Graphical abstract
Keywords: Pesticides, Organochlorine, Cocoa beans, Ondo, Ile-Ife, Nigeria
Highlights
-
•
Organochlorine pesticides (OCPs) were determined in dried cocoa beans in Nigeria.
-
•
Qualitative and quantitative evaluation of the extracted OCPs was done using GC-ECD.
-
•
Levels of OCPs in cocoa beans from Ondo range from ND to 82.17 ± 54.53 ng/g.
-
•
The OCPs levels in cocoa beans from Ondo are higher than that from Ile-Ife town.
-
•
OCPs in the cocoa beans were within the Maximum Residue Limit (MRLs) set by WHO.
Abstract
Levels of organochlorine pesticides (OCPs) were determined in dried cocoa beans obtained from cocoa produce stores at Ondo and Ile-Ife, Southwestern Nigeria. Cocoa beans samples were sun dried to a constant weight, pulverized and soxhlet extracted with dichloromethane to obtain the OCPs. Qualitative identification and quantitative evaluation of the extracted OCPs after clean-up on silica gel were accomplished with the aid of a Gas Chromatography coupled with an Electron Capture Detector (GC-ECD). Levels of OCPs in cocoa beans from Ondo had a mean range of ND (p, p’-DDE) to 82.17 ± 54.53 ng/g (p, p’-DDT) were higher than the OCPs levels in cocoa beans from Ile-Ife with a mean range of 0.37 ± 0.63 ng/g (Endrin) to 57.76 ± 81.48 ng/g (p, p’-DDT). The higher levels of OCPs detected in the cocoa beans from Ondo could be an indication of higher volume of OCPs application by cocoa farmers in Ondo and its environs since cocoa plantations were more concentrated than Ile-Ife environs. Levels of OCPs determined in the cocoa beans were within the Maximum Residue Limit (MRLs) for OCPs set by the World Health Organization/Food and Agricultural Organization. The study established the presence of OCPs in an important crop of Nigeria. Hence, there is the need to keep monitoring ecotoxicological chemical substances in agricultural food products of Nigeria so as to take steps that ensure health safety of end users.
1. Introduction
Organochlorine pesticides which have long been widely used in agriculture and public health as highly effective pest control agents [1], [2] have also been found to constitute health hazards to humans, organisms and the environment [3], [4], [5]. For example, they are known to cause prostate cancer [6], [7], liver cancer [8], diabetes [9], reproductive and developmental defects [10], [11], [12] and act as endocrine disruption [13] with acute immunotoxicity [14] and neurotoxicity [15]. This has led to the prescription of tolerance levels such as no-observable-adverse-effect-level (NOAEL) and maximum residue level (MRL) for various pesticides in food and water by the Codex Alimentarius Commission [16].
In Nigeria, before the advent of the oil boom, cocoa production had contributed tremendously to the infrastructural development of the country [17]. The oil boom of the eighties made the government to neglect this agricultural sector which had before then been the country’s major foreign exchange earner. This automatically implied a downward trend in cocoa production, thus, causing a substantial fall in the quantity available for export. However, there have been renewed efforts on the part of government and private individuals in the recent times to resuscitate the cocoa industry in Nigeria to its now fledgling status, where it has regained its major economic base for Nigeria [18]. Cocoa is the leading non-oil foreign exchange earner in Nigeria [19]. Nigeria has the capacity to produce over 300,000 t of cocoa, but only produces about 248,000 metric tonnes annually partly because of the scrapping of Cocoa Board in Nigeria. Generally, cocoa contributes over 26% of the Gross Domestic Product of the non-oil export in Nigeria, and 19% contribution to the world market [20]. Nigeria is the world’s fourth largest producer of cocoa after Ivory Coast, Indonesia and Ghana, and third largest exporter after Ivory Coast and Ghana. As it were, Ondo state in Southwestern Nigeria is the highest producer of cocoa in Nigeria. In 2007, it produced about 40% of the total cocoa production in the country [21], [22].
In order to limit losses from insect pests and diseases, Nigerian cocoa farmers employ the use of a wide range of pesticides, such as copper sulphate, benzene hexachloride (BHC), Aldrex 40 [17]. Others include diazinon, chlorpyrifos, fenitrothion and cuprous oxide. Farmers in Nigeria use various kinds of pesticides such as Gammalin 20 (Lindane), DDT, DD-force, Weed-Off, Termicot, Atrazin, Glyphosphate, Metaclors-plus, Alaclhlor 2–4 Amcine and aldrin to formulate local insectides that are applied on farm products [23]. Earlier workers maintained that OCPs are still used in agriculture clandestinely under unknown trade names in developing countries such as Nigeria [24], [25].
Organochlorine pesticides continue to experience widespread use by farmers in Nigeria due to the high efficacy and lower cost of the OCPs despite the ban that has been placed on the continuous use of this class of chemicals [26]. The presence of OCPs has been reported in top soils of some agricultural farmlands and settlements in Nigeria [27], [28], [29]; in cocoa beans [30]; and cowpea grains and dried yam chips [31], [32].
The aim of this study was to carry out a comparative investigation of the quality of dried cocoa beans with respect to occurrence and levels of OCP residues in samples obtained from two prominent cocoa-cultivating areas of Nigeria, namely: Osun and Ondo States. This comparative study is important in evaluating the current status of OCPs usage in the Nigerian environment.
2. Materials and methods
2.1. Reagents used and their sources
The reagents used in this study are dichloromethane (GFS Chemicals, Columbus), n–Hexane (Ultrafine Limited, Marlborough House, London), acetone (GFS Chemicals, Columbus), silica gel 60–200 mesh (Labtech Chemicals), and anhydrous sodium sulphate (Merck, Germany). These reagents were procured through various sales representatives of the producing companies resident in Nigeria. Solvents, such as dichloromethane, n-Hexane and acetone, were doubly distilled to obtain higher purity, while silica gel and anhydrous sodium sulphate were heated in an oven at 120 °C for 12 h to ensure that no adsorbed water element remained as part of the clean-up materials.
2.2. Study area and sample collection
Prior to the sample collection, a preliminary survey of cocoa farmers in the study area was carried out to gather information on types of pesticides commonly used them for pests and diseases in the cocoa plantations. Cocoa beans were sampled from thirteen randomly selected cocoa produce stores (O–1 to O–13) in Ondo and twelve other produce stores (I–1 to I–12) in Ile-Ife. Thus, a total of 25 samples were collected for analysis. Ondo town (in Ondo West Local Government) is known as a major player in the plantation and export of cocoa beans with several plantations at its surburbs, such as Ile-Oluji, Oke-Igbo, Epe, Laje, Litaye, Bagbe, Igunsin, Ayetoro, Igbindo, Lamu, Ajue, Ilekere, and so on. Dried cocoa beans from these suburbs are transported to cocoa produce stores in Ondo with international affiliations for further processing and subsequent export.
Similarly, Ile-Ife in Osun state is an agrarian community with substantial focus on cocoa plantation as one of the major cash crops. The most common category of soils in Ife area is the Itagunmodi series, which is well known for its significance in cocoa culture; soils belonging to this series are some of the best cocoa soils in western Nigeria [33]. Several cocoa-producing communities surrounding Ile-Ife, such as Ifetedo, Modakeke, Olode, Owode, Akintola, Mefoworade, Amula and Omifunfun, among others, bring their dried cocoa beans to various cocoa produce stores in Ile-Ife for further processing and onward transmission to local and foreign processing industries.
Samples of cocoa beans collected from various produce stores at Ondo and Ile-Ife were further sun-dried to reduce the moisture contents until constant weights were obtained. The dried cocoa bean samples were manually decorticated and the resultant nibs were ground to powdery form with the aid of Agate pestle and mortar.
2.3. Extraction of organochlorine pesticide residues
A 20 g portion of the ground cocoa bean sample was weighed into a Whatman extraction thimble pre-extracted with n-hexane and dichloromethane (DCM) to remove extraneous organic contaminants that might be adhering to the surface or pores of the thimble. Using the method described elsewhere [34], the sample was soxhlet extracted for an average period of 5 h using DCM as the extracting solvent. The resulting extract was concentrated by distilling off the solvent using a rotary evaporator at about 41 °C to about 3 mL. The concentrated extract was cooled down to room temperature and then concentrated further to about 2 mL under a stream of nitrogen gas of high percentage purity of 99.99%, in preparation for the clean – up procedure.
2.4. Clean-up procedure
A column of about 15 cm (length) × 1 cm (internal diameter) was packed first with glass wool and then with about 7.5 g activated silica gel prepared in a slurry form in DCM. About 5 g of anhydrous sodium sulfate was placed at the top of the column to absorb any water in the sample or the solvent. Pre-elution was done with 15 mL of DCM, without exposing the sodium sulfate layer to air, so as to prevent the drying up of the silica gel adsorbent. The reduced extract was run through the column and allowed to sink below the sodium sulfate layer. Elution was done with 3 × 10 mL portions of the extracting solvent (DCM). The eluate was collected, dried with anhydrous sodium sulfate and evaporated to dryness under a stream of analytical grade nitrogen (99.99%).
