Much attention has been focused on the barrier to T cell activation posed by the forest of bulky glycoproteins forming the “glycocalyx” of the cell surface, but there are even deeper potential barriers to signaling on the other side of the membrane that have only recently started coming into focus 1. The cell cortex comprises a cross-linked network of filamentous (F)-actin, myosin, and other proteins lying directly underneath the plasma membrane, forming a barrier typically 100nm thick (Fig. 1A) 2. The cortex offers resistance to externally applied forces and has a key role in cell-shape change. In this issue of Nature Immunology, Weiss and colleagues test a new tool for kick-starting T-cell activation and discover a new F-actin dependent barrier to T cell receptor (TCR) signaling in thymocytes 3. The barrier is breached at least in part by CD28 engagement and thus has the potential to be important for the generation of CD28-dependent T cell lineages such as FoxP3+ regulatory T-cells 4.
Figure.
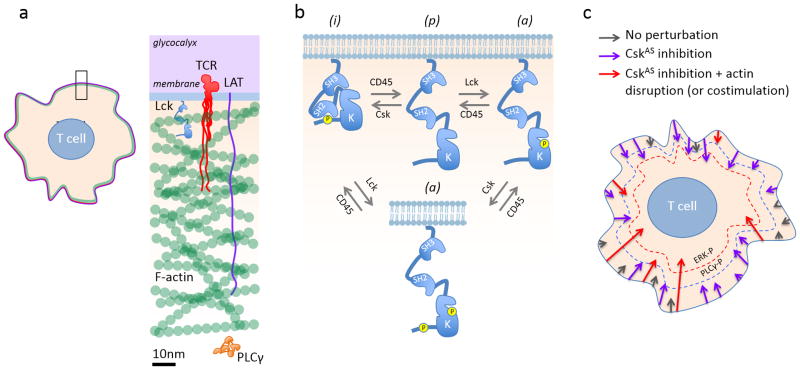
Effects of Csk inhibition and actin disruption on TCR signaling. (a) The actin cortex forms a physical barrier between the membrane and the rest of the cytoplasm. Only the F-actin part of the cortex is shown. The proteins are drawn to scale; the TCR/CD3 and LAT cytoplasmic domains, even at the theoretical length maximum (~3.5Å/residue), are occluded by the actin cortex. (b) Role of Csk in Lck regulation. The active (a), inactive (i) and ‘primed’ (p) isoforms of Lck interconvert via Csk- and Lck auto-phosphorylation, and via CD45-mediated dephosphorylation (re-drawn from ref 4). (c) Signaling thresholds and the effects of global receptor perturbation. Signaling cascades initiated by Lck-mediated phosphorylation of individual TCRs in the resting state are represented by arrows of varying length: basal signaling, grey; CskAS inhibition-initiated signaling (purple); CskAS inhibition- and actin disruption-initiated signaling (or costimulation, red). Arrow length corresponds to the depth of the signaling cascade reached. For a given condition the lengths of the arrows vary, reflecting stochastic effects. The blue and red dashed lines symbolize signaling thresholds corresponding to PLCγ (PLCγ-P) and ERK (ERK-P) phosphorylation. In the unperturbed cell, the signaling cascades reach neither threshold. CskAS inhibition is likely to globally enhance Lck activity and TCR phosphorylation, increasing the number of signaling cascades and the probability that one or more cascades breaches the threshold required for PLCγ phosphorylation. ERK phosphorylation also requires actin disruption, enhancing PLCγ access to substrates in the membrane.
