Graphical abstract
Abbreviations: CAT, catalase; GPx, glutathione peroxidase; H2O2, hydrogen peroxide; MDA, malondialdehyde; NaCl, sodium chloride; ROS, reactive oxygen species; –SH, sulfhydryl groups; SOD, superoxide dismutase
Keywords: Vinblastine, Gastrointestinal disorders, Constipation, Oxidative stress, Intracellular mediators, Rat
Highlights
-
•
Vinblastine, an anticancer drug, induced constipation in rat.
-
•
Vinblastine induced oxidative stress in intestine in rat.
-
•
Vinblastine provoked a disturbance of intracellular mediators in rat.
Abstract
The purpose of this study is to examine the gastrointestinal disorders after injection of vinblastine (2 mg kg−1 b.w. i.v.) in rats. Animals were divided into two equal groups: Group 1 was considered as a control group (NaCl, 0.9%). Group 2 was treated with intravenous injection of vinblastine for 7 days. Loperamide (2 mg kg−1) was injected in a saline solution subcutaneously to induce constipation in another group of rats during the same period. Fecal parameters of the different groups have been determined. At the end of the experiment, animals were anaesthetized and sacrificed by decapitation. The intestinal mucosa specimens were examined for lipid peroxidation, sulfhydryl groups (−SH) and protein carbonylation as well as antioxidant enzyme activities and intracellular mediators. Gastrointestinal motility was realized by the test meal (10% charcoal in 5% gum arabic). In result, statistically significant decreases in the fecal number and water content collected during 24 h were detected in the vinblastine group, but less important than loperamide control group. The animals treated with vinblastine, showed also a significant decrease (13%) of GIT, lower than that of loperamide (34%). The intestinal tissues from vinblastine-treated rats were showed a significant increase in lipoperoxydation and H2O2 production as well as a significant depletion of enzymatic and non-enzymatic antioxidants. Added to that, a disruption of intracellular iron and calcium levels was observed. Therefore, the present study provide the first strong evidence that vinblastine induced numerous disruptions in gastrointestinal which are related to oxidative stress and intracellular mediators disorders.
1. Introduction
The magnitude of use of chemotherapeutic agents has demonstrated therapeutic benefits in a wide variety of cancers including gastrointestinal (GI) cancers from the esophagus to the large intestine [1]. However, the gastrointestinal disturbance produced by these xenobiotics is occurring more frequently and with greater toxicological significance than previously thought. Although there are some preliminary clinical studies and reports, there does not appear to be an extensive examination of gastrointestinal damage of numerous chemotherapeutic agents in the rat [2]. Among these agents, include vinblastine which is an anti-cancer or chemotherapy drug used to prevent and treat numerous cancers such as lung cancer, breast cancer and lymphoma. However, it is well known to be associated with various side effects, with toxicity to the GI tract being a major clinical concern. In addition, the chemotherapy can result in the generation of excess ROS/RNS in the cytochrome P450 monooxygenase system. On another hand, the enzyme systems, such as the xanthine-oxidase system, and non-enzymatic mechanisms also play a role in creating excess oxidative stress during chemotherapy [3], [4].
Constipation is a common problem in public health which marked with feces remain in the colon for prolonged periods of time, leading to water absorption, hardening of stool and excessive straining [5]. It is a risk factor of colorectal cancer [6]. As a drug inducing constipation, the loperamide inhibits intestinal water secretion [7] and colonic peristalsis [8], which extends the fecal evacuation time and delays intestinal luminal transit [9].
The present research was therefore undertaken to study the effect of vinblastine on gastrointestinal motility in relation to oxidative stress in rat.
2. Materials and methods
2.1. Chemicals and reagents
Charcoal meal, Gum arabic, Hydrochloric acid (HCl), trichloroacetic acid (TCA), 2-Thio-barbituric acid (TBA), nicotinamide adenine dinucleotide, sodium pentobarbital, p-hydroxybenzoic acid and butylated hydroxytoluene (BHT) were procured from Sigma chemicals Co (Germany). The vinblastine and loperamide were purchased from local pharmacy. All other chemicals used were of analytical grade.