2.5. Qualitative identification and quantitative evaluation of the OCPs
The dried eluate was reconstituted with 1 mL 2, 2, 4–trimethylpentane (isooctane). With the aid of a microsyringe, 1 μL of the 1.0 mL purified extract was injected into the injection port of a gas chromatograph coupled with a 63Ni electron capture detector (GC-ECD, Hewlett Packard 7890A series II). The column consisted of a DB-17 fused silica capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness). The temperatures of the injector and detector were 250 °C and 300 °C (held for 5 min), respectively. Oven temperature programme started from 60 °C (1 min) and continued at 20 °C/min to 150 °C and at 5 °C/minute to 280 °C held for 4 min; injected sample volume was 1 μL. The injection was carried out on a splitless injector at 200 °C and the purge activation time was 30 s. The carrier gas was N2 at 30 mL/min; and the splitless flow rate was 19.6 mL/min. The run time was 23 min. The individual OCPs were identified by comparing the elution time of standard OCPs with those in the samples, while each OCP was quantified by comparing the peak areas of the OCPs in samples with those in standard. Gas chromatographic analysis of the samples was carried out at the Nigerian Institute of Oceanography and Marine Research (NIOMR) Central Laboratory, Victoria Island, Lagos, Nigeria.
2.6. Recovery experiment
Since no certified pesticide reference materials were available during the course of this study, recovery analysis was performed in order to evaluate the precision and efficiency of the analytical procedures using standard addition method. A 20 g sample of pulverized cocoa beans was divided into two. One part was spiked with 10 ppm standard mixture consisting of some of the available organochlorine insecticides of interest, namely: HCH, γ-BHC, aldrin, p,p′-DDE, α-chlordane, p,p′-DDD, dieldrin, heptachlor, endrin and p,p′-DDT. These were thoroughly mixed, while the other (control) group was left unspiked. The samples were taken through the extraction and clean up protocols, following the procedures of earlier workers [34]. Also, 10 mL of the standard 1000 mg/L mixture of the OCPs, in spectra grade n- hexane, was put into a clean, oven-dried sample bottle. This was dried at ambient temperature by passing a stream of high purity nitrogen gas through it, and the residue reconstituted using 2, 2, 4 – trimethylpentane. With the aid of a microsyringe, 1.0 μL of each of the spiked, unspiked (control) and standard mixture was injected into the GC column for GC-ECD analysis. The recoveries of OCPs were determined by comparing the peak areas of the OCPs after spiking with those obtained from the evaporated standard residues. The percentage recovery was evaluated from the relationship:
where A′ is the amount of an OCP in the spiked sample, A is the amount of OCP in the unspiked sample and B is the amount of OCP used for spiking.
2.7. Response factor (RF) determination
The response factor of some of the available standard OCPs was obtained by the method of Ogunfowokan et al. [35]. This was determined by injecting 1.0 μL of 1000 ppm stock solution of the standard mixture containing the internal standard (I.S.) into the GC. The internal standard used for this work was hexachlorohexane (HCH). The response factor for each of the available samples was obtained using the expression:
3. Results and discussion
The percent recovery values of 80.13% to 109.21% presented in Table 1 fell within the 70–110% as the acceptable range for recovery stipulated by the EU Guidelines for evaluating accuracy and precision of a method [36] indicating that the procedures used for the assessment of OCPs in this work were efficient and reliable. The 0.902–1.859 range of response factors of the OCPs indicated that the programmed method for their GC-ECD identification and quantification was efficient as these values implied distinctive non-overlapping chromatograms of the detected OCPs.
Table 1.
Response Factor and Percent Recovery for OCPs.
| OCPs | Response factor | Amount (μg/g) of OCPs used for spiking | Mean amount of OCPs recovered | Percent recovery* | EU-MRL (μg/g) in food items** |
|---|---|---|---|---|---|
| HCH | – | 10 | 9.056 | 90.56 ± 4.28 | |
| γ-BHC | 1.208 ± 0.023 | 10 | 8.202 | 82.02 ± 2.56 | 0.01−0.05 |
| Aldrin | 1.527 ± 0.012 | 10 | 9.451 | 94.51 ± 5.27 | 0.006−1 |
| p, p'- DDE | 0.902 ± 0.007 | 10 | 8.909 | 89.09 ± 6.23 | 0.02−5 |
| α-Chlordane | 1.776 ± 0.126 | 10 | 8.013 | 80.13 ± 4.88 | 0.002−0.5 |
| p, p'- DDD | 1.859 ± 0.214 | 10 | 10.686 | 106.86 ± 5.07 | 0.02−5 |
| Dieldrin | 1.105 ± 0.005 | 10 | 10.921 | 109.21 ± 3.95 | 0.006−1 |
| Heptachlor | 1.188 ± 0.015 | 10 | 9.174 | 91.74 ± 4.36 | 0.006−0.5 |
| Endrin | 1.436 ± 0.133 | 10 | 8.596 | 85.96 ± 4.83 | 0.05−0.1 |
| p, p'- DDT | 0.984 ± 0.008 | 10 | 9.339 | 93.39 ± 4.31 | 0.02−5 |
*Values are means of triplicate analysis ± s.d.
**Values are for edible plants and animals generally.
The field survey questionnaire administered to the farmers showed that all the 31 farmers have applied, at least, one pesticide on his cocoa plantation at one time or the other in the past (Table 2). The least applied pesticide was glyphosate (Touch down®), to which 5.56% of the responses was positive while the most regularly applied pesticide was cuprous oxide + metalaxy-M (Ridomil Gold EC®), to which about 89% of the farmers admitted regular usage of. Other pesticides that enjoyed fairly high percentage application patronage by the farmers were chlorpyrifos (Rocket®) (33%); (Perfect Killer 20EC®) (44%), copper hydroxide (Champ DP®) (39%); Fungurah OH® (44%), Ultimax Plus (44%), and lindane (Gamalin 20EC®) (61%). Further interactions with the farmers revealed that pesticides were applied by farmers for purposes other than the ones indicated by the manufacturers. For example, some farmers claimed that they use a mixture of aldrex (aldrin dust) and lindane to ward of many insects from their crops.
Table 2.
Commonly used pesticide types by cocoa farmers.
| Trade name | Active ingredients | Number of farmers | Percentage (%) |
|---|---|---|---|
| Gammalin 20 EC | Lindane | 19 | 61.1 |
| Ridomil Gold EC | Cuprous oxide + metalaxyl-M | 28 | 88.9 |
| Donwell | 7 | 22.2 | |
| Weed Off | Glyphosate | 5 | 16.7 |
| Ultimax Plus | 14 | 44.4 | |
| Funguran OH | Copper hydroxide | 14 | 44.4 |
| Red Force | 9 | 27.8 | |
| Perfect Killer 20EC | Chloryrifos | 14 | 44.4 |
| Kocide 101WP | Cuprous oxide | 3 | 11.1 |
| Champ DP | Copper hydroxide | 12 | 38.9 |
| Touch down | Glyphosate | 2 | 5.6 |
| Rocket 20EC | Chlorpyrifos | 10 | 33.3 |
Total number of farmers interviewed = 31.
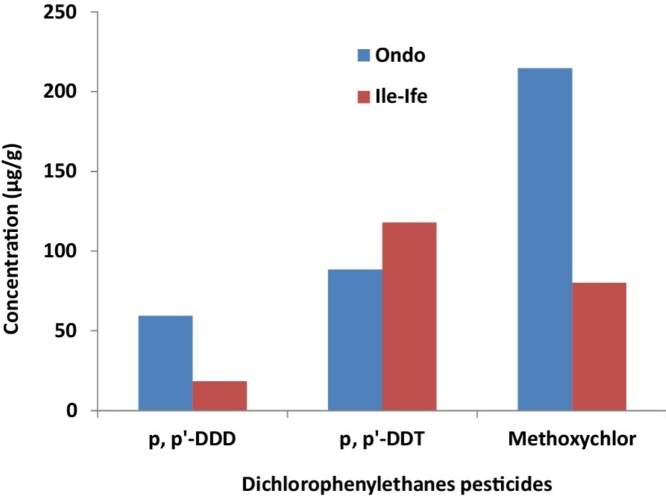
The OCPs investigated in this study belong to three broad classes, namely: dichlorodiphenylethanes, cyclodienes and chlorinated benzenes or cyclohexanes. Many of the OCPs species evaluated are breakdown products of the parent pesticide. For example, breakdown products of DDT include DDD and DDE. Levels of dichlorodiphenylethanes in cocoa beans from Ondo and Ile-Ife are presented in Table 3. The limit of detection determined ranged from 0.017 μg/g delta BHC to 0.405 μg/g methoxychlor. The levels (μg/g) of dichlorodiphenylethanes in cocoa beans from Ondo fell within the range of not detected (ND) as seen in p, p'-DDE and p, p'-DDD to 214.58 ± 3.40 for methoxychlor, while in cocoa beans from Ile-Ife, the range was from ND for p, p'-DDE to 236.54 ± 5.16 for p, p'-DDT. The total burden of dichlorodiphenylethanes (μg/g) in the cocoa bean samples obtained from Ondo ranged from 9.24 to 364.12, while in samples from Ile-Ife, the range was from 1.97 to 412.58. From their mean values, it was obvious that the most predominant dichlorodiphenylethane in cocoa beans from the two towns was p, p'-DDT, while the least occurring was p, p'-DDE.
