Highlights
-
•
Co-cultures of liver and immune cells can be used to detect iDILI compounds.
-
•
Pro-inflammatory factors are involved in the development of iDILI.
-
•
The co-exposure of a drug candidate with TNF might be sufficient to predict iDILI.
Abbreviations: CD, cluster of differentiation; DAMP, damage-associated molecular pattern; EC, effective concentration; EpCAM, epithelial cellular adhesion molecule; HSP, heat shock protein; iDILI, idiosyncratic drug-induced liver injury; JNK, c-Jun N-terminal kinase; LPS, bacterial lipopolysaccharide; NF-κB, nuclear factor kappa B; NPC, non-parenchymal cell; NSAID, nonsteriodal anti-inflammatory drug; PAMP, pathogen-associated molecular pattern; SD, standard deviation; TNF, tumor necrosis factor
Keywords: Idiosyncratic, Drug-induced liver injury, Co-culture model, Inflammation, Preclinical research
Abstract
Interactions between hepatocytes and immune cells as well as inflammatory episodes are frequently discussed to play a critical role in the alteration of the individual susceptibility to idiosyncratic drug-induced liver injury (iDILI). To evaluate this hypothesis and to face the urgent need for predictive in vitro models, we established two co-culture systems based on two human cell lines in presence or absence of pro-inflammatory factors (LPS, TNF), i.e. hepatoma HepG2 cells co-cultured with monocytic or macrophage-like THP-1 cells. HepG2 monocultures served as control scenario. Mono- or co-cultures were treated with iDILI reference substances (Troglitazone [TGZ], Trovafloxacin [TVX], Diclofenac [DcL], Ketoconazole [KC]) or their non-iDILI partner compounds (Rosiglitazone, Levofloxacin, Acetylsalicylic Acid, Fluconazole). The liver cell viability was subsequently determined via WST-Assay. An enhanced cytotoxicity (synergy) or a hormetic response compared to the drug effect in the HepG2 monoculture was considered as iDILI positive. TGZ synergized in co-cultures with monocytes without an additional pro-inflammatory stimulus, while DcL and KC showed a hormetic response. All iDILI drugs synergized with TNF in the simple HepG2 monoculture, indicating its relevance as an initiator of iDILI. KC showed a synergy when co-exposed to both, monocytes and LPS, while TVX and DcL showed a synergy under the same conditions with macrophages. All described iDILI responses were not observed with the corresponding non-iDILI partner compounds. Our first results confirm that an inflammatory environment increases the sensitivity of liver cells towards iDILI compounds and point to an involvement of pro-inflammatory factors, especially TNF, in the development of iDILI.
1. Introduction
Idiosyncratic drug-induced liver injury (iDILI), which accounts for up to 17% of all cases of acute liver failure [1], [2], remains a serious problem for public health due to the inability to predict those rare but severe adverse drug reactions. The prognosis for patients suffering from iDILI is very poor, ranging from 60 to 70% mortality without liver transplantation [3]. In addition, iDILI is the most frequent cause for the non-approval of a drug or its withdrawal from the market [4], [5], [6] and therefore represents a major issue for the drug development process and the marketing of drugs that are safe for the broad population. Consequently, the US Food and Drug Administration (FDA) demanded the majority of post-marketing restrictions (e.g. black box warnings) for drugs that induce idiosyncratic reactions [7]. What makes idiosyncratic reactions so difficult to predict, are their elusive characteristics [8]. A very low incidence of about 19 cases per 100,000 per year [9] makes it nearly impossible to recognize iDILI during pre-marketing trials, which typically cover only 1000–3000 subjects for a new drug application. Only the most evident hepatotoxicants can be expected to show cases of such severe outcomes as iDILI in those small subject numbers. Therefore, it is also understandable that iDILI cannot be predicted from regulatory animal toxicity studies. At present, iDILI can only be identified when a lot more patients are exposed to a certain drug post-marketing. Moreover, idiosyncratic reactions occur sporadically and show a variable onset relative to the start of exposure, this meaning that some patients develop toxicities soon after the start of exposure and some not until after a longer exposure period [10]. Drug concentrations that are able to induce iDILI are in the therapeutic dose range and are generally safe to the majority of the population. In addition, iDILI reactions are reported to be of a more dose-independent nature and unrelated to the pharmacologic action of the drug, but appear to reflect host factors and individual susceptibility [11], [4], [12]. To date it is not clear which factor(s) cause(s) this individual susceptibility and why some individuals develop iDILI and some do not. It seems possible that the susceptibility of a patient is triggered by an erratically occurring event that appears during the running drug therapy. In this case, the low incidence as well as the sporadic occurrence of iDILI would be explained. Whether the susceptibility factors are based on genetic differences or environmental factors is not yet understood, but it is most likely that the underlying mechanisms are multifactorial [8]. Amongst the variety of hypotheses that aim to explain the origin of iDILI, the inflammatory stress hypothesis has become the most studied and most promising approach. Roth et al. [13] suggested, that a mild inflammatory stress might render an individual susceptible to develop hepatotoxicity at an otherwise safe dose of the drug. Because inflammation often occurs in humans, but erratically and to a varying degree throughout the whole lifespan of an individual, it fulfills the requirements for a susceptibility factor that might account for iDILI. Therefore, a promising interplay to be studied in connection with inflammation-associated iDILI is that of liver parenchymal cells (PCs) and non-parenchymal immune cells (NPCs), such as liver resident macrophages (Kupffer cells) and infiltrating monocytes. It is well known that in particular Kupffer cells have a central function in hepatotoxicity by initiating and assembling local and systemic responses to liver injury and, together with recruited monocytes, rule the complex process of inflammation [14], [15]. Xenobiotics can activate Kupffer cells directly or indirectly upon an initial hepatic insult, thereby resulting in the release of a variety of inflammatory mediators such as cytokines (e.g. TNF and interleukins), which can trigger a secondary response that appears to exacerbate the initial hepatocyte damage [14], [11], [16]. Moreover, an existing moderate inflammation due to the activation of Kupffer cells via e.g. bacterial lipopolysaccharide (LPS) appears to sensitize hepatocytes to toxic substances and can lower the threshold for hepatotoxicity [17], [11]. An increasing number of recently developed in vivo and in vitro models for the prediction of (i)DILI incorporate immune cells and/or pro-inflammatory factors such as LPS and TNF, thus attempting to provide evidence for the inflammatory stress hypothesis. Most in vivo studies are based on rodents which are co-exposed to idiosyncratic drugs and LPS to induce a mild inflammatory background during drug exposure [18], [19], [20], [17], [21]. In vitro models are either based on the parenchymal cell itself and a co-exposure to pro-inflammatory factors [22], [23], [24] or a co-culture of hepatocytes and macrophages or monocytes including pro-inflammatory factors in most but not all cases [25], [26], [27]. All these studies confirm the suggestion that inflammation and the involved immune cells play a role in the development of iDILI. Unfortunately, most published studies are limited to one drug or one exposure scenario and are therefore not suitable for the establishment of a general iDILI testing approach that is applicable to structurally and mechanistically diverse iDILI compounds. In vivo animal studies in general lack predictability for hepatotoxicity in humans [28], mainly due to interspecies variations, and do not allow a reasonably high throughput for the screening of drugs in the preclinical development process. A simple well-controlled in vitro system, which saves time, money and animals, would strongly improve the early screening process for iDILI. In addition, a system that combines parenchymal with non-parenchymal cells and thereby allows intercellular communication is required to reflect multicellular phenomena like drug-induced toxicity and to understand how these interactions contribute to hepatotoxicity. Only a co-culture model can help to determine whether the communication to immune cells is necessary to predict iDILI or if (single) secreted pro-inflammatory factors might suffice to mirror iDILI in single PC cultures in vitro. Importantly, co-cultures represent a closer approximation to the in vivo situation and therefore have a higher relevance than models that are only based on the PC itself [29].
To this end, we developed an inflammatory in vitro liver co-culture model combining the human hepatoma cell line HepG2 with monocytic or macrophage-like THP-1 cells separated by a porous membrane. Monocytes were added to mimic the infiltration of immune cells during liver injury and inflammation and the macrophage-like cells as a surrogate for Kupffer cells. For the validation of this liver model we tested a panel of four drug pairs (Troglitazone – Rosiglitazone; Trovafloxacin – Levofloxacin; Diclofenac – Acetylsalicylic acid and Ketoconazole − Fluconazole), each consisting of a drug that is known to induce iDILI or a non-iDILI partner compound from the same substance class that has no potential to induce iDILI as a control [30], [4], [8], [31], [32]. Drugs were tested in mono- or co-culture and in the presence or absence of a pro-inflammatory background (induced by LPS or TNF) for comparison, resulting in nine different exposure scenarios per examined drug. Based on these tests, we aimed to identify whether the addition of immune cells and/or an additional pro-inflammatory environment to liver cell cultures could improve the detection of iDILI drugs and therefore represent a more sensitive liver model for the prediction of iDILI.
2. Materials and methods
2.1. Materials
All drugs except Diclofenac sodium salt were purchased from Sigma (Taufenkirchen, Germany). Diclofenac sodium salt (DcL) was obtained from Cayman Chemical (Ann Arbor, MI, USA). Lipopolysaccharides (LPS) from Escherichia coli 0111:B4 and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma and dimethyl sulfoxide (DMSO) from Carl Roth GmbH + Co. KG (Karlsruhe, Germany). DMEM (low glucose), fetal bovine serum (FBS), trypsin/EDTA (0.25%/0.02%) and phosphate buffered saline (PBS) were obtained from Biochrom GmbH (Berlin, Germany). Gentamycin (50 mg/mL) and UltraPure™ 0.5 M EDTA solution were bought from Thermo Fisher Scientific (Waltham, MA, USA). Accutase solution was purchased from PromoCell GmbH (Heidelberg, Germany). Human TNF-α, premium grade, was obtained from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany). Cell Proliferation Reagent WST-1 was purchased from Roche Deutschland Holding GmbH (Penzberg, Germany) and the DuoSet® ELISA for human CXCL8/IL-8 from R&D Systems (Minneapolis, MN, USA). The antibodies (mouse anti-human CD32-PE; mouse anti-human CD11b-APC; mouse anti-human CD14-APC) and the fixative for FACS measurements were obtained from Beckman Coulter (Brea, CA, USA), while the mouse anti-human EpCAM-FITC (CD326) and mouse anti-human CD45-BV510 were from BD Biosciences (Franklin Lakes, NJ, US). Isotype controls (EpCAM-FITC: mouse IgG1 FITC; CD14-BV510: mouse IgG1 BV510; CD11b-APC: mouse IgG1 APC; CD14-APC: mouse IgG2a APC) were purchased from BD Biosciences (Franklin Lakes, NJ, US). Mouse IgG2a PE, the isotype control for CD32-PE, was bought from eBioscience (San Diego, CA, USA). Titriplex® III for the FACS buffer was obtained from Merck KGaA (Darmstadt, Germany) and the BD Horizon™ Brilliant Stain Buffer as well as the Human BD Fc Block™ from BD Biosciences. The THP-1 cell line (ACC 16) and the HepG2 cell line (ACC 180) were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ) (Braunschweig, Germany). The Falcon Cell Culture Inserts (0.4 μm pore size) and the corresponding Falcon™ Companion Plates for Cell Culture Inserts and Transwell® Permeable Supports (3 μm pore size) incl. corresponding Corning® Costar® cell culture plates were purchased from Corning Life Sciences (Amsterdam, The Netherlands) in the 12-well format. 48-Well plates were obtained from Sigma.
