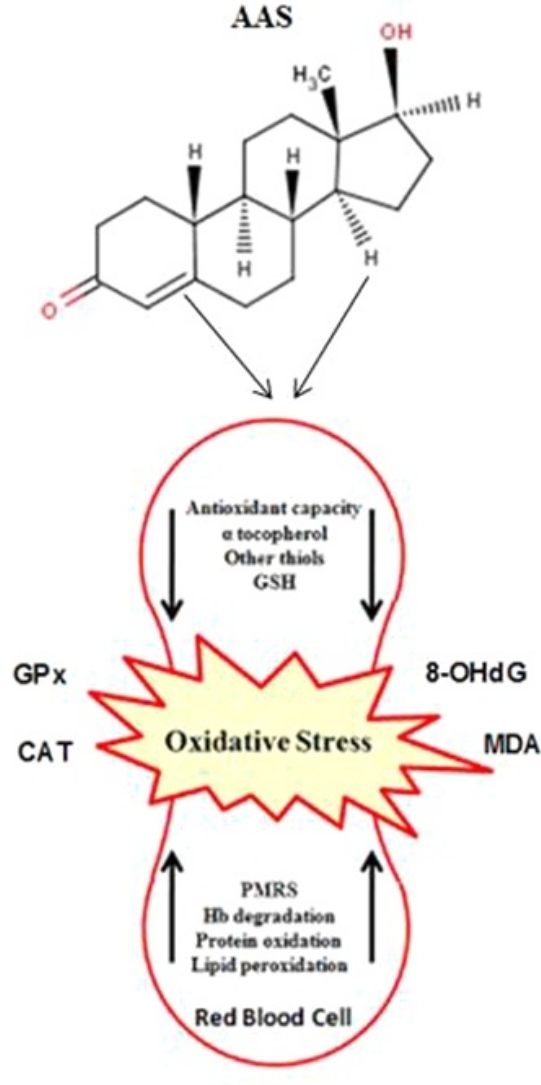
Graphical abstract
Keywords: ROS, Strength exercise, Anabolic
Highlights
-
•
Single session of high-intensity RE on trained bodybuilder men had effect on DNA damage and oxidative stress.
-
•
The AAS users showed greater response than NAAS users. At pre-RE, the levels of oxidant were greater for the AAS group.
-
•
The study has reported a range of negative redox status consequence of AAS use in conjunction with resistance training.
-
•
The alteration of oxidative stress status with AAS use likely increase the injury risks in some organs in athletes.
Abstract
The aim of this study was to determine the effect of anabolic androgenic steroids (AAS) use on oxidative stress responses to a single session of resistance exercise in strength-trained men. Twenty-three strength trained men, with 11 self-reporting regular AAS use and 12 self-reporting never taking AAS (NAAS) volunteered to participate in this study. Blood draws were obtained pre and post resistance exercise in order to evaluate changes in oxidative stress biomarkers levels (i.e., 8-hydroxy-2-deoxyguanosine [8-OHdG], malondialdehyde [MDA], and nitric oxide [NO]), antioxidant defense systems (i.e., glutathione peroxidase [GPx] and catalase [CAT]), and glucose (GLU) levels. The AAS users had higher level of 8-OHdG (77.3 ± 17 vs. 57.7 ± 18.2 ng/mg), MDA (85.6 ± 17.8 vs. 52.3 ± 15.1 ng/mL), and GPx (9.1 ± 2.3 vs. 7.1 ± 1.3 mu/mL) compared to NAAS at pre exercise (p < 0.05). Both the experimental groups showed increases in 8-OHdG (p = 0.001), MDA (p = 0.001), GPx (p = 0.001), NO (p = 0.04), CAT (p = 0.02) and GLU (p = 0.001) concentrations after resistance exercise, and the AAS group indicated significant differences in 8-OHdG (p = 0.02) and MDA (p = 0.05) concentrations compared with NAAS users at post exercise. In conclusion, use of AAS is associated with alterations in immune function resulting in oxidative stress, and cell damage; however, high-intensity resistance exercise could increase greater oxidative stress biomarkers in strength-trained men.
1. Introduction
Anabolic androgenic steroids (AAS) are one of the most commonly used drugs among athletes, frequently in combination with resistance training to improve physical performance, lean body mass, muscle size, strength, protein metabolism, bone metabolism, and collagen synthesis [1], [2], [3], [4]. Although amounts of some AAS may be useful in some pathological conditions, they are often abused by competing athletes desiring to build muscle mass and enhance physical performance, and by non-athletes aiming to improve their personal appearance [5].