Table 3.
Levels (μg/g) of Dichlorodiphenylethanes in Cocoa Beans Samples from Ondo and Ile-Ife.
| Ondo Samples | Ile-Ife Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | p, p'-DDE | p, p'-DDD | p, p'-DDT | Methoxychlor | Total OCPs burden | Code | p,p'-DDE | p, p'-DDD | p, p'-DDT | Methoxychlor | Total OCPs burden |
| O–1 | ND | ND | 18.86 ± 0.06 | 33.16 ± 1.02 | 52.02 ± 1.02 | I–1 | ND | 10.85 ± 0.15 | 40.62 ± 2.09 | 38.97 ± 1.04 | 90.44 ± 2.34 |
| O–2 | ND | ND | 25.03 ± 0.11 | 72.54 ± 1.14 | 97.57 ± 1.15 | I–2 | ND | 13.59 ± 0.18 | 85.99 ± 2.32 | 56.22 ± 1.22 | 155.80 ± 2.63 |
| O–3 | ND | ND | 141.37 ± 2.31 | 46.21 ± 0.12 | 187.58 ± 2.31 | I–3 | ND | 2.22 ± 0.04 | 24.23 ± 0.13 | 5.69 ± 0.08 | 32.14 ± 0.16 |
| O–4 | ND | 59.40 ± 2.14 | 88.38 ± 0.12 | 214.58 ± 3.40 | 362.36 ± 4.02 | I–4 | ND | 18.44 ± 0.16 | 117.88 ± 3.24 | 80.20 ± 2.03 | 216.52 ± 3.83 |
| O–5 | ND | 12.63 ± 0.12 | 143.81 ± 3.54 | 23.37 ± 0.15 | 179.81 ± 3.55 | I–5 | 6.78 ± 0.22 | 24.29 ± 0.19 | 236.54 ± 5.16 | 21.64 ± 1.02 | 289.25 ± 5.27 |
| O–6 | ND | ND | 89.96 ± 0.17 | 17.34 ± 0.24 | 107.30 ± 0.29 | I–6 | ND | 18.27 ± 0.17 | 183.22 ± 3.07 | 211.09 ± 5.21 | 412.58 ± 6.05 |
| O–7 | ND | 10.33 ± 0.12 | 72.43 ± 1.12 | 11.33 ± 0.08 | 94.09 ± 1.13 | I–7 | 1.32 ± 0.04 | 2.04 ± 0.12 | 2.14 ± 0.08 | 2.24 ± 0.07 | 7.74 ± 0.17 |
| O–8 | ND | 9.52 ± 0.13 | 80.44 ± 1.04 | 118.37 ± 4.14 | 208.33 ± 4.27 | I–8 | 0.29 ± 0.06 | 0.40 ± 0.03 | 0.61 ± 0.04 | 0.68 ± 0.03 | 1.98 ± 0.08 |
| O–9 | ND | 13.35 ± 0.13 | 138.84 ± 2.02 | 37.37 ± 1.03 | 189.56 ± 2.27 | I–9 | 0.42 ± 0.03 | 0.57 ± 0.08 | 0.57 ± 0.08 | 0.41 ± 0.02 | 1.97 ± 0.12 |
| O–10 | ND | 0.52 ± 0.04 | 2.33 ± 0.11 | 6.39 ± 0.21 | 9.24 ± 0.24 | I–10 | 0.45 ± 0.05 | 0.74 ± 0.05 | 0.56 ± 0.02 | 0.44 ± 0.03 | 2.19 ± 0.08 |
| O–11 | ND | 2.29 ± 0.08 | 16.09 ± 0.12 | 9.36 ± 0.26 | 27.74 ± 0.30 | I–11 | 0.46 ± 0.08 | 0.78 ± 0.03 | 0.47 ± 0.02 | 0.42 ± 0.04 | 2.13 ± 0.10 |
| O–12 | ND | 14.55 ± 0.15 | 165.52 ± 3.23 | 184.05 ± 3.49 | 364.12 ± 4.76 | I–12 | 0.20 ± 0.02 | 0.38 ± 0.02 | 0.28 ± 0.02 | 2.51 ± 0.08 | 3.37 ± 0.09 |
| O–13 | ND | 6.17 ± 0.11 | 85.15 ± 2.15 | 12.94 ± 0.04 | 104.26 ± 2.15 | Mean ± s.d. | 0.83 ± 1.91 | 7.71 ± 8.86 | 57.76 ± 81.48 | 35.04 ± 61.38 | 101.34 ± 102.41 |
| Mean ± s.d. | ND | 9.90 ± 5.93 | 82.17 ± 54.53 | 60.54 ± 69.19 | 152.61 ± 88.29 | Range | ND–6.78 | 0.38–24.29 | 0.28–236.54 | 0.41–211.09 | 1.97–412.58 |
| Range | ND | ND–59.40 | 2.33–165.52 | 6.39–214.58 | 9.24–364.12 | CV | 230.12 | 114.92 | 141.07 | 175.17 | 101.06 |
| CV | 160.91 | 66.36 | 114.29 | 73.82 | |||||||
The mean levels of p, p'-DDE detected in cocoa samples from Ondo (ND) and Ile-Ife (0.83 ± 1.91 μg/g) were higher than the 0.058 μg/g mean value of p, p'-DDE reported by Nsikak and Aruwajoye [37] in similar samples obtained from a local market in Ota, western Nigeria. Factors responsible for the observed difference might include difference in the families of plants involved, and hence, their systemic abilities to store the pesticides probably differ. Soil nature, season of planting, amount of pesticides applied and mode of contamination are some of the other factors that could account for the differences of pesticides found in a plant species. However, the 82.17 ± 54.53 μg/g mean concentration of p, p'-DDT detected in cocoa samples from Ondo was comparatively and significantly higher than the mean value (0.08 mg/kg) reported in an earlier finding [31] for cocoa beans from Ondo state. This confirmed that large volume of OCPs were possibly included in the pesticides used in cocoa plantations in the state. Levels of p, p'-DDT in the cocoa beans from Ondo and Ile-Ife were higher than that of its metabolites (p,p'-DDD and p,p'-DDD) suggesting that p, p'-DDT was one of the components of pesticides recently applied on the cocoa farms, and hence, was yet to undergo any appreciable transformation. Total dichlorodiphenylethanes burden in cocoa samples from Ondo ranged from 9.24 to 364.12 μg/g, while the range was between 1.97–412.58 μg/g in Ile-Ife samples. These values indicated an uneven distribution of dichlorodiphenylethanes in cocoa samples. The values of the coefficient of variation that were not less than 66 and were as high as 161 for Ondo samples or 230 for Ile-Ife samples further buttressed the wide variations of dichlorodiphenylethanes values from one sample to another.