2.2. Selection, classification and pharmacokinetic properties of iDILI drugs
Drugs with the potential to induce the severe idiosyncratic form of DILI were selected by literature research. For the establishment and a first validation of our in vitro model four iDILI drugs, namely Troglitazone (TGZ), Trovafloxacin (TVX), Diclofenac (DcL) and Ketoconazole (KC) were investigated. Non-iDILI partner compounds of the corresponding iDILI drug were used as ‘control’ drugs for the comparison of a treatment that leads to iDILI with a treatment that lacks the potential to induce the idiosyncratic form of DILI. The selected non-iDILI partner compounds belong to the same substance class as its corresponding iDILI drug and show, whenever possible, a similar chemical structure, to exclude a substance class-specific effect. The four non-iDILI partner compounds were Rosiglitazone (RGZ), Levofloxacin (LVX), Acetylsalicylic acid (ASS) and Fluconazole (FC).
The selected compounds and their non-iDILI partner compounds, some of which induce DILI but not the idiosyncratic form, were classified according to two classification models (Table 1). Firstly, Chen et al. [34] (Table 1(a)) developed a classification model that utilizes FDA-approved labels and the severity of the DILI reaction to subdivide drugs into three LTKB classes: most, less or no DILI concern. Secondly, according to Xu et al. [33] (Table 1(b)) a drug was considered as DILI positive if it was either withdrawn, not marketed in the US, received a Black Box Warning (BBW) or Warnings and Precautions (WP) or had a significant number (>10) of clinical reports of serious hepatotoxicity that meet the criteria of Hy’s Law [36], while DILI negative drugs do not meet any of the above-mentioned criteria. According to both classification models, all selected iDILI compounds are classified as DILI positive and are of most DILI concern. Non-iDILI partner compounds were classified as DILI negative, except for FC, which was DILI positive and of most DILI concern.
Table 1.
Detailed information on the reference substances used in this study. Listed are the iDILI drugs with their corresponding non-iDILI partner compound from the same substance class. Cmax values were taken from Xu et al. [33] if not stated otherwise. WDN = Withdrawn; BBW = Black Box Warning; WP = Warnings and Precautions; N/Av = Not available.
| DILI classification |
||||||||
|---|---|---|---|---|---|---|---|---|
| Substance class | Drug | iDILI/non-iDILI | FDA status* | (a) | (b) | Cmax (μg/mL) | 100 x Cmax (μM) | Used conc. (μM) |
| Thiazolidine-dione | TGZ | iDILI | WDN | Most DILI-concern | positive | 2.82 | 639 | 32 (5xCmax) |
| RGZ | Non-iDILI | WP | N/Av | negative | 0.37 | 104 | 32 (31xCmax) | |
| Fluoro-quinolone | TVX | iDILI | WDN | Most DILI-concern | positive | 2.09 | 408 | 25 (6xCmax) |
| LVX | Non-iDILI | WP | N/Av | negative | 5.70 | 1577 | 25 (2xCmax) | |
| NSAID | DcL | iDILI | WP | Most DILI-concern | positive | 2.37 | 744 | 125 (17xCmax) |
| ASS | Non-iDILI | N/Av | N/Av | negative | 1.00 | 552 | 125 (23xCmax) | |
| Imidazole | KC | iDILI | BBW | Most DILI-concern | N/Av | 4.22** | 794 | 20 (2xCmax) |
| FC | Non-iDILI | WP | Most DILI-concern | positive | 2.70 | 882 | 20 (2xCmax) | |
aLTKB class (Liver Toxicity Knowledge Base; developed by FDA) was taken from Chen et al. [34].
bDILI classification according to Xu et al. [33]; drugs are classified as positive or negative for DILI.
*FDA-approved labels were taken from DailyMed (http://dailymed.nlm.nih.gov/dailymed/index.cfm).
**Cmax value was taken from Huang et al. [35].
To make sure that the drugs were tested in vitro in a therapeutically relevant dose range, a concentration of 100-fold the average plasma maximum concentration (Cmax) was never exceeded (Table 1) and concentrations of up to 100-fold Cmax were considered as therapeutically relevant, as previously discussed by Xu et al. [33] and applied by Cosgrove et al. [22] in their iDILI study. Cmax values were obtained from a list compiled by Xu et al. [33], based on values obtained in humans at commonly recommended therapeutic doses, as well as by literature searches. Non-iDILI partner compounds were dosed in about the same or higher Cmax-ratio(s) as their corresponding iDILI compound.
2.3. Cell culture and exposure protocol
2.3.1. Permanent culture
The human monocytic cell line THP-1 [37] was grown in suspension in DMEM medium supplemented with 10% v/v heat-inactivated (h.i.) FBS and 5 μg/mL gentamycin. Cells were grown permanently at a concentration of 0.8–1 × 106 cells/mL, which was crucial because lower cell densities are known to diminish the responsiveness to PMA [38]. THP-1 cells were sub-cultured three times a week and were used for a maximum of three months. The human hepatoma cell line HepG2 [39] was grown in DMEM medium supplemented with 10% v/v FBS and 5 μg/mL gentamycin. HepG2 cells were sub-cultured twice a week, using 0.05% EDTA in PBS before trypsinization to ensure cell dissociation and homogeneous cell seedings. Cells were used at passages 8–28.
2.3.2. THP-1 differentiation
Differentiation of THP-1 cells into their mature macrophage-like state was induced by exposing THP-1 monocytes to 50 nM PMA in growth medium for 72 h and an additional resting phase in PMA-free growth medium for another 24 h to enhance the differentiation status of the cells [40]. Thereafter up to 99% of the cells became adherent and showed typical macrophage-like morphology. At this stage, cells were used for co-culture experiments or FACS measurements. For additional information on the differentiation protocol and the PMA-concentration selection see Supplementary Methods 1.
2.3.3. Monocultures
For the treatment of monocultures, for the preparation of dose-response curves or for the comparison with the co-cultures, 7.5 × 105 HepG2 cells in 1.5 mL cell culture medium were seeded into 12-well plates from Corning® Costar® or Falcon™. After 24 h, cells reached 95–100% confluency and were ready for exposure. For the dose-response curves, 3 × 105 (drugs, LPS) or 5 × 105 (TNF) THP-1 monocytes were seeded and exposed simultaneously in 0.5 mL in 48-well plates. For the same purpose, 7.5 × 104 THP-1 macrophages were exposed in the membrane-insert system in a final volume of 2.0 mL. For each exposure to drugs and/or pro-inflammatory factors in mono- as well as co-cultures, the medium was substituted by a protein-reduced exposure medium (DMEM + 2% h.i. FBS + 5 μg/mL gentamycin), resembling a more physiologically relevant test environment, as the albumin concentration in human blood is approximately 2% [41]. Exposure medium contained the indicated concentrations of the drug and/or the pro-inflammatory factor or vehicle.
2.3.4. Co-culture
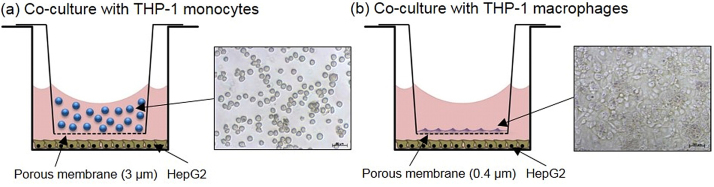
For co-cultures, HepG2 cells were seeded into the lower compartment of the membrane-insert systems (12-well) at a density of 7.5 × 105 one day before the co-culture system was established and the exposure was planned. THP-1 differentiation was started 4d (72 h + 24 h) before. At the day of exposure, adherent THP‐1 macrophages (7.5 × 104 cells) on the membrane in the Falcon-insert or THP-1 monocytes in suspension (3 × 105 cells for co-cultures without additional stimulation of the cells and co-exposure to LPS or 5 × 105 cells for co-cultures with co-exposure to TNF) were transferred into the Transwell® Permeable Supports (membrane with a 3 μm pore size) and were added to the HepG2 cells (Fig. 1a and b). Cells were thereby separated by a porous membrane, which allowed the exchange of e.g. secretion products. In the upper compartment, the insert, 0.5 mL exposure medium and in the lower compartment 1.5 mL exposure medium were present (final volume: 2.0 mL). The ratio of THP-1 macrophages to HepG2 cells was therefore 1:10, which perfectly correlates with the Kupffer cell (liver resident macrophage) content of about 10% in the human liver [42]. THP-1 monocytes represented a greater part (29–40% of all cells in the system) in our model, which reflects the high influx of monocytes into the liver during liver injury and inflammation [43]. The effect of the immune cells themselves on the HepG2 cell viability was evaluated to further characterize our co-culture model systems (see Supplementary Results 1).
Fig. 1.
Schematic construction of the two in vitro co-culture liver inflammation models combining human hepatoma (HepG2) cells with human (a) monocytic or (b) macrophage-like (THP-1) cells. The cells are separated by a porous membrane (0.4 or 3 μm pore size) at a 1 mm distance.
2.4. Cytotoxicity
To prepare stock solutions, all drugs were dissolved in DMSO except for DcL, which was dissolved in PBS. LPS was reconstituted in non-supplemented DMEM and TNF in aqua dest. Stock concentrations were 30 mg/mL for TGZ and RGZ, 50 and 36 mg/mL for TVX and LVX, 9 and 40 mg/mL for DcL and ASS as well as 20 and 33 mg/mL for KC and FC. The LPS stock concentration was 1 mg/mL and that of TNF 100 ng/mL. Aliquots of the stocks were stored at −20 °C until use. The maximal final DMSO concentration was 0.56% for the preparation of the dose-response curves and 0.056% for the co-culture experiments. Cells were treated with the indicated drug concentrations in single culture or co-culture as previously described. Drug concentrations for the co-culture experiments were selected based on the criteria that they were high enough to induce a moderate drug-only toxicity of about 20% (=EC80, the concentration at which 80% of the cells are still viable) in the HepG2 cells and simultaneously did not lead to a total loss of the viability of the more sensitive THP-1 cells. In addition, a possible masking of effects, when choosing too high concentrations, was also taken into account. The criteria were fulfilled by all drugs except for DcL, which showed no cytotoxicity in HepG2 cells at the used concentration. Non-iDILI partner compounds were dosed at the same concentration that was selected for the corresponding iDILI compounds. For all experiments in which a co-exposure with a pro-inflammatory factor was conducted, non-toxic concentrations of 1 μg/mL LPS and 10 ng/mL TNF were used. Cells were treated for the indicated times, but not longer than for 48 h.
As a measure of viability, the metabolic activity of HepG2 cells was examined by using the WST-1 assay according to the manufacturer’s protocol. Briefly, inserts with THP-1 monocytes or macrophages as well as the exposure medium were removed and the remaining HepG2 cells on the bottom of the plate were incubated with 10% of the tetrazolium salt in fresh exposure medium for 30 min at 37 °C. The formed water-soluble formazan was measured with the SpectraMax 340 PC (Molecular Devices, CA, US) at 450 nm. Two to three biological replicates from the same 12-well plate were measured with three technical replicates per biological replicate.