The AAS abuse may be a serious problem in the United States, United Kingdom as well as other parts of the world [5], [6], [7], and during the past 2 decades the number of AAS users increased more than 2000% in the world [5], [6], [8]. It seems that AAS abuse have some adverse effects on organs and it is associated with detrimental side-effects on the hepatic [9], [10], [11], [12], endocrine, and cardiovascular systems [11], [12]. For example, previous studies have reported that AAS induced pathological left ventricular hypertrophy [13] with disproportional extracellular collagen accumulation and/or interstitial fibrosis [14]. For the immune function, researchers reported that use of AAS reduced immune cell number and function [15], [16]. On the other hand, AAS have been shown to adversely influence lymphocyte differentiation and proliferation, antibody production, Natural Killer Cytotoxic activity and the production of certain cytokines, thereby altering the immune reaction [11], [12], [13], [14], [15], [16].
Although previous authors reported side effects of AAS use on physiological variables, the influence of this drug on oxidative stress responses is unclear. Oxidative stress is a condition in which the delicate balance between free radicals production and their subsequent amelioration via the antioxidant defense system becomes skewed in favor of free radical expression [17], [18], [19]. Since longitudinal administration of AAS provides dysfunction of mitochondrial respiratory chain complexes (major source of ROS) and mono-oxygenase systems [20], it would be possible that these alterations were accompanied by an increased ROS generation resulting in oxidative stress and cell damage.
With review in literature, some studies have investigated the effects of AAS on oxidative stress responses in liver [21], brain [22], and cardiac [5] in rats and all of them reported negative effects of AAS. For instant, Camiletti-Moiron et al. [22] examined the effects of high-intensity exercise and AAS use on brain redox status in Wistar rats and found impairments in brain redox status after stranazolol administration.
Since previous studies have used rats to identify the effects of AAS on oxidative stress biomarkers, no information is available on the effects of AAS use on changes in oxidative stress biomarkers and antioxidant defense systems after resistance exercise (RE) in human. Therefore, the purpose of this investigation was to determine the influence of longitudinal use of AAS on changes in oxidative stress biomarkers and antioxidant defense systems after high-intensity RE in men. We hypothesized that the oxidative stress responses to RE is higher in strength-trained men who used AAS.
2. Methods
2.1. Participants
Twenty-three strength-trained men were recruited through local gyms, and personal contacts (Table 1). For inclusion in the study, participants had to have a minimum of 2 yr of resistance training experience with four to five training sessions per week and age between 18 and 25 yr. Exclusion criterions for the study was the presence of known respiratory, cardiovascular, or musculoskeletal disease. A specific inclusion criterion for the AAS-using group (AAS: n = 11) was a documented self-reported history of AAS use for at least 1 yr (Table 2). An inclusion criterion for the non-AAS (NAAS) group (n = 12) was self-reported history of never taking AAS. The participants in the AAS group had similar dose and type of steroids through the 1 yr ago and all of them were during the methenolone enanthat use. Before taking part in the study, the participants were notified about the potential risks involved and gave their written consent. This study was approved by the university’s human research ethics committee.
Table 1.
Participant characteristics.
| Variable | Group |
|
|---|---|---|
| AAS (n = 11) | NAAS (n = 12) | |
| Age (yr) | 19.4 ± 2.3 | 20.8 ± 3.6 |
| Height (cm) | 179.5 ± 6.7 | 181.5 ± 5.5 |
| Weight (kg) | 88.1 ± 8.3 | 87.1 ± 9.4 |
| BMI (kg/m2) | 27.3 ± 2.6 | 25.9 ± 3.6 |
| Strength-training experience (yr) | 3.2 ± 2.1 | 3.2 ± 2.1 |
AAS: Anabolic-androgenic steroids, NAAS: Non AAS.
Table 2.