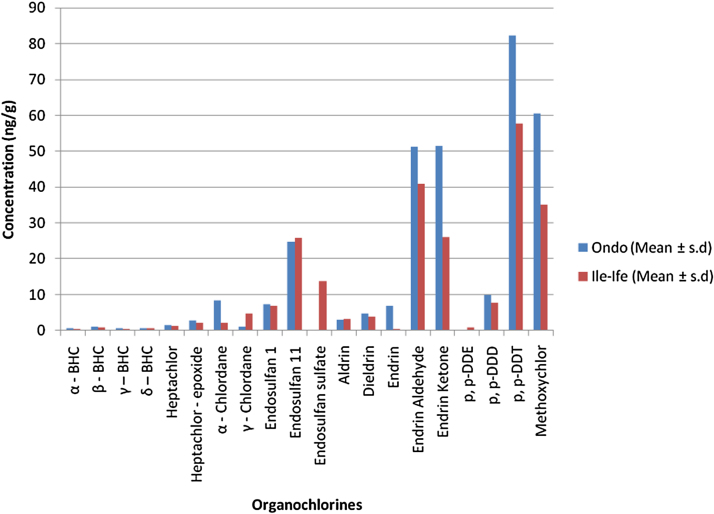
Table 4, Table 5 present the levels of cyclodienes (μg/g) in cocoa beans from Ondo and Ile-Ife, respectively. The mean levels of cyclodienes in cocoa beans obtained from Ondo ranged from 1.18 μg/g γ-chlordane to 51.56 μg/g endrin ketone, while the range was from 1.20 μg/g Heptachlor to 40.87 μg/g endrin aldehyde in cocoa beans from Ile-Ife. Obviously, endrin aldehyde and endrin ketone were the most predominant cyclodienes detected in the cocoa beans from Ondo and Ile-Ife, while the least occurring was either γ-chlordane (in Ondo samples) and Endrin (in Ife samples). The predominance of endrin aldehyde and endrin ketone could probably be as a result of biochemical transformations of parent OCPs to these metabolites. Also, levels of aldrin in the cocoa beans from Ondo and Ile-Ife were lower than those of its metabolite, dieldrin. This might imply that there had been in vivo metabolism of the original aldrin into dieldrin [38]. Mean concentration of endosulfan I (7.42 μg/g) obtained in cocoa beans samples from Ondo was higher than the 6.83 μg/g obtained in cocoa beans from Ile-Ife while the endosulfan II mean concentration value of 24.78 μg/g in samples from Ondo was lower than the 25.91 μg/g value in samples from Ile-Ife. Compared to the 0.55 mg/kg endosulfan I and 0.08 mg/kg endosulfan II average values in cocoa beans from Ondo state [31], the values obtained in the present study were significantly higher at p < 0.05. The similar and higher level of endosulfan recorded in cocoa samples in both location may be attributed the fact that the insecticide is now being used as a replacement for lindane which has been the only known insecticide for insect pest control in cocoa plantations in Nigeria for a long time.
Table 4.
Levels (μg/g) of cyclodienes in Cocoa Beans Samples from Ondo.
| Code | Heptachlor | Heptachlor – epoxide | α – Chlordane | γ – Chlordane | Endosulfan I | Endosulfan II | Endosulfan sulfate | Aldrin | Dieldrin | Endrin | Endrin Aldehyde | Endrin Ketone | Total OCP burden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O–1 | 0.80 ± 0.05 | 1.86 ± 0.05 | 4.83 ± 0.14 | ND | 3.09 ± 0.05 | 9.44± 0.06 | 6.42 ± 0.10 | 1.21 ± 0.04 | ND | 2.35 ± 0.08 | 15.49 ± 0.32 | 50.49 ± 3.05 | 95.98 ± 3.07 |
| O–2 | 0.81 ± 0.05 | 1.93 ± 0.06 | 5.33 ± 0.16 | 1.08 ± 0.04 | 6.07 ± 0.10 | 6.01 ± 0.04 | 4.81 ± 0.08 | 3.48 ± 0.10 | 3.72 ± 0.08 | 22.50 ± 2.12 | 39.16 ± 2.03 | 15.53 ± 1.12 | 110.43 ± 3.15 |
| O–3 | 1.61 ± 0.10 | 3.96 ± 0.15 | 20.48 ± 0.21 | 1.54 ± 0.06 | 12.97 ± 0.19 | 15.22 ±0.08 | 43.84 ± 3.10 | 3.36 ± 0.10 | ND | 52.36 ± 4.10 | 95.31 ± 5.11 | 299.41 ± 16.15 | 550.06 ± 7.71 |
| O–4 | 0.90 ± 0.06 | 2.35 ± 0.10 | 17.41 ± 0.20 | 2.84 ± 0.12 | ND | ND | 125.38 ± 5.13 | 4.45 ± 0.15 | 3.23 ± 0.06 | ND | ND | 58.87 ± 3.15 | 215.43 ± 6.03 |
| O–5 | 2.16 ± 0.22 | 3.45 ± 0.13 | 7.82 ± 0.16 | 3.10 ± 0.14 | 10.89 ± 0.16 | 48.89 ± 3.01 | 25.40 ± 2.00 | 5.28 ± 0.18 | 6.55 ± 0.14 | ND | 87.98 ± 5.06 | 39.67 ± 2.02 | 241.19 ± 6.55 |
| O–6 | 2.13 ± 0.20 | 4.58 ± 0.15 | 6.18 ± 0.16 | ND | 9.77 ± 0.15 | 39.09 ± 2.20 | 25.96 ± 2.03 | 3.25 ± 0.10 | 9.68 ± 0.21 | 10.38 ± 0.40 | 71.24 ± 4.04 | 37.10 ± 2.01 | 219.36 ± 5.44 |
| O–7 | 1.64 ± 0.12 | 3.10 ± 0.11 | 5.30 ± 0.15 | 0.86 ± 0.02 | 9.70 ± 0.15 | 34.59 ± 2.15 | 27.14 ± 2.04 | 3.78 ± 0.12 | 4.96 ± 0.12 | ND | 58.71 ± 3.13 | 30.82 ± 1.30 | 180.60 ± 4.51 |
| O–8 | 1.80 ± 0.15 | 3.20 ± 0.11 | 5.87 ± 0.15 | 0.91 ± 0.03 | 9.53 ± 0.14 | 36.20 ± 2.16 | 19.38 ± 1.31 | 3.09 ± 0.09 | 10.38 ± 0.24 | ND | 63.50 ± 3.01 | 28.92 ± 1.19 | 182.78 ± 4.12 |
| O–9 | 2.27 ± 0.25 | 3.62 ± 0.15 | 19.19 ± 0.21 | 1.52 ± 0.06 | 12.11 ± 0.17 | 46.69 ± 3.00 | 38.05 ± 3.05 | 3.30 ± 0.10 | ND | ND | 87.74 ± 5.04 | 45.15 ± 3.01 | 259.64 ± 7.28 |
| O–10 | 0.63 ± 0.04 | 0.59 ± 0.02 | 0.82 ± 0.02 | 0.39 ± 0.01 | 1.68 ± 0.02 | 3.75 ± 0.02 | 1.14 ± 0.02 | 0.95 ± 0.04 | 0.99 ± 0.02 | 0.99 ± 0.03 | 3.87 ± 0.08 | 6.07 ± 0.02 | 21.87 ± 0.12 |
| O–11 | 0.86 ± 0.05 | 1.21 ± 0.04 | 3.31 ± 0.11 | 1.54 ± 0.05 | 1.97 ± 0.02 | 7.44 ± 0.05 | 1.26 ± 0.02 | 1.02 ± 0.04 | 1.18 ± 0.03 | ND | 11.79 ± 0.23 | 4.61 ± 0.01 | 36.19 ± 0.28 |
| O–12 | 2.12 ± 0.20 | 3.92 ± 0.60 | 7.76 ± 0.17 | 1.54 ± 0.06 | 12.56 ± 0.17 | 49.29 ± 3.05 | 26.71 ± 2.02 | 3.49 ± 0.70 | 12.62 ± 0.25 | ND | 85.34 ± 5.04 | 33.38 ± 2.00 | 238.73 ± 6.62 |
| O–13 | 0.90 ± 0.05 | 2.60 ± 0.11 | 4.35 ± 0.12 | ND | 6.08 ± 0.10 | 25.57 ± 2.01 | 19.57 ± 1.30 | 1.82 ± 1.06 | 7.44 ± 0.16 | ND | 45.61 ± 2.13 | 20.23 ± 1.22 | 134.17 ± 3.60 |
| Mean ± s.d. | 1.43 ± 0.63 | 2.80 ± 1.17 | 8.36 ± 6.37 | 1.18 ± 1.00 | 7.42 ± 4.53 | 24.78 ± 18.55 | 28. 08 ± 32.19 | 2.96 ± 1.35 | 4.67 ± 4.34 | 6.81 ± 15.16 | 51.21 ± 34.49 | 51.56 ± 76.21 | 191.26 ±93.23 |
| Range | 0.63–2.27 | 0.59–3.96 | 0.82–20.48 | ND–0.39 | ND–12.97 | ND–49.29 | 1.14–125.38 | 0.95–5.28 | ND–12.62 | ND–52.36 | ND–87.98 | 4.61–299.41 | 36.19–550.06 |
| CV | 44.06 | 41.79 | 76.20 | 84.75 | 61.05 | 74.86 | 114.64 | 45.61 | 92.93 | 222.61 | 67.35 | 147.81 | 48.75 |
Table 5.
