Graphical abstract
Keywords: Resistance spot welding, Aerosol generation, Inhalation, Zinc, Methyl methacrylate
Highlights
-
•
An automated welder was designed to expose animals to galvanized spot welding fume.
-
•
Generated particles generally were in the submicron and ultrafine size ranges.
-
•
The primary metals present in galvanized spot welding fumes were iron and zinc.
-
•
Zinc appeared to be the causative agent in the development of acute lung inflammation.
Abstract
Resistance spot welding is a common process to join metals in the automotive industry. Adhesives are often used as sealers to seams of metals that are joined. Anti-spatter compounds sometimes are sprayed onto metals to be welded to improve the weldability. Spot welding produces complex aerosols composed of metal and volatile compounds (VOCs) which can cause lung disease in workers. Male Sprague-Dawley rats (n = 12/treatment group) were exposed by inhalation to 25 mg/m3 of aerosol for 4 h/day × 8 days during spot welding of galvanized zinc (Zn)-coated steel in the presence or absence of a glue or anti-spatter spray. Controls were exposed to filtered air. Particle size distribution and chemical composition of the generated aerosol were determined. At 1 and 7 days after exposure, bronchoalveolar lavage (BAL) was performed to assess lung toxicity. The generated particles mostly were in the submicron size range with a significant number of nanometer-sized particles formed. The primary metals present in the fumes were Fe (72.5%) and Zn (26.3%). The addition of the anti-spatter spray and glue did affect particle size distribution when spot welding galvanized steel, whereas they had no effect on metal composition. Multiple VOCs (e.g., methyl methacrylate, acetaldehyde, ethanol, acetone, benzene, xylene) were identified when spot welding using either the glue or the anti-spatter spray that were not present when welding alone. Markers of lung injury (BAL lactate dehydrogenase) and inflammation (total BAL cells/neutrophils and cytokines/chemokines) were significantly elevated compared to controls 1 day after exposure to the spot welding fumes. The elevated pulmonary response was transient as lung toxicity mostly returned to control values by 7 days. The VOCs or the concentrations that they were generated during the animal exposures had no measurable effect on the pulmonary responses. Inhalation of galvanized spot welding fumes caused acute lung toxicity most likely due to the short-term exposure of particles that contain Zn.
1. Introduction
Resistance spot welding joins metals at surfaces that are made to fit together for the purpose of making a joint (Stout, 1987). During the process, two electrodes are used to clamp metal sheets together and to pass high levels of current, but at low voltage, through the metal pieces. Extremely high temperatures are generated due to the high electrical resistance where the metal surfaces contact each other. When the current is stopped, the electrode tips cool the spot weld and cause the metal to solidify under pressure. Spot welding is used for light gauge metal parts. A spot weld has the mechanical characteristics like those of a rivet, but with a strength and soundness much greater. Resistance spot welding is most commonly used in the automotive and aircraft industries where relatively thin metal sections are welded and high-speed, repetitive welds are needed (Stout, 1987). In some cases, different epoxy adhesives are applied as sealers to the seams and joints of the spot metal pieces that are joined. Also, the sheet metal pieces that are to be welded are sometimes treated with anti-spatter chemicals before welding to improve weldability.
Because of the process and the different chemicals used, resistance spot welding can produce complex aerosols that are composed of metal oxide particles and volatile organic compounds (VOCs). Air sampling of different automotive assembly plants indicated that resistance spot welding is a major particle emitting source and can produce among the highest concentrations of particles (>1 mg/m3) compared to other processes in these facilities (Buonanno et al., 2011, Liu and Hammond, 2010). During a NIOSH Health Hazard Evaluation performed at an automotive assembly plant (Kanwal and Boylstein, 2006), numerous body shop chemicals (e.g., methyl methacrylate, formaldehyde, acetic acid, styrene), some of which are known to cause respiratory irritation, asthma, and bronchitis, were detected in the air of the plant. Also, several workers in the body shop were determined to have developed some type of respiratory illness. Furthermore, other investigations have demonstrated that workers who been exposed to aerosols generated during resistance spot welding using glues in automotive assembly and manufacturing plants can develop respiratory symptoms and disease (Loukzadeh et al., 2009, Luo et al., 2006, Hammond et al., 2005, Lee et al., 1990). However, little is known regarding the composition of aerosols generated during resistance spot welding of metals treated with glues and other chemicals and their potential effects on health.
Previously, we have developed a resistance spot welding fume generator and inhalation system by which different target aerosol concentrations can be maintained within an exposure chamber over an extended period of time (Afshari et al., 2014). The initial study examined the pulmonary effects of mild steel spot welding fume exposure with or without a glue in rats (Zeidler-Erdely et al., 2014). Mild steel spot welding particles were composed of almost entirely of iron (Fe). Acute inhalation exposure to this Fe-rich spot welding fume at a relatively high concentration produced only mild, transient lung injury and no inflammation. The primary objective of the current study was to assess the pulmonary effects as well as the physical and chemical characterization of the aerosols generated in the breathing zone of exposed animals during spot welding performed on strips of zinc (Zn)-containing galvanized steel sheet metal in the presence or absence of either a glue or an anti-spatter spray. Inhalation exposure to Zn-rich particles have been shown to cause both acute lung injury and inflammation (Gordon et al., 1992). To date, no studies, as far as we can determine, have been performed that have exclusively examined the pulmonary toxicity of Zn-containing galvanized steel spot welding aerosols and compared the potential lung effects to other common spot welding fumes, such as Fe-rich mild steel fume.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats from Hilltop Lab Animals (Scottdale, PA, USA), weighing 250–300 g and free of viral pathogens, parasites, mycoplasmas, Helicobacter, and CAR Bacillus, were used. The rats were acclimated for one week after arrival and were provided HEPA-filtered air, irradiated Teklad 2918 diet, and tap water ad libitum. All animal procedures used during the study were reviewed and approved by the National Institute for Occupational Safety and Health (NIOSH) Animal Care and Use Committee. The animal facilities are specific pathogen-free, environmentally controlled, and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.2. Galvanized steel resistance spot welding animal exposure
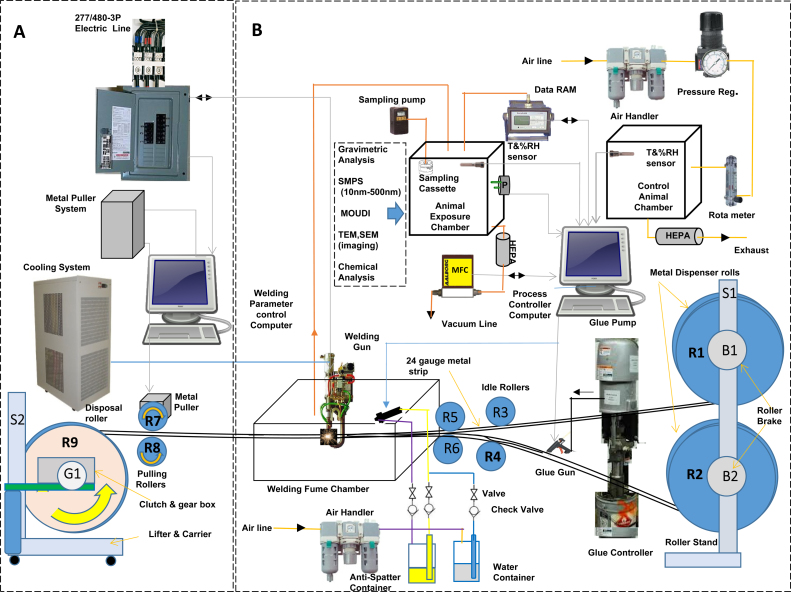
Animals (n = 12/treatment group) were housed individually and exposed for 4 h/day for 8 days by whole-body inhalation using a resistance spot welder and inhalation exposure system (Fig. 1) as previously described (Afshari et al., 2014). In brief, two rolls that contained strips of galvanized steel sheet metal were directed by a pair of rollers to copper-tipped electrodes of the welder, and spot welded at a determined distance of 20 mm between each spot weld by an automated, computer-controlled resistance spot welding gun (Small new modified “C” style Trans-gun 136 kva-AC; Milco Manufacturing Company, Warren, MI, USA). The spot welder was set at 7.5–10 kA with a welding time of 140 ms, a post-weld holding time of 50 ms, and a clamping force of 2.6 kN (600 lbs). The time between welds and amount of dilution air entering the exposure chamber was gradually increased or decreased automatically through software to maintain a constant exposure chamber aerosol target concentration of 25 mg/m3. In some cases, a glue (Fusor 108B/109B Metal Bonding Adhesive; LORD Corp., Cary, NC, USA) or an anti-spatter agent (Parco AWS-100; Henkel Surface Corporation, Madison, Heights, MI, USA), substances commonly used in spot welding, were applied to the two strips of metal before spot welding.