Anabolic-androgenic steroids used by participants.
| Drug | Number of Times Reported | |
|---|---|---|
| Testosterone cypionate | (Depo testosterone) | 3 |
| Nandrolone phenpropionate | (Durabolin) | 1 |
| Methandrostenolone | (Dianabol) | 2 |
| Oxandrolone | (Anavar) | 1 |
| Methenolone enanthate | (Depo premabalon) | 3 |
2.2. Study design
A cross-sectional design was used for this study. The participants recruited to the laboratory four times. Initially, participants completed self-report questionnaires related to general health, training status, and history of AAS use. During this session, height (Seca 222, Terre Haute, IN) and weight (Tanita, BC-418MA, Tokyo, Japan) for each athlete was measured and then the participants performed one repetition of maximum (1RM) test for the bench press and lat-pull down exercise. On day two, 1RM of arm curl and back squat were assessed. On day three, 1RM of knee extension and knee flexion were measured. On day four, the participants performed a single session of high-intensity RE. Pre and post RE blood samples were obtained to analyze changes in oxidative stress biomarkers and antioxidant defense systems. All tests and RE sessions were conducted on the same time of day (i.e., morning, 9:00–12:00 A.M.), under similar weather and laboratory conditions. The subjects were advised to avoid any vigorous activities on the day before the test and during the study.
2.3. Muscle strength assessment
For prescription of RE, the 1RM of each exercise (i.e., bench press, lat-pull down, arm curl, back squat, knee extension and knee flexion) was determined. One RM testing was performed using procedures described in detail elsewhere [23]. Spotters were present to provide verbal encouragement and ensure safety.
2.4. Resistance exercise program
A trained researcher supervised exercise session carefully so that exercise prescriptions were correctly administered during session (i.e., rest and or velocity of movement). Before the exercise session, the participants performed a general warm-up consisting of 10 min running, 5 min dynamic stretching, and 5 min of ballistic movements to increase blood circulation and temperature of the involved muscle groups. Also, a specific warm-up of 1 set of 5 repetitions at 50–60% of 1RM was performed before an exercise. The participants performed high-intensity RE including 5 sets with 80% of 1RM to failure for the bench press, lat-pull down, arm curl, back squat, knee extension and knee flexion, respectively. The rest period between exercises and sets were 60 and 30 s, respectively.
2.5. Blood sampling and analysis
At before (pre exercise) and after (at least 1 min post exercise) RE, venous blood samples (5 mL) were collected from a forearm vein. Then, the blood was collected into EDTA-containing tubes and centrifuged immediately at 1370 × g for 10 min at 4 °C, and the plasma was collected. The blood was allowed to clot at room temperature for 30-min and centrifuged at 1500 × g for 10 min. The serum layer was removed and frozen at −20° C in multiple aliquots for further analyses.
2.6. Markers of oxidative stress
Oxidative DNA damage, 8-OHdG excretion, was measured by using the enzyme-linked immunosorbent assay (ELISA). The assay was carried out in duplicate using the manufacturer’s instructions (New 8-OHdG Check, ELISA, Japan Institute for the Control of Aging; Catalog No: KO G-200S/E). Plasma MDA was measured by using a spectrophotometric assay for MDA. The assay was carried out in duplicate; intraassay coefficients of variances were 3.4%, and detection limit was 0.0088 μM, using themanufacturer’s instructions (Bioxytech®MDA-586TM, Spectrophotometric Assay for MDA. Catalog Number 21044; OXIS Research, Portland, OR, USA). NO was measured using ELISA kit (Nitrate/Nitrite Colorimetric Assay Kit, Cayman Chemical, and Ann Arbor, Catalog: 780001 USA) and manufacturer’s instructions.
2.7. Markers of defense system
GPx were analysed by using a Glutathione Peroxidase Assay Kit (catalog no. 703102; Cayman Chemical Co., Ann Arbor, Michigan, USA) according to the manufacturer’s instruction. The CAT assay kit was obtained from Cayman Chemical (catalog no. 707002, Cayman Chemical Co., Ann Arbor, Michigan, USA) and the assays were conducted according to their instructions. The kit consists of assay buffer, sample buffer, formaldehyde standard, catalase, potassium hydroxide, methanol, hydrogen peroxide, purpald, and potassium periodate. After reaction of the enzyme with methanol in the presence of H2O2, formaldehyde is produced, and is measured by a spectrophotometric method with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole as the chromogen. Purpald forms a bicyclic heterocycle with aldehydes, and this complex changes from colorless to purple upon oxidation.
Serum GLU level was measured with Shimadzu UV/Vis 1208 spectrophotometer using commercially available kits (Eastbiopharm Co., Ltd, China).
