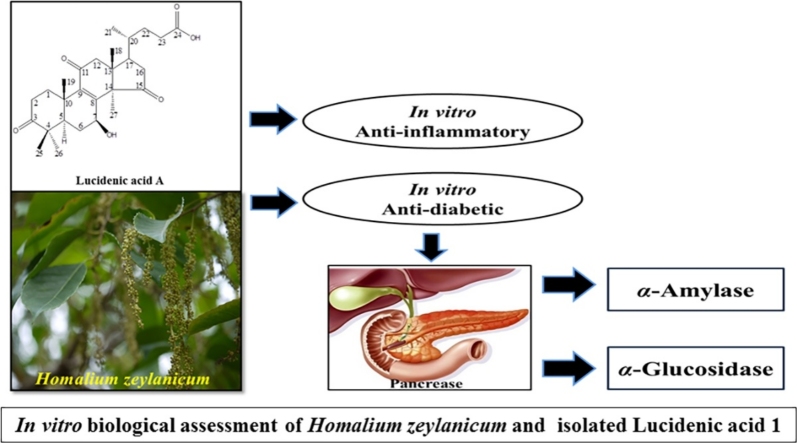
Graphical abstract
Abbreviations: HAHZ, hydro alcoholic extract of Homalium zeylanicum; TLC, thin layer chromatography; Rf, Retardation factor; TPC, total phenolic contents; TFC, total flavonoid contents; DPPH, 2,2-diphenyl-1-picrylhydrazyl; NO, Nitric oxide; OH, hydroxyl; SOD, superoxide anion; GAeqv/g, gallic acid equivalents per gram; Queqv/g, Quercetin equivalents per gram; NBT, nitroblue tetrazolium; PMS, phenazine methosulphate; IC50, half maximal inhibitory concentration; pNPG, p-nitrophenyl-α-d-glucopyranoside; DNS, dinitrosalicylic; ROS, reactive oxygen species; PBS, phosphate buffer saline; NSAIDs, nonsteroidal anti-inflammatory drugs
Chemical compounds studied in this article: Lucidenic acid A (PubChem CID: 14109375), gallic acid (PubChem CID: 370), Quercetin (PubChem CID: 5280343), EDTA (PubChem CID: 6049), acarbose (PubChem CID: 41774), Diclofenac sodium (PubChem CID: 5018304)
Keywords: Homalium zeylanicum, Lucidenic acid a, Antidiabetic, Antiinflammatory, &alpha, -glucosidase, &alpha, -amylase
Highlights
-
•
Current investigation supports antioxidant, anti-diabetic activities of H. zeylanicum.
-
•
Current investigation also supports anti-inflammatory activity of H. zeylanicum.
-
•
The research successfully isolated and analysed structure of lucidenic acid A.
-
•
Lucidenic acid A reported first time in the bark of H. zeylanicum.
-
•
Lucidenic acid A produces a significant anti-inflammatory activity.
Abstract
Homalium zeylanicum (Gardner) Benth. (Flacourtiaceae) is a medicinal plant useful in controlling rheumatism, inflammation and diabetes. The objective of this work evaluates in vitro antioxidant, antidiabetic, and antiinflammatory properties of hydroalcohol extract of bark of H. zeylanicum (HAHZ). It also describes isolation and structure determination of lucidenic acid A, which is the first report in this plant. In order to explain the role of antioxidant principles, DPPH, nitric oxide, hydroxyl, superoxide and metal chelating assays were performed. Antidiabetic and anti-inflammatory activities were investigated by quantifying α-amylase, α-glucosidase and protein denaturation inhibitory activities of HAHZ. Biochemical estimations were performed. The chemical structure of the triterpenoid was elucidated using 1H, 13C NMR and high resolution-MS. IC50 of DPPH, nitric oxide, hydroxyl, superoxide and metal chelating activities were of 36.23 ± 0.27, 40.11 ± 0.32, 35.23 ± 0.57, 43.34 ± 0.22 and 11.54 ± 0.08 μg/mL, respectively. IC50 of α-amylase and α-glucosidase activities were 29.12 ± 0.54, and 18.55 ± 0.15 μg/mL. Total phenolic and total flavonoid contents were recorded at 233.65 mg/g GAE and 172.7 mg/g QE. Regarding kinetic behaviour, HAHZ showed competitive inhibition on α-glucosidase and mixed competitive inhibition on α-amylase. Lucidenic acid A was confirmed by spectroscopic studies. Anti-inflammatory activity of lucidenic acid A was determined by using protein denaturation assay with IC50 13 μg/mL but HAHZ showed 30.34 ± 0.13 μg/mL. Phenols and flavonoids could be attributed to inhibition of intestinal carbohydrases for anti-diabetic activities whereas triterpenoids could be responsible for anti-inflammatory activity of H. zeylanicum.