Fig. 1.
Diagram of the resistance spot welding fume generation and exposure system (adapted from Afshari et al., 2014). The system is divided into separated areas: (A) enclosed control room with the welding computer controller, the sheet metal puller system controller, electric power supply, and the spot welding gun cooling system; (B) resistance spot welding gun, air-tight welding fume chamber, sheet metal pulling system (sheet metal rolls and assorted rollers), anti-spatter spray system, glue pump and gun dispenser system, and animal exposure chambers for control and spot welding fume-exposed animals with assorted aerosol characterization devices. Abbreviations: DataRam = real-time aerosol monitor; MOUDI = Micro-Orifice Uniform Deposit Impactor; SEM = scanning electron microscopy; TEM = transmission electron microscopy; T = temperature; RH = relative humidity; SMPS = scanning mobility particle sizer; MFC = mass flow controller.
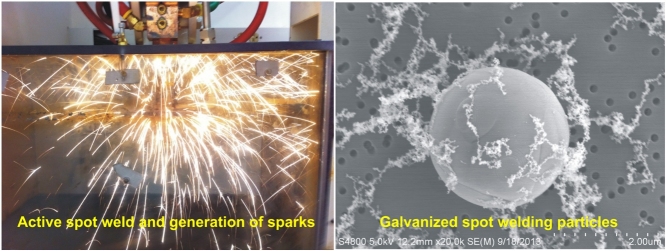
When a spot weld is made during resistance spot welding, sparks or the expulsion of molten metal are often generated (Fig. 2). After completion of the spot weld, the sparks subside and the resulting aerosol, a combination of metal fume derived from the welding of the strips of metal and VOCs from the heating of the adhesive applied between the metal strips, fills the sealed welding fume chamber. The aerosol that is generated is transported via tubing from the spot welding fume chamber to an animal exposure chamber. The temperature and relative humidity (Vaisala Temperature-Humidity Probe, model# HMP233; Woburn, MA, USA) inside the exposure chamber were measured and continuously recorded. The target concentration of 25 mg/m3 represents a dose between 15 and 40 mg/m3 of well-characterized metal inert gas welding fume inhalation concentrations used previously (Antonini et al., 2007, Antonini et al., 2009) and coincides with studies that examined the pulmonary and neurotoxicity of mild steel spot welding fumes (Zeidler-Erdely et al., 2014, Sriram et al., 2014). Sham controls were exposed to filtered air.
Fig. 2.
Images of an active spot weld and generation of sparks.
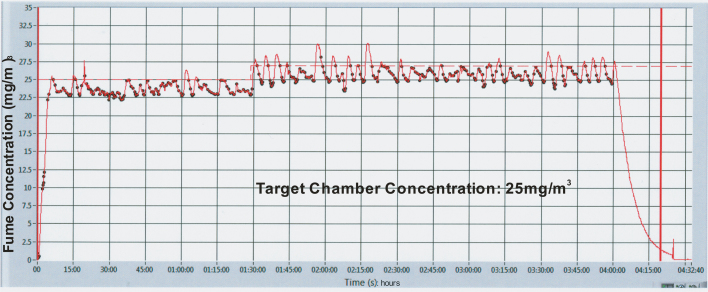
To maintain a consistent fume concentration in the exposure chamber, fume was collected through an aerosol delivery line above the spot welding system at a flow rate of 25 l/min by maintaining a slight negative pressure within the exposure chamber (Afshari et al., 2014). The aerosol delivery line mated with dilution air and was allowed to mix before entering the exposure chamber. Dilution airflow rate was adjusted by a 0–20 l/min mass flow controller. The exposure system control software would automatically make adjustments to the dilution air and the time between welds to provide a desired mass concentration (25 mg/m3) in the exposure chamber. The mass concentration in the chamber was monitored in real time by a real time aerosol monitor (DataRAM, MIE, Inc. DR-2000, Bedford, MA, USA). By controlling the number of spot welds performed over time, a target aerosol concentration could be maintained within the animal exposure chamber during the 4-h exposure period (Fig. 3). The dips on the DataRAM readings indicate the time at which a spot weld is initiated and a spark is formed. The peaks on the DataRAM readings indicate the rise in aerosol concentration after the completion of a spot weld and the disappearance of the spark. See Table 1 for actual aerosol exposure concentrations during animal exposures. Shams were housed in an air-tight animal chamber that was located in close proximity to the spot welding exposure chamber and received conditioned, filtered air. The control chamber had the same dimensions and was made from identical materials as the spot welding fume animal exposure chamber. The supplied air originated from a water-seal compressor, in-house air-line and was conditioned through a dryer, charcoal filter, and HEPA filter.
Fig. 3.
Representative exposure chamber aerosol concentration (mg/m3) recording during a 4 h welding exposure as determined by a DataRAM. The figure indicates that by controlling the number of spot welds performed over time a target aerosol concentration (25 mg/m3) could be maintained at fairly constant levels during the 4-h exposure period.
Table 1.
Actual galvanized steel spot welding exposure chamber concentrations.
| Exposure Condition | Fume Concentration (mg/m3)a |
|---|---|
| Galvanized steel, no glue | 24.6 ± 2.6 |
| Galvanized steel, plus glue | 23.8 ± 2.8 |
| Galvanized steel, plus anti-spatter spray | 22.9 ± 4.0 |
Mean daily fume concentrations in the exposure chamber ± standard error (n = 8). Measurements were made in duplicate every 30 min during the daily 4-h exposure for each day. Target fume concentration was 25 mg/m3. Inhalation exposures were for 4 h/day for 8 days.
2.3. Galvanized steel resistance spot welding aerosol characterization
All generated aerosols were collected in the breathing zone of the animals in the exposure chamber during the daily 4-h exposure. Particle morphology, size distribution, and metal profile did not change during the 4-h exposure.
2.3.1. Particle morphology
Spot welding fume was collected onto 47-mm Nuclepore polycarbonate filters (Whatman, Clinton, PA, USA). The filters were mounted onto aluminum stubs with silver paste. The collected welding particles were viewed using a Hitachi S4800 field emission scanning electron microscope (Bruker, Madison, WI, USA).
2.3.2. Particle size distribution
The size distribution of the aerosols inside the exposure chamber was determined using a Micro-Orifice Uniform Deposit Impactor (MOUDI, MSP Model 110, MSP Corporation, Shoreview, MN, USA) that is intended for general purpose aerosol sampling, and a Nano-MOUDI (MSP Model 115). Using the two MOUDI impactors in tandem, particles were collected between the size ranges of 0.010–18 μm that were separated into 15 fractions. The 50% cutoff particle diameter for each stage is as follows: stage 1 (inlet): 18 μm; stage 2: 10 μm; stage 3: 5.6 μm; stage 4: 3.2 μm; stage 5: 1.8 μm; stage 6: 1.0 μm; stage 7: 0.56 μm; stage 8: 0.32 μm; stage 9: 0.18 μm; stage 10: 0.10 μm; stage 11: 0.056 μm; stage 12: 0.032 μm; stage 13: 0.018 μm; stage 14: 0.010 μm; stage 15 (back-up filter). The mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) of the spot welding fume were determined from gravimetric measurements.