2.8. Statistical analyses
Descriptive statistics (mean ± standard deviation [SD]) for each of the variables were calculated. The distribution of each variable was examined using the Shapiro-Wilk test. To determine the effect of RE on oxidative stress changes a 2 (time) × 2 (group) ANOVA was applied. When a significant F value was achieved, Bonfferoniʼs post-hoc procedures were performed to identify the pairwise differences between the means. Effect sizes (ESs) were calculated using Cohenʼs d. Threshold values for assessing magnitudes of ES were 0.2, 0.6, 1.2 and 2.0 for small, moderate, large and very large, respectively [24]. The level of significance was set at p ≤ 0.05.
3. Results
There was a significant difference between groups at baseline in oxidative stress biomarkers (i.e., 8-OHdG, p = 0.005 and MDA, p = 0.004) and antioxidant defense system (GPx, p = 0.007). The RE induced a significant change in oxidative stress biomarker levels for the AAS and NAAS groups (p < 0.05). The AAS group also indicated significant differences compare to NAAS group in 8-OHdG (p = 0.02) and MDA (p = 0.05) at post exercise. Both the experimental groups showed significant elevations in antioxidant defense systems after high-intensity RE (p < 0.05). However, no significant group by time interaction effects were observed in the GPx (p = 0.09), NO (p = 0.41), and CAT (p = 0.89). GLU level significantly increased after resistance exercise compared to the pre-exercise level for both the groups (p = 0.001). However, no significant group by time interaction effect was observed for the elevation of GLU level (p = 0.7) (Table 3).
Table 3.
Oxidative stress and antioxidant changes before and after resistance exercise.
| AAS (n = 11) | NAAS (n = 12) | P value | |
|---|---|---|---|
| 8-OHdG (ng/mg) | |||
| Before | 77.3 ± 17 | 57.7 ± 18.2 | T = 0.001 |
| After | 92.9 ± 20.1 | 85.6 ± 17.8 | G = 0.005 |
| % of change | 20.1 | 48.3 | T × G = 0.02 |
| ES | 0.91b | 1.5c | |
| MDA (ng/mL) | |||
| Before | 85.6 ± 17.8 | 52.3 ± 15.1 | T = 0.001 |
| After | 101.7 ± 22.4 | 63.2 ± 19.9 | G = 0.004 |
| % of change | 18.8 | 21.1 | T × G = 0.05 |
| ES | 0.90b | 0.72b | |
| NO (μm) | |||
| Before | 4.8 ± 1.1 | 4.8 ± 2.2 | T = 0.04 |
| After | 5.6 ± 1.4 | 5.3 ± 1.4 | G = 0.83 |
| % of change | 16.6 | 10.4 | T × G = 0.41 |
| ES | 0.72b | 0.22a | |
| GPx (mu/mL) | |||
| Before | 9.1 ± 2.3 | 7.1 ± 1.3 | T = 0.001 |
| After | 11.3 ± 3.2 | 8.4 ± 1.6 | G = 0.007 |
| % of change | 24.1 | 18.3 | T × G = 0.09 |
| ES | 0.95b | 1b | |
| CAT (mu/mL) | |||
| Before | 8 ± 1.6 | 8.1 ± 1.2 | T = 0.02 |
| After | 8.7 ± 0.9 | 8.9 ± 1.9 | G = 0.81 |
| % of change | 8.7 | 9.8 | T × G = 0.89 |
| ES | 0.43a | 0.66b | |
| GLU (mg/dL) | |||
| Before | 100.6 ± 14.1 | 93.7 ± 7.2 | T = 0.001 |
| After | 115.8 ± 14.5 | 111.3 ± 8 | G = 0.06 |
| % of change | 15.1 | 18.7 | T × G = 0.7 |
| ES | 1b | 2.4d | |
AAS: Anabolic-androgenic steroids, NAAS: Non AAS, T: Time, G: Group, ES: Effect size.
a,b,c,dDenote small, moderate, large and very large ESs, respectively.
Regarding percentage of change between the two groups after RE, the AAS group indicated more changes than NAAS group in NO (16.6% vs. 10.4%) and GPx (24.1% vs. 18.3%), while the NAAS group showed greater changes compared to AAS group in 8-OHdG (48.3% vs. 20.1), MDA (21.1% vs. 18.8%), CAT (9.8% vs. 8.7%) and CLU (18.7 vs. 15.1%).