1. Introduction
Oxidative stress is the major cause of a number of chronic diseases such as diabetes, rheumatic arthritis, cancer, atherosclerosis, hematological and neurodegenerative disorders. Generation of free radicals due to oxidative stress factors associated with inflammation and other diseases became major health issues in recent years. The oxi-flammation effect is induced by oxidative stress factors which may affect various organ systems, and progression of insulin-resistance in the body. Increased inflammation, oxidative stress, dyslipidemia, and glucotoxicity are interlinked with each other which may cause an extra demand on β-cells to stimulate insulin. In this process β-cells are no longer able to meet the over increasing demand of insulin, resulting in the development of frank diabetes and may contributes to several diabetes-associated complications like cardiovascular diseases, nephropathy, neuropathy, retinopathy, urological diseases, and cancer [1], [2]. India stands first in the whole world, having the highest number of diabetes patients and this disorder is increasing very fast across the globe. The global incidence stress induced diabetes for all age groups would reach to 4.4% in 2030 CE [3]. Within few decades; it will become one of the world’s commonest forms of disease. As per ethnobotanical reports, more than 800 plant species having antidiabetic and antioxidant activities are found in literature. This protective roles can be mainly attributed to the presence of secondary metabolites, which are defined as bioactive phenols, flavoniods and alkaloids in fruits, vegetables, grains, and other parts of plants [4], [5]. Various pharmacological approaches have been introduced in diabetes treatment. These treatments include different modes of action of herbal drugs including stimulation of insulin release, inhibition of gluconeogenesis, increasing the number of glucose transporters and reduction of glucose absorption from the intestine [6]. One of the beneficial therapies is to impair the glucose absorption by the inhibition of carbohydrate hydrolyzing enzymes such as α-amylase and α-glucosidase in the digestive organs.
Homalium zeylanicum (Gardner) Benth. (Flacourtiaceae), commonly known as ‘Kalladamba', is distributed in Western Ghats, Andhra Pradesh, Tamil Nadu, and Kerala of India. Ethnobotanically, H. zeylanicum is used in many ailments such as diabetes, rheumatism, and wound healing activities [7], [8]. Our earlier report on antioxidant activities of Indian species of Homalium established that the ethyl acetate extract of leaves and bark of H. nepalense, H. tomentosum and H. zeylanicum were found to be better active than other successive extracts [9]. This work is presented here to establish further the antioxidant, anti-diabetic, and anti-inflammatory activity of hydroalcohol extract of H. zeylanicum. The kinetic behaviour of H. zeylanicum on α-glucosidase and α-amylase were performed while evaluating the antidiabetic properties of this plant. The current investigation reported first the presence of a triterpenoid lucidenic acid A in the bark of H. zeylanicum.
2. Materials and methods
2.1. General
1H spectra was measured on high resolution 700 MHz NMR Spectroscopy (Agilent DD2700 MHz NMR), 13C NMR was measured on Bruker AvIII HD–300 MHz FT NMR with low and high temperature facility −90 °C to 80 °C in CDCl3, with tetramethylsilane (TMS) as an internal standard. Mass spectra data was recorded on a JMS-T100L; AccuTOF Mass spectrometer. FT-IR was recorded on Agilent Cary 630. All the spectroscopic analysis of compound 1 had done at Central Drug Research Institute (CDRI), Lucknow, India. Column chromatography was performed by using a glass column (Borosil, 500 × 18 mm) and it was filled with silica gel (100–200 mesh, Himedia, India). TLC was performed using silica gel 60 F254 (Merck, India) precoated plates and detection was visualized at 254 nm and 365 nm UV. Other chemicals and reagents used in the study were of analytical grades and procured from Himedia, India, Sigma-Aldrich, India and SRL, India.
2.2. Plant collection and identification
Barks of Homalium zeylanicum were collected from Tirumala Hills, Chittoor District, Andhra Pradesh, India. The plant was botanically identified by Dr. P.C. Panda, Principal Scientist, Regional Plant Resource Centre, Bhubaneswar, Odisha. Voucher specimen was deposited in the herbarium of RPRC for future references (Voucher No. 7545/T).
2.3. Extraction and isolation
The dried powdered bark materials of H. zeylanicum (1 kg) were extracted with 70% hydro-alcoholic (HAHZ) (3 L × 4) by cold maceration. HAHZ was concentrated (9.6%; w/w) and preliminary phytochemical investigation was carried out for HAHZ in order to assess the presence of different phytochemicals [10]. HAHZ (20 g) was chromatographed on a column eluted successively with stepwise gradients of hexane (100%), followed by hexane:chloroform in the proportion of 99:1, 98:2 and continued upto 0:100 with chloroform. Then the elution was followed by chloroform:methanol in the proportion of 99:1, 98:2, 97:3 and continued to 90:10. Around 415 fractions were collected with each fraction collection capacity was of 15 mL and accordingly similar fractions were re-pooled into a single fraction by TLC profiling with same Rf values. Each fraction was tested by following in vitro anti-inflammatory protein denaturation activity study by using multimode microplate reader (Synergy H1 M, BioTEK, USA). It was found that the fraction no. 106–113 with the eluent of hexane:chloroform (66:34%), were shown better activities than other fractions. Further purification of the fraction no. 106–113 was done by washing number times with methanol and a powdered isolated pure compound 1 was obtained (6.7 mg; with respect to HAHZ of 0.0022%). Purification was further cross checked by performing TLC with hexane:chloroform (1:1, v/v; Rf 0.20).
2.4. Estimation of total phenolic contents (TPC)
TPC of HAHZ was determined by using the Folin-Ciocalteu reagent [11]. About 10 μL of 1 mg/mL HAHZ, 450 μL of distilled water and 2.5 mL of 0.2 N Folin-Ciocalteu reagents was added. After 5 min, 2 mL of 10% sodium carbonate was added. The absorbance of the resulting blue-colored solution was measured at 765 nm after incubation at 37 °C for 30 min by using a multimode micro plate reader (SynergyH1MF, BioTek, USA). Gallic acid was used as a reference drug and phenolic content was expressed as mg/g gallic acid equivalents (GAE) per gram of dried extract (mg GAeqv/g).