2.2. Experimental animal groups and ethic statement
Wistar rats weighing 200–230 g (SIPHAT, Ben-Arours, Tunisia) were kept in cages under standard laboratory conditions with tap water and standard ad libitum, in a 12-h/12-h light/dark cycle at a temperature between 21 and 23 °C. All experiments were performed according with the local ethics committee of Tunis University for the use and care of animals in accordance with the NIH recommendations. All experiments were performed at the same time of day (9 h), to avoid the circadian rhythm impacts.
To evaluate the effect of vinblastine on small intestinal motility, the animals were randomly divided into a control group (group I, 10 mL kg−1 of saline solution) and two other experimental groups, a standard (loperamide) and vinblastine groups. There were eight animals assigned to each group. The rats (group II) were injected with loperamide (2 mg kg−1 of body weight) in a saline solution subcutaneously once per day for 7 days. In parallel, animals of group III were injected by vinblastine (2 mg kg−1 of body weight) in a saline solution intravenously under the same conditions as loperamide. We measured the body weight gain, food intake and water intake during the same period.
On day 5 and during 24 h, the wet and dry weights of fecal pellets of the animals were collected and measured. The total number, wet weight and water content of the fecal pellets were determined. The water content was determined by drying fecal pellets at 70 °C for 24 h in an oven and calculating the difference between the weight before and after drying [10]. The water content was calculated as follows:
| Fecal water content (%) = [(fecal wet weight − fecal dry weight)/fecal wet weight] × 100. |
On the last day, animals were anaesthetized by intraperitoneal injection of sodium pentobarbital (40 mg kg−1) and sacrificed by decapitation. The mucosal intestinal specimens were scraped and then placed in a phosphate buffered saline (PBS) solution, homogenized and centrifuged for 15 min at 9000 × g. Supernatants were stored at −80 °C for the determination of biochemical parameters.
2.3. Gastrointestinal transit
The effect of vinblastine and loperamide on movement rate in the intestinal tract was evaluated by the method elucidated by Yu et al. [11] with some changes. Animals fasted for 16 h prior the experiment, but consumed water ad libitum. At 15 min after treatment, different groups of rats received the charcoal meal (10% charcoal in 5% gum arabic). After 30 min, the rats were scarified and the intestinal tract was excised. The distance traveled by the charcoal meal from the pylorus was measured and expressed as a percentage of the total length of the small intestine from the gastro-pyloric junction to the ileocecal junction as follows:
| T (%) = B/A × 100 |
Where T is the intestinal tract motility percent, A is the total length of the intestinal tract, and B is the moving distance of the most distal end portion of the charcoal.
2.4. Serum lipids
To evaluate the content of lipids in the serum such as triglycerides, total cholesterol and high-density lipoprotein (HDL) cholesterol, the experimental animals were anesthetized on the last day of the experiment, and blood was collected from the abdominal vein via laparotomy. Serum was obtained from the collected blood by centrifugation to 2000 × g for 10 min. Triglycerides, total cholesterol, and HDL cholesterol were measured using commercially available kits from Biomaghreb, Tunisia.
2.5. MDA, −SH and protein carbonyls measurement
In intestine mucosa the MDA level was measured according to the double heating method [12]. Briefly, the aliquots from intestine tissue homogenates were mixed with BHT–TCA solution containing 1% BHT (w/v) dissolved in 20% TCA (w/v) and centrifuged at 1000 × g for 5 min at 4 °C. The supernatant was blended with 0.5 N HCl and 120 mM TBA in 26 mM Tris and then heated at 80 °C for 10 min. Afer cooling, absorbance of the resulting chromophore was determined at 532 nm using a UV–vis spectrophotometer (Beckman DU 640B). MDA levels were determined by using an extinction coefficient for the MDA–TBA complex of 1.56 × 105 M−1 cm−1. Thiol groups (−SH) level was performed according to Ellman’s method [13]. The results were expressed as nmol of thiol groups per mg of proteins. Oxidative damage to proteins induced by vinblastine in mucosal intestine was assessed by estimating the protein carbonylation according to Levine et al. [14]. Results were expressed as μmol carbonyl residues/mg proteins.