4. Discussion
It has been reported that AAS have adverse physiological effects; however, a large number of athletes and non-athletes used AAS to enhance their physical performance and quality of life. In fact, AAS users are engaged in typical strength exercise to improve their muscle mass by hypertrophy [4]. Yet, when administered chronically (i.e., 1 year), AAS could be associated with adverse effects resulting in oxidative stress.
In relation to changes in oxidative stress biomarkers, 8-OHdG was evaluated as a biomarker of oxidative DNA damage [20], [21]. In this study, 8-OHdG excretion significantly increased after RE for both the AAS and NAAS groups, which is in line with the study of Radak et al. [25] who reported significant increases in the 8-OHdG level after 200 eccentric contractions with knee extensors. In contrast to our findings, Bloomer et al. [26] did not find significant changes in plasma concentrations of 8-OHdG after squat exercise with 70% of 1RM. Our results are in agreement with previous studies which showed a session of RE could increase 8-OHdG level [19], [25], [26], [27], [28], [29]. In contrast, other studies indicated that no increase in DNA damage was occurred after submaximal or maximal exercise [7], [30]. The reason for these apparent discrepancies is unclear, but it could be in relation to duration and intensity of exercise, fitness level, athletic ability and environmental conditions that all have an impact on the occurrence or severity of oxidative stress and damage. Although the exact mechanism for ROS production, oxidative stress and DNA damage following RE is not clear, previous investigators reported that a relatively anoxic state occurred after every RE set in the exercising muscle following rapid blood reperfusion and it was similar to an ischemia-reperfusion state [28], [29]. Another possible mechanisms responsible for the RE-induced ROS formation could be xanthine–xanthine oxidase pathway, respiratory burst of neutrophils, catecholamine autooxidation, local muscle ischemia-hypoxia, conversion of the weak superoxide to the strong hydroxyl radical by lactic acid, and alteration of calcium homeostasis [19], [25], [27].
In addition to DNA damage, lipid peroxidation (damage in cellular compartments) occur during RE. Thus, measurement of MDA levels in the plasma or serum provides a convenient in vivo index of lipid peroxidation and represents a non-invasive biomarker of oxidative stress [31]. In this study MDA significantly increased after RE for both the AAS and NAAS groups which is in accordance with the finding of Ramel et al. [31] who reported a significant increase in plasma MDA concentration after a circuit type RE. Enhancements of MDA after submaximal, high-intensity, exhaustive maximal, cycling stage, and half-marathon have been supported in previous studies [7], [31], [32].
However, our findings are inconsistent with those of previous studies [25], [31] which did not observe significant changes in MDA after RE. The possible reasons for this discrepancy could be due to type of RE protocols and exercise intensity. The increases in MDA concentrations after RE may be due to muscle damage and increases in creatine kinase (CK) activity [27]. Because of cellular membranes have polyunsaturated fatty acids, the exercise-induced muscle damage increased concentration of polyunsaturated fatty acids in the blood, and it could be one of the important mechanisms to increase lipid peroxidation (i.e., MDA) after exercise in the blood; however, we did not directly measure the muscle damage indicators (i.e., CK and or LDH), thus it could be a speculation [3], [27].
In the present study, significant change was seen in NO levels after the RE for both the AAS and NAAS groups. In accordance with our findings, previous studies have reported that NO increased in venous plasma after prolonged running, cycling [20], [33], [34] and incremental cycling exercise to VO2max [10]. In contrast, some authors did not report any changes in NO metabolism following an incremental treadmill test to exhaustion [14]. The contradictory results may be caused by some variables including sample characteristics type and intensity of exercise. Vascular formation of NO is directly facilitated by increased shear stress [27], [35], [36]. During physical exercise (i.e., RE), cardiac output increased and blood redistributed to the exercising muscles [37], [38], [39]. The exercise-induced increase of blood flow produced shear stress, thereby providing a possible coupling between exercise and endogenous NO formation resulting in elevation of NO [28].