2.5. Estimation of total flavonoid contents (TFC)
TFC of HAHZ was assayed according to standard protocol with a slight modification to it [11]. About 500 μL of 1 mg/mL HAHZ was mixed with 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminium chloride hexahydrate, 0.1 mL of 1 M potassium acetate and 2.8 mL of deionized water. After incubation at room temperature for 30 min, the absorbance of the reaction mixture was measured at 415 nm by using a multimode micro plate reader (SynergyH1MF, BioTek, USA). Quercetin was used as reference drug and the results were expressed as mg/g Quercetin equivalents (mg Queqv/g).
2.6. DPPH free radical scavenging assay of H. zeylanicum
The scavenging activity of HAHZ on the stable free radical DPPH was assayed using the modified protocol in which the bleaching rate of DPPH was monitored at a characteristic wavelength in the presence of the sample [12]. Stock solution of 1 mg/mL was prepared in methanol. Various concentrations (10–100 μg/mL) of HAHZ were mixed with 0.1 mL of a 0.15% DPPH solution in methanol. The mixture was kept for 30 min in the darkness, and then the absorbance was read at 517 nm (SynergyH1MF, BioTek, USA).% of decrease in DPPH absorbance was calculated by measuring the absorbance of the sample by applying the following equation:
| Inhibition (%) = (AControl-ASample/AControl) X 100 |
Different concentrations of ascorbic acid as a reference drug were used as positive controls.
2.7. Nitric oxide (NO) free radical scavenging activity of H. zeylanicum
The reaction mixture (3 mL) containing sodium nitroprusside (10 mM) in phosphate buffer saline (PBS) and different concentrations of HAHZ (10–100 μg/mL) were incubated at 25 °C for 150 min. Then 1 mL of Griess reagent (1% sulphanilamide, 2% H3PO4 and 0.1% naphthyl ethylene diaminedihydrochloride) was added. The absorbance of the chromophore formed was measured at 546 nm (SynergyH1MF, BioTek, USA).% of inhibition of nitric oxide generated was measured by comparing the absorbance values of control and test compounds, whereas quercetin was taken as reference drug. The procedure followed here was the modified [13].
| Inhibition (%) = (AControl-ASample/AControl) X 100 |
2.8. Hydroxyl (OH) free radical scavenging activity of H. zeylanicum
The scavenging activity of HAHZ on hydroxyl radical was measured according to the method of Klein et al. [14]. Different concentrations of HAHZ (1 mL) were added with 1 mL of iron-EDTA solution (200 μM ferrous ammonium sulfate and 1 mM EDTA), 100 μL of hydrogen peroxide (1 mM), 360 μL of deoxyribose (28 mM) in 50 mM sodium phosphate buffer, pH 7.4). The reaction was initiated by adding 100 μL of ascorbic acid (0.22%) and incubated at 80–90 °C for 15 min in a water bath. After incubation the reaction was terminated by the addition of 1 mL of ice-cold TCA (10% w/v). 3 mL of Nash reagent (75 g of ammonium acetate, 3 mL of glacial acetic acid, and 2 mL of acetyl acetone were mixed and raised to 1 L with distilled water) was added, and left at room temperature for 15 min. The reaction mixture without sample was used as control. The intensity of the color formed was measured with spectrophotometer at 412 nm against reagent blank (SynergyH1MF, BioTek, USA). Ascorbic acid was served as a control.
| Inhibition (%) = (AControl-ASample/AControl) X 100 |
2.9. Superoxide anion (SOD) free radical scavenging activity of H. zeylanicum
Measurement of SOD anion scavenging activity of different HAHZ was performed by following the protocol described by Yen and Chen [15]. About 1 mL of nitroblue tetrazolium (NBT) solution (156 μM NBT in 100 mM phosphate buffer, pH 7.4), 1 mL NADH solution (468 μM in 100 mM phosphate buffer, pH 7.4) and 0.1 mL of HAHZ in methanol were mixed. The reaction started by adding 100 μL of phenazine methosulphate (PMS) solution (60 μM PMS in 100 mM phosphate buffer, pH 7.4) to the mixture. The reaction mixture was incubated at 25 °C for 5 min and the absorbance at 560 nm was measured against blank samples (SynergyH1MF, BioTek, USA). Decreased absorbance of the reaction mixture indicated increased SOD anion scavenging activity.% of inhibition was calculated by considering quercetin as a reference drug.
| Inhibition (%) = (AControl-ASample/AControl) X 100 |
2.10. Metal chelating activity of H. zeylanicum
The chelation of ferrous ions by HAHZ was performed according to the method developed by Dinis et al. [16]. Briefly, 50 μL of 2 mM FeCl2 was added to 1 mL of different concentrations of HAHZ (10–100 μg/mL). The reaction was initiated by the addition of 0.2 mL of 5 mM ferrozine solution. The mixture was vigorously shaken and left to stand at room temperature for 10 min. The absorbance of the solution was thereafter measured at 562 nm (SynergyH1MF, BioTek, USA).% of inhibition of ferrozine-Fe2+ complex formation was calculated by following the above equation. EDTA was used as positive control.
| Inhibition (%) = (AControl-ASample/AControl) X 100 |
2.11. Antidiabetic study of H. zeylanicum
2.11.1. α-glucosidase inhibition study of H. zeylanicum
The procedure was followed according to the method of with slight modifications of the previous protocol [17]. α-glucosidase type I (1 U/mL) (20 μL) was premixed with HAHZ/acarbose at varying concentrations made up in 50 mM phosphate buffer at pH 6.8 and incubated for 5 min at 37 °C. 1 mM pNPG (20 μL) in 50 mM of phosphate buffer was added to initiate the reaction, and the mixture was further incubated at 37 °C for 20 min. The reaction was terminated by the addition of 50 μL of 1 M Na2CO3, and the final volume was made up to 150 μL. α-glucosidase activity was determined spectrophotometrically at 405 nm (SynergyH1MF, BioTek, USA) by measuring the quantity of p-nitrophenol released from pNPG. The assay was performed in triplicate. IC50 value of HAHZ was calculated by following the above equation. Acarbose was used as positive control.