2.3.3. Particle metal analysis
Spot welding aerosols were collected inside the exposure chamber onto 5 μm polyvinyl chloride membrane filters in 37-mm cassettes during welding. The particle samples were digested and the metals analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) by Bureau Veritas North America, Inc. (Novi, MI, USA), according to NIOSH method 7303 modified for hot block/HCl/HNO3 digestion (National Institute for Occupational Safety and Health, 1994). Metal content of blank filters also were analyzed for control purposes.
2.3.4. Volatile organic compounds (VOCs) identification
Evacuated canisters (6L) were equipped with stainless steel particulate filters and used to sample VOCs during the spot welding process, by LeBouf et al. (2012). The air samples were analyzed using a pre-concentrator/gas chromatograph/mass spectrometer system with the following modifications: the pre-concentrator was an Entech 7200; six additional analytes (acetonitrile, acetaldehyde, styrene, 2,3-butanedione, 2,3-pentanedione, and 2,3-hexanedione) were included; and qualitatively identified compounds were compared to NIST 2011 Mass Spectral Library and included in the report if the quality factor was greater than 75%. The canister method is only partially validated, but is in the process of being reviewed for incorporation into the NIOSH Manual of Analytical Methods.
2.4. In vivo toxicity after galvanized spot welding
2.4.1. Body temperature
Due to the presence of Zn in the fumes and the possible development of metal fume fever, animal body temperatures were measured before and after each exposure using a Bat-10 thermometer coupled to a RET-2 rat rectal probe (Physitemp Inc, Clifton, NJ).
2.4.2. Partial lung bronchoalveolar lavage (BAL)
Based on the set-up of the exposure system, three separate exposure experiments were performed as depicted in Fig. 7, Fig. 8, Fig. 9: (1) air vs. galvanized spot welding; (2) air vs. galvanized spot welding + glue; (3) air vs. galvanized spot welding + anti-spatter spray. For each experiment, n = 12 rats/treatment for a total of 24 rats. Because two time points (1 and 7 days post-exposure) were examined, n = 6 rats/treatment at each time point. At 1 and 7 days after galvanized spot welding aerosol exposure, rats (n = 6/treatment group at each time point) were deeply anesthetized with an intraperitoneal injection of sodium pentobarbital (>100 mg/kg body weight, IP; Fatal-Plus Solution, Vortech Pharmaceutical, Inc., Dearborn, MI, USA) and then exsanguinated by severing the abdominal aorta. The left bronchus was clamped and BAL was performed on the right lung lobes with Ca2+ and Mg2+-free phosphate buffered saline (PBS). The right lung was lavaged with 4 ml of PBS, kept separate on ice, followed by 4 washes (5 ml/wash). Both lavage fractions were then centrifuged (500 × g, 10 min, 4 °C) and the acellular supernatant of the first lavage used for evaluation of lung toxicity (below). Finally, the cell pellets of the first and subsequent washes were combined then suspended in 1 ml PBS for cell counts and differentials.
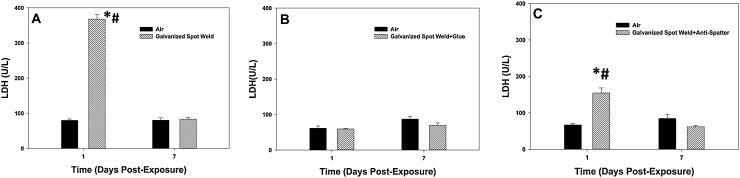
Fig. 7.
LDH activity in BAL after galvanized spot welding (A) with no glue, (B) with glue, and (C) with anti-spatter spray at 1 and 7 days after exposure to 25 mg/m3 for 4 h/day for 8 days. Controls were exposed to filtered air. Values are means ± standard error (n = 6 animals/treatment group); *significantly different from air control within a time point, p < 0.05; #significantly different from corresponding spot weld group at day 7, p < 0.05.
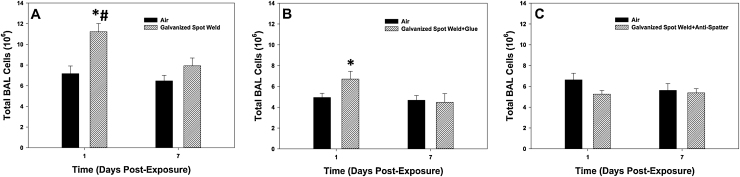
Fig. 8.
Total BAL cells recovered after galvanized spot welding (A) with no glue, (B) with glue, and (C) with anti-spatter spray at 1 and 7 days after exposure to 25 mg/m3 for 4 h/day for 8 days. Controls were exposed to filtered air. Values are means ± standard error (n = 6 animals/treatment group); *significantly different from air control within a time point, p < 0.05; #significantly different from corresponding spot weld group at day 7, p < 0.05.
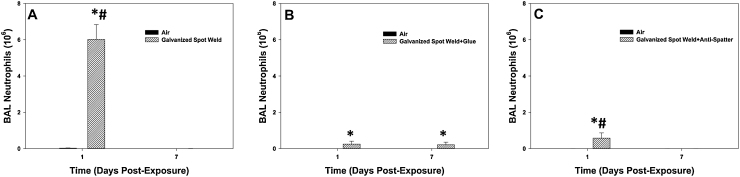
Fig. 9.
BAL neutrophils recovered after galvanized spot welding (A) with no glue, (B) with glue, and (C) with anti-spatter spray at 1 and 7 days after exposure to 25 mg/m3 for 4 h/day for 8 days. Controls were exposed to filtered air. Values are means ± standard error (n = 6 animals/treatment group); *significantly different from air control within a time point, p < 0.05; #significantly different from corresponding spot weld group at day 7, p < 0.05.
2.4.3. Assessment of lung inflammation and injury
Harvested BAL cells were counted using a Coulter Multisizer II and AccuComp software (Beckman Coulter Inc., Hialeah, FL, USA) and a hemocytometer. Cytospin slides for BAL cell differentials were prepared as described previously (Zeidler-Erdely et al., 2011). A minimum of 300 cells were identified and counted under light microscopy. Alterations in alveolar-capillary barrier permeability and cytotoxicity in the lung were assessed by measuring albumin levels and lactate dehydrogenase (LDH) activity, respectively, in the acellular BAL fluid. Measurements were performed with a COBAS c111 analyzer (Roche Diagnostic Systems, Indianapolis, IN, USA) as previously described (Zeidler-Erdely et al., 2011).
2.4.4. Multiplex analysis of cytokines
Because of the signficant inflammatory response observed after exposure to galvanized spot welding fume in the absence of glue or anti-spatter spray, we quantified 27 cytokine/chemokine biomarkers simultaneously in the BAL by using a Discovery Assay® called the Rat Cytokine Array/Chemokine Array 27-Plex (Eve Technologies Corp, Calgary, AB, Canada). The multiplex assay was performed at Eve Technologies by using the Bio-Plex™ 200 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and a Milliplex rat cytokine kit (Millipore, St. Charles, MO, USA) according to their protocol. The 27-Plex consisted of epidermal growth factor (EGF), eotaxin, fractalkine, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), GRO/KC/CINC-1, interferon-gamma (IFNγ), interleukin 1-alpha (IL-1α), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, IL-17A, IL-18, IP-10, Leptin, LIX, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP-1α), MIP-2, regulated on activation, normal T cell expressed and secreted (RANTES), tumor necrosis factor-alpha (TNFα), and vascular endothelial growth factor (VEGF). The assay sensitivities of these markers range from 0.1- 15.7 pg/ml. Individual analyte values and other assay details are available on Eve Technologies' website or in the Milliplex protocol.