Levels (μg/g) of cyclodienes in cocoa beans samples from Ile-Ife.
| Code | Heptachlor | Heptachlor – epoxide | γ – Chlordane | α – Chlordane | Endosulfan I | Endosulfan II | Endosulfan sulfate | Aldrin | Dieldrin | Endrin | Endrin Aldehyde | Endrin Ketone | Total OCP burden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I–1 | 1.73 ± 0.14 | 3.26 ± 0.30 | 1.41 ± 0.08 | 6.43 ± 0.12 | 8.91 ± 1.01 | 38.9 ± 2.00 | 8.67 ± 0.36 | 2.18 ± 0.12 | 9.28 ± 0.24 | ND | 62.28 ± 2.14 | 25.24 ± 1.32 | 168.29 ± 3.83 |
| I–2 | 0.84 ± 0.06 | 3.94 ± 0.32 | 2.41 ± 0.10 | 7.84 ± 0.14 | 12.29 ± 1.03 | 48.22 ± 2.10 | 33.43 ± 0.02 | 9.82 ± 0.36 | 2.70 ± 0.15 | ND | 81.14 ± 2.23 | 40.22 ± 2.28 | 242.85 ± 3.99 |
| I–3 | 0.84 ± 0.06 | 1.00 ± 0.10 | 0.66 ± 0.04 | 3.53 ± 0.07 | 2.57 ± 0.07 | 9.14 ± 0.11 | 3.55 ± 0.15 | 1.77 ± 0.14 | ND | ND | 12.41 ± 0.18 | 13.77 ± 1.21 | 49.24 ± 1.26 |
| I–4 | 0.96 ± 0.07 | 5.01 ± 0.35 | 7.22 ± 0.31 | 11.51 ± 0.20 | 16.83 ± 1.23 | 68.96 ± 3.20 | 48.04 ± 2.05 | 10.24 ± 1.03 | 14.46 ± 1.02 | ND | 86.67 ± 2.31 | 73.14 ± 3.01 | 343.04 ± 5.72 |
| I–5 | 3.96 ± 0.31 | 6.13 ± 0.41 | 3.67 ± 0.15 | 12.67 ± 0.22 | 25.03 ± 2.01 | 83.8 ± 3.22 | 18.72 ± 1.01 | 5.52 ± 0.34 | 5.28 ± 0.17 | ND | 144.48 ± 3.14 | 88.38 ± 3.07 | 397.64 ± 5.93 |
| I–6 | 3.16 ± 0.30 | 5.32 ± 0.36 | 2.55 ± 0.12 | 11.07 ± 0.21 | 14.39 ± 1.20 | 58.54 ± 3.14 | 45.24 ± 2.04 | 4.06 ± 0.32 | 10.37 ± 0.33 | ND | 99.57 ± 2.25 | 48.58 ± 2.36 | 302.85 ± 5.16 |
| I–7 | 0.30 ± 0.02 | ND | 3.18 ± 0.15 | 1.48 ± 0.06 | 0.94 ± 0.04 | 1.53 ± 0.14 | 3.49 ± 0.13 | 2.40 ± 0.17 | 1.97 ± 0.10 | 2.18 ± 0.12 | 1.75 ± 0.09 | 11.39 ± 1.13 | 30.61 ± 1.18 |
| I–8 | 0.48 ± 0.03 | 0.30 ± 0.02 | 0.12 ± 0.04 | ND | 0.44 ± 0.02 | 0.56 ± 0.04 | 0.93 ± 0.06 | 0.54 ± 0.08 | 1.36 ± 0.06 | 0.82 ± 0.05 | 0.84 ± 0.05 | 1.99 ± 0.04 | 8.38 ± 0.16 |
| I–9 | 0.38 ± 0.02 | 0.30 ± 0.02 | 0.37 ± 0.03 | 0.43 ± 0.03 | 0.59 ± 0.03 | 0.37 ± 0.03 | 0.77 ± 0.04 | 0.29 ± 0.05 | ND | 0.39 ± 0.01 | 0.46 ± 0.04 | 1.89 ± 0.02 | 6.24 ± 0.10 |
| I–10 | 0.81 ± 0.06 | 0.33 ± 0.03 | 1.44 ± 0.07 | 0.49 ± 0.04 | ND | 0.37 ± 0.03 | 0.65 ± 0.04 | 0.26 ± 0.04 | ND | 0.42 ± 0.04 | 0.30 ± 0.02 | 2.23 ± 0.06 | 7.30 ± 0.14 |
| I–11 | 0.94 ± 0.07 | 0.37 ± 0.03 | 1.63 ± 0.08 | 0.51 ± 0.04 | ND | 0.38 ± 0.04 | 0.60 ± 0.03 | 0.36 ± 0.06 | ND | 0.43 ± 0.04 | 0.35 ± 0.04 | 2.38 ± 0.06 | 7.95 ± 0.16 |
| I–12 | 0.02 ± 0.01 | 0.14 ± 0.01 | 0.64 ± 0.03 | 0.26 ± 0.02 | ND | 0.20 ± 0.02 | 0.48 ± 0.02 | 0.13 ± 0.02 | ND | 0.25 ± 0.01 | 0.21 ± 0.02 | 4.60 ± 0.10 | 6.93 ± 0.11 |
| Mean ± s.d. | 1.20 ± 1.19 | 2.17 ± 2.37 | 2.11 ± 1.96 | 4.69 ± 4.96 | 6.83 ± 8.50 | 25.91 ± 31.73 | 13.71 ± 18.26 | 3.13 ± 3.63 | 3.79 ± 4.97 | 0.37 ± 0.63 | 40.87 ± 51.23 | 26.15 ± 30.04 | 130.94 ± 70.80 |
| Range | 0.02–3.96 | ND–6.13 | 0.12–7.22 | ND–12.67 | ND–25.03 | 0.20–68.96 | 0.48–48.04 | 0.13–10.24 | ND–14.46 | ND–2.18 | 0.21–144.48 | 1.89–88.38 | 6.24–397.64 |
| CV | 99.17 | 109.22 | 92.89 | 105.76 | 124.45 | 122.46 | 133.19 | 115.97 | 131.14 | 170.27 | 125.35 | 114.88 | 54.07 |
The total burden of cyclodienes (μg/g) in cocoa beans from Ondo and Ile-Ife had the respective ranges of 21.87–550.06 and 6.24–397.64. Generally, the mean levels of cyclodienes in the cocoa samples from both towns were in the order: Σendrins > Σendosulfans > Σchlordanes > dieldrin > Σheptachlors > aldrin. This somewhat similar trend might be indicative of similarity in the types and usages of pesticides by cocoa farmers in the two towns and proxy villages from where the cocoa beans were harvested. It has been noted that cyclodienes and some other OCPs are associated with severe seizures and possibility of inducing hepatic microsomal drug-metabolizing enzymes [39], [40]. At tissue levels of between 3 and 9 ppm in field animals, cyclodienes could lead to sublethal neurotoxic effects and death [41]. Although the levels of total cyclodienes were lower than those that produced sublethal neurotoxic effects and death in field animals, it must be stated that possible bioaccumulation could manifest in some health disorders. Besides, a single pesticide may be safe at a particular level, but concurrent occurrence of different pesticides may lead to unpleasant health effects of multiple exposures.
The levels of chlorinated benzenes in cocoa beans from Ondo and Ile-Ife are presented in Table 6. The mean levels (μg/g) of chlorinated benzenes ranged from 0.64 γ-BHC to 1.13 β-BHC in samples from Ondo town, while the range was between 0.41 α-BHC to 0.86 β-BHC in samples from Ile-Ife. The least predominant of the detected chlorinated benzenes was lindane (γ-BHC) with values of 0.64 ± 0.33 μg/g and 0.47 ± 0.32 μg/g for samples from Ondo and Ile-Ife, respectively. This shows that the use of lindane (Gammalin 20EC®, Capistox 20EC® and Kokotine 20EC®) have reduced drastically due to the introduction of endosulfan (Thiodan 35EC®, Endocel 35EC®), endosulfan + deltamethrin (Decis-Dan/Cracker 282EC®) and other insecticides. Lindane is one of the OCPs associated with severe seizures and fatalities [40] at elevated levels, but its rapid metabolic dispositions might lead to its low detection as witnessed in the present study. Lindane residues in samples from both Ondo and Ile-Ife were higher than the values reported by Owusu–Ansah et al. [42] for cocoa beans from the Twifo Praso district of Ghana, where there was no detectable lindane residue. This probably indicated that cocoa farmers around Ile-Ife and Ondo were using lindane for cocoa pest control. From Table 1, it can be seen that most of the OCPs detected had their levels below the recommended WHO/FAO-MRL values of between 0.006–1 μg/g in animal and plant food products [43]. Dichlorodiphenylethanes had values that fell within the range 0.006–1 μg/g (6–1000 μg/g) of WHO/FAO-MRL in most cases, a few of the cyclodienes from both Ondo and Ile-Ife had their value within the given WHO/FAO-MRL range, whereas majority of the cyclodienes fell below the range. Virtually all the chlorinated benzenes had values that were lower than the 0.006–1 μg/g range. It was noted from this study that the cocoa beans investigated contained dichlorodiphenylethanes and cyclodienes at a much higher level than chlorinated benzenes. From Fig. 1 it was observed that OCPs levels in cocoa beans from Ondo were higher than those found in cocoa beans from Ile-Ife. This further confirmed the earlier report [31] that Ondo state, being the highest producer of cocoa in Nigeria, might have attracted more pesticide marketers thereby leading to an influx of cocoa pesticides into the state.