| Inhibition (%) = (AControl-ASample/AControl) X 100 |
2.11.2. Mode of α-glucosidase inhibition by H. zeylanicum
The mode of inhibition of α-glucosidase by HAHZ was determined according to the modified method of Ali et al. [18], by means of substrate concentration at 1/2 Vmax of v and HAHZ concentration and Lineweaver-Burk plot over pNPG. Briefly, 50 μL of HAHZ (500 μg/mL) was preincubated with 100 μL of α-glucosidase solution (1 U/mL) for 10 min at 25 °C in one set of tubes. In another set of tubes α-glucosidase was preincubated with 50 μL of phosphate buffer (pH 6.9). 50 μL of pNPG at increasing concentrations in the range of 10–50 mM (1/pNPG = 0.1-0.5 mM−1), was added to both sets of reaction mixtures to start the reaction. The mixture was then incubated for 10 min at 25 °C, and 500 μL of Na2CO3 was added to stop the reaction. The amount of reducing sugars released was determined spectrophotometrically by using acarbose standard curve and converted to reaction velocities. A double reciprocal (Lineweaver-Burk) plot (1/v versus 1/[S]) where v is reaction velocity and [S] is substrate pNPG concentration was plotted to determine the mode of inhibition. All graphs were plotted by Microsoft Excel [19].
2.11.3. α-amylase inhibition study of H. zeylanicum
Porcine pancreatic α-amylase inhibition referred to the method of Kwon et al. [20]. A total of 200 μL of sample solution and 500 μL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing α-amylase solution (0.5 mg/mL) were incubated at 25 °C for 10 min. After pre-incubation, 500 μL of a 1% starch solution in 0.02 M sodium phosphate buffer was added. The reaction mixture was then incubated at 25 °C for 10 min. The reaction was stopped with 1 mL of dinitrosalicylic (DNS) acid, color reagent. The reaction mixture was then incubated in a boiling water bath for 5 min, and cooled to room temperature. The reaction mixture was then diluted after adding 10 mL of water, and absorbance was measured at 540 nm (SynergyH1MF, BioTek, USA). Acarbose was used as positive control.
| Inhibition (%) = (AControl-ASample/AControl) X 100 |
2.11.4. Mode of α-amylase inhibition by H. zeylanicum
The mode of inhibition of α-amylase by HAHZ was determined using the bark extract with the lowest IC50 according to the modified method [18] by means of substrate concentration at 1/2 Vmax of v and HAHZ concentration and Lineweaver-Burk plot over starch. Briefly, 250 μL of the (100 μg/mL) HAHZ was preincubated with 250 μL of α-amylase solution (1 U/mL) for 10 min at 25 °C in one set of tubes. In another set of tubes α-amylase was preincubated with 250 μL of phosphate buffer (pH 6.9). 250 μL of starch at increasing concentrations in the range of 10–50 mM (1/starch = 0.1-0.5 mM−1), was added to both sets of reaction mixtures to start the reaction. The mixture was then incubated for 10 min at 25 °C, and 500 μL of DNS was added to stop the reaction. The amount of reducing sugars released was determined spectrophotometrically using acarbose standard curve and converted to reaction velocities. A double reciprocal (Lineweaver-Burk) plot (1/v versus 1/[S]) where v is reaction velocity and [S] is substrate starch concentration was plotted to determine the mode of inhibition. All graphs were plotted by Microsoft Excel [19].
2.12. Protein denaturation study of H. zeylanicum
The reaction mixture (5 mL) consisted of 0.2 mL of egg albumin (from fresh hen’s egg), 2.8 mL of phosphate buffered saline (pH 6.4) and 2 mL of varying concentrations of HAHZ. Similar volume of double-distilled water served as control. Then the mixtures were incubated at 37 ± 2 °C for 15 min and then heated at 70 °C for 5 min after vigorous shaking. After cooling, the absorbance was measured at 660 nm (SynergyH1MF, BioTek, USA). Diclofenac sodium was used as reference drug [21].% of inhibition of protein denaturation was calculated by using the formula as above. The extract/drug concentration for 50% inhibition (IC50) was determined from the dose response curve by plotting% of inhibition with respect to control against treatment concentration.
2.13. Statistical analysis
A minimum of three independent experiments were carried and the results are presented as mean ± standard deviation by using SPSS, Version 11. Calibration curves of the standards were considered as linear if R2 > 0.99.
3. Results and discussions
3.1. Phytochemical analysis and biochemical estimation of H. zeylanicum
HAHZ showed the presence of phenols, flavonoids, tannins, saponins, steroids, and carbohydrates. Studies on polyphenols have shown a wide range of antibacterial, antiviral, antiinflammatory, anticancer, anti-allergic, and antidiabetic activities [6], [22]. HAHZ contains substantial polyphenols, and flavonoids. Biochemical analysis of HAHZ were investigated and found to contain the higher level of TPC, TFC content of 233.65 mg GAE/g and 172.7 mg Queqv/g, respectively. Plant materials rich in phenolic and flavonoid compounds have potent antioxidant activities and are thought to have positive effects on human health [23]. Results suggested that TPC and TFC may be the major contributors for the antioxidant activity of H. zeylanicum, and exhibited significant correlation in the radical scavenger activities.