An important finding of the present study was greater level of oxidative stress biomarker for the AAS users at pre-exercise. Moreover, these differences continued at post-exercise and the AAS bodybuilders indicated more changes than NAAS in oxidative stress. We found that prolonged AAS (i.e., 1 year) treatment could induced an oxidative stress situation by enhanced lipid peroxidation and DNA damage. Interestingly, greater NO elevations after RE caused ROS production and this response was greater for the AAS users resulting in elevation of MDA and 8-OHdG levels. Thus, it is tempting to speculate with the possibility that the observed changes in prooxidant/antioxidant status could be causally related with the adverse effects of AAS. Mitochondria might be an important cellular target for oxidative damage since the mitochondrial membrane is rich in polyunsaturated fatty acids; alteration in the lipid environment of respiratory chain complexes may cause a decrease in their activity leading, in turn, to a perturbation of energetic metabolism. On the other word, lipid peroxidation-derived aldehydes are generally stable and can diffuse within the cell [5], [16]; hence they could attack targets far from the site of the original free radical-initiated event expanding oxidative damage to a wide range of molecules (i.e., DNA). Thus, lipid peroxidation (i.e., MDA), DNA damage (i.e., 8-OHdG) and NO elevations might constitute an initial event of a multi-step process leading finally to injury in some organs. Further works are necessary to establish the relationship between changes in oxidative stress and AAS use in different organs of human subjects.
The CAT and GPx are the primary defense against ROS generated during exercise, and these enzymes activity are known to increase in response to exercise in both the animal and human studies [16], [21]. In this study, GPx activity was higher for the AAS users at pre-exercise and these elevations maintained at post-exercise. However, both the AAS and NAAS groups showed increases in the GPx concentrations at post-RE. Although there were no significant differences between groups in the CAT activity at pre-exercise, both the groups indicated significant increases in the CAT concentration at post-RE. Our findings are in accordance with previous studies that have reported incremental effects of exercise on GPx and CAT concentrations [9], [21]. In contrast, a few studies did not find any changes in the GPx activity with acute maximal exercise [4], [39]. Hudson et al. [38] reported that RE intensity plays a critical role to exercise-induced oxidative stress and it seems that following high-intensity RE greater changes in oxidative stress could be made.
Our concern was to characterize the potentially adverse effects of prolonged use of AAS on redox status. It appears that continuous and prolonged use of AAS provide a local and sustained oxidative stress state could lead to increased expression of antioxidant enzymes through free radical-mediated induction of redox sensitive signal cascades. It seems that greater GPx activity at pre-exercise for the AAS group could be in relation to overproduction of ROS and exceeding antioxidant defenses systems. After RE, exercise-induced oxidative stress promotes defense system by elevation of CAT and GPx for both the AAS and NAAS groups. In view of such findings, we can conclude that the proliferation in the antioxidant activity is linked as an auto-defense system against oxidative damage. Therefore greater oxidative damage is in accordance with higher antioxidant activity produced as an auto-defense mechanism [16], [38], [40].
The results of this study indicated that GLU levels significantly increased after the RE compared to baseline level for both the AAS and NAAS groups. The increases in the GLU level could be due to enhancement of catecholamines [29], [41]. Similarly, the higher values of GLU levels were found after exercise compare to pre-exercise. Blood GLU levels are maintained by the liver through glycogenolysis and glyconeogenesis during exercise [29]. This increase may be explained by hormonal factors which may be effective on GLU usage during exercise.
5. Conclusions
In summary, our results indicated that a session of high-intensity RE on strength-trained men had meaningful effects on antioxidant capacity, DNA damage and oxidative stress. Likewise, the AAS users showed greater responses than NAAS users. At pre-RE, the level of oxidant and antioxidant levels were greater for the AAS group. The present study has reported a range of negative redox status consequence of AAS use in conjunction with resistance training. The alteration of oxidative stress status and antioxidant enzyme activities with AAS use likely increase the injury risks in some organs in athletes.
Conflict of interests
None.
Funding
No.
Acknowledgements
The authors wish to thank all participants who took part in the study and the Human Performance Laboratory team for their technical support.