Table 6.
Levels (μg/g) of Chlorinated Benzenes in Cocoa Beans Samples from Ondo and Ile-Ife.
| Ondo Samples | Ile-Ife Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | α-HCB | β-BHC | γ-BHC | δ-BHC | Total OCPs burden | Code | α-BHC | β-BHC | γ-BHC | δ-BHC | Total OCPs burden | |
| O–1 | 0.10 ± 0.01 | 0.32 ± 0.02 | 0.33 ± 0.02 | 0.15 ± 0.01 | 0.90 ± 0.03 | I–1 | 0.63 ± 0.04 | 1.51 ± 0.14 | 0.56 ± 0.04 | 0.66 ± 0.05 | 3.36 ± 0.16 | |
| O–2 | 0.16 ± 0.02 | 0.83 ± 0.04 | 0.33 ± 0.02 | 0.42 ± 0.04 | 1.74 ± 0.06 | I–2 | 0.48 ± 0.02 | 1.31 ± 0.12 | 0.73 ± 0.06 | 0.50 ± 0.04 | 3.02 ± 0.14 | |
| O–3 | 0.17 ± 0.02 | 1.54 ± 0.05 | 0.69 ± 0.04 | 3.37 ± 0.12 | 5.77 ± 0.14 | I–3 | 0.13 ± 0.01 | 0.31 ± 0.03 | 0.25 ± 0.01 | 1.57 ± 0.02 | 2.26 ± 0.04 | |
| O–4 | 0.46 ± 0.04 | 1.12 ± 0.05 | 0.45 ± 0.03 | 0.43 ± 0.03 | 2.46 ± 0.08 | I–4 | 0.21 ± 0.02 | 1.26 ± 0.12 | 0.63 ± 0.05 | 0.83 ± 0.07 | 2.93 ± 0.15 | |
| O–5 | 0.93 ± 0.08 | 1.77 ± 0.07 | 0.99 ± 0.06 | 0.32 ± 0.03 | 4.01 ± 0.13 | I–5 | 0.96 ± 0.08 | 1.73 ± 0.06 | 1.11 ± 0.01 | 0.58 ± 0.04 | 4.38 ± 0.11 | |
| O–6 | 3.10 ± 0.15 | 1.87 ± 0.07 | 1.3 ± 0.08 | 0.71 ± 0.15 | 6.98 ± 0.24 | I–6 | 1.67 ± 0.10 | 2.87 ± 0.21 | 0.92 ± 0.08 | 1.21 ± 0.09 | 6.67 ± 0.26 | |
| O–7 | 0.64 ± 0.07 | 1.37 ± 0.05 | 0.50 ± 0.05 | 0.65 ± 0.05 | 3.16 ± 0.11 | I–7 | 0.23 ± 0.12 | 0.63 ± 0.01 | 0.31 ± 0.03 | 1.28 ± 0.10 | 2.45 ± 0.16 | |
| O–8 | 1.37 ± 0.10 | 1.34 ± 0.02 | 0.88 ± 0.06 | 0.53 ± 0.04 | 4.12 ± 0.12 | I–8 | 0.08 ± 0.01 | 0.14 ± 0.01 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.50 ± 0.03 | |
| O–9 | 0.35 ± 0.04 | 1.59 ± 0.08 | 0.96 ± 0.08 | 0.33 ± 0.02 | 3.23 ± 0.12 | I–9 | 0.17 ± 0.02 | 0.13 ± 0.02 | 0.23 ± 0.03 | 0.13 ± 0.01 | 0.66 ± 0.04 | |
| O–10 | 0.94 ± 0.08 | 0.56 ± 0.04 | 0.37 ± 0.04 | 0.35 ± 0.03 | 2.22 ± 0.10 | I–10 | 0.14 ± 0.02 | 0.23 ± 0.02 | 0.35 ± 0.05 | 0.22 ± 0.02 | 0.94 ± 0.06 | |
| O–11 | 0.34 ± 0.04 | 0.46 ± 0.04 | 0.34 ± 0.04 | 0.46 ± 0.05 | 1.60 ± 0.09 | I–11 | 0.11 ± 0.01 | 0.17 ± 0.02 | 0.36 ± 0.04 | 0.22 ± 0.02 | 0.86 ± 0.05 | |
| O–12 | 0.70 ± 0.04 | 1.07 ± 0.06 | 0.86 ± 0.08 | 0.62 ± 0.08 | 3.25 ± 0.13 | I–12 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.23 ± 0.03 | 0.43 ± 0.03 | |
| O–13 | 0.14 ± 0.01 | 0.87 ± 0.06 | 0.30 ± 0.03 | 0.06 ± 0.01 | 1.37 ± 0.07 | Mean ± s.d. | 0.41 ± 0.48 | 0.86 ± 0.88 | 0.47 ± 0.32 | 0.63 ± 0.50 | 2.37 ± 1.16 | |
| Mean ± s.d | 0.72 ± 0.81 | 1.13 ± 0.50 | 0.64 ± 0.33 | 0.65 ± 0.84 | 3.14 ± 1.31 | Range | 0.07–1.67 | 0.05–2.87 | 0.08–1.11 | 0.12–1.57 | 0.43–6.67 | |
| Range | 0.10–3.10 | 0.32–1.87 | 0.30–1.30 | 0.06–3.37 | 0.90–6.98 | CV | 117.07 | 102.33 | 68.09 | 79.37 | 48.95 | |
| CV | 112.5 | 44.25 | 51.56 | 129.23 | 41.72 | |||||||
Fig. 1.
The Mean Concentration Values (ng/g) of Organochlorines in Cocoa Beans obtained from Ondo and Ile-Ife.
The correlation coefficients of OCPs in cocoa beans from Ondo (Table 7) showed positive significant correlations in 54 out of 190 correlation pairs at both 0.01 and 0.05 levels, amounting to 28.4%, while the correlation coefficients of OCPs in cocoa beans from Ile-Ife (Table 8) showed positive significant correlations in 131 out of 210 correlation pairs at both 0.01 and 0.05 levels, amounting to 62.4%. Positive significant correlations existing between a pair of OCPs imply that the affected OCPs probably have the same sources, or were proportionally affected by similar environmental factors.
Table 7.
Correlation coefficient of OCPs in cocoa beans from Ondo.
| OPCs | α – BHC | β – BHC | γ – BHC | δ – BHC | Heptachlor | Hept achlor epoxide | α – Chlordane | γ – Chlordane | Endosulfan I | Endosulfan II | Endosulfan sulfate | Aldrin | Dieldrin | Endrin | Endrin Aldehyde | Endrin Ketone | p, p'-DDD | p, p'-DDT | Methoxychlor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α – BHC | 1.000 | ||||||||||||||||||
| β – BHC | 0.526 | 1.000 | |||||||||||||||||
| γ – BHC | 0.729** | 0.845** | 1.000 | ||||||||||||||||
| δ – BHC | −0.066 | 0.337 | 0.160 | 1.000 | |||||||||||||||
| Heptachlor | 0.466 | 0.848** | 0.914** | 0.189 | 1.000 | ||||||||||||||
| Heptachlor epoxide | 0.466 | 0.862** | 0.827** | 0.387 | 0.880** | 1.000 | |||||||||||||
| α – Chlordane | −0.213 | 0.499 | 0.293 | 0.560* | 0.369 | 0.487 | 1.000 | ||||||||||||
| γ – Chlordane | −0.222 | 0.331 | 0.170 | 0.129 | 0.264 | 0.136 | 0.516 | 1.000 | |||||||||||
| Endosulfan I | −0.217 | 0.744** | 0.709** | 0.435 | 0.874** | 0.852** | 0.358 | 0.094 | 1.000 | ||||||||||
| Endosulfan II | 0.390 | 0.690** | 0.780 | −0.084 | 0.918** | 0.764** | 0.094 | 0.109 | 0.822** | 1.000 | |||||||||
| Endosulfan sulfate | −0.042 | 0.334 | 0.103 | 0.168 | 0.088 | 0.258 | 0.703** | 0.570* | −0.110 | −0.110 | 1.000 | ||||||||
| Aldrin | 0.170 | 0.771** | 0.528 | 0.169 | 0.612* | 0.637* | 0.501 | 0.688** | 0.508 | 0.476 | 0.557* | 1.000 | |||||||
| Dieldrin | 0.541 | 0.360 | 0.492 | −0.216 | 0.475 | 0.511 | −0.296 | −0.053 | 0.392 | 0.608* | −0.026 | 0.346 | 1.000 | ||||||
| Endrin | −0.124 | 0.217 | 0.027 | 0.895** | 0.002 | 0.261 | 0.456 | 0.005 | 0.326 | −0.246 | 0.034 | 0.122 | −0.290 | 1.000 | |||||
| Endrin Aldehyde | 0.222 | 0.786** | 0.739** | 0.437 | 0.884** | 0.871** | 0.406 | 0.170 | 0.988** | 0.823** | −0.059 | 0.552 | 0.381 | 0.337 | 1.000 | ||||
| Endrin Ketone | −0.187 | 0.324 | 0.124 | 0.952** | 0.164 | 0.396 | 0.684** | 0.176 | 0.396 | −0.097 | 0.288 | 0.203 | −0.309 | 0.863** | 0.418 | 1.000 | |||
| p, p'-DDD | −0.112 | 0.142 | −0.020 | −0.167 | −0.006 | 0.044 | 0.469 | 0.630* | −0.276 | −0.100 | 0.917** | 0.499 | 0.067 | −0.303 | −0.235 | −0.056 | 1.000 | ||
| p, p'-DDT | 0.097 | 0.746** | 0.668* | 0.362 | 0.817** | 0.844** | 0.634* | 0.475 | 0.792** | 0.735** | 0.400 | 0.687** | 0.400 | 0.168 | 0.835** | 0.421 | 0.271 | 1.000 | |
| Methoxychlor | −0.074 | 0.036 | 0.073 | −0.002 | 0.082 | 0.172 | 0.366 | 0.457 | −0.043 | −0.020 | 0.660* | 0.421 | 0.368 | −0.083 | −0.048 | 0.035 | 0.739** | 0.341 | 1.000 |
*Correlation is significant at the 0.05 level (two – tailed); ** Correlation is significant at the 0.01 level (two – tailed); n = 13.