2.6. Antioxidant enzyme activities estimations
The activity of superoxide dismutase (SOD) was determined by the method of inhibition of the nicotinamide adenine dinucleotide (reduced) phenazinemetho-sulphate-nitroblue-tetrazolium reaction system as adapted by Kakkar et al., [15] and the results have been expressed as units (U) of SOD activity per mg proteins. Catalase (CAT) activity was estimated by the method of Aebi [16] and the results are expressed as nmol min−1 mg−1 proteins. GPx activity was measured by the procedure of Flohé and Günzler [17] and the results are expressed as nmol GSH min−1 mg−1 proteins.
2.7. Intracellular mediator determinations
H2O2 level in small intestine mucosa was performed according to Dingeon et al., [18]. The non-haem iron was measured using ferrozine as described by Leardi et al., [19]. Calcium level was measured using a colorimetric method according to Stern and Lewis [20].
2.8. Statistical analysis
The results were analyzed by one-way analysis of variance (ANOVA) and were expressed as means ± standard error of the mean (S.E.M.). All statistical tests were two-tailed, and a p value of 0.05 or less was considered significant.
3. Results
3.1. Effects of vinblastine and loperamide on body weight and food and water intake
As shown in Table 1, a statistically significant (0.05) decrease in weight gain was detected in the loperamide group (31.97%), when compared with the vehicle groups. But, no significant change in this last was detected when comparing the vinblastine-treated groups (4.30%) with the vehicle groups. The same results were found for food intake. While there was no difference observed concerning the water intake (Table 1).
Table 1.
Effect of vinblastine and loperamide on food and water intake and weight gain in rat.
| Groups | Feed intake (g) | Water intake (mL/7days/rat) | Weight gain (g/7days/rat) |
|---|---|---|---|
| Control (NaCl, 5 mL kg−1 b.w.) | 25.34 ± 3.26a | 13.24± 1.04a | 18.36 ± 2.10a |
| Vinblastine (2 mg kg−1 b.w.) | 21.91 ± 2.55a | 10.11± 0.45a | 17.57 ± 1.15a |
| Loperamide (2 mg kg−1 b.w.) | 16.43 ± 0.72b | 9.73± 0.22a | 12.49 ± 0.39b |
Data are means ± SD (n = 8): different letters from each other are significantly different (P < 0.05).
3.2. Effects of vinblastine and loperamide on fecal parameters
In this context, significant (P < 0.05) decreases in the fecal number and water content collected during 24 h were detected in the loperamide control group and vinblastine-treated groups when compared with the vehicle control group (Table 2). Indeed, the total number of fecal pellets collected over 24 h on D5 decreased by 12.88% in the vinblastine group which is less important than the loperamide control group (24.33%). On another hand, the water content of the fecal pellets collected over 24 h on D5 decreased by 25.9% in the vinblastine group and by 45.56% in the loperamide control group when compared with the vehicle control group.
Table 2.
