 2012;76(3):263–270. doi: 10.1111/j.1365-3083.2012.02724.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W., Meng L., Jiang C., Hou W., Xu J., Wang B., Lu S. Induction of toll-like receptor 2 positive antigen-presenting cells in spleen of pristane-induced arthritis in rats. Mol. Biol. Rep. 2012;39(4):3667–3673. doi: 10.1007/s11033-011-1141-3. [DOI] [PubMed] [Google Scholar]
- 27.Ulmansky R., Turjeman K., Baru M., Katzavian G., Harel M., Sigal A., Barenholz Y. Glucocorticoids in nano-liposomes administered intravenously and subcutaneously to adjuvant arthritis rats are superior to the free drugs in suppressing arthritis and inflammatory cytokines. J. Control. Release. 2012;160(2):299–305. doi: 10.1016/j.jconrel.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Fayaz Ahmad S., Sultan P., Ashour A.E., Khan T.H., Attia S.M., Bakheet S.A., Abd-Allah A.R. Modulation of Th1 cytokines and inflammatory mediators by Euphorbia hirta in animal model of adjuvant-induced arthritis. Inflammopharmacology. 2013;21(5):365–375. doi: 10.1007/s10787-012-0161-5. [DOI] [PubMed] [Google Scholar]
- 29.Liu M., Mao W., Guan H., Li L., Wei B., Li P. Effects of taurochenodeoxycholic acid on adjuvant arthritis in rats. Int. Immunopharmacol. 2011;11(12):2150–2158. doi: 10.1016/j.intimp.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Halldorsdottir H.D., Jonsson T., Thorsteinsson J., Valdimarsson H. A prospective study on the incidence of rheumatoid arthritis among people with persistent increase of rheumatoid factor. Ann. Rheum. Dis. 2000;59(2):149–151. doi: 10.1136/ard.59.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebert A.G., Schleifer C.R., Hess S.M. Absorption, disposition, and excretion of 3H-mineral oil in rats. J. Pharm. Sci. 1966;55(9):923–929. doi: 10.1002/jps.2600550911. [DOI] [PubMed] [Google Scholar]