Table 8.
Correlation coefficient of OCPs in cocoa beans from Ile-Ife.
| OCs | α – BHC | β – BHC | γ – BHC | δ – BHC | Heptachlor | Hept.epoxide | α − Chlordane | γ − Chlordane | Endosulfan I | Endosulfan II | Endo.sulfate | Aldrin | Dieldrin | Endrin | Endrin aldehyde | Endrin Ketone | p,p'-DDE | p,p'-DDD | p,p'-DDT | Methoxychlor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α – BHC | 1.000 | |||||||||||||||||||
| β – BHC | 0.939** | 1.000 | ||||||||||||||||||
| γ – BHC | 0.815** | 0.882** | 1.000 | |||||||||||||||||
| δ – BHC | 0.358 | 0.436 | 0.257 | 1.000 | ||||||||||||||||
| Heptachlor | 0.857** | 0.808** | 0.894** | 0.246 | 1.000 | |||||||||||||||
| Hept.epoxide | 0.755** | 0.885** | 0.939** | 0.285 | 0.810** | 1.000 | ||||||||||||||
| α – Chlordane | 0.726** | 0.876** | 0.919** | 0.401 | 0.781** | 0.989** | 1.000 | |||||||||||||
| γ – Chlordane | 0.228 | 0.482 | 0.569 | 0.319 | 0.310 | 0.650* | 0.711** | 1.000 | ||||||||||||
| Endosulfan I | 0.678* | 0.805** | 0.933** | 0.252 | 0.814** | 0.976** | 0.971** | 0.677* | 1.000 | |||||||||||
| Endosulfan II | 0.690* | 0.840** | 0.926** | 0.274 | 0.784** | 0.992** | 0.988** | 0.704** | 0.991** | 1.000 | ||||||||||
| Endo.sulfate | 0.620* | 0.801** | 0.728** | 0.351 | 0.477 | 0.846** | 0.860** | 0.742** | 0.764** | 0.824** | 1.000 | |||||||||
| Aldrin | 0.313 | 0.575 | 0.664* | 0.275 | 0.305 | 0.772** | 0.798** | 0.792** | 0.765** | 0.799** | 0.860** | 1.000 | ||||||||
| Dieldrin | 0.558 | 0.768** | 0.629* | 0.339 | 0.499 | 0.791** | 0.808** | 0.750** | 0.716** | 0.787** | 0.820** | 0.652* | 1.0000 | |||||||
| Endrin | −0.323 | −0.354 | −0.414 | 0.105 | −0.419 | −0.555 | −0.502 | −0.061 | −0.478 | −0.512 | −0.415 | −0.319 | −0.338 | 1.000 | ||||||
| Endrin aldehyde | 0.754** | 0.863** | 0.958** | 0.255 | 0.845** | 0.987** | 0.972** | 0.610* | 0.990** | 0.987** | 0.773** | 0.740** | 0.712** | −0.505 | 1.000 | |||||
| Endrin Ketone | 0.597* | 0.750** | 0.885** | 0.298 | 0.748** | 0.947** | 0.962** | 0.776** | 0.984** | 0.976** | 0.783** | 0.798** | 0.736** | −0.421 | .954** | 1.000 | ||||
| p,p'-DDE | 0.297 | 0.232 | 0.553 | −0.027 | 0.654* | 0.412 | 0.403 | 0.239 | 0.579* | 0.470 | −0.008 | 0.133 | 0.012 | 0.000 | 0.536 | 0.568 | 1.000 | |||
| p,p'-DDD | 0.737** | .869** | 0.947** | 0.287 | 0.815** | 0.992** | 0.987** | 0.693* | 0.991** | 0.996** | 0.822** | 0.775** | 0.776** | −0.473 | 0.992** | 0.971** | 0.494 | 1.000 | ||
| p,p'-DDT | 0.805** | .849** | 0.943** | 0.296 | 0.898** | 0.943** | 0.936** | 0.577* | 0.957** | 0.938** | 0.745** | 0.636* | 0.639* | −0.451 | 0.961** | 0.934** | 0.604* | 0.956** | 1.000 | |
| Methoxychlor | 0.839** | .872** | 0.627* | 0.407 | 0.553 | 0.686* | 0.681* | 0.388 | 0.542 | 0.611* | 0.834** | 0.463 | 0.712** | −0.356 | 0.606* | 0.513 | −0.152 | 0.639* | 0.646* | 1.000 |
*Correlation is significant at the 0.05 level (2-tailed); **Correlation is significant at the 0.01 level (2-tailed); n = 12.
4. Conclusion
This study has shown that harvested cocoa beans from Ondo and Ile-Ife towns were contaminated with OCPs in amounts that indicated that the pesticides are still widely in use among cocoa farmers in these regions. Most of the OCPs were found to be at higher levels in cocoa beans from Ondo than in cocoa beans from Ile-Ife. The occurrence of OCPs in cocoa beans from Ondo and Ile-Ife corroborates the fact that cocoa farmers in these areas use high volume of pesticides to control pests and diseases in their plantations. Further work on pesticide residues analysis of blood serum of the farmers, their municipal water sources and cocoa-based food products are recommended.
References
- 1.United Nations Environment Programme (UNEP) 2003. Regionally Based Assessment of Persistent Toxic Substances. Mediterranean Regional Report, United Nations Environment Programme on Chemicals. www.chem.unep.ch/pts/regreports/mediterranean.pdf (Accesed April, 2015. [Google Scholar]
- 2.Aiyesanmi A.F., Idowu G.A. Organochlorine pesticides residues in soil of cocoa farms in Ondo state central district, Nigeria. Environ. Nat. Resour. Res. 2012;2(2):65–73. [Google Scholar]
- 3.Colborn T., Dumanoski D., Meyers J.P. Dutton; New York, USA: 1996. Our Stolen Future. [Google Scholar]
- 4.Helberg M., Bustnes J.O., Erikstad K.E., Kristiansen K.O., Skaare J.U. Relationships between reproductive performance and organochlorine contaminants in great black- backed gulls (Larus marinus) Environ. Pollut. 2005;134:475–483. doi: 10.1016/j.envpol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Dos Santos J.S., Schwanz T.G., Coelho A.N., Heck-Marques M.C., Mexia M.M., Emanuelli T., Costabeber I. Estimated daily intake of organochlorine pesticides from dairy products in Brazil. Food Control. 2015;53:23–28. [Google Scholar]
- 6.Alavanja M., Samanic C., Dosemici M., Lubin J., Tarone R., Lynch C., Knott C., Thomas K., Hoppin J., Barker J., Coble J., Sandler D., Blair A. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am. J. Epidemiol. 2003;157(9):800–814. doi: 10.1093/aje/kwg040. [DOI] [PubMed] [Google Scholar]
- 7.Settimi L.A., Mashina A., Andrion A., Axelson O. Prostate cancer and exposure to pesticides in agricultural setting. Int. J. Cancer. 2003;104:458–461. doi: 10.1002/ijc.10955. [DOI] [PubMed] [Google Scholar]
- 8.Figa-Talamanca I., Mearelli I., Valente P., Bascherini S. Cancer mortality in a cohort of rural licensed pesticide users in the province of Rome. Int. J. Epidemiol. 1993;22:579–583. doi: 10.1093/ije/22.4.579. [DOI] [PubMed] [Google Scholar]
- 9.Beard J., Sladden T., Morgan G., Berry G., Brooks L., McMicheal A. Health impacts of pesticide exposure in a cohort of outdoor workers. Environ. Health Perspect. 2003;111:724–730.. doi: 10.1289/ehp.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toft G., Hagmar L., Giwercman A., Bonde J.P. Epidemiological evidence on reproductive effects of persistent organochlorines in humans. Reprod. Toxicol. 2004;19:5–26. doi: 10.1016/j.reprotox.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Colborn T. A case for visiting the safety of pesticides: a closer look at neurodevelopment. Environ. Health Perspect. 2006;114(1):10–17. doi: 10.1289/ehp.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yucra S., Rubio J., Casco M., Gonzales C., Steenland K., Gonzales G.F. Semen quality and reproductive sex hormones levels in Peruvian Pesticide sprayers. Int. J. Occup. Res. 2006;63(2):295–300. doi: 10.1179/oeh.2006.12.4.355. [DOI] [PubMed] [Google Scholar]
- 13.Barlow S.M. Agricultural Chemicals and Endocrine mediated chronic toxicity or carcinogenicity. Scand. J. Work Environ. Health. 2005;31:119–122. [PubMed] [Google Scholar]
- 14.Galloway T., Handy R. Immunotoxicology of organophosphorus pesticides. Ecotoxiology. 2003;12(1–4):343–363. doi: 10.1023/a:1022579416322. [DOI] [PubMed] [Google Scholar]
- 15.Kamel F., Hoppin J.A. Association of Pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect. 2004;112(9):950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CODEX, Report of the twenty-seven session, Geneva, Switzerland, (2004) 28 June-3 July.