2.5. Statistics
Results are means ± standard error of measurement. Statistical analysis was performed using SigmaStat (Systat Software, Inc., SigmaPlot for Windows, San Jose, CA, USA). The significance of difference between exposure groups (Fig. 6, Fig. 7, Fig. 8, Fig. 9) was determined using an analysis of variance (ANOVA) and the Tukey post-hoc test. The criterion of significance was set at p < 0.05.
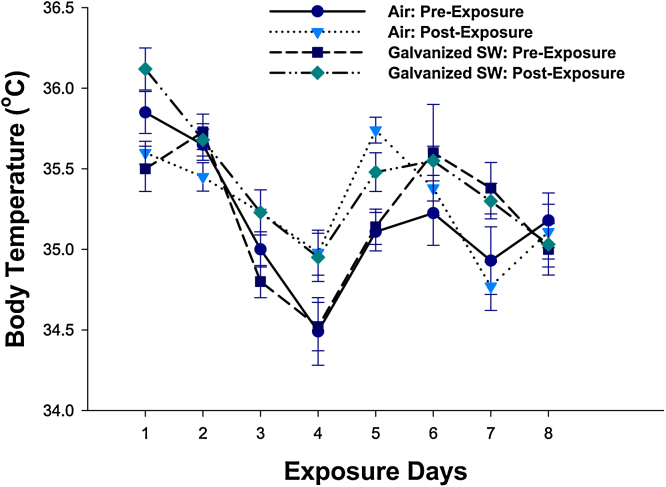
Fig. 6.
Daily rat body temperatures before and after each 4-h exposure of galvanized spot welding fume in the absence of glue or anti-spatter spray. Values are means ± standard error of measurement (n = 12 rats/treatment group).
3. Results
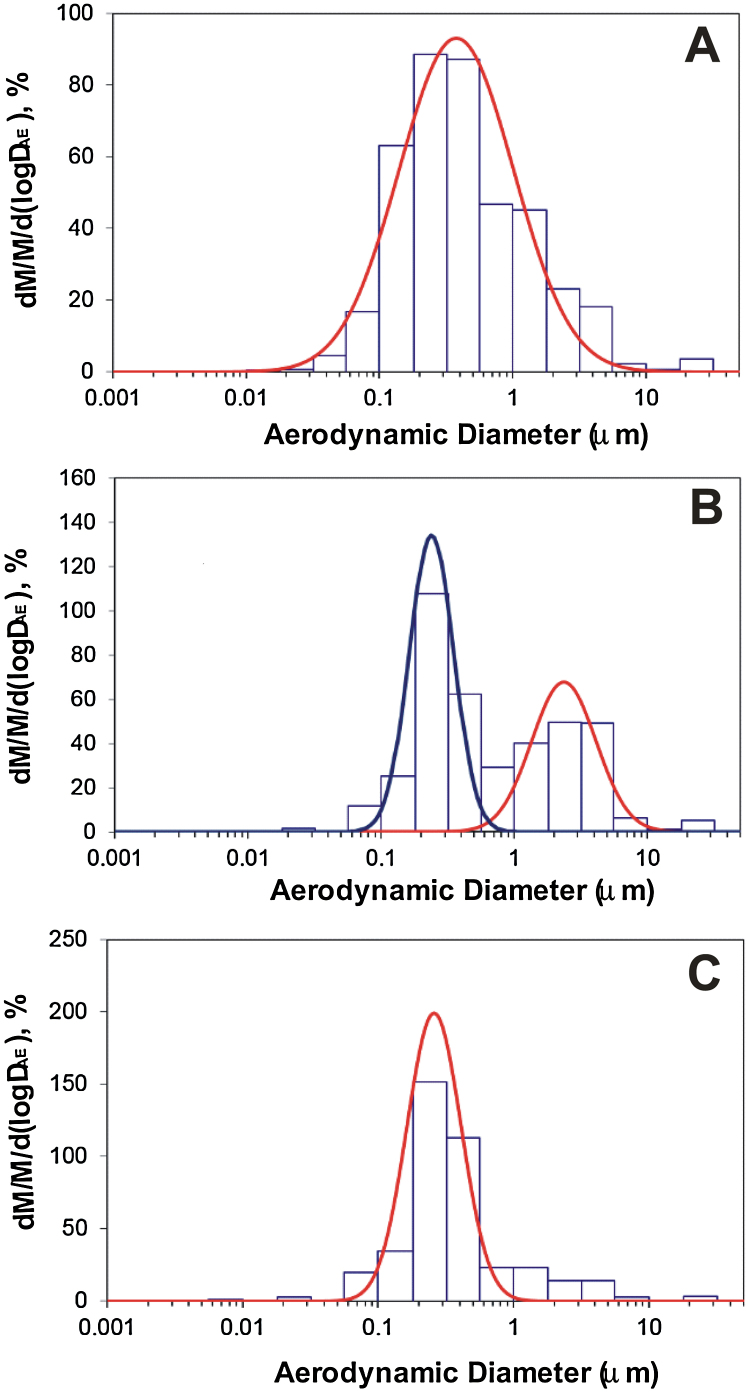
In the determination of size distribution, spot welding of galvanized steel generated a single, submicron distribution mode with a mass median aerodynamic diameter (MMAD) of 0.38 μm and a geometric standard deviation (GSD) of 2.7 (Fig. 4A). In the presence of a glue, a bimodal distribution curve was observed with galvanized spot welding (Fig. 4B). Of the mass of particles generated when welding with the glue, 57% was in the submicron mode (MMAD: 0.24 μm; GSD: 1.5), whereas 42% was in the micron mode (MMAD: 2.4 μm; GSD: 1.7). When spot welding after treating the galvanized metal with anti-spatter spray, a single, submicron distribution mode re-emerged with a MMAD of 0.26 μm and a GSD of 1.6 (Fig. 4C).
Fig. 4.
Particle size distribution of galvanized spot welding (A) with no glue, (B) with glue, and (C) with anti-spatter spray, comparing% mass concentration versus aerodynamic diameter as measured by a Moudi and Nano-Moudi impactor system.
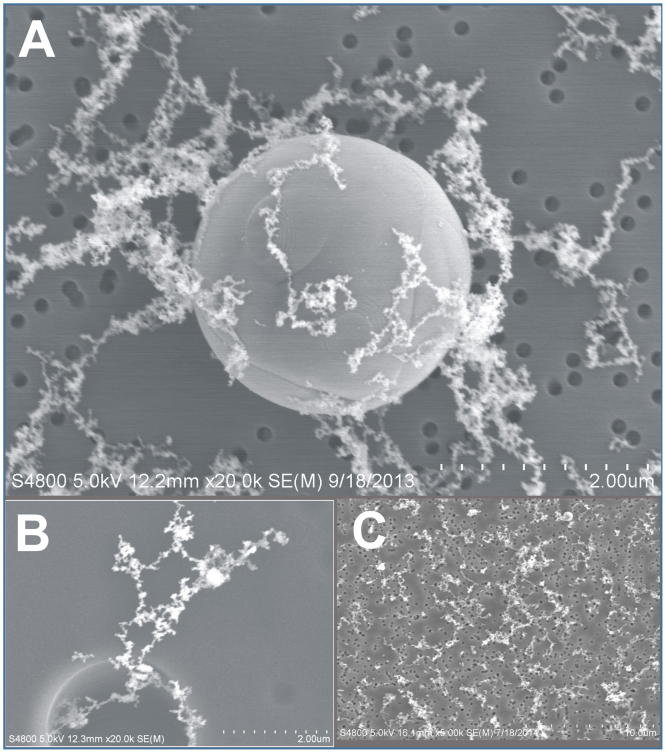
Morphology of the particles generated during galvanized spot welding was typical for welding fume (Fig. 5). The majority of particles were arranged as agglomerated, chain-like structures of nanometer-size primary particles. Larger, micron-size spherical particles that were derived from spatter produced during the welding process also were observed (Fig. 5A&B). When using an anti-spatter spray, it appears the presence of these spherical, larger particles was greatly reduced (Fig. 5C).