Comparison of fecal parameters following injection of vinblastine in rats with loperamide-induced constipation.
| Fecal parameters on Day 5 (collection for 24) |
||||
|---|---|---|---|---|
| Groups | Number of fecal pellet (n) | Wet weight (g/24 h/rat) | Dry weight (g/24 h/rat) | Water content (%) |
| Control (NaCl, 5 mL kg−1 b.w.) | 62.42 ± 5.91a | 8.23 ± 1.12a | 4.52 ± 0.21a | 45.08 ± 5.10a |
| Vinblastine (2 mg kg−1 b.w.) | 54.38 ± 3.33b | 5.63 ± 0.71b | 3.75 ± 0.76a | 33.40 ± 3.24b |
| Loperamide (2 mg kg−1 b.w.) | 47.23 ± 4.52c | 3.26 ± 0.03c | 2.46 ± 0.04b | 24.54 ± 2.51c |
Data are means ± SD (n = 8): different letters from each other are significantly different (P < 0.05).
3.3. Effects of vinblastine and loperamide on the intestinal charcoal transit
The data of gastrointestinal transit are shown in Table 3. As expected, the intestinal charcoal transit was significantly (P < 0.05) decreased by 11.16% in the vinblastine treated-group when compared with the vehicle control group. However, it decreased by 32.95% in the loperamide control group as compared with the vehicle control group.
Table 3.
Comparison of gastrointestinal motility following injection of vinblastine in rats with loperamide-induced constipation.
| Gastrointestinal motility (during 30 min) | |||
|---|---|---|---|
| Groups | Total small intestine length (cm) | Transit distance of charcoal meal (cm) | Gastrointestinal charcoal transit ratio (%) |
| Control (NaCl, 5 mL kg−1 b.w.) | 96.16 ± 7.45a | 66.00 ± 4.55a | 68.64 ± 4.36a |
| Vinblastine (2 mg kg−1 b.w.) | 94.45 ± 6.32a | 56.18 ± 5.28b | 59.48 ± 3.16b |
| Loperamide (2 mg kg−1 b.w.) | 97.92 ± 9.92a | 43.76 ± 3.66c | 44.69 ± 4.37c |
Data are means ± SD (n = 8): different letters from each other are significantly different (P < 0.05).
3.4. Effects of vinblastine and loperamide on serum lipid levels
Endogenous metabolites (serum lipids) were also examined in the serum of the drugs induced constipation in rats and the results are presented in the Table 4, but there were no significant differences.
Table 4.
Effect of vinblastine and loperamide on serum lipids in rat.
| Triacylglycerol (mg/dL) | Total cholesterol (mg/dL) | HDL cholesterol(mg/dL) | |
|---|---|---|---|
| Control (NaCl, 5 mL kg−1 b.w.) | 72.34 ± 6.40a | 61.24 ± 7.21a | 21.47 ± 4.46a |
| Vinblastine (2 mg kg−1 b.w.) | 67.00 ± 5.75a | 62.66 ± 4.22a | 21.33 ± 2.73a |
| Loperamide (2 mg kg−1 b.w.) | 60.45 ± 6.07b | 65.32 ± 5.32a | 22.63 ± 1.12a |
Data are means ± SD (n = 8): different letters from each other are significantly different (P < 0.05).
3.5. Effects of vinblastine and loperamide on lipids, thiols group level and proteins in intestinal mucosa
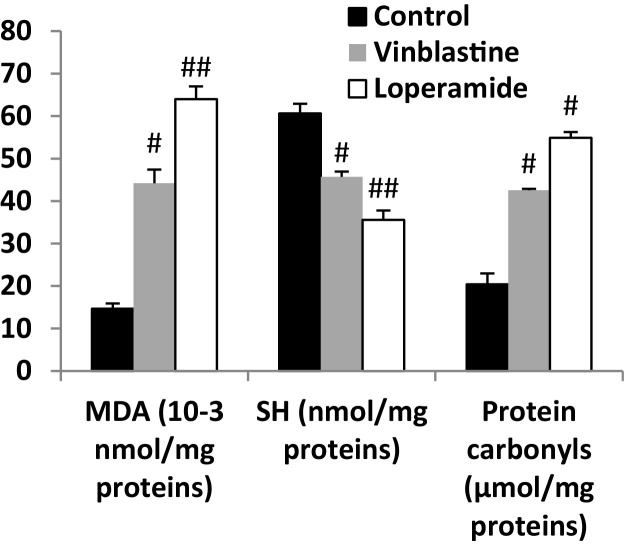
The MDA (an end product of lipid peroxidation) is widely used as a marker of lipid peroxidation. Vinblastine-injection resulted a significant increase of MDA in the intoxicated group (201.71%) compared to the vehicle control group. The same effect was observed following the injection of loperamide, but with a greater increase (336.86%) than that detected with chemotherapy agent. While, the treatment of rats with both drugs provoked the reduction of the level of thiols group which is one of the most relevant markers of oxidative stress in the intestine mucosa. This latter effect was confirmed also by the oxidation of proteins and elevation of the carbonyl groups under the influence of these drugs (Fig. 1).