References
- 1.Evans N.A. Current concepts in anabolic-androgenic steroids. Am. J. Sports Med. 2004;32:534–542. doi: 10.1177/0363546503262202. [DOI] [PubMed] [Google Scholar]
- 2.Ferrando A.A., Tipton K.D., Doyle D. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am. J. Physiol. 1998;275:E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 3.Kanayama G., Hudson J.I., Pope H. Illicit anabolic-androgenic steroid use. Horm. Behav. 2010;58(1):111–121. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha-Hikim I., Artaza J., Woodhouse L. Testosterone induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2002;283:E154–E164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 5.Angell P., Chester J.N., Green D.J. Anabolic steroid use and longitudinal, radial, and circumferential cardiac motion. Med. Sci. Sports Exerc. 2012;44(4):583–590. doi: 10.1249/MSS.0b013e3182358cb0. [DOI] [PubMed] [Google Scholar]
- 6.Parkinson A.B., Evans N.A. Anabolic androgenic steroids: a survey of 500 users. Med. Sci. Sports Exerc. 2006;38(4):644–651. doi: 10.1249/01.mss.0000210194.56834.5d. [DOI] [PubMed] [Google Scholar]
- 7.Umegaki K., Higuchi M., Inoue K. Influence of one bout of intensive running on lymphocyte micronucleus frequencies in endurance-trained and untrained men. Int. J. Sports Med. 1998;19(8):581–585. doi: 10.1055/s-2007-971963. [DOI] [PubMed] [Google Scholar]
- 8.Yesalis C.E., Kennedy N.J., Kopstein A.N. Anabolic androgenic steroid use in the United States. JAMA. 1993;270:1217–1221. [PubMed] [Google Scholar]
- 9.Neri M., Bello S., Bonsignore A. Anabolic androgenic steroids abuse and liver toxicity. Mini Rev. Med. Chem. 2011;11:430–437. doi: 10.2174/138955711795445916. [DOI] [PubMed] [Google Scholar]
- 10.Riezzo I., Di Paolo M., Neri M. Anabolic steroid- and exercise-induced cardio-depressant cytokines andmyocardial β1 receptorexpression in CD1 mice. Curr. Pharm. Biotechnol. 2011;12:275–284. doi: 10.2174/138920111794295792. [DOI] [PubMed] [Google Scholar]
- 11.Dornelles G.L., Bueno A., de Oliveira J.S. Biochemical and oxidative stress markers in the liver and kidneys of rats submitted to different protocols of anabolic steroids. Mol. Cell. Biochem. 2017;425:181–189. doi: 10.1007/s11010-016-2872-1. [DOI] [PubMed] [Google Scholar]
- 12.Steven S., Daiber A., Dopheide J.F. Peripheral artery disease, redox signaling, oxidative stress – basic and clinical aspects. Redox Biol. 2017;12:787–797. doi: 10.1016/j.redox.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nottin S., Nguyen L.D., Terbah M. Cardiovascular effects of androgenic anabolic steroids in male bodybuilders determined by tissue Doppler imaging. Am. J. Cardiol. 2006;97:912–915. doi: 10.1016/j.amjcard.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Rocha F.L., Carmo E.C., Roque F.R. Anabolic steroids induce cardiac renin–angiotensin system and impair the beneficial effects of aerobic training in rats. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3575–H3583. doi: 10.1152/ajpheart.01251.2006. [DOI] [PubMed] [Google Scholar]
- 15.Hughes T.K., Fulep E., Juelich T. Modulation of immune responses by anabolic androgenic steroids. Int. J. Immunopharmacol. 1995;17:857–863. doi: 10.1016/0192-0561(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 16.Marshall-Gradisnik S., Green R., Brenu E.W. Anabolic androgenic steroids effects on the immune system: a review. Cent. Eur. J. Biol. 2009;4:19–33. [Google Scholar]
- 17.de Mattos A.M., da Costa J.A.C., Jordão Júnior A.A. Omega-3 Fatty acid supplementation is associated with oxidative stress and dyslipidemia, but does not contribute to better lipid and oxidative status on hemodialysis patients. J. Ren. Nutr. 2017 doi: 10.1053/j.jrn.2017.02.006. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; pii: S1051-2276(17)30067-5
- 18.Gao X., Gào X., Zhang Y. Associations of self-reported smoking, cotinine levels and epigenetic smoking indicators with oxidative stress among older adults: a population-based study. Eur. J. Epidemiol. 2017 doi: 10.1007/s10654-017-0248-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Bloomer R.J., Goldfarb A.H. Anaerobic exercise and oxidative stress: a review. Can. J. Appl. Physiol. 2004;29:245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 20.Molano F., Saborido A., Delgado J. Rat liver lysosomal and mitochondrial activities are modified by anabolic-androgenic steroids. Med. Sci. Sports Exerc. 