Fig. 5.
Representative scanning electron micrographs depicting particles collected on filters from selected stages of MOUDI/Nano-MOUDI particle impactors after galvanized spot welding (A) with no glue, (B) with glue, and (C) with anti-spatter spray.
The metal composition of the particles generated during spot welding of galvanized steel was determined (Table 2). The particles were composed of mostly Fe (72.5%) and Zn (26.3%) with small amounts of manganese (Mn) and copper (Cu) present. Spot welding in the presence of the glue or the anti-spatter agent did not alter the metal composition of the generated fumes (data not shown). In nearly all spot welding test conditions, low levels (<10 ppb) of different VOCs were generated (Table 3). Many different VOCs were produced when spot welding was performed in the presence of the glue or anti-spatter agent as compared when spot welding without them. The glue component had the greatest influence on VOC production as the concentrations for o-xylene, acetaldehyde, acetone, benzene, methyl methacrylate, and m,p-xylene were all measured above 500 ppb. The high level of methyl methacrylate was specific for spot welding with the glue as it was not observed in any other welding test condition where glue was absent.
Table 2.
Metal composition of galvanized steel spot welding fume.
| Metal | Weight% of metalsa |
|---|---|
| Iron (Fe) | 72.5 ± 1.8 |
| Zinc (Zn) | 26.3 ± 1.8 |
| Manganese (Mn) | 0.649 ± 0.01 |
| Copper (Cu) | 0.539 ± 0.04 |
Relative to all metals analyzed. Values are means ± standard error; n = 4 different welding collection periods of 60 min.
Table 3.
Concentrations (ppb) of volatile organic compounds measured in the animal exposure chamber.
| Test Condition | <10 ppb | 11–100 ppb | 101–500 ppb | 501–1000 ppb | >1000 ppb |
|---|---|---|---|---|---|
| Before welding | Acetaldehyde Acetone 2,3-Pentadione |
||||
| Welding only | Ethanol Acetonitrile Isopropyl alcohol Benzene 2,3-Pentadione Toulene |
Acetaldehyde Acetone |
|||
| Welding with glue | Acetonitrile Isopropyl alcohol 2,3-Butanedione n-Hexane |
Ethanol 2,3-Pentadione Toluene Ethylbenzene Styrene |
o-Xylene | Acetaldehyde Acetone Benzene Methyl methacrylate m,p-Xylene |
|
| Welding with anti-spatter | Acetonitrile Isopropyl alcohol Methylene chloride 2,3-Butanedione n-Hexane Chloroform Benzene 2,3-Pentadione Toluene Ethylbenzene m,p-Xylene Styrene o-Xylene |
Ethanol Acetone |
Acetaldehyde | ||
| Glue only, no welding | Acetaldehyde Acetone Benzene m,p-Xylene |
Methyl methacrylate |
|||
| Anti-spatter only, no welding | Acetonitrile Isopropyl alcohol Methylene chloride Chloroform Benzene 2,3-Pentadione Toluene Ethylbenzene m,p-Xylene Styrene o-Xylene Limonene |
Acetone | Ethanol | Acetaldehyde |
Because of the presence of Zn, the metal believed to be the primary causative agent in the development of metal fume fever in welders after inhalation, animal body temperature was monitored before and after each daily exposure throughout the 8-day exposure regimen (Fig. 6). Slight day-to-day variations were observed in body temperature of animals from the different treatment groups. However, exposure to the galvanized spot welding fumes had no significant effect on body temperature compared to air controls before or after each daily exposure. Spot welding in the presence of the glue or the anti-spatter agent also did not alter body temperature compared to controls (data not shown).
In the assessment of lung injury after exposure to fumes generated during galvanized spot welding, LDH activity was measured in the acellular BAL fluid at 1 and 7 days after the 8-day exposure period (Fig. 7). Galvanized spot welding caused a significant increase in LDH activity compared to air control at 1 day after exposure (Fig. 7A). By 7 days, this elevation had returned to control values. When galvanized spot welding was performed in the presence of a glue or anti-spatter spray, the elevation in lung injury was either completely attenuated (Fig. 7B) or reduced (Fig. 7C), respectively.
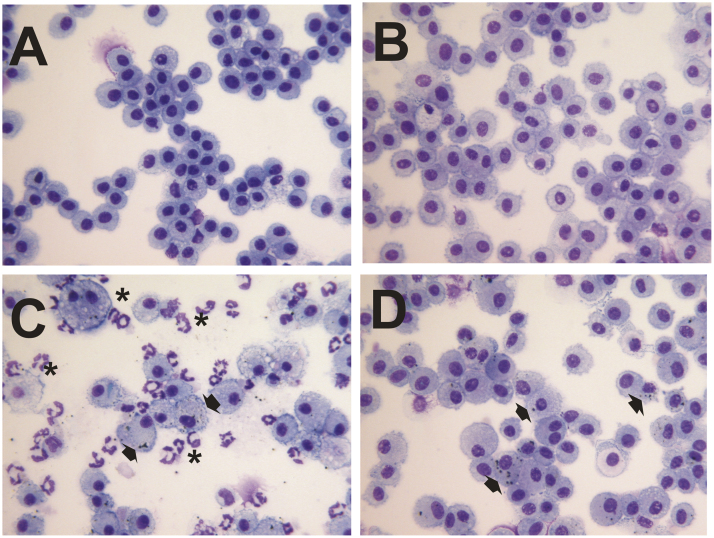
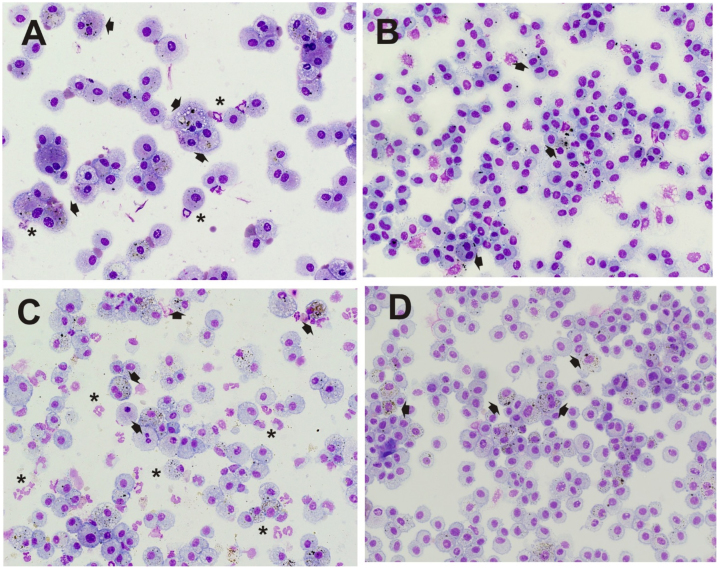
For the measurement of lung inflammation, galvanized spot welding with or without glue increased total cell recovery by BAL at 1 day after exposure compared to control, but had no effect at day 7 after exposure (Fig. 8A&B). When an anti-spatter spray without glue was used, no significant differences were seen when comparing the groups at either time point (Fig. 8C). In all situations, BAL neutrophil number was significantly elevated in the spot welding groups compared to air control at 1 day after exposure (Fig. 9). The neutrophil response when welding using either a glue or anti-spatter spray was reduced when compared with spot welding alone (Fig. 9B&C). Cytospin-stained images of recovered BAL cells confirmed cell counting results (Fig. 10, Fig. 11). Significantly greater numbers of neutrophils were observed at 1 day after exposure to fumes when spot welding without the glue and anti-spatter spray (Figs. 10C vs. 11A&C). In all spot welding scenarios, very few neutrophils were observed after 7 days of exposure (Figs. 10D, 11B&D).