Fig. 1.
Vinblastine-induced changes in intestinal mucosa of MDA, protein carbonyls and thiol groups level in rats. Animals were treated with NaCl, challenged with intravenous injection of vinblastine (2 mg kg−1b.w.), loperamide (2 mg kg−1 b.w., subcutaneously) or (NaCl, 0.9%). #: p < 0.05 compared to NaCl group. ##: p < 0.01 compared to NaCl group. Values are means ± SEM (n = 8).
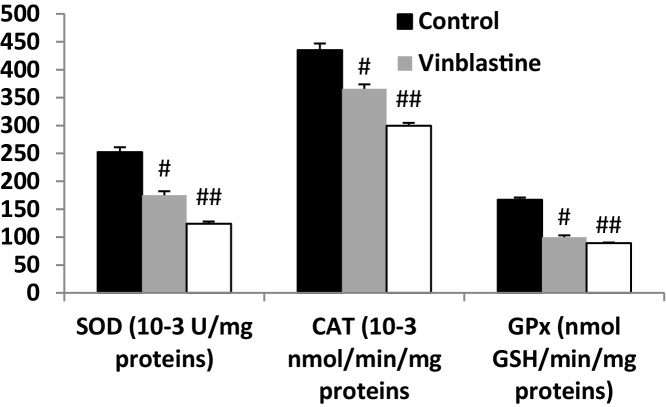
3.6. Effects of vinblastine and loperamide on antioxidant enzymes activities
The influence of vinblastine treatment on the intestinal mucosa antioxidant enzyme activities was also examined and the results were presented in Fig. 2. In this respect, the treatment for one week of animals was accompanied by a significant depletion in the activities of antioxidant enzymes such as SOD (30.54% inhibition) and CAT (15.93%) and GPx (40.12%). In addition, as expected, loperamide as a constipation agent causes a strong decline in these activities. These inhibitions were 50.84%, 31.14% and 46.50%, respectively.
Fig. 2.
Vinblastine-induced changes in intestinal mucosa of antioxidant enzymes activities in rats. Animals were treated with NaCl, challenged with intravenous injection of vinblastine (2 mg kg−1 b.w. i.v.), loperamide (2 mg kg−1 b.w., subcutaneously) or (NaCl, 0.9%). #: p < 0.05 compared to NaCl group. ##: p < 0.01 compared to NaCl group. Values are means ± SEM (n = 8).
3.7. Effects of vinblastine and loperamide on intracellular mediator levels in intestinal mucosa
The cytotoxic effect in intestinal mucosal cells of this chemotherapeutic agent was evaluated by the perturbation of intracellular mediators such as hydrogen peroxide, free iron and calcium. In this context, the vinblastine (2 mg kg−1 b.w.) injection increased these mediators in enterocytes (Table 5).
Table 5.