2.5. Flow cytometry
Flow cytometric measurements were performed using a ten color, three laser Navios™ 10/3 flow cytometer (Beckman Coulter GmbH, Krefeld, Germany). THP-1 monocytes in suspension were collected by centrifugation (800 rpm, 5 min, 4 °C), while PMA-differentiated adherent THP-1 macrophages were collected by detaching them gently by a 5 min incubation with accutase solution, thereby leaving the cell surface proteins intact. After being washed in ice-cold PBS, 3–6 × 105 cells were dissolved in FACS buffer (PBS supplemented with 2 mM Titriplex® III and 5% h.i. FBS) and centrifuged (400g, 5 min, 4 °C). Cell pellets were resuspended in 100 μL Horizon™ Brilliant Stain Buffer with Human BD Fc Block™ in order to minimize non-specific binding of immunoglobulins to Fc receptors and incubated for 10 min at 4 °C. Thereafter, the corresponding amount of antibody was added (20 μL for EpCAM-FITC and CD32-PE; 10 μL for CD14-APC and CD11b-APC; 5 μL for CD45-BV510; volumes per 1 × 106 cells) and the cell suspension was incubated for another 20 min at 4 °C. After adding 1 mL of FACS buffer to the stained cell suspension, cells were washed by centrifugation at 400g for 5 min at 4 °C. Subsequently, cells were fixed in FACS buffer containing 10% fixative for 15 min at RT. Thereafter, the pelleted cells (400 g, 5 min, 4 °C) were resuspended in 0.5 mL FACS buffer and 100,000 events were recorded. Additionally single stained compensation controls and isotype controls for each conjugated antibody were measured simultaneously. All data were analyzed using the Kaluza® Flow Analysis Software (Beckman Coulter GmbH, Krefeld, Germany).
2.6. Statistics
All experiments were repeated at least three times and all data were recorded as mean ± SD. Statistical analyses were performed using Prism 4 software version 4.03 (GraphPad Software Inc., CA, USA). The statistical tests used to evaluate the results of each experiment are mentioned in the figure legends. When comparing three or more groups, one-way ANOVA was performed with Tukey’s post hoc analysis to compare pairs of group means. In order to compare the mono- with the co-cultures under the different experimental conditions, a two-way ANOVA followed by the Bonferroni post hoc test was performed. When using unpaired two-tailed Student’s t test, a parallel performed F-test was used to decide whether the test had to be repeated on the basis of Welch’s correlation. This was the case when variances of the groups were tested as being not equal. A p-value of <0.05 was considered significant.
3. Results
3.1. Concentration selection of iDILI drugs for optimal dosage in co-culture experiments
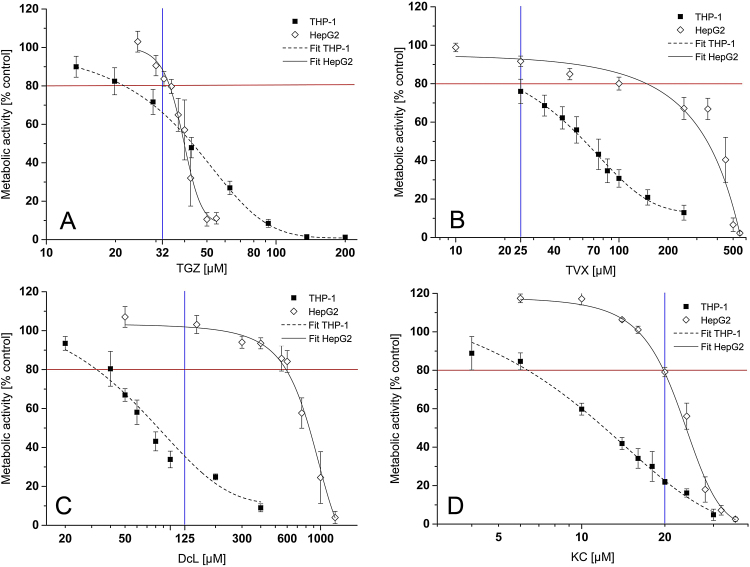
During the development of the present in vitro model system for the prediction of inflammation-associated iDILI, in which we co-exposed human HepG2 cells to known iDILI drugs or their corresponding non-iDILI partner compound and immune cells and/or pro-inflammatory factors, it became clear that the concentration selection of the iDILI drug was crucial to obtain clear synergistic or hormetic effects. It was assumed that at least HepG2 cells had to exhibit a modest drug-only cytotoxicity of about 20% (EC80) to obtain clear-cut effects (data not shown). This preceding damage seemed to be necessary to elicit a decrease of the threshold for hepatotoxicity. The dose-response relationship of the four iDILI drugs was recorded in HepG2 cells and THP-1 monocytes and depicted in an overlay for better comparison (Fig. 2). It was further assumed that THP-1 macrophages are as sensitive or even more sensitive as THP-1 monocytes to iDILI drugs, so that no additional dose-response curves were obtained for this cell type, and this assumption was taken into account for the concentration selection.
Fig. 2.
Dose-dependent cytotoxicity of four iDILI drugs. HepG2 cells and THP-1 monocytes were exposed to increasing concentrations of the respective drug (A = TGZ; B = TVX; C = DcL; D = KC) or the vehicle for 24 h. DMSO concentration never exceeded 0.56%. Thereafter, the metabolic activity of the cells was assessed by performing the WST-1 assay. Data are expressed as% of vehicle control activity and are depicted in an overlay for better comparison. Red lines indicate the concentrations at which a moderate toxicity of 20% (EC80) was observed; blue lines indicate the viability of each cell type at the concentration used for the co-culture experiments. All data (n = 3–4) are presented as mean ± SD. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Single cell types were treated with increasing concentrations of the respective iDILI drug for 24 h and cytotoxicity was measured by using the WST-1 assay (Fig. 2). The evaluation of the EC50 values revealed clear discrepancies of cytotoxicity profiles between both cell types for all iDILI drugs, except for TGZ. Generally, THP-1 cells respond more sensitively to the drugs than HepG2 cells. Consequently, for the concentration selection compromises had to be made, in most cases to the detriment of the viability of the THP-1 cells (compare Table 2). The concentration selection for DcL and KC especially appeared to be more difficult. For both drugs, the EC80 values in HepG2 cells, which are crucial as previously mentioned, were far apart from the THP-1 EC80 values. Therefore, the highest possible concentrations that induced a cytotoxic effect in HepG2 cells and did not decrease the THP-1 viability by more than 70–80% after 24 h exposure had to be chosen. Only then one can assume that THP-1 cells remained functional during exposure. The metabolic activity of HepG2 cells was not affected by the selected DcL concentration. However, HepG2 cells showed morphological changes (Supplementary Fig. 1), which indicated that liver cells were damaged to a certain extent. The selected concentrations (TGZ, 32 μM; TVX, 25 μM; DcL, 125 μM; KC, 20 μM), all of which led to a modest cytotoxicity in the HepG2 cells, were used in all subsequent experiments. Non-iDILI partner compounds were used at the same concentrations at which their corresponding iDILI drug was tested. The tested concentrations did not show any cytotoxicity in both cell lines except for RGZ in THP-1 monocytes, which caused a minor viability loss of 10% (Supplementary Fig. 2).
Table 2.
EC50 and EC80 values for HepG2 cells and THP-1 monocytes. The third row per drug depicts the viability of the respective cell type after a 24 h exposure to the indicated concentration of the iDILI drug, expressed as% of the vehicle control activity.
| Drug | Concentration | THP-1 | HepG2 |
|---|---|---|---|
| TGZ | EC50 | 42 μM | 40 μM |
| EC80 | 27 μM | 34 μM | |
| Used conc. (32 μM) | ∼70% | ∼85% | |
| TVX | EC50 | 60 μM | 370 μM |
| EC80 | 23 μM | 100 μM | |
| Used conc. (25 μM) | ∼76% | ∼92% | |
| DcL | EC50 | 76 μM | 775 μM |
| EC80 | 30 μM | 604 μM | |
| Used conc. (125 μM) | ∼30% | ∼100% | |
| KC | EC50 | 12 μM | 24 μM |
| EC80 | 7 μM | 18 μM | |
| Used conc. (20 μM) | ∼20% | ∼80% | |
3.2. Verification of the immune cell functionality and concentration selection of pro-inflammatory factors for the optimal cell stimulation
To verify the differentiation status of the cells and to check whether THP-1 macrophages, which differentiated on the insert membranes, showed the same quality as cells differentiated in normal cell culture flasks, the expression of differential cell surface markers in THP-1 macrophages and monocytes was analyzed by flow cytometry (Fig. 3). Besides typical monocytic and macrophage markers, we also tested the cells regarding the expression of the epithelial surface marker EpCAM to make sure that the used immortalized THP-1 cell line had not transformed. EpCAM expression was not detected in THP-1 monocytes and macrophages. CD45, a surface marker present in almost all haematolymphoid cells including monocytes and macrophages, was detected in both cell types. PMA-differentiated THP-1 cells showed a significant higher expression of CD45 than THP-1 monocytes. Moreover, a significant up-regulation of the macrophage-markers CD14 and CD11b was observed in THP-1 macrophages, while the monocyte-marker CD32 was down-regulated. No significant differences between THP-1 macrophages differentiated and cultured on the membranes and in the cell culture flasks were observed.
Fig. 3.
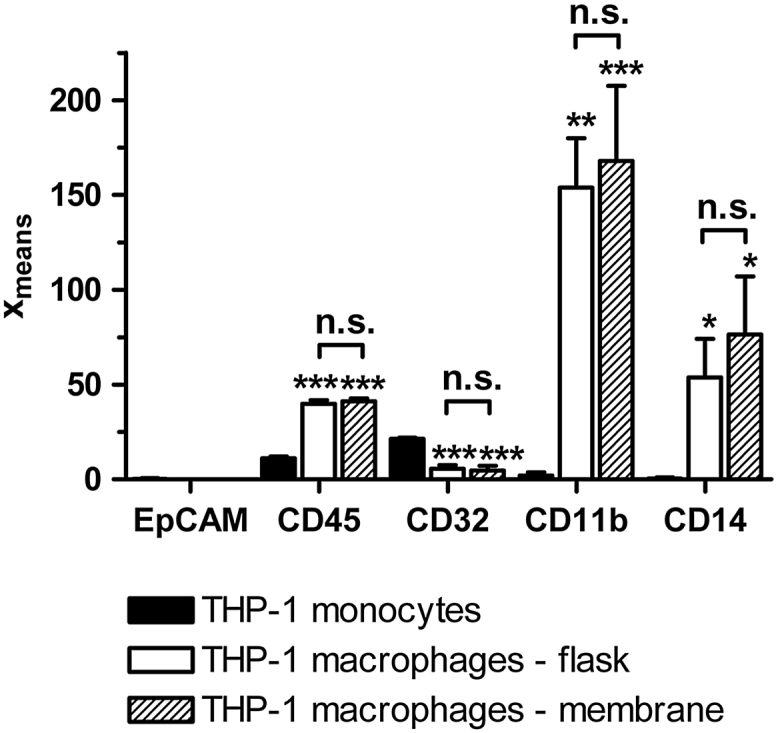
Cell surface marker expression in THP-1 monocytes and macrophages. Expression levels of the marker proteins were examined by flow cytometry and are depicted as xmeans, the averaged and weighted logarithm of the fluorescence intensity. All data (n = 3) are presented as mean ± SD. Statistical analysis was performed using a one-way ANOVA and the Tukey post hoc test. * indicates the significance in comparison to monocytes. p < 0.05*; p < 0.01**; p < 0.001***.