3.2. DPPH free radical scavenging assay of H. zeylanicum
When antioxidants react with DPPH, which is a stable free radical becomes paired off in the presence of a hydrogen donor and is reduced to the DPPHH and as a consequence, the absorbance decreases resulting in decolorization (yellow color) with respect to the number of electrons captured. HAHZ significantly and dose dependently reduced DPPH radical, and the IC50 was recorded at 36.23 ± 0.27 μg/mL and the result was comparable with the reference drug ascorbic acid (25.12 ± 0.07 μg/mL) (Table 1). Free radicals are known to play a definite role in a wide variety of pathological manifestations [24]. In the present study HAHZ showed significantly higher percentage of inhibition of DPPH and positively correlated with the higher amount of phenols and flavonoids presence in the bark of HAHZ that are capable of donating hydrogen to a free radical to scavenge the potential damage.
Table 1.
In vitro antioxidant, anti-diabetic, and anti-inflammatory studies of hydroalcohol extract of bark of Homalium zeylanicum and lucidenic acid A.
|
In vitro antioxidant studies (μg/mL) | ||
|---|---|---|
| In vitro assays | HAHZ | Standard |
| DPPH | 36.23 ± 0.27 | 25.12 ± 0.07 |
| NO | 40.11 ± 0.32 | 24.13 ± 0.11 |
| OH | 35.23 ± 0.57 | 28.24 ± 0.17 |
| SOD | 43.34 ± 0.22 | 26.21 ± 0.13 |
| Metal chelating | 11.54 ± 0.08 | 12.27 ± 0.04 |
| In vitro anti-diabetic studies (μg/mL) | ||
| α-amylase | 29.12 ± 0.54 | 19.89 ± 0.21 |
| α-glucosidase | 18.55 ± 0.15 | 16.02 ± 0.24 |
| In vitro anti-inflammatory study (μg/mL) | ||
| Protein Denaturation | 30.34 ± 0.13 | 10.16 ± 0.12 |
| 13.56 ± 0.10 (Lucidenic acid A) | ||
Standard drug ascorbic acid was considered for DPPH and, hydroxyl radical scavenging assays. For nitric oxide, superoxide dismutase assays, quercetin was considered as a standard drug. For metal chelating assay, EDTA was considered as a standard drug to perform the assay. For anti-diabetic study, acarbose was considered as a standard drug for both α-amylase and α-glucosidase assays. For protein denaturation anti-inflammatory study, diclofenac sodium was considered as a standard drug to perform the assay. HAHZ is the hydroalcohol extract of bark of H. zeylanicum. However, the isolated Lucidenic acid A did not show in vitro antioxidant and anti-diabetic activities.
3.3. Nitric oxide (NO) free radical scavenging activity of H. zeylanicum
The procedure is based on the principle that, sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitrite ions that can be estimated using Griess reagent. Scavengers of nitric oxide compete with oxygen, leading to reduced production of nitrite ions and other ROS like NO2, N2O4 and peroxynitrite. Accumulation of large amounts of those radicals may lead to tissue damage by causing oxidative damage to lipids, proteins, nucleic acids and carbohydrates [25], [26]. HAHZ was a good scavenger of nitric oxide as it acted against oxygen, leading to reduced production of nitrite ions. Less the production of nitrite ions less is the absorbance value. The IC50 value of HAHZ was found to be 40.11 ± 0.32 μg/mL and the result was comparable with the reference drug quercetin (24.13 ± 0.11 μg/mL) (Table 1). The presence of phenols and flavonoids in HAHZ were responsible for the scavenging activity.
3.4. Hydroxyl (OH) free radical scavenging activity of H. zeylanicum
One of the most reactive oxygen radical is the OH radical as it can destroy the bio-molecules in the body, such as protein and DNA, resulting into mutagenesis, carcinogenesis and cytotoxicity [27]. Hydroxyl ions are generated from dihydrogen peroxide by Fenton reaction; Fe2+ + H2O2, and produced Fe3+ + OH. + OH−. It is the iron-salt-dependent degradation of dihydrogen peroxide, producing the highly reactive OH radical. On addition of a reducing agent, biological molecules get damaged [28]. In this study HAHZ showed appreciable potential to scavenge OH radicals. IC50 was recorded at 35.23 ± 0.57 μg/mL, and was found comparable to ascorbic acid (28.24 ± 0.17 μg/mL) (Table 1).
3.5. Superoxide (SOD) free radical scavenging activity of H. zeylanicum
SOD radical scavenging potential of HAHZ was determined and the IC50 found at 43.34 ± 0.22 μg/mL (Table 1). The result was comparable with the reference drug quercetin (26.21 ± 0.13 μg/mL). Radical scavenging activity was observed to be in increased fashion with the increase in the concentration of HAHZ. In PMS/NADH-NBT system, SOD anion is produced from dissolved oxygen in PMS/NADH coupling reaction. This anion leads to the reduction of NBT. Consequently, SOD anion in the reaction mixture gets consumed leading to the reduction in absorbance. Based on earlier report, it may be inferred that the presence of polyphenols was responsible for neutralizing the radicals generated by SOD thus suggesting the antioxidant potential of H. zeylanicum [29].
3.6. Metal chelating activity of H. zeylanicum
There are certain antioxidants that do not convert free radicals to more stable products but slow the rate of oxidation by several different mechanisms. Chelation of pro-oxidant metals is one of such activities. Iron and other transition metals (copper, chromium, cobalt, vanadium, cadmium, arsenic, and nickel) promote oxidation by acting as catalysts of free radical reactions. These redox-active transition metals transfer single electrons during changes in oxidation states. Metal ion chelating capacity of HAHZ was significant since it reduces the concentration of the transition metal that catalyzes lipid peroxidation [30]. Metal chelating activity of HAHZ recorded at 11.54 ± 0.08 μg/mL whereas EDTA, the reference drug IC50 was recorded at 12.27 ± 0.04 μg/mL (Table 1).