Fig. 10.
Representative images of cytospin-stained lung BAL cells after exposure to filtered air (A, B) or 25 mg/m3 of galvanized spot welding fume (C, D) for 4 h/day for 8 days: (A) air, 1-day post-exposure; (B) air, 7-day post-exposure; (C) galvanized spot welding fume, 1-day exposure; (D) galvanized spot welding fume, 7-day spot welding fume. Welding for this set of exposures did not use glue or anti-spatter spray. Asterisks indicate neutrohils; arrows point to particles within macrophages; magnification is 40×.
Fig. 11.
Representative images of cytospin-stained lung BAL cells after exposure to 25 mg/m3 of galvanized spot welding fume in the presence of glue (A, B) or anti-spatter spray (C, D) for 4 h/day for 8 days: (A) galvanized spot welding fume with glue, 1-day post-exposure; (B) galvanized spot welding fume with glue, 7-day post-exposure; (C) galvanized spot welding fume with anti-spatter, 1-day exposure; (D) galvanized spot welding fume with anti-spatter, 7-day post-exposure. Asterisks indicate neutrophils; arrows point to particles within macrophages; magnification is 40×.
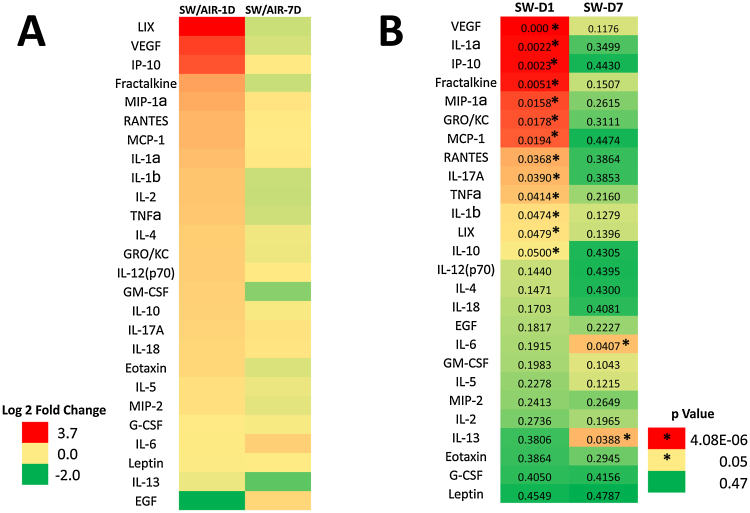
To further assess the inflammatory response after inhalation exposure to galvanized spot welding fumes, multiple cytokines and chemokines associated with the inflammatory response were measured in the recovered BAL fluid (Fig. 12). At 1 day after the 8-day exposure period, nearly all of the BAL inflammatory mediators were increased for the spot welding group compared to control (Fig. 12A). After a 7-day recovery period, the elevation observed in many of these mediators had returned to control values. In the assessment of which of these cytokines and chemokines were statistically different, 13 mediators (VEGF, IL-1α, IP-10, fractalkine, MIP-1α, GRO/KC/CINC-1, MCP-1, RANTES, IL-17A, TNFα, IL-1β, LIX, and IL-10) were significantly different at day 1, but only 2 (IL-6 and IL-13) persisted at day 7 post-exposure (Fig. 12B). Of the mediators that were statistically different, all were significantly increased at the two time points except for IL-13 which was significantly downregulated at 7 days after exposure.
Fig. 12.
Heat maps of (A) fold change and (B) p value comparisons for galvanized spot welding (SW) exposure versus air controls for a multiplex of different cytokines and chemokines in BAL fluid at 1 and 7 days post-exposure (n = 6 animals/treatment group). Welding for this set of exposures did not use glue or anti-spatter spray. In (A), red, yellow, and green represent overexpression, no expression, and under expression, respectively (see color legend in Figure). In (B), red, yellow, and green represent highly significant p value, significant at p value of 0.05, and not significant p value, respectively (*significantly different from air control, p < 0.05; n = 6 animals/treatment group).
4. Discussion and conclusions
Respiratory effects have been observed in workers exposed to fumes generated during resistance spot welding, a common joining process used in the automotive industry (Loukzadeh et al., 2009, Luo et al., 2006, Hammond et al., 2005, Lee et al., 1990). Particle concentrations have been measured to be quite high in different automotive processing plants where resistance spot welding is performed (Buonanno et al., 2011, Liu and Hammond, 2010). Toxicology studies assessing the health effects associated with the inhalation of resistance spot welding aerosols are limited (Sriram et al., 2014, Zeidler-Erdely et al., 2014). Previously, a resistance spot welding fume generator and inhalation exposure system has been developed (Afshari et al., 2014). The system can produce complex aerosols, and different target aerosol concentrations can be maintained within an animal exposure chamber over an extended period of time. The previous study characterized and evaluated the toxicity of Fe-rich fumes from spot welding of mild steel. The goal of the current study was to assess the pulmonary effects as well as the physical and chemical characterization of the aerosols generated during spot welding of Zn-containing galvanized steel in the presence and absence of a glue and anti-spatter compound, two substances that are often used in the process.
In the measurement of the physical properties of the galvanized spot welding aerosols, particle morphology and size distribution were typical for what has been observed during standard arc welding processes (Antonini et al., 2006, Jenkins et al., 2005, Zimmer and Biswas, 2001). Particle size distribution was observed to be multi-modal. The MMAD of the spot welding fumes for all galvanized spot welding conditions was similar and ranged between 0.24–0.38 μm, depending on whether or not the glue or anti-spatter agent were used. The majority of the particles were agglomerated, chain-like structures of nanometer-size primary particles collected in the submicron size range. The presence of non-agglomerated ultrafine particles accounted for only a very small amount (<5%) of the particles generated. Some much larger, micron-size spherical particles also were observed. In the assessment of the size distribution of particles collected in spot welding work areas at different automotive processing plants, Dasch and D’Arcy (2008) found similar results, observing a bimodal particle size distribution of both super-micron particles (>20 μm) and an agglomeration mode of smaller particles (∼1 μm). The larger, micron-size spherical particles likely were derived from spatter produced during the spot welding process. Spatter is caused by sparking and metal expulsion during welding which leads to the formation of large spherical particles formed from the solidification of molten material after emission into the air. Interestingly, when spot welding using galvanized metal strips that had been treated with the anti-spatter compound in the current study, lower amounts of these larger spherical particles were generated.
The galvanized welding particles were composed of mostly Fe and Zn. Welding in the presence of the glue or anti-spatter agent had no effect on metal composition of the fume. Because of a significant amount of Zn present in the generated fumes, animal body temperature was determined throughout the exposure period as one measure of metal fume fever. Zn has been implicated as the primary causative metal responsible for the development of fume fever, a common acute health effect observed in welders (Wardhana and Datau, 2014, Wong et al., 2012). Metal fume fever is a flu-like condition that presents within 48 h and resolves in 1–2 days after onset. No significant changes were observed in animal body temperature before and after exposure to galvanized welding fume when compared with exposure to filtered air. This result in rats was not surprising as rodent models of toxin-induced (e.g., Zn exposure) fever as determined by changes in body temperature have been a challenge to develop and interpret (Gordon and Rowsey, 1998, Briese, 1998). In addition, metal fume fever, even in humans, seems to affect only especially susceptible individuals. The likeliness to find such susceptible animals in inbred strains may prove difficult.