The concentrations of LPS and TNF used for the co-exposure experiments in mono- or co-cultures were derived from initial dose titration studies performed in single cultures of the three cell types. Concentrations of 1 μg/mL and 10 ng/mL were chosen in the case of LPS and TNF, respectively. The concentrations induced a stable pro-inflammatory state in the cells, were located in the linear increasing segment of the stimulation curves, so that a further stimulation via a drug or DAMPs will not be masked, and reflect the levels humans are exposed to during inflammation [44], [45]. For more detail see Supplementary Results 2.
3.3. All iDILI drugs, but not their non-iDILI partner compounds, show immune cell- and/or pro-inflammatory factor-dependent synergistic or hormetic effects, each of them in at least two exposure scenarios
To determine whether the exclusive addition of immune cells or an additionally induced acute inflammatory stress might render livers cells more sensitive to iDILI drugs, we exposed (i) HepG2 single cultures, (ii) co-cultures of HepG2 cells with THP-1 monocytes and (iii) co-cultures of HepG2 cells with THP-1 macrophages to a total of four iDILI/non-iDILI partner compounds and co-exposed them with or without a pro-inflammatory factor (LPS, TNF). Hence, each drug was examined on the basis of nine different exposure scenarios. After the respective exposure period, the viability of the HepG2 cells was assessed. The monoculture of HepG2 cells was always tested in parallel as a control scenario and to examine whether the pro-inflammatory factor alone could induce synergistic effects. In case that the iDILI drug showed no synergetic or hormetic effect in one of exposure scenarios, the corresponding non-iDILI drug was not tested in this particular exposure scenario because a comparison was not needed. All results are listed as an overview in Table 3.
Table 3.
Mono- and co-culture experiments of HepG2 cells with human (a) monocytic or (b) macrophage-like (THP-1) cells. Cells were exposed to known iDILI substances and their corresponding non-iDILI partner compound. Results are based on the assessment of the viability of the HepG2 cells after 24 h or 48 h exposure to 32 μM TGZ/RGZ, 25 μM TVX/LVX, 125 μM DcL/ASS or 20 μM KC/FC with or without a co-exposure to a pro-inflammatory stimulus (LPS, 1 μg/mL, or TNF, 10 ng/mL) compared to the vehicle control. Green check marks (√) indicate synergistic effects (increased cytotoxicity) between the drug and the co-cultured cells and/or the pro-inflammatory factor; Violet arrows (↗) indicate an increased metabolic activity of the HepG2 cells; Black crosses (X) indicate no effect and (./.) indicates not performed experiments. Each experiment was performed at least three times. Evaluation of the significance of the effects was performed using a two-way ANOVA and the Bonferroni post hoc test or a one-way ANOVA and the Tukey post hoc test (see single graphs per experiment for significance levels). Mono = Monoculture of HepG2; Co = Co-culture of HepG2 with the indicated THP-1 cell type.
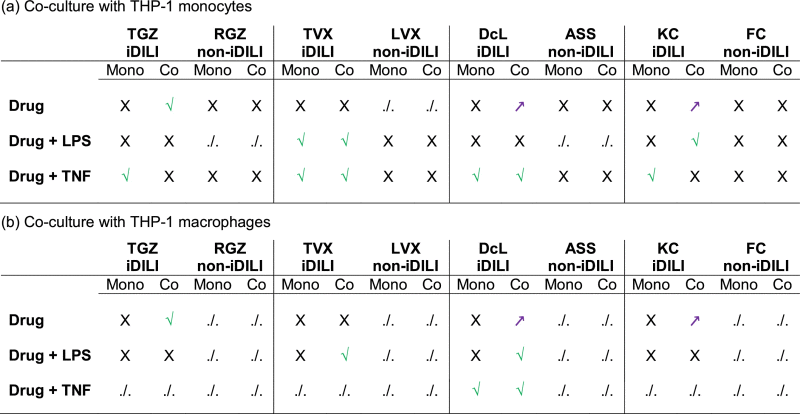 |
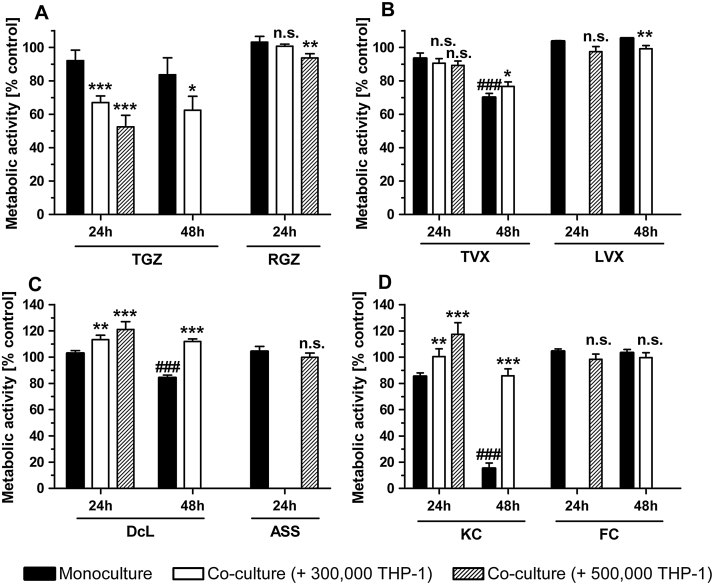
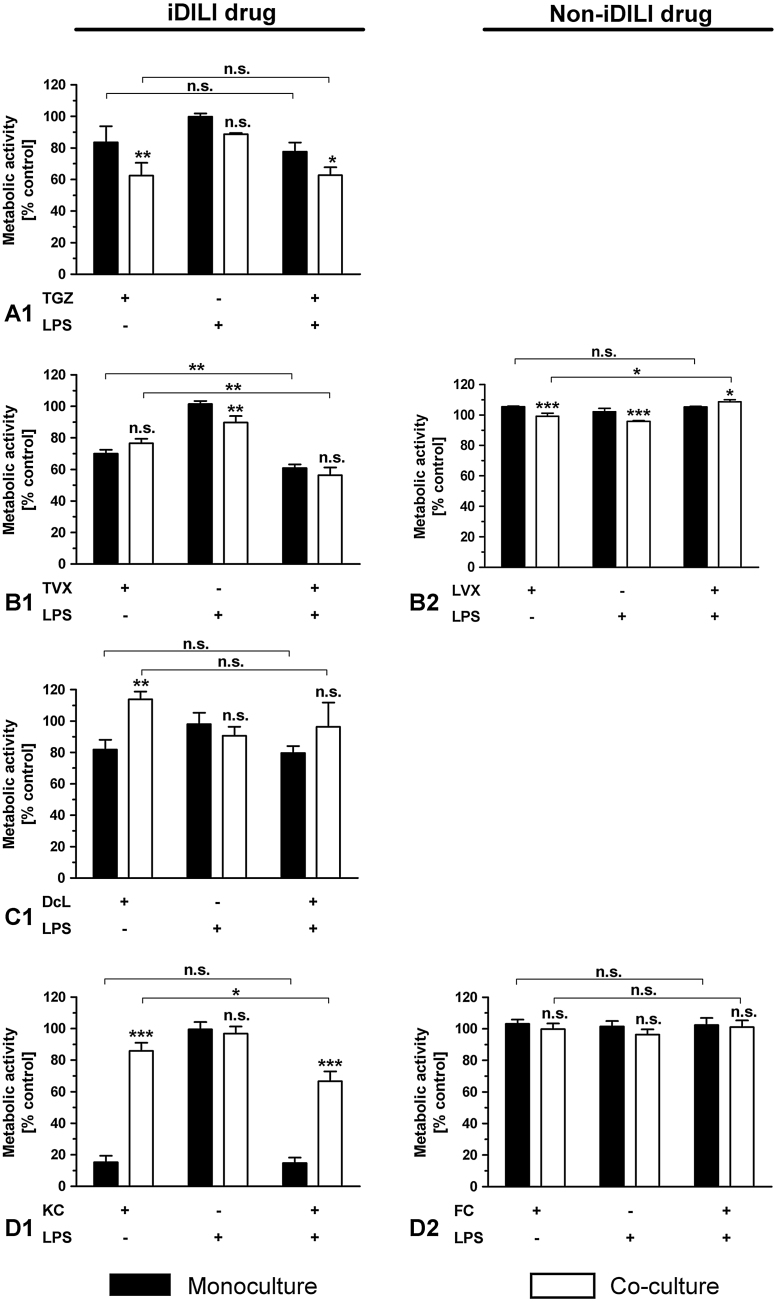
To determine whether the presence of immune cells alone is sufficient to sensitize liver cells to known iDILI drugs, HepG2 cells were co-cultured with 3–5 × 105 THP-1 monocytes and treated with the iDILI/non-iDILI drugs for 24 h or 48 h (Fig. 4). The exposure of the co-cultures to TGZ led to a significant decrease in HepG2 viability when compared to the moderate toxic effect of TGZ in HepG2 monocultures after 24 h. Importantly, this synergistic effect was enhanced at higher THP-1 cell numbers, but was not further increased after a 48 h exposure. RGZ at the same concentration did not cause any significant cell death after 24 h and only negligible effects in the co-culture with 5 × 105 THP-1 monocytes (Fig. 4A). A significant increase in metabolic activity, the so-called hormesis Mattson et al., 2008, was observed in co-cultures treated with DcL and KC if compared to the treated HepG2 monocultures. This effect was also enhanced by higher numbers of immune cells. Furthermore, the increased cytotoxicity of both drugs seen in the HepG2 monocultures after a prolonged exposure of 48 h, was even compensated by the immune cells in the co-culture, reaching activity levels that were seen in HepG2 single cultures after only 24 h exposure. In both cases, THP-1 monocytes seemed to act protective against drug-only toxicities. The non-iDILI partner compounds ASS and FC at the same concentrations did not induce comparable effects and showed viabilities at the control levels (Fig. 4C&D). The iDILI drug TVX did not show any clear synergistic or hormetic effects, except for a slight but significant increase in metabolic activity after 48 h exposure. LVX, as in the case of all other non-iDILI partner compounds, did not induce considerable effects (Fig. 4B).
Fig. 4.
Identification of immune cell-dependent synergistic effects of iDILI drugs on HepG2 cell viability without further stimulation of the immune cells. Mono- and co-cultures of HepG2 cells with different numbers of THP-1 monocytes were exposed to (A) 32 μM TGZ/RGZ, (B) 25 μM TVX/LVX, (C) 125 μM DcL/ASS and (D) 20 μM KC/FC or the respective vehicle control for 24 h or 48 h. Thereafter, the metabolic activity of the HepG2 cells was assessed as a viability parameter. All data (n = 3–9) are presented as% of the vehicle control activity and mean ± SD. The statistical analysis was performed using a one-way ANOVA and the Tukey post hoc test or Student’s t test (unpaired, two-tailed). * indicates the level of significance when compared to the HepG2 monoculture at the corresponding point in time and for the corresponding drug. # indicates the level of significance when compared to the 24 h value for the corresponding drug. p < 0.05*/#; p < 0.01**/##; p < 0.001***/###.