3.7. Inhibition and the mode inhibition of H. zeylanicum on α-glucosidase
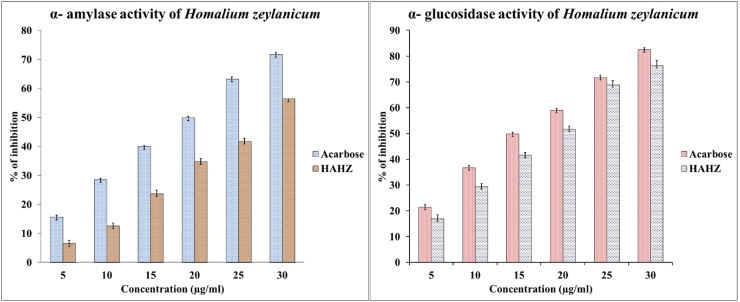
α-glucosidase inhibitory activity of HAHZ was investigated by using p-nitrophenyl-α-d-glucopyranoside (pNPG) as a substrate. Under specified conditions of pH 6.8 and at 7 °C, α-glucosidase catalyzes the conversion of the substrate 4-nitrophenyl-α-d-glucopyranoside (pNPG) to α-d-glucopyranoside and 4-nitrophenol. The yellow color developed by the latter product is measured spectrophotometrically at 405 nm. Our present study indicated the dose-dependent inhibitory activity of HAHZ against α-glucosidase with the IC50 of 18.55 ± 0.15 μg/mL and it showed remarkable inhibition on α-glucosidase suggesting the presence of potential enzyme inhibiting compound in the extract (Fig. 1). As an inhibitor of α-glucosidase, HAHZ delays the breaking down of carbohydrate in the small intestine and diminish the postprandial blood glucose excursion in a person suffering from diabetes [19]. From the kinetic study, it was found that HAHZ competitively inhibits α-glucosidase. As a competitive inhibitor, HAHZ blocks small intestine brush border enzymes which are necessary to hydrolyze oligo and polysaccharides to monosaccharide. Inhibition of this enzyme slows the absorption of carbohydrates as a result the postprandial rise in plasma glucose is blunted in both normal and diabetic subjects [31]. To find this mechanism of inhibition, we have formulated double reciprocal plot from the kinetics data by plotting the Lineweaver-Burk plot and the result indicates the competitive mode of inhibition of HAHZ similar to acarbose. In this study, we also found the inhibitory action of HAHZ on α-glucosidase to be reversible as the enzyme activity which was recovered intact after dialysis as the process of dialysis cleared the inhibitors from the enzyme.
Fig. 1.
In vitro anti-diabetic activity of hydroalcohol extract of bark of Homalium zeylaicum (HAHZ). Reducing power was measured at different concentration of HAHZ (10–100 μg/mL) for α-amylase and α-glucosidase assays. Acarbose was considered as control for both assays. Values were the mean of triplicate experiments for both α-amylase and α-glucosidase assays. The result represents as mean ± SEM (n = 3). IC50 value of HAHZ was found to be significantly compared (p < 0.05) to the standard drug acarbose in one way ANOVA test. Student’s t-test was performed to analyze this data set (SPSS, Version 11).
3.8. Inhibition and the mode inhibition of H. zeylanicum on α-amylase
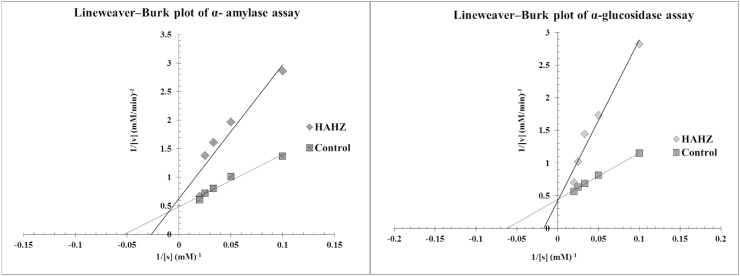
α-amylase inhibitors prevent dietary starches from being digested and absorbed by the body. This feature is useful for treating diabetes mellitus. α-amylase inhibitors act as an anti-nutrient that obstruct the digestion and absorption of carbohydrates and potentially useful in control of obesity and diabetes. Acarbose is complex oligosaccharides that delay the digestion of carbohydrates. It inhibits the action of pancreatic amylase in breakdown of starch [32]. Synthetic inhibitors cause side effects such as abdominal pain, diarrhoea and soft faces in the colon. Acarbose serves as a reference drug for α-amylase inhibitor assay. As can be seen in Fig. 1 and Table 1, acarbose at a concentration of (10–100 μg/mL) showed α-amylase inhibitory activity at 19.89 ± 0.21 μg/mL whereas the IC50 value of HAHZ was recorded at 29.12 ± 0.54 μg/mL. The mode of inhibitions by HAHZ on carbohydrate digesting enzymes as α-amylase as shown in Fig. 3. The mode of inhibition activities were determined by analysis of the double reciprocal (Lineweaver-Burk) plot. The graph depict that HAHZ displayed a mixed competitive inhibition of α-amylase activity (Fig. 3). This suggests that the active component of the HAHZ binds to a site other than the active site of the enzyme and thereby preventing the breaking down of oligosaccharides to disaccharides. This result is in agreement with previous reports which indicated that excessive inhibition of pancreatic α-amylase could result in the abnormal bacterial fermentation of undigested carbohydrates in the colon and therefore mild α-amylase inhibition activity is desirable [33]. Lineweaver-Burk plot also showed that HAHZ inhibits α-amylase mixed competitively. This suggests that the active components in the extract compete with the substrate for binding to the active sites of the enzyme there by preventing the breaking down of oligosaccharides to disaccharides [34].