Injection of vinblastine during one week induced disturbance of intracellular mediator levels in mucosal intestine.
| H2O2 (μmol/mg proteins) | Free Iron (nmol/mg proteins) | Calcium (10−3 nmol/mg proteins) | |
|---|---|---|---|
| Control (NaCl, 5 mL kg−1 b.w.) | 7.23 ± 1.06a | 5.83 ± 0.37a | 28.31 ± 3.69a |
| Vinblastine (2 mg kg−1 b.w.) | 12.22 ± 1.70b | 12.72 ± 2.18b | 37.54 ± 4.56b |
| Loperamide (2 mg kg−1 b.w.) | 17.42 ± 2.53c | 13.48 ± 2.26b | 42.03 ± 4.82c |
Data are means ± SD (n = 8): different letters from each other are significantly different (P < 0.05).
4. Discussion
Constipation may arise from a variousness of causes, including the use of chemical compounds, such as morphine, dietary habits and psychological stress [21]. Recently, it was reported that many chemotherapy agents are the cause of an acute or chronic constipation. In this regard, we are interested to examine the effect of one chemotherapeutic agent such as vinblastine on gastrointestinal transit as well as associated disturbances.
Firstly, fecal parameters such as the fecal pellet number and fecal water content have been used as indices of the effect of this chemotherapy agent. In fact, as a result of vinblastine treatment, a decrease was detected in the fecal pellet number and water content discharged over 24 h (on day 5). Also, the results obtained for the fecal pellet numbers and fecal water content showed that the fecal output, measured by the fecal pellet number and water content, was significantly decrease by the injection of loperamide. These data provide the first strong evidence that the chemotherapeutic drug can be considered an important candidate for provoking constipation in patients who received vinblastine in the course of chemotherapy.
Secondly, the transit process of the entire gastrointestinal tract reflects the overall gastrointestinal motor activity. Thus, measuring the gastrointestinal charcoal transit ratio is also useful in the diagnosis of constipation [10]. A decrease in the gastrointestinal charcoal transit ratio indicates constipation [22]. In the present study, a reduction in the gastrointestinal charcoal transit ratio was observed in the rats treated with vinblastine and loperamide compared to healthy rats, providing also evidence that vinlastine exerts constipation in rats. Therefore, these observations indicated a successful model of constipation was established.
In addition, our results showed that the triglyceride, total cholesterol, and HDL cholesterol levels were not significantly different among the vinblastine group as compared with the normal vehicle control. By contrast, a decrease was observed only in triglyceride level in loperamide group.
On another hand, numerous studies have reported an increased oxidative stress and imbalance in antioxidant enzymes following the use of antineoplastic agents in patients who receive these drugs during cancer chemotherapy [23], [24], [25]. In this respect, we showed that vinblastine was provoked an oxidative-stress status in intestinal mucosa as assessed by high MDA and protein carbonyls as well as deleterious effects on non-enzymatic antioxidants such as sulfhydryl groups level. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins and lipids [26]. Our results are in agreement with those obtained by Li et al. who showed that the content of MDA increases in constipated rats [27].
Added to that, the current study suggests that injection of vinblastine causes depletion of the activity of antioxidant enzymes such as SOD, CAT and GPx. These data fully corroborate previous reports, indicating that chronic constipation can cause potential oxidative stress in children and depletion of antioxydant enzymes activities [28].
Finally, the chemotherapeutic agent used in this study induced a disturbance in intracellular mediators (increase of free iron, H2O2 production and Ca2+ level) in small intestine. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling. In fact, free iron and hydrogen peroxide catalyzed the highly toxic hydroxyl radical production via the Fenton reaction, leading to membrane lipoperoxidation [29]. Further, it is well known that ROS can cause a rapid rise in calcium levels in the cytoplasm of different cell types [30], [31].
5. Conclusion
Collectively, these findings clearly indicate that vinblastine, as chemotherapy drug, caused constipation by reduction of gastrointestinal motility and perturbation of fecal parameters in experimental rats. These changes were accompanied by a shift in the balance between prooxidants and antioxidants and intracellular mediators interruption.
Declaration of interest
Only the authors are responsible for the content of this paper.