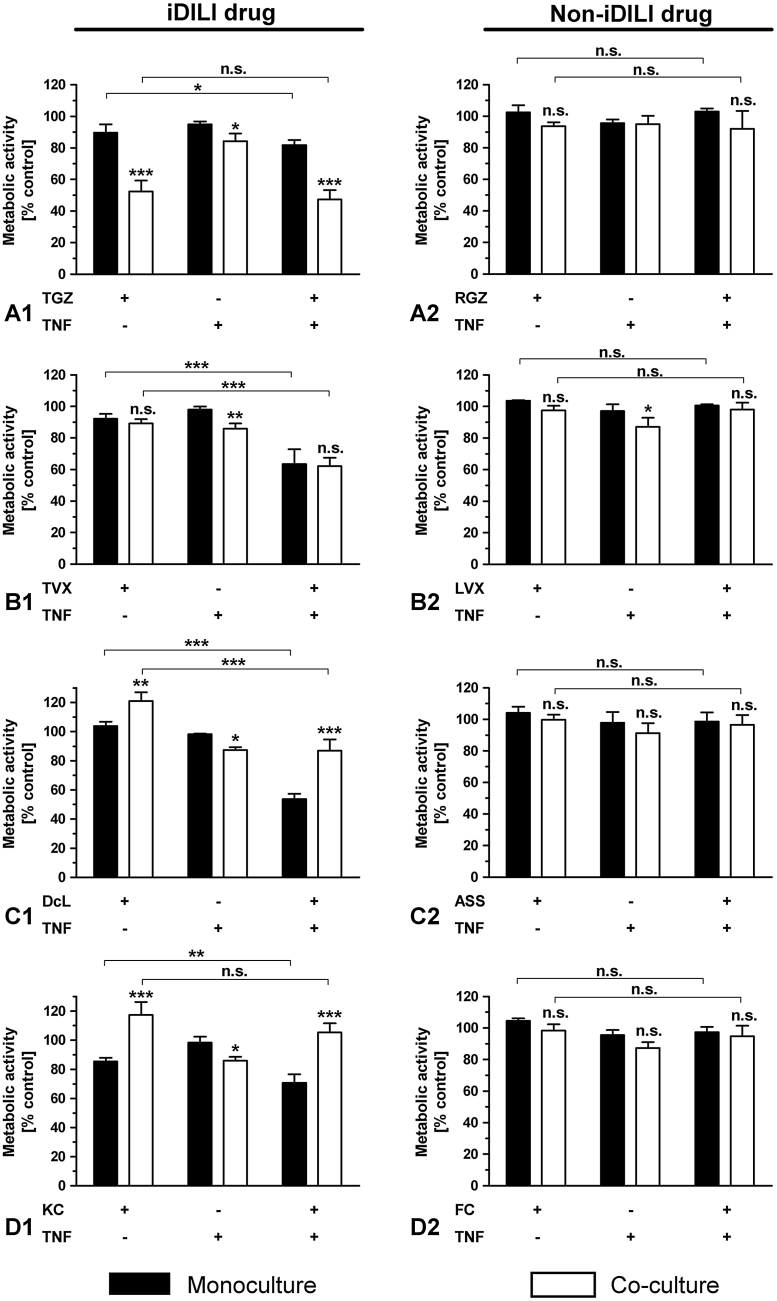
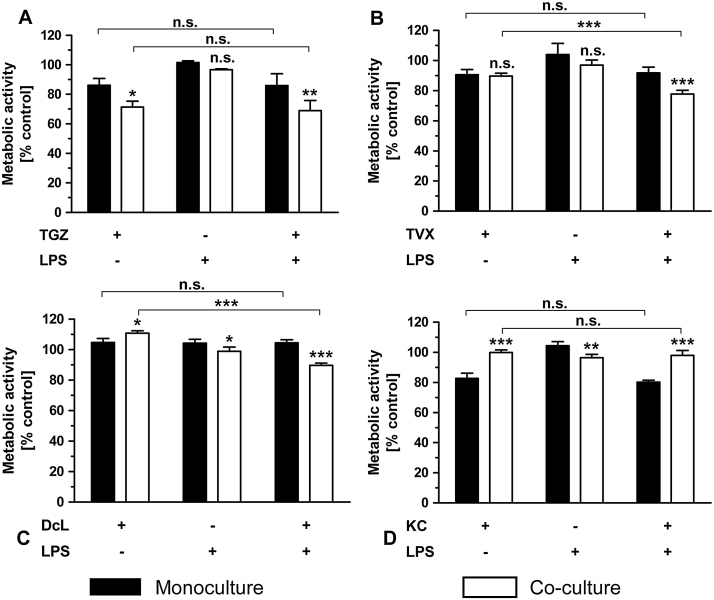
The co-exposure of each iDILI drug with the pro-inflammatory factor TNF for 24 h resulted in a significant synergistic effect by inducing enhanced cellular death in the HepG2 monoculture (Fig. 5A1-D1), while the corresponding non-iDILI partner compounds showed no effect (Fig. 5A2-D2). These synergistic effects were not intensified by the addition of THP-1 monocytes. In contrast, the enhanced toxicity of DcL and KC was compensated in the co-culture system. TNF exposure in the co-culture system alone had a slight but significant detrimental effect on HepG2 cells, thereby inducing a moderate preceding damage. Synergies observed in the case of TVX and DcL were stronger than in the case of TGZ and KC.
Fig. 5.
Identification of immune cell and/or TNF-dependent synergistic effects of iDILI drugs on HepG2 cell viability. Mono- and co-cultures of HepG2 cells with THP-1 monocytes were exposed to (A1-2) 32 μM TGZ/RGZ, (B1-2) 25 μM TVX/LVX, (C1-2) 125 μM DcL/ASS or (D1-2) 20 μM KC/FC with or without co-exposure to 10 ng/mL TNF or the corresponding vehicle control for 24 h. Thereafter, the metabolic activity of the HepG2 cells was assessed by performing the WST-1. All data (n = 3-4) are presented as% of the vehicle control activity and mean ± SD. The statistical analysis was performed by using a two-way ANOVA and the Bonferroni post hoc test or one-way ANOVA and the Tukey post hoc test. * indicates the level of significance when compared to the HepG2 monoculture at the corresponding exposure scenario when not stated otherwise. p < 0.05*; p < 0.01**; p < 0.001***.
The co-exposure with LPS identified two out of four iDILI drugs (TVX and KC) that induced a significantly enhanced cell death in HepG2 cells after 48 h treatment (Fig. 6B1&D1). While TVX showed a synergy in the HepG2 monoculture, which was not intensified by immune cells, KC showed a synergy only in the presence of both, the immune cells and the pro-inflammatory factor. The corresponding non-iDILI partner compounds LVX and FC did not show any relevant effects. In the co-culture system LPS alone only affected HepG2 cell viability to a minor extent without reaching significance.
Fig. 6.
Identification of immune cell and/or LPS-dependent synergistic effects of iDILI drugs on HepG2 cell viability. Mono- and co-cultures of HepG2 cells with THP-1 monocytes were exposed to (A1) 32 μM TGZ, (B1-2) 25 μM TVX/LVX, (C1) 125 μM DcL or (D1-2) 20 μM KC/FC with or without co-exposure to 1 μg/mL LPS or the corresponding vehicle control for 48 h. Thereafter, the metabolic activity of the HepG2 cells was assessed by performing the WST-1 assay. All data (n = 3–4) are presented as% of the vehicle control activity and mean ± SD. The statistical analysis was performed by using a two-way ANOVA and the Bonferroni post hoc test or one-way ANOVA and the Tukey post hoc test. * indicates the level of significance when compared to the HepG2 monoculture at the corresponding exposure scenario when not stated otherwise. p < 0.05*; p < 0.01**; p < 0.001***.
To determine whether the use of the more mature THP-1 macrophages could be advantageous in the prediction of inflammation-associated iDILI, we examined the effect of a co-exposure of the iDILI drugs with LPS when co-culturing HepG2 cells with 7.5 × 104 THP-1 macrophages (Fig. 7). In this setting, the cell number of THP-1 macrophages was about one quarter the cell number used for the co-cultures with THP-1 monocytes, but resembles the optimal macrophage to hepatocyte ratio. Despite the small cell numbers, all tested iDILI drugs showed the same immune cell-dependent effects that were seen in the co-culture experiments with the THP-1 monocytes (synergy for TGZ, hormesis for DcL and KC). Most importantly, two iDILI drugs, namely TVX and DcL, showed synergistic effects in the presence of the THP-1 macrophages as well as the pro-inflammatory factor LPS. No synergies were observed when one of the conditions was missing (Fig. 7B&C). These two synergies were not observed in the exposure scenarios with the THP-1 monocytes.
Fig. 7.
Identification of immune cell and/or LPS-dependent-dependent synergistic effects of iDILI drugs on HepG2 cell viability. Mono- and co-cultures of HepG2 cells with THP-1 macrophages were exposed to (A) 32 μM TGZ, (B) 25 μM TVX, (C) 125 μM DcL or (D) 20 μM KC with or without co-exposure to 1 μg/mL LPS or the corresponding vehicle control for 24 h. Thereafter, the metabolic activity of the HepG2 cells was assessed by performing the WST-1 assay. All data (n = 3–4) are presented as% of the vehicle control activity and mean ± SD. The statistical analysis was performed using a two-way ANOVA and the Bonferroni post hoc test or one-way ANOVA and the Tukey post hoc test. * indicates the level of significance when compared to the HepG2 monoculture at the corresponding exposure scenario when not stated otherwise. p < 0.05*; p < 0.01**; p < 0.001***.
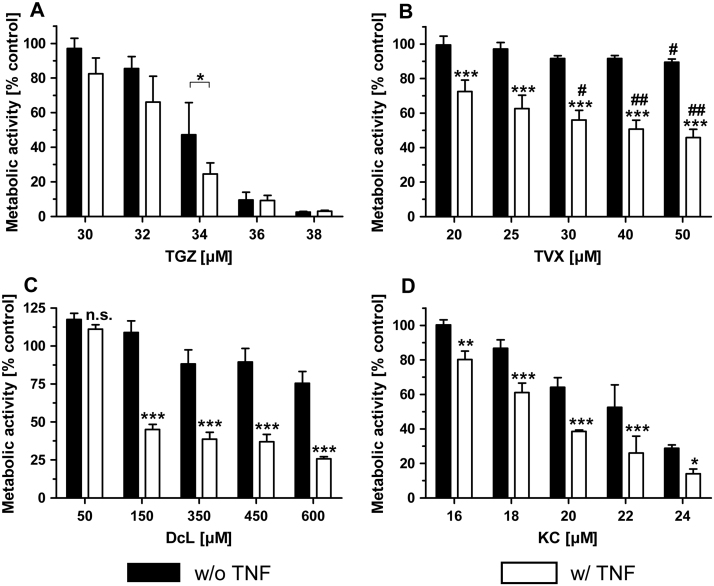
3.4. Each iDILI drug synergizes with TNF in HepG2 monocultures
Our experiments show that all tested iDILI drugs synergized with TNF in HepG2 monocultures. To verify these TNF-dependent synergistic effects, we co-exposed HepG2 cells with increasing concentrations of the corresponding iDILI drug, including concentrations that were lower or higher than the single concentrations used for the co-culture experiments, and TNF. The statistical analyses revealed significant differences between the two treatment groups (with and without TNF) for all iDILI drugs tested (Fig. 8). Furthermore it was shown, that DcL at a concentration as low as 50 μM, was not able to induce a synergistic effect when co-exposed with TNF. While the extent of the synergetic effect was relatively consistent with increasing concentrations for three out of the four tested iDILI drugs, the co-exposure of TVX with TNF produced synergies that were dose-dependently increased (Fig. 8B). The synergistic effects of TVX and DcL were again shown to be stronger than those of TGZ and KC. This observation also correlated with alterations in the morphology of the HepG2 cell cultures that were exposed to both, TVX or DcL and TNF, indicating pronounced hepatocellular injury (Supplementary Fig. 1).