1999;31:243–250. doi: 10.1097/00005768-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Pey A., Saborido A., Blazquez I. Effects of prolonged stanozolol treatment on antioxidant enzyme activities, oxidative stress markers and heat shock protein HSP72 levels in rat liver. J. Steroid Biochem. Mol. Biol. 2003;87:269–277. doi: 10.1016/j.jsbmb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Camiletti-Moiron D., Aparicio V.A., Nebot E. High intensity exercise modified the effects of stanozolol on brain oxidative stress in rats. Int. J. Sport Med. 2015;36:984–991. doi: 10.1055/s-0035-1548941. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer W.J., Fry A.C. 6th ed. Human Kinetics; Champaign, IL: 1995. ACSM’s Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 24.Hopkins W.G., Marshall S.W., Batterham A.M. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009;41:3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 25.Radak Z., Pucsok J., Meeseki S. Muscle soreness-induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic. Biol. Med. 1999;26:1059–1063. doi: 10.1016/s0891-5849(98)00309-8. [DOI] [PubMed] [Google Scholar]
- 26.Bloomer R.J., Goldfarb A.H., Wideman L. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength Cond. Res. 2005;19:276–285. doi: 10.1519/14823.1. [DOI] [PubMed] [Google Scholar]
- 27.Pozzi R., Rosa J.C., Eguchi R. Genetic damage in multiple organs of acutely exercised rats. Cell Biochem. Funct. 2010;28(8):632–636. doi: 10.1002/cbf.1700. [DOI] [PubMed] [Google Scholar]
- 28.Sakai Y., Iwamura Y., Hayashi J. Acute exercise causes mitochondrial DNA deletion in rat skeletal muscle. Muscle Nerve. 1999;22(2):258–261. doi: 10.1002/(sici)1097-4598(199902)22:2<258::aid-mus15>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Tsai K., Hsu T.G., Hsu K.M. Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic. Biol. Med. 2001;31(11):1465–1472. doi: 10.1016/s0891-5849(01)00729-8. [DOI] [PubMed] [Google Scholar]
- 30.Demirbag R., Yılmaz R., Güzel S. Effects of treadmill exercise test on oxidative/antioxidative parameters and DNA damage. Anadolu Kardiyol Derg. 2006;6:135–140. [PubMed] [Google Scholar]
- 31.Ramel A., Wagner K.H., Elmadfa I. Plasma antioxidants and lipid oxidation after submaximal resistance exercise in men. Eur. J. Nutr. 2004;43:2–6. doi: 10.1007/s00394-004-0432-z. [DOI] [PubMed] [Google Scholar]
- 32.Nikolaidis M.G., Mougios V. Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med. 2004;34:1051–1076. doi: 10.2165/00007256-200434150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Goto C., Nishioka K., Umemura T. Acute moderate intensity exercise induces vasodilation through an increase in nitric oxide bioavailiability in humans. Am. J. Hypertens. 2007;20(8):825–830. doi: 10.1016/j.amjhyper.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Dixon C.B., Robertson R.J., Goss F.L. The effect of acute resistance exercise on serum malondialdehyde in resistance-trained and untrained collegiate men. J. Strength Cond. Res. 2006;20:693–698. doi: 10.1519/R-15854.1. [DOI] [PubMed] [Google Scholar]
- 35.Node K., Kitakaze M., Sato H. Effect of acute dynamic exercise on circulating plasma nitric oxide level and correlation to norepinephrine release in normal subjects. Am. J. Cardiol. 1997;79:526–528. doi: 10.1016/s0002-9149(96)00804-1. [DOI] [PubMed] [Google Scholar]
- 36.Miranda K.M., Espey M.G., Wink D.A. A rapid simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:67–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 37.Gül M., Demircan B., Taysi S. Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006;143:239–245. doi: 10.1016/j.cbpa.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Hudson M.B., Hosick P.A., McCaulley G.O. The effects of resistance exercise on hormonal markers of oxidative stress. Med. Sci. Sport Exerc. 2008;40:542–548. doi: 10.1249/MSS.0b013e31815daf89. [DOI] [PubMed] [Google Scholar]
- 39.Coker R.H., Kjaer M. Glucoregulation during exercise – the role of the neuroendocrine system. Sports Med. 2005;35:575–583. doi: 10.2165/00007256-200535070-00003. [DOI] [PubMed] [Google Scholar]
- 40.Sawka M.N., Burke L.M., Eichner E.R. American College of Sports Medicine position stand: exercise and fluid replacement. Med. Sci. Sports Exerc. 2007;39:377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 41.St Croix C.M., Wetter T.J., Pegelow D.F. Assessment of nitric oxide formation during exercise. Am. J. Res. Crit. Care Med. 1999;159:1125–1133. doi: 10.1164/ajrccm.159.4.9806144. [DOI] [PubMed] [Google Scholar]