Src family kinases such as Lck undergo allosteric regulation driven by tyrosine phosphorylation (Fig. 1B). SFK all share a unique N-terminal domain that is involved in membrane interactions, an SH3 domain, an SH2 domain, a linker with a polyproline motif, a kinase domain (SH1) and a C-terminal extension containing a phosphorylatable tyrosine. The tertiary structure of these kinases allows for two inhibitory intramolecular interactions: the SH3 domain with the polyproline motif and the SH2 domain with the phosphorylated C-terminal tyrosine. The aptly named, cytosolic C-terminal Src kinase (Csk) phosphorylates this C-terminal tyrosine, encouraging intramolecular SH2 domain binding and kinase inhibition. The phosphate is removed by the transmembrane tyrosine kinase CD45 in the case of Lck, favouring activation. Unlike the Src-family kinases, Csk lacks a membrane-recruitment domain and thus needs to bind through its SH2 domain to membrane-anchored adapters such as Csk binding protein (CBP).
Weiss and colleagues asked what would happen if Csk could be rapidly turned off. Earlier studies had used overexpression of dominant negative forms of Csk 5, but approaches involving stable gene expression are subject to compensation and other caveats. To acutely and specifically inhibit Csk, Weiss et al. teamed up with the Shokat laboratory to generate a bulky ATP analog-sensitive mutant of Csk (CskAS)6. In work published previously, when a membrane-anchored form of CskAS was expressed in the Jurkat T-cell line, inhibition with the bulky analogue resulted in the full activation of TCR signaling including ERK phosphorylation and cytoplasmic Ca2+ elevation 6. Now, Tan et al have made a Bac transgenic mouse so that the impact of acute Csk inhibition in primary cells could be gauged. When Csk is acutely inhibited in primary thymocytes, the early TCR activation cascade is triggered, including activating phosphorylation of Lck, ZAP-70, LAT and PLC-γ. These results indicate that the TCR proximal elements of the tyrosine kinase cascade worked as expected, with the acute inhibition of Csk pushing the equilibrium toward greater activation of Lck, driving the assembly of the classical proximal signalosome without TCR engagement. Unexpectedly, however, whereas Csk inhibition generated robust TCR proximal signals, no ERK activation or cytoplasmic Ca2+ elevation ensued, identifying a hitherto unsuspected signaling checkpoint comprising the cleavage of phosphatidylinositol-4,5-bisphosphate by phospholipase C-γ (PLC-γ) 3. The checkpoint could however be overcome by disrupting F-actin with cytochalasin D or by engaging CD28 in thymocytes with dendritic cells or beads coated with CD86, which resulted in dramatic F-actin recruitment to the synapse. Mature T cells had a more complex response to F-actin manipulation following Csk inhibition so the existing view that F-actin has a positive role in mature T cells still holds 7.
Little is known about the actin cortex of lymphocytes. At the typical depth of 100nm (and a mesh-size of 15–30nm), the cortex of T-cells would be much deeper than the maximum theoretical reach of the TCR or the PLCγ adapter, linker of activated T cells (LAT; Fig. 1a). This is why processes like secretion need to generate holes in the actin cortex to allow vesicles to reach the plasma membrane 8. Cortical actin is argued to be a diffusion barrier in BCR signaling and the work of Tan et al now shows that the cortical F-actin acts as a barrier apparently constraining the access of otherwise active PLC-γ to phosphoinositide lipids. Exactly how the barrier function is achieved is unclear since there is at least some access of PLC-γ to the signalosome because it is phosphorylated following Csk inhibition without actin disruption. Bringing CD28 into the equation as a remodeler of actin nevertheless has interesting functional implications as a phosphorylated tyrosine in the cytoplasmic domain of CD28 may also directly modulate Lck activity through binding to Lck’s SH2 domain in competition with the terminal tyrosine phosphorylated by Csk 9. During thymic differentiation of Treg, therefore, CD28 may both overcome the actin barrier and contribute to Lck activation mechanisms that are critical for additional down-stream signaling.