Fig. 8.
Verification of TNF-dependent synergistic effects of iDILI drugs on HepG2 cell viability. Monocultures of HepG2 cells were exposed to increasing concentrations of the respective drug (A = TGZ; B = TVX; C = DcL; D = KC) with or without co-exposure to 10 ng/mL TNF or the corresponding vehicle control for 24 h. The metabolic activity of the HepG2 cells was assessed by performing the WST-1 assay. All data (n = 3) are presented as% of the vehicle control activity and mean ± SD. The statistical analysis was performed by using a two-way ANOVA and the Bonferroni post hoc test or one-way ANOVA and the Tukey post hoc test. * indicates the level of significance compared to the drug exposure without co-exposure to TNF; # indicates the level of significance compared to the smallest drug concentration used in the corresponding experiment. p < 0.05*/#; p < 0.01**/##; p < 0.001***/###.
4. Discussion
Due to its low predictability idiosyncratic drug-induced liver injury (iDILI) represents not only a major issue for the pharmaceutical industry but also for the broad population, which is exposed to drugs with severe side effects. Unfortunately, up to now, no valid liver models that are able to predict those rare side effects in the preclinical screening procedure are available. Based on a study by Edling et al. [25] reporting that co-cultures of human hepatoma cells and monocytes might be a better system to predict the cytotoxicity mediated by potential hepatotoxins and recent studies indicating that inflammatory episodes, immune cells and of these secreted factors are involved in the development of iDILI (e.g., [11], [46], [22], we established and evaluated an immune competent human in vitro hepatocyte–NPC co-culture model system between human HepG2 and monocytic or macrophage-like THP-1 cells for the prediction of inflammation-associated iDILI.
Our co-culture model was established on the basis of the human HepG2 cell line and offers the advantage that it does not rely on the use of human primary hepatocytes, which although being the ‘gold standard’ for the prediction of hepatotoxicity, are only available to a limited extent and show strong donor-to-donor variations. Although HepG2 cells are known to exhibit only little metabolic competence and hence an immune response to protein adducts with reactive drug metabolites cannot be depicted in this co-culture model, we were mainly interested in the effects of the parent compound on the different cell types itself. In this regard we aimed to investigate whether the parent compound can induce an immune cell-mediated response that is damaging to the liver cells or can act on the liver cells during an existing inflammation in such way, that the liver cells are rendered sensitive to e.g. cytotoxic cytokines released from the immune cells as then shown by a loss of viability of the liver cells. Other working groups showed that all used iDILI drugs in this study are able to influence intracellular signaling pathways (e.g. JNK- and Nf-κB-activation) in HepG2 cells [47], [48], [24], [23], which might influence the response of liver cells to inflammatory secretion products. In addition, Gerets et al. [49] found out that a high metabolic capacity in liver cell lines does not necessarily correlate with a high sensitivity for the detection of hepatotoxic drugs. Moreover, human primary hepatocytes can only be obtained by a complicated procedure including an enzymatic digestion, which can alter the cellular phenotype [50]. This is also the case for primary liver macrophages (Kupffer cells), which, due to the tissue disintegration, can release damage-associated molecular patterns (DAMPs), which may induce a DAMP-induced activation of the isolated cells [51]. Although we wanted to achieve a pro-inflammatory environment in our co-culture model, it is crucial that the immune cells are not in a pronounced inflammatory status, which may mask the effect of a weak stimulus from e.g. a drug. Our differentiation protocol for THP-1 cells on membrane-inserts resulted in macrophages with an only moderate basal inflammatory state, but showed a strong inflammatory response to the endotoxin LPS, a pathogen-associated molecular pattern (PAMP), and to the pro-inflammatory cytokine TNF, a DAMP. The differentiated state of the THP-1 macrophages was confirmed by their distinct surface marker expression and we were able to show, that in spite of the different culture conditions of macrophages in a cell culture flask and on membranes, there are no significant differences regarding the differentiation status between macrophages differentiated and cultured in a cell culture flask and those differentiated and cultured on the used membrane-inserts. Therefore, PMA-differentiated THP-1 macrophages prepared according to our differentiation protocol can be used in co-culture without any limitations. Furthermore, PMA-differentiated THP-1 cells were found to show significant expression of Toll-like receptors and nucleotide-binding oligomerization domain (NOD)-like receptors for the recognition of both PAMPs (e.g. LPS and exogenous stimuli) and DAMPs (e.g. HMGB1, TNF) [52], [53]. The products (e.g., TNF) secreted by PAMP- or DAMP-activated macrophages are reported to contribute to hepatotoxicity by acting directly on the hepatocyte to cause cell death or indirectly by activating or recruiting other cells, such as monocytes and neutrophils [14]. Moreover, monocytes also play an important role in hepatotoxicity by linking the systemic and local hepatic compartments during liver injury and mostly generate a more intense inflammatory response to danger signals (e.g., DAMPs) than macrophages do [54], [55]. All these responses are considered to possibly contribute to iDILI and cannot be reproduced in systems that only focus on the liver cell itself or incorporate incompetent immune cells.
In general, all tested drug concentrations (TGZ, 32 μM; TVX, 25 μM; DcL, 125 μM; KC, 20 μM) reflect clinically relevant exposure levels, while not exceeding 100-fold the Cmax. In the present study, we observed effects at lower exposure concentrations than in a number of previous iDILI reports, thereby indicating that our liver co-culture models seem to be more sensitive to (i)DILI. Exposure concentrations used by other working groups were in the range of 50–500 μM for TGZ [25], [56], [57], [58], 100–800 μM for TVX [59], [22], [26], 250–450 μM for DcL [60], [61], [23], [20] and >20–75 μM for KC [62], [23].
Generally, there are two assumptions related to the inflammatory stress hypothesis: (1) A drug-only hepatotoxicity could be enhanced by a secondary response of immune cells (resulting in inflammation) or (2) a drug could increase an otherwise harmless (already existing) inflammatory stress that hereupon results in liver damage [46], [17]. The first trigger requires the release of DAMPs by the hepatocytes, which in turn affect the immune cell behavior. To better understand the development of iDILI, it is helpful to have an experimental model that depicts both mechanisms. In contrast to studies that only focus on the liver cell itself, we compared both, the effect on the single liver cell culture and the effect on the co-culture. Thereby, it was possible to distinguish between the two above-mentioned triggers.
The first trigger, a (moderate) drug-only hepatotoxicity, was studied by exposing co-cultures of HepG2 cells and different numbers of THP-1 monocytes to the iDILI drugs and by comparing the effects to those of HepG2 monocultures. No further pro-inflammatory stimulus was set. Thereby, it was tested whether the drug alone is able to induce stress signals that can initiate a secondary (inflammatory) response, which in turn lowers the threshold for hepatotoxicity and thus leads to a synergistic effect resulting in cell death. We defined an effect as synergistic if the drug-only toxicity in the HepG2 monoculture was increased by different circumstances, such as the addition of immune cells. Only TGZ showed a synergy by the sole addition of monocytes, as previously described by Edling et al. [25] in a similar cell culture model. However, we are the first to show that the increased HepG2 cell death can be enhanced by the addition of higher monocyte numbers and is thus clearly immune cell-dependent. Therefore, TGZ must be able to induce a cytotoxic response by THP-1 monocytes, generated by a direct interaction or a secondary signal triggered by the drug-induced liver cell injury and the release of intercellular DAMPs. Which DAMPs these might be, remains to be elucidated. Several DAMPs, which can activate immune cells and induce a ‘sterile’ inflammation (inflammation in the absence of an infection) [63], have been suggested to play a role in the development of liver injury (HSPs, HMGB1, TNF) [64], [65], [66]. Kegel et al. [67] confirmed that components released from hepatocytes due to the treatment with subtoxic concentrations of hepatotoxic substances can activate the macrophage/monocytes system. We failed to identify the cytokine pattern in the co-culture exposed to TGZ, although a highly sensitive immunoassay (MSD® Multi-Spot Assay System; V-Plex™) was used (data not shown), so that we cannot determine if the triggered immune-mediated cytotoxic response of TGZ is of an inflammatory nature, as assumed. However, gene expression studies by Edling et al. [25] also indicate the involvement of inflammation in the initiation of the synergistic effect. Furthermore, our results suggest that the higher the number of activated immune cells during liver injury is, the more severe the outcome becomes. Importantly, the non-iDILI partner compound of TGZ, RGZ, did not show any adverse effects, i.e., our model is able to distinguish between an iDILI and a non-iDILI drug. In addition, we demonstrate, that iDILI drugs not only induce synergistic effects but also increase the metabolic activity of HepG2 cells. Such increases might result from a so-called hormesis effect, meaning that the liver cells are in an activated state trying to compensate damaging processes [68], [69]. When compensation fails, cell homeostasis collapses and leads to cell death. A hormesis is often seen in dose-response curves at concentrations close to the break-down of cell health. Such hormetic effects, which were enhanced by increasing the monocyte cell numbers, were shown in the case of DcL and KC. In these cases, HepG2 cells are more stressed by the iDILI drug in co-culture than in HepG2 monoculture. Unexpectedly, the increased drug-only toxicity of DcL and KC was compensated in the co-culture with THP-1 monocytes after a prolonged exposure of 48 h, thereby indicating a protective effect mediated by the immune cells. However, this observation is in line with the finding that Kupffer cells, besides their central contribution to liver injury, can also be protective in many cases, including drug-induced liver injury [14], [53]. For example, Ju et al. [70] described a protective role of Kupffer cells in acetaminophen-induced hepatotoxicity. ASS and FC, as non-iDILI partner compounds of DcL and KC, did not show a hormetic effect, again supporting the high predictability of our model. TVX was the only drug in which case the sole addition of immune cells was not sufficient to induce a synergistic or hormetic effect after 24 h. Therefore, only TVX is not able to produce a stress signal that was sufficient to initiate a secondary response that in turn damaged or stressed the liver cells. In total, we show that three out of four reference iDILI drugs were detected without an additional pro-inflammatory stimulus, on the basis of the first trigger. The above-mentioned effects were reproducible in co-cultures with macrophages.