- 17.Asogwa E.U., Dongo L.N. Problems associated with pesticide usage and application in Nigerian cocoa production: a review. Afr. J. Agric. Res. 2009;4(8):675–683. [Google Scholar]
- 18.Faturoti B.O., Madukwe M.C., Ogunedojutimi O., Anyanwu L. Socioeconomic Impact of SARO agro-allied organic cocoa programme on beneficiary cocoa farmers in Nigeria. J. Agric. Ext. Rural Dev. 2012;4(16):435–445. [Google Scholar]
- 19.Hassan O.M. Impact of non-oil sector on economic growth in Nigeria. Ilorin J. Marketing. 2015;1(1):183–194. [Google Scholar]
- 20.United Nations Conference on Trade and Development (UNCTAD) 2004. World Investment Report 2004: The Shift Towards Services (Overview) www.unctad.org/wir. [Google Scholar]
- 21.Aikpokpodion P.E. Nutrients dynamics in cocoa soils, leaf, and beans in Ondo State, Nigeria. J. Agric. Sci. 2010;1(1):1–9. [Google Scholar]
- 22.Ajayi J.R., Afolabi M.O., Ogunbodede E.F., Sunday A.G. Modeling rainfall as a constraining factor for cocoa yield in Ondo State. Am. J. Sci. Ind. Res. 2010;1(2):127–134. [Google Scholar]
- 23.Sonwa D.J., Weise S., Adesina A., Nkongmeneck A.B., Tchatat M., Ndoye O. Production constraints on cocoa agroforestry systems in west and central Africa: the need for integrated pest management and multi-institutional approaches. Forest. Chron. 2005;81(3):345–349. [Google Scholar]
- 24.World Cocoa Foundation . 2014. Cocoa Market Update. http://worldcocoafoundation.org/wp-content/uploads/Cocoa-Market-Update-as-of-4-1-2014.pdf. [Google Scholar]
- 25.Aikpokpodion P., Lajide L., Aiyesanmi A.F., Lacorte S. Residues of Dichlorodiphenylethane (DDT) and its metabolites in cocoa beans from three Cocoa Ecological zones in Nigeria. Eur. J. Appl. Sci. 2012;4(2):52–57. [Google Scholar]
- 26.Ogunfowokan A.O., Oyekunle J.A.O., Torto N., Akanni M.S. A study on persistent organochlorine pesticide residues in fish tissues and water from an agricultural fish pond. Emir. J. Food Agric. 2012;24(2):165–184. [Google Scholar]
- 27.Awofolu R.O., Fatoki O.S. Persistent organochlorine pesticide residues in freshwater systems and sediments from Eastern Cape, South Africa. Water SA. 2003;29(3):323–330. [Google Scholar]
- 28.Sosan M.B., Akingbohungbe A.E., Ojo I.A.O., Durosinmi M.A. Insecticide residue in the blood serum and domestic water source of cacao farmers in southwestern Nigeria. Chemosphere. 2008;72:781–784. doi: 10.1016/j.chemosphere.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Adeyemi D., Anyakora C., Ukpo G., Adebayo A. Evaluation of the levels of Organochlorine Pesticide Residues in water samples of Lagos Lagoon using solid phase extraction method. J. Environ. Chem. Ecotoxicol. 2008;3(6):160–166. [Google Scholar]
- 30.Bentum J.K., Essumang D.K., Dodoo D.K. Lindane and Propoxur Residues in the top soil of some cocoa growing areas in five districts of the central region of Ghana. Bull. Chem. Soc. Ethiop. 2006;20(2):193–199. [Google Scholar]
- 31.Aikpokpodion P., Lajide L., Aiyesanmi A.F., Lacorte S., Grimalt J., Carretero J. Residues of Diazinon and Endosulfan in Cocoa beans from three Cocoa Ecological zones in Nigeria. Eur. J. Appl. Sci. 2012;4(6):265–272. [Google Scholar]
- 32.Oyekunle J.A.O., Ogunfowokan A.O., Torto N., Akanni M.S. Determination of organochlorine pesticides in the agricultural soil of Oke-Osun farm settlement, Osogbo, Nigeria. Environ. Monit. Assess. 2011;175:51–61. doi: 10.1007/s10661-010-1617-y. [DOI] [PubMed] [Google Scholar]
- 33.Okoya A.A., Torto N., Ogunfowokan A.O., Asubiojo O.I. Organochlorine (OC) pesticide residues in soils of major cocoa plantations in Ondo State, Southwestern Nigeria. Afr. J. Agric. Res. 2013;8(28):3842–3848. [Google Scholar]
- 34.Olufade Y.A., Sosan M.B., Oyekunle J.A.O. Levels of organochlorine insecticide residues in cowpea grains and dried yam chips from markets in Ile-Ife, Southwestern Nigeria: a preliminary survey. Ife J. Sci. 2014;16(2):161–170. [Google Scholar]
- 35.Ogunfowokan A.O., Asubiojo O.I., Fatoki O.S. Isolation and determination of polycyclic aromatic hydrocarbons in surface runoff and sediment. Water Air Soil Pollut. 2003;147:245–261. [Google Scholar]
- 36.European Union . Laying down Minimum Standards for the Protection. 1999. European union directive 1999/74/EC. [Google Scholar]
- 37.Nsikak U.B., Aruwajoye O.I. Assessment of contamination by organochlorine pesticides in Solanum lycopersicum L. and Capsicum annuum: a market survey in Nigeria. Afr. J. Environ. Sci. Technol. 2011;5(6):437–442. [Google Scholar]
- 38.Fucks R.A., Schröder R. Agents for control of animal pests. In: Büchel K.H., editor. Chemistry of Pesticides. John Wiley & Sons; NY: 1983. pp. 9–226. [Google Scholar]
- 39.Hunter J., Maxwell J.D., Stewart D.A., Williams R., Robinson J., Richardson A. Increased hepatic microsomal enzyme activity from occupational exposure to certain organochlorine pesticides. Nature. 1972;237(5355):399–401. doi: 10.1038/237399a0. [DOI] [PubMed] [Google Scholar]
- 40.Echobichon D.J. Toxic effects of pesticides. In: Klaassen C.D., editor. Casarett & Doull’s Toxicology: The Basic Science of Poisons. 5th edition. McGraw-Hill; New York: 1996. pp. 649–655. [Google Scholar]
- 41.Walker C.H. second edition. CRC, Press, Taylor & Francis Group; 2009. Organic Pollutants: An Ecotoxicological Perspective. [Google Scholar]
- 42.Owusu-Ansah E., Koranteng- Addo J.E., Boampnsem L.K., Menlah E., Abole E. Assessment of Lindane pesticide residue in cocoa beans in the Twifo Praso district of Ghana. J. Chem. Pharm. Res. 2010;2(4):580–587. [Google Scholar]
- 43.WHO/FAO-MRL Codex Alimentarius . Pesticide Residues in Food and Feed. 2015. International food standards. Online Database, www.fao.org/fao-who-codexalimentarius/standards/pesticides. [Google Scholar]