It is necessary, however, to bring just one note of caution to the interpretation of the new data. This is because, whereas the types of perturbation imposed by Tan et al. i.e. global actin disruption and acute Csk inhibition will both impact on all the TCRs present simultaneously, the T-cell is capable of responding in a physiological setting to just a few TCR ligands10, indicating that individual TCRs acting locally are capable of strong signalling. Perturbations that are imposed on very large numbers of TCRs simultaneously, the individual effects of which might otherwise be relatively weak, will tend to increase the overall probability of signalling, invoking responses that might be comparable in strength to those primed by “strong” ligands acting through just a few TCRs, partly due to stochastic effects (Fig. 1c) 11. Among the challenges remaining it will be necessary to show that local actin re-modelling, perhaps mediated by costimulation, contributes significantly to local signaling responses to conventional ligands, versus other local processes.
The classical feedback model for Csk is that active SFKs phosphorylate sites on adapter proteins at the membrane that then recruit Csk, which in turn inhibits the active SFK by phosphorylating its C-terminal tyrosine 12. It has, however, been shown that the Csk docking site in CBP is transiently dephosphorylated during TCR triggering 5, 13, 14. Nevertheless, the role of CBP in control of Csk localization appears to be somewhat redundant, as CBP-deficient mice have no T-cell phenotype 15. While Tan et al clearly show that inhibiting Csk is a potent means to trigger the TCR, as the authors argue elsewhere it is difficult to envisage how Csk inhibition might be co-opted in vivo, in a way that initiates or promotes de novo signaling 14. Afterall, Tan et al only observe a relatively slow decay in Lck terminal tyrosine phosphorylation following Csk inhibition. On the other hand, as the authors also note, given the scale of the effects of Csk blockade in the absence of TCR engagement, it is difficult to envisage how conformational rearrangements, e.g. the dissociation of CD3 ITAMs from the membrane 16, could be considered necessary elements of the TCR triggering mechanism. In addition, ligand dependent mechanical distortion17 is also largely out of the question. Mechanical feedback on T cell responses may instead by integrated through changes in the f-actin cortex.
The elegant experiments of Tan et al have two important take home messages. First, along with their previous studies of CskAS, the new data show that the TCR signalosome can be activated in primary thymocytes (and Jurkat cell lines) without receptor engagement simply by acutely increasing SFK activation, vividly illustrating the sensitivity of the TCR to changes in the balance of kinase and phosphatase activities. Second, cortical actin can form a functional barrier between active PLCγ and its substrate. It’s not so much the quantity of F-actin that matters, perhaps, but also its organization, as CD28 engagement increased F-actin accumulation at the site of engagement, whilst still overcoming the barrier to full signaling breached also by F-actin disruption. The work of Tan et al thus significantly expands the possibilities for how cortical F-actin could regulate signaling in T-lineage cells.
References
- 1.Treanor B, et al. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity. 2010;32:187–199. doi: 10.1016/j.immuni.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts B, et al. Molecular Biology of the Cell. 5. Garland Science; 2007. [Google Scholar]
- 3.Tan YX, et al. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol. 2013 doi: 10.1038/ni.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, et al. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torgersen KM, et al. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J Biol Chem. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 6.Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A. Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Sci Signal. 2011;4:ra59. doi: 10.1126/scisignal.2001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AC, et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol. 2011;9:e1001152. doi: 10.1371/journal.pbio.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong KF, et al. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011;12:1105–1112. doi: 10.1038/ni.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 11.Aoki K, et al. Stochastic ERK Activation Induced by Noise and Cell-to-Cell Propagation Regulates Cell Density-Dependent Proliferation. Mol Cell. 2013;52:529–540. doi: 10.1016/j.molcel.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Kawabuchi M, et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 13.Brdicka T, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol Cell Biol. 2003;23:2017–2028. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobenecker MW, Schmedt C, Okada M, Tarakhovsky A. The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Mol Cell Biol. 2005;25:10533–10542. doi: 10.1128/MCB.25.23.10533-10542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Z, Janmey PA, Finkel TH. The receptor deformation model of TCR triggering. FASEB J. 2008;22:1002–1008. doi: 10.1096/fj.07-9331hyp. [DOI] [PMC free article] [PubMed] [Google Scholar]