The second trigger, a concurrent inflammatory stress, was investigated by co-exposing co-cultures of HepG2 and THP-1 monocytes to the iDILI drugs and non-toxic doses of the pro-inflammatory stimuli TNF or LPS and comparing the effects to those obtained in HepG2 monocultures. In summary, all reference iDILI drugs showed a significant synergistic effect when co-exposing them to TNF in the HepG2 monoculture for 24 h, while their corresponding non-iDILI partner compounds did not induce any significant damage under these conditions. This finding is in line with the results of a few research groups, which mostly focus on only one iDILI drug and often do not compare the results to a non-iDILI partner compound. Synergies with TNF in vitro or in vivo were shown for TVX [24], [21], DcL [23], [20] and KC [23]. We are the first to show a synergy in the case of TGZ and TNF as well. Importantly, TVX, which did not show a synergy when HepG2 cells and THP-1 monocytes were co-cultured, as well as DcL and KC, which showed a hormesis rather than a synergy, needed a concurrent inflammatory stress in the form of the pro-inflammatory cytokine TNF to elicit a synergistic effect. Thereby, we demonstrate that TNF seems to be an important initiator of and contributor to inflammation-associated iDILI and that the simple co-exposure of a drug candidate with TNF might be sufficient to predict iDILI with a great probability in the early preclinical screenings. The verification of the TNF-dependent synergistic effects of all four iDILI drugs on HepG2 viability by not only exposing HepG2 monocultures to a single concentration, but to increasing concentrations of the respective drug, confirmed the relevance of TNF to predict inflammation-associated iDILI. There were significant differences between the two treatment groups (with and without TNF) for each substance. In addition, recent studies suggest that also iDILI reactions are dose-dependent, because drugs at doses lower than 50 mg per day are very rarely associated with iDILI [71] and a plasma cmax,total > 1 μM showed a good correlation with an increased likelihood of (i)DILI [72]. Our data support this hypothesis by showing that the onset of a synergy by DcL, in which case doses >50 μM were necessary to elicit enhanced toxicities, is dose-dependent. It is likely that in the case of the other three iDILI drugs lower concentrations than those we looked at would also not be able to induce a synergy. The synergistic effect of all four drugs was not intensified in the co-culture system with THP-1 monocytes. It is possible that the TNF-stimulated monocytes were not able to secrete significantly more TNF that would add to the already existing TNF-concentration (10 ng/mL), and thereby could not further contribute to the synergistic effect. Generally, one might suppose that it is logical that the non-iDILI drugs show no effects when co-exposed to TNF or immune cells, because they were tested at concentrations that were non-toxic for the HepG2 cells. This assumption, however, can be refuted by the example of DcL, which was also tested at a non-toxic dose and showed clear synergistic effects.
Synergies when co-exposed to LPS were found in the case of two of the tested iDILI drugs (TVX and KC) after a pro-longed exposure of 48 h, while their corresponding non-iDILI partner compounds from the same substance class (LVX and FC) did not show any effects. In the case of TVX, the stimulus LPS was able to generate an environment that induces a synergy in the basic HepG2 monoculture. An explanation for this observation might be the induction of TNF via the LPS-stimulation of the HepG2 cells, which express the required receptor TLR4 [73], albeit only to a limited extent. Potentially, the secreted TNF can induce a synergy in the same way as it does in the co-exposure scenario. However, this enhanced toxicity was not further augmented by the monocytes in the co-culture system. In contrast to TVX, the synergy in the case of KC was only observed in the presence of both, the pro-inflammatory factor LPS and the monocytes. In this case, not only the pro-inflammatory stimulus was sufficient to induce a synergy but the LPS-activated monocytes seem to set up the required environment that enables the detection of the iDILI drug via a synergy. Under the same conditions and only under these (both, co-culture with immune cells and co-exposure to the pro-inflammatory stimulus LPS), a synergistic effect was observed in co-cultures with macrophages after only a 24 h exposure to TVX or DcL. Hence, also in these two cases, the immune cells are necessary to enhance the inflammatory stress on the liver cells and to provide a pro-inflammatory environment that leads to the development of an iDILI reaction. In the scientific literature, synergies with LPS in vitro or in vivo were reported for TVX (e.g., [18], [26]), DcL [20] and KC [60]. However, this was only possible in much more complex models, such as animal, primary co-culture or ex vivo models. We demonstrate that these findings are reproducible in a much more simple and feasible model. Interestingly, most research groups that reported a synergistic effect with LPS in their corresponding model, showed that the synergistic effect was always accompanied by an elevated TNF level [18], [60], [20], [26]. Taken together, these results point out to the pro-inflammatory factor TNF as an initiator for inflammation-associated iDILI.
Healthy hepatocytes as well as HepG2 cells are normally resistant to TNF-induced toxicity [74], so that one can assume that the hepatotoxicants sensitize hepatocytes to TNF-mediated cell death by disturbing intracellular biochemical processes and inducing various stress signaling or toxicity pathways. It is likely that the induction of perturbing signaling pathways, albeit to a small extent, might lead to an interaction and a crosstalk with the TNF signaling pathway, thereby resulting in a severe outcome like cell death. To date it is unclear, which and how drug-induced toxicity pathways crosstalk and affect the TNF signaling cascade, rendering it toxic to hepatocytes in (i)DILI. Fredriksson et al. [23] carried out pioneering work in this research field by showing, that drug-induced PERK/ATF4/CHOP-dependent ER stress/UPR program and oxidative stress independently sensitize hepatocytes to TNF-mediated hepatotoxicity by enhancing the activation of the apoptotic signaling downstream of the TNF-R1 receptor. Thus, the elucidation of the “toxic” crosstalk between cytokine- and drug-induced stress-related signaling pathways is a promising approach that would enhance our understanding of this type of iDILI.
5. Conclusion
In conclusion, we developed a promising in vitro test system, based on the use of simple and convenient mono- and co-culture models of immortalized liver and immune cell lines for the detection of drugs that have the potential to induce inflammation-associated iDILI. With our different exposure scenarios, which mimic distinct in vivo conditions like a drug exposure in an inflammatory environment during infection or a ‘sterile’ inflammation, it was possible to detect the selected reference iDILI drugs in a minimum of two exposure scenarios. Furthermore, our results support the inflammatory stress hypothesis and point to an involvement of pro-inflammatory factors, especially the pro-inflammatory cytokine TNF, in the development of iDILI.
Funding
This work was exclusively performed and funded by the Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e. V.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2017.02.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Ostapowicz G., Fontana R.J., Schiødt F.V., Larson A., Davern T.J., Han S.H., McCashland T.M., Shakil A.O., Hay J.E., Hynan L., Crippin J.S., Blei A.T., Samuel G., Reisch J., Lee W.M., U.S. Acute Liver Failure Study Group Results of a prospective study of acute liver failure at 17 tertiary carecenters in the United States. Ann. Intern. Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Björnsson E., Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig. Liver Dis. 2006;38(1):33–38. doi: 10.1016/j.dld.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle J.H., Carithers R.L., Jr., Shapiro C., Nascher H. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 4.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov. 2005;4(6):489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 5.Kaplowitz N. Rules and laws of drug hepatotoxicity. Pharmacoepidemiol. Drug Saf. 2006;15(4):231–233. doi: 10.1002/pds.1212. [DOI] [PubMed] [Google Scholar]
- 6.Hepatotoxicity Working Group . FDA/CDER und CBER; 2007. Premarketing Evaluation of Drug-Induced Liver Injury - Concept Paper. [Draft]http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/ucm073046. pdf (Accessed 18 July 2016) [Google Scholar]
- 7.Lasser K.E., Allen P.D., Woolhandler S.J., Himmelstein D.U., Wolfe S.M., Bor D.H. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287(17):2215–2220. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- 8.Li A.P. A review of the common properties of drugs with idiosyncratic hepatotoxicity and the multiple determinant hypothesis for the manifestation of idiosyncratic drug toxicity. Chem. Biol. Interact. 2002;142(1-2):7–23. doi: 10.1016/s0009-2797(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 9.Björnsson E.S. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin. Liver Dis. 2014;34(2):115–122. doi: 10.1055/s-0034-1375953. [DOI] [PubMed] [Google Scholar]
- 10.Roth R.A., Ganey P.E. Intrinsic versus idiosyncratic drug-induced hepatotoxicity-two villains or one? J. Pharmacol. Exp. Ther. 2010;332(3):692–697. doi: 10.1124/jpet.109.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganey P.E., Luyendyk J.P., Maddox J.F., Roth R.A. Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem. Biol. Interact. 2004;150(1):35–51. doi: 10.1016/j.cbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Uetrecht J. Idiosyncratic drug reactions: past, present, and future. Chem. Res. Toxicol. 2008;21(1):84–92. doi: 10.1021/tx700186p. [DOI] [PubMed] [Google Scholar]
- 13.Roth R.A., Luyendyk J.P., Maddox J.F., Ganey P.E. Inflammation and drug idiosyncrasy-is there a connection? J. Pharmacol. Exp. Ther. 2003;307(1):1–8. doi: 10.1124/jpet.102.041624. [DOI] [PubMed] [Google Scholar]
- 14.Roberts R.A., Ganey P.E., Ju C., Kamendulis L.M., Rusyn I., Klaunig J.E. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol. Sci. 2007;96:2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- 15.Adams D.H., Ju C., Ramaiah S.K., Uetrecht J., Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol. Sci. 2010;115(2):307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng X., Luyendyk J.P., Ganey P.E., Roth R.A. Inflammatory stress and idiosyncratic hepatotoxicity: hints from animal models. Pharmacol. Rev. 2009;61(3):262–282. doi: 10.1124/pr.109.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganey P.E., Roth R.A. Concurrent inflammation as a determinant of susceptibility to toxicity from xenobiotic agents. Toxicology. 2001;169(3):195–208. doi: 10.1016/s0300-483x(01)00523-6. [DOI] [PubMed] [Google Scholar]
- 18.Shaw P.J., Hopfensperger M.J., Ganey P.E., Roth R.A. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol. Sci. 2007;100(1):259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- 19.Waring J.F., Liguori M.J., Luyendyk J.P., Maddox J.F., Ganey P.E., Stachlewitz R.F., North C., Blomme E.A., Roth R.A. Microarray analysis of lipopolysaccharide potentiation of trovafloxacin-induced liver injury in rats suggests a role for proinflammatory chemokines and neutrophils. J. Pharmacol. Exp. Ther. 2006;316(3):1080–1087. doi: 10.1124/jpet.105.096347. [DOI] [PubMed] [Google Scholar]