Acknowledgements
Financial support of the Tunisian Ministry of Higher Education and the Scientific Research is gratefully acknowledged; financial disclosures: none declared.
References
- 1.Sung C.P., Hoon J.C. Chemotherapy for advanced gastric cancer: review and update of current practices. Gut Liver. 2013;7:385–393. doi: 10.5009/gnl.2013.7.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaime A.Y., Xiao W.T., Kathryn A.R., Marc W.F., Neal M.D. Chemotherapy induced gastrointestinal toxicity in rats: involvement of mitochondrial DNA, gastrointestinal permeability and cyclooxygenase −2. J. Pharm. Pharmaceut. Sci. 2003;6:308–314. [PubMed] [Google Scholar]
- 3.Conklin K.A. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr. Cancer. 2000;37:1–18. doi: 10.1207/S15327914NC3701_1. [DOI] [PubMed] [Google Scholar]
- 4.Conklin K.A. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 5.Mostafa S.M., Bhandari S., Ritchie G., Gratton N., Wenstone R. Constipation and its implications in the critically ill patient. Br. J. Anaesth. 2003;91:815–819. doi: 10.1093/bja/aeg275. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro N., Budhathoki S., Ohnaka K., Toyomura K., Kono S., Ueki T., Tanaka M., Kakeji Y., Maehara Y., Okamura T., Ikejiri K., Futami K., Maekawa T., Yasunami Y., Takenaka K., Ichimiya H., Terasaka R. Constipation and colorectal cancer risk: the fukuoka colorectal cancer study. Asian Pac. J. Cancer Prev. 2011;12:2025–2030. [PubMed] [Google Scholar]
- 7.Hughes S., Higgs N.B., Turnberg L.A. Loperamide has antisecretory activity in the human jejunum in vivo. Gut. 1984;25:931–935. doi: 10.1136/gut.25.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohji Y., Kawashima K., Shimizu M. Pharmacological studies of loperamide, an anti-diarrheal agent. II. Effects on peristalsis of the small intestine and colon in guinea pigs (author's transl) Nih.Yakurig. Zass. 1978;74:155–163. doi: 10.1254/fpj.74.155. [DOI] [PubMed] [Google Scholar]
- 9.Yamada K., Onoda Y. Comparison of the effects of T-1815, yohimbine and naloxone on mouse colonic propulsion. J. Smooth Muscle Res. 1993;29:47–53. doi: 10.1540/jsmr.29.47. [DOI] [PubMed] [Google Scholar]
- 10.Wintola O.A., Sunmonu T.O., Afolayan A.J. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010;10:95–99. doi: 10.1186/1471-230X-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L.L., Liao J.F., Chen C.F. Anti-diarrheal effect of water extract of Evodiae fructus in mice. Ethnopharmacology. 2000;73:39–45. doi: 10.1016/s0378-8741(00)00267-1. [DOI] [PubMed] [Google Scholar]
- 12.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 13.Ellman G.E. Tissue sulfhydryl groups. Arch. Biochem. Bio-Phys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Levine L.R., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Method Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 15.Kakkar P., Das B., Viswanathan P.N. Modified spectrophotometric assay of SOD. Indian J. Biochem. Biophys. 1984;2:130–132. [PubMed] [Google Scholar]
- 16.Aebi H. Catalase. In: Bergmeyer H.U., editor. Methods in Enzymatic Analysis. Academic Press Inc.; New York: 1974. pp. 673–686. [Google Scholar]
- 17.Flohé L., Günzler W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 18.Dingeon B., Ferry J.P., Roullet A. Auto analysis of blood sugar by Trinder's method. Ann. Biol. Clin. 1975;33:3–13. [PubMed] [Google Scholar]