Fig. 3.
Lineweaver-Burk plots of α-amylase and α-glucosidase (Vmax of 0.55 approx.) and (Vmax of 0.45 approx.) activities over a range of substrate concentrations (10–50 mM) in the absence (Control) or presence of hydroalcohol extract of bark of Homalium zeylaicum (HAHZ). The graph depicts competitive (reversible) mode of inhibition of α-glucosidase and mixed competitive mode of inhibition of α-amylase.
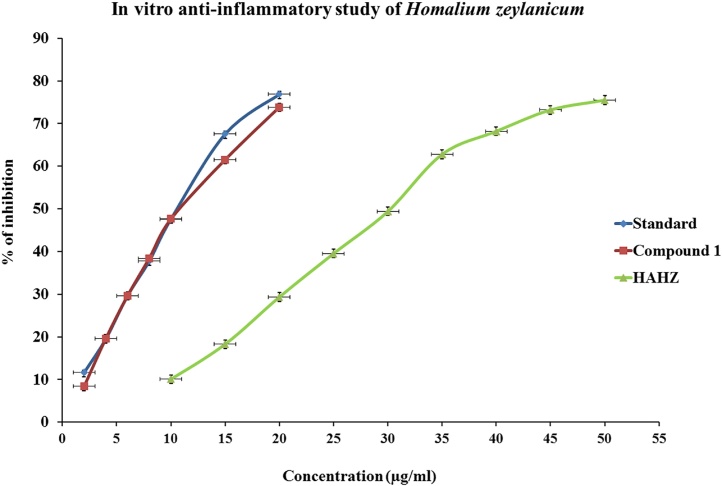
3.9. In vitro anti-inflammatory activity study of H. zeylanicum
Denaturation of proteins is a well documented cause of inflammation in conditions like rheumatoid arthritis. The protection against protein denaturation was the main mechanism of action of NSAIDs before the discovery of their inhibitory effect on cyclooxygenase, may play an important role in the anti-rheumatic activity of NSAIDs [35]. HAHZ produces a significant anti-inflammatory activity in dose dependent manner by inhibiting the protein denaturation at 30.34 ± 0.13 μg/mL (Fig. 2). For this assay diclofenac sodium was considered as a reference drug (IC50 10.16 ± 0.12 μg/mL). Further the isolated compound 1 was evaluated for protein denaturation study by following the above procedure and found that the drug has shown a better inhibitor of protein denaturation at concentration of 13.56 ± 0.10 μg/mL and was comparable with the reference drug (Table 1).
Fig. 2.
In vitro anti-inflammatory activities of hydroalcohol extract of bark of Homalium zeylaicum (HAHZ). The protein denaturation was measured at different concentration of HAHZ (10–100 μg/mL) and compound 1 (10–100 μg/mL). Diclofenac sodium was taken as standard for this assay. Values are the mean of triplicate experiments for this assay and represented as mean ± SEM (n = 3). IC50 values of two different groups as HAHZ and compound 1 were significantly compared (p < 0.05) to diclofenac sodium standard group in one way ANOVA test. Student’s t-test was performed to analyze this data set (SPSS, Version 11).
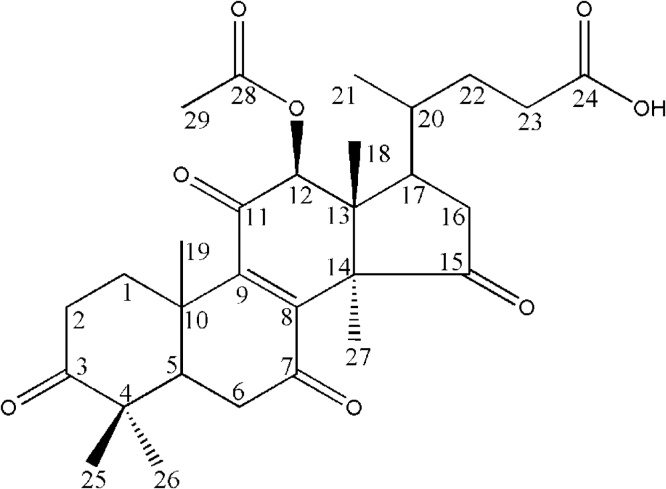
3.10. Compound-1
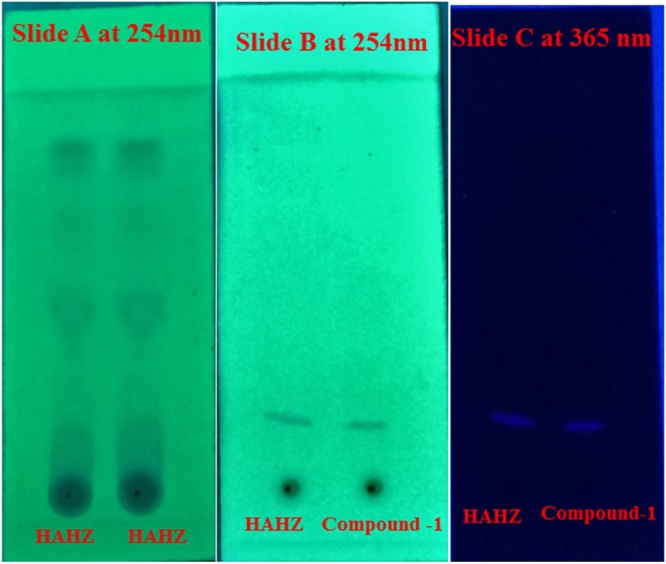
Lucidenic acid A (1); 7β-hydroxy, 4,4,14 trimethyl-3,11,15-tri-oxochol-8-en-24-oic acid was obtained as a white powder, and passed the terpenoid chemical test. Melting point was recorded at 284–285 °C, and further the purity of the compound 1 was confirmed by TLC (Rf 0.20; hexane:chloroform; 1:1 at 254 and 366 nm) as shown in Fig. 4 along with the fingerprint of HAHZ (chloroform:ethylacetate:formic acid; 4:5:1 at 254 nm). The UV spectrum with absorption maxima was recorded (EtOH) λmax at 233 nm, 254 nm and indicated the presence of hetero annular diene in compound 1 (Fig. 5).