Other possible measures of metal fume fever are the assessments of the cytokine/chemokine response and inflammatory cell influx into the lungs. It has been hypothesized that metal fume fever is caused by the release of specific cytokines which induce a systemic and pulmonary inflammatory response (Greenberg and Vearrier, 2015, Wardhana and Datau, 2014, Blanc et al., 1993). Using a rat model of fume fever, Gordon et al. (1992) observed a significant elevation in both total BAL cells and BAL LDH at 24 h after inhalation exposure of 2.5 or 5.0 mg/m3 of ZnO. In agreement, inhalation exposure to galvanized spot welding fumes in the current study caused a significant increase in total BAL cells and neutrophils compared to air control at 1 day after exposure. BAL LDH also was elevated at 1 day in the galvanized spot welding group, indicating an increase in lung injury. These significant increases in BAL LDH and inflammatory cell influx were not observed when performing mild steel spot welding in a previous study at the same concentration (Zeidler-Erdely et al., 2014). Specific pro-inflammatory chemokines (e.g., IP-10, fractalkine, MIP-1α, GRO/KC/CINC-1, MCP-1, RANTES, LIX), cytokines (e.g., IL-1α, IL-17A, TNFα, IL-1β, IL-10), and growth factors (e.g., VEGF) also were elevated in the BAL at 1 day after galvanized spot welding fume exposure. This elevated pulmonary response was transient as the lung injury and inflammation mostly returned to control values by 7 days after exposure. Interestingly, IL-13, an important cytokine in mediating allergic disease, was the only inflammatory factor in the current study to be significantly downregulated at 7 days after exposure. Further investigation may be warranted to examine the role of IL-13 related to exposure of Zn-containing particles.
When galvanized spot welding was performed in the presence of either the glue or anti-spatter spray, the pulmonary inflammatory response was somewhat dampened, possibly due to a physical coating of the surface of the newly generated particles by the increased volatile components that were produced. Any coating, paint, or the use of cleaning, degreasing, of anti-spatter/slag agents on the metal pieces to be welded can influence the physical and chemical composition of the resultant welding fume and potentially alter its toxicity potential (Martin et al., 1997). Previously, we have shown that the surface of freshly formed welding particles are highly reactive with a greater concentration of reactive oxygen species and induced greater lung inflammation and injury compared to aged particles (Antonini et al., 1998, Leonard et al., 2010). Thus, any coating of the surface of the welding particle could potentially lessen their reactivity and potential for acute pulmonary toxicity. Indeed, adding a silica precursor to the weld shield gas in different arc welding processes has been shown to form a coat on the surface of freshly formed welding particles, reducing hexavalent chromium emission and reactive oxygen species formation (Paulson et al., 2011, Topham et al., 2010).
However, it does not mean these substances are biologically or toxicologically inert. Significantly high levels of VOCs were observed after galvanized spot welding, specifically when the glue and anti-spatter spray were used. Inhalation of VOCs has been known to cause numerous health effects, including a variety of pulmonary and neurological effects (Environmental Protection Agency, 2012). Although it doesn’t appear that the VOCs produced by spot welding in the current study increased the lung injury or inflammation endpoints, the potential neurological impact remains to be investigated. Acute central nervous system effects, such as dizziness, headache, unconsciousness, and seizures, have been observed after inhalation VOCs (White and Proctor, 1997). In addition, loss of coordination, memory impairment, and mood and personality changes have been associated with chronic or high-dose exposures to VOCs (Dick, 2006). Previously, we observed the volatile component of aerosols generated during mild steel spot welding using a glue induced neurotoxic changes in different brain areas, such as altered neurotransmitter levels, decreased mRNA of the olfactory marker protein and tyrosine hydroxylase in the olfactory bulb, and reduced expression of dopamine transporters and receptors in the olfactory bulb (Sriram et al., 2014). Specifically, exposure to methyl methacrylate, which was generated in very high concentrations when spot welding with the glue in the current study, has been reported to cause local neurological symptoms (Chan et al., 1994, Leggat et al., 2009). Discrete brain areas from the animals exposed in the current study were recovered and are being assessed for markers of neurotoxicity. The results of neurotoxicity part of the project will be reported in a separate manuscript.
In summary, an automated welder was designed to characterize the aerosols generated during resistance spot welding of galvanized steel in the presence and absence of a glue or an anti-spatter spray. The generated particles generally were in the submicron size range with a significant number of ultrafine particles formed. The primary metals present in the fumes were Fe and Zn. The addition of the anti-spatter spray and glue did affect particle size distribution when spot welding galvanized steel, whereas they had no effect on metal composition. Significant elevations in lung injury (BAL lactate dehydrogenase) and inflammation (total BAL cells and neutrophils) were observed at 1 day, but not 7 days, after an 8-day exposure to galvanized spot welding fumes compared to air control. Exposure to Zn appeared to be the causative agent in the development of acute lung inflammation as this effect was not observed when compared to Fe-rich spot welding fumes from a previous study (Zeidler-Erdely et al., 2014). Multiple VOCs were identified when spot welding using either the glue or the anti-spatter spray that were not present when welding alone. It did not appear that the VOCs (or the concentrations of VOCs that were generated during the animal exposures) had a measurable effect on the pulmonary responses assessed in the current study. The effect of the galvanized spot welding on neurological responses in discrete brain regions in the presence or absence of the glue or anti-spatter spray currently is being investigated.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Acknowledgement
This study was funded by National Institute for Occupational Safety and Health project #927ZLEG.
References
- Afshari A., Zeidler-Erdely P.C., McKinney W., Chen B.T., Jackson M., Schwegler-Berry D., Friend S., Cumpston A., Cumpston J.L., Leonard H.D., Meighan T.G., Frazer D.G., Antonini J.M. Development and characterization of a resistance spot welding aerosol generator and inhalation exposure system. Inhal. Toxicol. 2014;26:708–719. doi: 10.3109/08958378.2014.941118. [DOI] [PubMed] [Google Scholar]
- Antonini J.M., Roberts J.R., Stone S., Chen B.T., Schwegler-Berry D., Frazer D.G. Short-term inhalation exposure to mild steel welding fume had no effect on lung inflammation and injury but did alter defense responses to bacteria in rats. Inhal. Toxicol. 2009;21:182–192. doi: 10.1080/08958370802360661. [DOI] [PubMed] [Google Scholar]