- 19.Leardi A., Caraglia M., Selleri C., Pepe S., Pizzi C., Notaro R., Fabbrocini A., De Lorenzo S., Music‘o M., Abbruzzese A., Bianco A.R., Tagliaferri P. Desferrioxamine increases iron depletion and apoptosis induced by ara-C of human myeloid leukaemic cells. Br. J. Haematol. 1998;102:746–752. doi: 10.1046/j.1365-2141.1998.00834.x. [DOI] [PubMed] [Google Scholar]
- 20.Stern J., Lewis W.H. The colorimetric estimation of calcium in serum with o-cresolphthalein complexone. Clin. Chim. Acta. 1957;2:576–580. doi: 10.1016/0009-8981(57)90063-3. [DOI] [PubMed] [Google Scholar]
- 21.Kakino M., Tazawa S., Maruyama H., Kazuhiro T., Yoko A., Masamitsu S., Hideaki H. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement. Altern. Med. 2010;10:68. doi: 10.1186/1472-6882-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagar L., Sehgal R., Ojha S. Evaluation of antimotility effect of Lantana camara L var. acuelata constituents on neostigmine induced gastrointestinal transit in mice. BMC Complement. Altern. Med. 2005;5:18. doi: 10.1186/1472-6882-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weijl N.I., Hopman G.D., Wipkink-Bakker A., Lentjes E.G.W.M., Berger H.M., Cleton F.J., Osanto S. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann. Oncol. 1998;9:1331–1337. doi: 10.1023/a:1008407014084. [DOI] [PubMed] [Google Scholar]
- 24.Clemens M.R., Ladner C., Ehninger G., Einsele H., Renn W., Bühler E., Waller H.D., Gey K.F. Plasma vitamin E and β-carotene concentrations during radiochemotherapy preceding bone marrow transplantation. Am. J. Clin. Nutr. 1990;51:216–219. doi: 10.1093/ajcn/51.2.216. [DOI] [PubMed] [Google Scholar]
- 25.Faure H., Coudray C., Mousseau M., Ducros V., Douki T., Bianchini F., Cadet J., Favier A. 5-Hydroxymethyluracil excretion, plasma TBARS and plasma antioxidant vitamins in adriamycin-treated patients. Free Radic. Biol. Med. 1996;20:979–983. doi: 10.1016/0891-5849(95)02187-6. [DOI] [PubMed] [Google Scholar]
- 26.Chandra K., Syed S.A., Abid M., Sweety R., Najam A.K. Protection against FCA induced oxidative stress induced DNA damage as a model of arthritis and In vitro anti-arthritic potential of costus speciosus rhizome extract. Int. J. Pharmacogn. Phytochem. Res. 2015;7:383–389. [Google Scholar]
- 27.Li Y., Zong Y., Qi J., Liu K. Prebiotics and oxidative stress in constipated rats. J. Pediatr. Gastroenterol. Nutr. 2011;53:447–452. doi: 10.1097/MPG.0b013e31821eed83. [DOI] [PubMed] [Google Scholar]
- 28.Jun-Fu Z., Jian-Guo L., Sheng-Li Z., Ji-Yue W. Potential oxidative stress in children with chronic constipation. World J. Gastroenterol. 2005;11:368–371. doi: 10.3748/wjg.v11.i3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hessa J.A., Khasawneh M.K. Cancer metabolism and oxidative stress: insights into carcinogenesis and chemotherapy via the non-dihydrofolate reductase effects of methotrexate. BBA Clin. 2015;3:152–161. doi: 10.1016/j.bbacli.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebai H., Jabri M.A., Souli A., Rtibi K., Selmi S., Tebourbi O., El-Benna J., Sakly M. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita) decoction in rats. J. Ethnopharmacol. 2014;152:327–332. doi: 10.1016/j.jep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Ishii K., Tamaoka A., Takeda T., Ishii K., Iwasaki N., Shoji S. Clinical and neurological features of organoarsenic compound (diphenylarsenic acid) intoxication in Kamisu. Japan Rinsho Shink. 2006;46:768. [PubMed] [Google Scholar]