- 20.Ramm S., Mally A. Role of drug-independent stress factors in liver injury associated with diclofenac intake. Toxicology. 2013;312:83–96. doi: 10.1016/j.tox.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Liguori M.J., Ditewig A.C., Maddox J.F., Luyendyk J.P., Lehman-McKeeman L.D., Nelson D.M., Bhaskaran V.M., Waring J.F., Ganey P.E., Roth R.A., Blomme E.A. Comparison of TNFα to lipopolysaccharide as an inflammagen to characterize the idiosyncratic hepatotoxicity potential of drugs: trovafloxacin as an example. Int. J. Mol. Sci. 2010;11(11):4697–4714. doi: 10.3390/ijms11114697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosgrove B.D., King B.M., Hasan M.A., Alexopoulos L.G., Farazi P.A., Hendriks B.S., Griffith L.G., Sorger P.K., Tidor B., Xu J.J., Lauffenburger D.A. Synergistic drug-cytokine induction of hepatocellular death as an in vitro approach for the study of inflammation-associated idiosyncratic drug hepatotoxicity. Toxicol. Appl. Pharmacol. 2009;237(3):317–330. doi: 10.1016/j.taap.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fredriksson L., Wink S., Herpers B., Benedetti G., Hadi M., de Bont H., Groothuis G., Luijten M., Danen E., de Graauw M., Meerman J., van de Water B. Drug-induced endoplasmic reticulum and oxidative stress responses independently sensitize toward TNFα-mediated hepatotoxicity. Toxicol. Sci. 2014;140(1):144–159. doi: 10.1093/toxsci/kfu072. [DOI] [PubMed] [Google Scholar]
- 24.Beggs K.M., Fullerton A.M., Miyakawa K., Ganey P.E., Roth R.A. Molecular mechanisms of hepatocellular apoptosis induced by trovafloxacin-tumor necrosis factor-alpha interaction. Toxicol. Sci. 2014;137(1):91–101. doi: 10.1093/toxsci/kft226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edling Y., Sivertsson L.K., Butura A., Ingelman-Sundberg M., Ek M. Increased sensitivity for troglitazone-induced cytotoxicity using a human in vitro co-culture model. Toxicol. In Vitro. 2009;23(7):1387–1395. doi: 10.1016/j.tiv.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Bonzo J.A., Rose K., Freeman K., Deibert E., Amaral K.B., Ferguson S.S., Andersen M.E., Witek R.P., LeCluyse E.L. Differential effects of trovafloxacin on TNF-α and IL-6 profiles in a rat hepatocyte-Kupffer cell coculture system. Appl. In Vitro Toxicol. 2015;1(1):45–54. [Google Scholar]
- 27.Tukov F.F., Maddox J.F., Amacher D.E., Bobrowski W.F., Roth R.A., Ganey P.E. Modeling inflammation-drug interactions in vitro: a rat Kupffer cell-hepatocyte coculture system. Toxicol. In Vitro. 2006;20(8):1488–1499. doi: 10.1016/j.tiv.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W., Dorato M., Van Deun K., Smith P., Berger B., Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 29.Maier P., Milosevic N. Cocultures between primary parenchymal and nonparenchymal liver cells improve the reliability of results from in vitro toxicity testing. ALTEX. 1999;16:87–89. [PubMed] [Google Scholar]
- 30.Temple R.J. Drug-Induced Liver Toxicity. Silver Spring; MD: 2001. Hepatotoxicity through the years impact on the FDA.http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/ucm122149.pdf (Available at: Accessed 06 July 2016) [Google Scholar]
- 31.Goldkind L., Laine L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: lessons learned from the bromfenac experience. Pharmacoepidemiol. Drug Saf. 2006;15(4):213–220. doi: 10.1002/pds.1207. [DOI] [PubMed] [Google Scholar]
- 32.Pandit A., Sachdeva T., Bafna P. Drug-Induced hepatotoxicity: a review. J. Appl. Pharm. Sci. 2012;2(5):233–243. [Google Scholar]
- 33.Xu J.J., Henstock P.V., Dunn M.C., Smith A.R., Chabot J.R., de Graaf D. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol. Sci. 2008;105(1):97–105. doi: 10.1093/toxsci/kfn109. [DOI] [PubMed] [Google Scholar]
- 34.Chen M., Vijay V., Shi Q., Liu Z., Fang H., Tong W. FDA-approved drug labeling for the study of drug-induced liver injury. Drug Discov. Today. 2011;16(15-16):697–703. doi: 10.1016/j.drudis.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y.C., Colaizzi J.L., Bierman R.H., Woestenborghs R., Heykants J. Pharmacokinetics and dose proportionality of ketoconazole in normal volunteers. Antimicrob. Agents Chemother. 1986;30(2):206–210. doi: 10.1128/aac.30.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol. Drug Saf. 2006;15(4):241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int. J. Cancer. 1980;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 38.Aldo P.B., Craveiro V., Guller S., Mor G. Effect of culture conditions on the phenotype of THP-1 monocyte cell line. Am. J. Reprod. Immunol. 2013;70(1):80–86. doi: 10.1111/aji.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakabayashi H., Taketa K., Miyano K., Yamane T. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. J. Cancer Res. 1982;42(9):3858–3863. [PubMed] [Google Scholar]
- 40.Daigneault M., Preston J.A., Marriott H.M., Whyte M.K., Dockrell D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5(1):e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters T. San Diego, CA; Academic Press: 1996. All About Albumin: Biochemistry, Genetics, and Medical Applications; pp. 251–284. [Google Scholar]
- 42.Jones E.A., Summerfield J.A. second edition. Raven Press, Ltd.; New York: 1988. The Liver: Biology and Pathobiology. Kupffer Cells, Chapter 37. [Google Scholar]
- 43.Lewis C.E., McGee J.O.’D. Oxford University Press; New York: 1992. The Natural Immune System: The Macrophage; pp. 3–74. [Google Scholar]
- 44.Opal S.M., Scannon P.J., Vincent J.L., White M., Carroll S.F., Palardy J.E., Parejo N.A., Pribble J.P., Lemke J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septicshock. J. Infect. Dis. 1999;180(5):1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 45.Taudorf S., Krabbe K.S., Berg R.M., Pedersen B.K., Møller K. Human models of low-grade inflammation: bolus versus continuous infusion of endotoxin. Clin. Vaccine Immunol. 2007;14:250–255. doi: 10.1128/CVI.00380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw P.J., Ganey P.E., Roth R.A. Idiosyncratic drug-induced liver injury and the role of inflammatory stress with an emphasis on an animal model of trovafloxacin hepatotoxicity. Toxicol. Sci. 2010;118(1):7–18. doi: 10.1093/toxsci/kfq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae M.A., Song B.J. Critical role of c-Jun N-terminal protein kinase activation in troglitazone-induced apoptosis of human HepG2 hepatoma cells. Mol. Pharmacol. 2003;63(2):401–408. doi: 10.1124/mol.63.2.401. [DOI] [PubMed] [Google Scholar]
- 48.Herpers B., Wink S., Fredriksson L., Di Z., Hendriks G., Vrieling H., de Bont H., van de Water B. Activation of the Nrf2 response by intrinsic hepatotoxic drugs correlates with suppression of NF-κB activation and sensitizes toward TNFα-induced cytotoxicity. Arch. Toxicol. 2016;90(5):1163–1179. doi: 10.1007/s00204-015-1536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerets H.H., Tilmant K., Gerin B., Chanteux H., Depelchin B.O., Dhalluin S., Atienzar F.A. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol. Toxicol. 2012;28(2):69–87. doi: 10.1007/s10565-011-9208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godoy P., Hewitt N.J., Albrecht U., Andersen M.E., Ansari N., Bhattacharya S., Bode J.G. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013;87(8):1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G.Y., Nunez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spachidou M.P., Bourazopoulou E., Maratheftis C.I., Kapsogeorgou E.K., Moutsopoulos H.M., Tzioufas A.G., Manoussakis M.N. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjögren's syndrome. Clin. Exp. Immunol. 2007;147(3):497–503. doi: 10.1111/j.1365-2249.2006.03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon L.J., Barnes M., Tang H., Pritchard M.T., Nagy L.E. Kupffer cells in the liver. Compr. Physiol. 2013;3(2):785–797. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinakar C., Malur A., Raychaudhuri B., Buhrow L.T., Melton A.L., Kavuru M.S., Thomassen M.J. Differential regulation of human blood monocyte and alveolar macrophage inflammatory cytokine production by nitric oxide. Ann. Allergy. Asthma. Immunol. 1999;82(2):217–222. doi: 10.1016/S1081-1206(10)62600-2. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann H.W., Trautwein C., Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front. Physiol. 2012;3:56. doi: 10.3389/fphys.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lloyd S., Hayden M.J., Sakai Y., Fackett A., Silber P.M., Hewitt N.J., Li A.P. Differential in vitro hepatotoxicity of troglitazone and rosiglitazone among cryopreserved human hepatocytes from 37 donors. Chem. Biol. Interact. 2002;142(1-2):57–71. doi: 10.1016/s0009-2797(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 57.Maniratanachote R., Minami K., Katoh M., Nakajima M., Yokoi T. Chaperone proteins involved in troglitazone-induced toxicity in human hepatoma cell lines. Toxicol. Sci. 2005;83(2):293–302. doi: 10.1093/toxsci/kfi022. [DOI] [PubMed] [Google Scholar]
- 58.Gunness P., Mueller D., Shevchenko V., Heinzle E., Ingelman-Sundberg M., Noor F. 3D organotypic cultures of human HepaRG cells: a tool for in vitro toxicity studies. Toxicol. Sci. 2013;133(1):67–78. doi: 10.1093/toxsci/kft021. [DOI] [PubMed] [Google Scholar]
- 59.Liguori M.J., Blomme E.A., Waring J.F. Trovafloxacin-induced gene expression changes in liver-derived in vitro systems: comparison of primary human hepatocytes to HepG2 cells. Drug Metab. Dispos. 2008;36(2):223–233. doi: 10.1124/dmd.107.017608. [DOI] [PubMed] [Google Scholar]
- 60.Hadi M., Westra I.M., Starokozhko V., Dragovic S., Merema M.T., Groothuis G.M. Human precision-cut liver slices as an ex vivo model to study idiosyncratic drug-induced liver injury. Chem. Res. Toxicol. 2013;26(5):710–720. doi: 10.1021/tx300519p. [DOI] [PubMed] [Google Scholar]
- 61.Gómez-Lechón M.J., Ponsoda X., O’Connor E., Donato T., Jover R., Castell J.V. Diclofenac induces apoptosis in hepatocytes. Toxicol. In Vitro. 2003;17(5-6):675–680. doi: 10.1016/s0887-2333(03)00105-x. [DOI] [PubMed] [Google Scholar]
- 62.Wang K., Shindoh H., Inoue T., Horii I. Advantages of in vitro cytotoxicity testing by using primary rat hepatocytes in comparison with established cell lines. J. Toxicol. Sci. 2002;27(3):229–237. doi: 10.2131/jts.27.229. [DOI] [PubMed] [Google Scholar]
- 63.Chen C.J., Kono H., Golenbock D., Reed G., Akira S., Rock K.L. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13(7):851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 64.Imaeda A.B., Watanabe A., Sohail M.A., Mahmood S., Mohamadnejad M., Sutterwala F.S., Flavell R.A., Mehal W.Z. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 2009;119(2):305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kono H., Rock K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8(4):279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antoine D.J., Jenkins R.E., Dear J.W., Williams D.P., McGill M.R., Sharpe M.R., Craig D.G., Simpson K.J., Jaeschke H., Park B.K. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J. Hepatol. 2012;56(5):1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Kegel V., Pfeiffer E., Burkhardt B., Liu J.L., Zeilinger K., Nüssler A.K., Seehofer D., Damm G. Subtoxic concentrations of hepatotoxic drugs lead to Kupffer cell activation in a human In vitro liver model: an approach to study DILI. Mediators Inflamm. 2015;2015:0–14. doi: 10.1155/2015/640631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calabrese E.J., Blain R. The occurrence of hormetic dose responses in the toxicological literature: the hormesis database: an overview. Toxicol. Appl. Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 69.Mattson M.P. Hormesis defined. Ageing Res. Rev. 2008;7(1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ju C., Reilly T.P., Bourdi M., Radonovich M.F., Brady J.N., George J.W., Pohl L.R. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem. Res. Toxicol. 2002;15(12):1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 71.Lammert C., Einarsson S., Saha C., Niklasson A., Bjornsson E., Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47(6):2003–2009. doi: 10.1002/hep.22272. [DOI] [PubMed] [Google Scholar]
- 72.Shah F., Leung L., Barton H.A., Will Y., Rodrigues A.D., Greene N., Aleo M.D. Setting clinical exposure levels of concern for Drug-Induced Liver Injury (DILI) using mechanistic in vitro assays. Toxicol. Sci. 2015;147(2):500–514. doi: 10.1093/toxsci/kfv152. [DOI] [PubMed] [Google Scholar]
- 73.Wei X.Q., Guo Y.W., Liu J.J., Wen Z.F., Yang S.J., Yao J.L. The significance of Toll-like receptor 4 (TLR4) expression in patients with chronic hepatitis B. Clin. Invest. Med. 2008;31(3):E123–30. doi: 10.25011/cim.v31i3.3469. [DOI] [PubMed] [Google Scholar]
- 74.Leist M., Gantner F., Bohlinger I., Germann P.G., Tiegs G., Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J. Immunol. 1994;153(4):1778–1788. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.