- Antonini J.M., Stone S., Roberts J.R., Chen B., Schwegler-Berry D., Afshari A.A., Frazer D.G. Effect of short-term stainless steel welding fume inhalation exposure on lung inflammation, injury, and defense responses in rats. Toxicol. Appl. Pharmacol. 2007;223:234–245. doi: 10.1016/j.taap.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Antonini J.M., Afshari A.A., Stone S., Chen B., Schwegler-Berry D., Fletcher W.G., Goldsmith W.T., Vandestouwe K.H., McKinney W., Castranova V., Frazer D.G. Design, construction, and characterization of a novel robotic welding fume generator and inhalation exposure system for laboratory animals. J. Occup. Environ. Hyg. 2006;3:194–203. doi: 10.1080/15459620600584352. [DOI] [PubMed] [Google Scholar]
- Antonini J.M., Clarke R.W., Krishna Murthy G.G., Sreekanthan P., Jenkins N., Eagar T.W., Brain J.D. Freshly generated stainless steel welding fume induces greater lung inflammation in rats as compared to aged fume. Toxicol. Lett. 1998;98:77–86. doi: 10.1016/s0378-4274(98)00103-9. [DOI] [PubMed] [Google Scholar]
- Blanc P., Boushey H.A., Wong H., Wintermeyer S.F., Bernstein M.S. Cytokines in metal fume fever. Am. Rev. Respir. Dis. 1993;174:134–138. doi: 10.1164/ajrccm/147.1.134. [DOI] [PubMed] [Google Scholar]
- Briese E. Normal body temperature of rats: the setpoint controversy. Neurosci. Neurobehav. Rev. 1998;22:427–436. doi: 10.1016/s0149-7634(97)00051-1. [DOI] [PubMed] [Google Scholar]
- Buonanno G., Morawska L., Stabile L. Exposure to welding particles in automotive plants. J. Aerosol Sci. 2011;42:295–304. [Google Scholar]
- Chan P.K.L., Meek M.E., Dormer W. Methyl methacrylate: evaluation of risk to health from environmental exposures in Canada. J. Environ. Sci. Health Part C. 1994;12:397–407. [Google Scholar]
- Dasch J., D’Arcy J. Physical and chemical characterization of airborne particles from welding operations in automotive plants. J. Occup. Environ. Hyg. 2008;5:444–454. doi: 10.1080/15459620802122720. [DOI] [PubMed] [Google Scholar]
- Dick F.D. Solvent neurotoxicity. Occup. Environ. Med. 2006;63:221–226. doi: 10.1136/oem.2005.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency . Volatile Organic Compounds (VOCs), US Environmental Protection Agency; 2012. An Introduction to Indoor Air Quality (IAQ) Available from: http://www.epa.gov/iaq/voc.html. [Google Scholar]
- Gordon C.J., Rowsey P.J. Poisons and fever. Clin. Exp. Pharmacol. Physiol. 1998;25:145–149. doi: 10.1111/j.1440-1681.1998.tb02194.x. [DOI] [PubMed] [Google Scholar]
- Gordon T., Chen L.C., Fine J.M., Schlesinger R.B., Su W.Y., Kimmel T.A., Amdur M.O. Pulmonary effects of inhaled zinc oxide in human subjects, guinea pigs, rats, and rabbits. Am. Ind. Hyg. Assoc. J. 1992;53:503–509. doi: 10.1080/15298669291360030. [DOI] [PubMed] [Google Scholar]
- Greenberg M.L., Vearrier D. Metal fume fever and polymer fume fever. Clin. Toxicol. 2015;53:195–203. doi: 10.3109/15563650.2015.1013548. [DOI] [PubMed] [Google Scholar]
- Hammond S.K., Gold E., Baker R., Quinlan P., Smith W., Pandya R., Balmes J. Respiratory health effects related to occupational spray painting and welding. J. Occup. Med. Environ. Med. 2005;47:728–739. doi: 10.1097/01.jom.0000165748.31326.e8. [DOI] [PubMed] [Google Scholar]
- Jenkins N.T., Pierce W.M.-G., Eagar T.W. Particle size distribution of gas metal and flux cored arc welding fumes. Welding J. 2005;84:156s–163s. [Google Scholar]
- Kanwal R., Boylstein R.J. DHHS/Center for Disease Control and Prevention; Washington, DC: 2006. NIOSH Health Hazard Evaluation Report: HETA# 2006-0059-3009, Daimler-Chrysler Jefferson North Assembly Plant, Detroit, MI, July 2006. [Google Scholar]
- LeBouf R.F., Stefaniak A.B., Abbas Virji M. Validation of evacuated canisters for sampling volatile compounds in healthcare settings. J. Environ. Monit. 2012;14:977–983. doi: 10.1039/c2em10896h. [DOI] [PubMed] [Google Scholar]
- Lee H.S., Chia S.E., Yap J.C.H., Wang Y.T., Lee C.S. Occupational asthma due to spot-welding. Singapore Med. J. 1990;31:506–508. [PubMed] [Google Scholar]
- Leggat P.A., Smith D.R., Kedjarune U. Surgical applications of methyl methacrylate: a review of toxicity. Arch. Environ. Occup. Health. 2009;64:207–212. doi: 10.1080/19338240903241291. [DOI] [PubMed] [Google Scholar]
- Leonard S.S., Chen B.T., Stone S.G., Schwegler-Berry D., Kenyon A.J., Frazer D.G., Antonini J.M. Comparison of stainless and mild steel welding fumes in generation of reactive oxygen species. Part. Fibre Toxicol. 2010;7:32. doi: 10.1186/1743-8977-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Hammond S.K. Mapping particulate matter at the body weld department in an automotive assembly plant. J. Occup. Environ. Hyg. 2010;7:593–604. doi: 10.1080/15459624.2010.509844. [DOI] [PubMed] [Google Scholar]
- Loukzadeh Z., Sharifian S.A., Aminian O., Shojaoddiny-Ardekani A. Pulmonary effects of spot welding in automobile assembly. Occup. Med. 2009;59:267–269. doi: 10.1093/occmed/kqp033. [DOI] [PubMed] [Google Scholar]
- Luo J.C.J., Hsu K.-H., Shen W.-S. Pulmonary function abnormalities and airway irritation symptoms of metal fumes exposure on automotive spot welders. Am. J. Ind. Med. 2006;49:407–416. doi: 10.1002/ajim.20320. [DOI] [PubMed] [Google Scholar]
- Martin C.J., Guidotti T.L., Langard S. Respiratory hazards of welding. Clin. Pulm. Med. 1997;4:194–204. [Google Scholar]
- National Institute for Occupational Safety and Health . NIOSH Manual of Analytical Methods. 4th edition, Issue 2. U.S. Department of Health and Human Services Publication No. 98–119; Washington, DC: 1994. Elements by ICP (Hot Block/HCl/HNO3 digestion): method 7303. [Google Scholar]
- Paulson K.M., Wang J., Topham N., Wu C.-Y., Alexandrov B.T., Lippold J.C., Es-Said O.S. Alternatives for joing stainless steel to reduce Cr(VI) emissions and occupational exposures. J. Ship Prod. Des. 2011;27:91–97. [Google Scholar]
- Sriram K., Jefferson A.M., Lin G.X., Afshari A., Zeidler-Erdely P.C., Meighan T.G., McKinney W., Jackson M., Cumpston A., Cumpston J.L., Leonard H.D., Frazer D.G., Antonini J.M. Neurotoxicity following acute inhalation of aerosols generated during resistance spot weld-bonding of carbon steel. Inhal. Toxicol. 2014;26:720–732. doi: 10.3109/08958378.2014.954654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R.D. Weldability of Steels. 4th edition. Welding Research Council; New York: 1987. The welding processes in relation to weldability; pp. 20–22. [Google Scholar]
- Topham N., Kalivoda M., Hsu Y.-M., Wu C.-Y., Oh S., Cho K. Reducing Cr6+ emission from gas tungsten arc welding using a silica precursor. J. Aerosol Sci. 2010;41:326–330. [Google Scholar]
- Wardhana, Wardhana Datau E.A. Metal fume fever among galvanized welders. Acta Med. Indones. 2014;46:256–262. [PubMed] [Google Scholar]
- White R.F., Proctor S.P. Solvents and neurotoxicity. Lancet. 1997;349:1239–1243. doi: 10.1016/S0140-6736(96)07218-2. [DOI] [PubMed] [Google Scholar]
- Wong A., Greene S., Robinson J. Metal fume fever: a case review of calls made to the Victorian Poisons Information Center. Aust. Fam. Physician. 2012;41:141–144. [PubMed] [Google Scholar]
- Zeidler-Erdely P.C., Meighan T.G., Erdely A., Fedan J.S., Thompson J., Bilgesu S., Waugh S., Anderson S., Marshall N.B., Afshari A., McKinney W., Frazer D.G., Antonini J.M. Effects of acute inhalation of aerosols generated during resistance spot welding with mild steel on pulmonary, vascular, and immune responses in rats. Inhal. Toxicol. 2014;26:697–707. doi: 10.3109/08958378.2014.944287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler-Erdely P.C., Battelli L.A., Stone S., Chen B.T., Frazer D.G., Young S.H., Erdely A., Kashon M.L., Andrews R., Antonini J.M. Short-term inhalation of stainless steel welding fume causes sustained lung toxicity but no tumorigenesis in lung tumor susceptible a/j mice. Inhal. Toxicol. 2011;23:112–120. doi: 10.3109/08958378.2010.548838. [DOI] [PubMed] [Google Scholar]
- Zimmer A.T., Biswas P. Characterization of the aerosols resulting from arc welding processes. J. Aerosol Sci. 2001;32:993–1008. [Google Scholar]