Fig. 4.
Fingerprint and standardisation of HAHZ. Slide A: Fingerprint of HAHZ at two different concentrations (chloroform:ethylacetate:formic acid; 4:5:1) at 254 nm Slide B: Standardisation of compound 1 with HAHZ (chloroform:hexane; 1:1; Rf = 0.2) at 254 nm Slide C: Standardisation of compound 1 with HAHZ (chloroform:hexane; 1:1; Rf = 0.2) at 365 nm. HAHZ is the hydroalcohol extract of bark of Homalium zeylaicum.
Fig. 5.
Lucidenic acid A.
The IR spectrum of compound 1 revealed absorption bands for hydroxyl (3391 cm−1), α,β-unsaturated carbonyl (1624 cm−1), C—O—H stretching (1401 cm−1), C C stretching (1450 cm−1) and C—O stretching (1066 cm−1) functionalities.
The proton nuclear magnetic resonance (1H NMR) spectrum of compound 1 analyzed by the aid of 1H–1H shift correlated spectroscopy (COSY) and 1H-detected multiple quantum coherence spectroscopy (HMQC) experiments showed signals for six methyls of δ 0.67–1.12 (including one vinyl methyl at δ 1.70), a methine proton at δ 3.19 (dd, J 10.2 and, 4.4 Hz), and three olefinic protons at δ 4.73 (d, J 8.3 Hz), δ 4.735 (br s) and δ 4.6 (br s, J 9.1, 4.2 Hz). In addition, a singlet at δ 7.26 for a COOH proton was also observed (Table 2).
Table 2.
1H NMR and 13C NMR spectral data of compounds 1.
| 1H NMR (δ in ppm) | 13C NMR (δ in ppm) | ||
|---|---|---|---|
| 1H (α) | 1.70 | C1 | 30.2 (2) |
| 1H (β) | 1.972 | C2 | 33.9 (2) |
| 2H (α) | 2.194 | C3 | 213.6 (0) |
| 2H (β) | 2.198 | C4 | 46.9 (0) |
| 5H | 2.999 (dd) | C5 | 49.5 (1) |
| 6H (α) | 2.248 (dd) | C6 | 26.9 (2) |
| 6H (β) | 2.267 (dd) | ||
| 7H | 4.603 | C7 | 65.5 (1) |
| 12H (α) | 1.282 | C8 | 156.8 (0) |
| C9 | 140.2 (0) | ||
| C10 | 37.8 (0) | ||
| C11 | 196.7 (0) | ||
| C12 | 51.3 (2) | ||
| C13 | 48.4 (0) | ||
| C14 | 55.9 (0) | ||
| C15 | 215.6 (0) | ||
| 16H (α)- | 1.280 | C16 | 42.7 (2) |
| 16H (β)- | 1.282 | ||
| 17H | 1.467 | C17 | 47.2 (1) |
| 18CH3 | 0.671 | C18 | 18.1 (3) |
| 19CH3 | 1.282 | C19 | 18.8 (3) |
| 20H | 1.48 (m) | C20 | 36.3 (1) |
| 21CH3 | 0.89 (d) | C21 | 17.3 (3) |
| 22H | 1.197-1.249 (m) | C22 | 31.5 (2) |
| 23H | 2.99 & 3.17 (m) | C23 | 37.3 (2) |
| 24H | 7.26 (s) | C24 | 181.6 (0) |
| 25CH3 | 1.18 (s) | C25 | 24.1 (3) |
| 26CH3 | 1.19 (s) | C26 | 21.5 (3) |
| 27CH3 | 0.96 (s) | C27 | 28.4 (3) |
The carbon-13 nuclear magnetic resonance (13C NMR) spectrum demonstrated signals characteristic for six methyls, seven olefinic carbons, a hydroxyl-bearing methine carbon and showed 26 carbon atoms. It showed the presence of hydroxyl at C7 position (δ 65.5), carbonyl group of C15, C3 and C11 positions recorded at δ 215.6, 213.6 and 196.7 respectively. The presence of carbonyl group on COOH at C24 position (δ 181.6). The signal at δ 156.8 and 140.2 indicated the presence of α, β-unsaturated carbonyl group at C8 and C9 position (Table 1).
From the mass spectral analysis of 1, molecular formulae C27H38O6 by ESI–MS and was recorded at 458.59 (M+). The intensities of ion peaks were observed at 457 [M-H, 20%], 439 [M-H2O; 100%], 424 [M-CH3; 15%], 395 [M-H2O-CO2; 43%]. Above spectral data analysis and previous literature could confirm that the compound 1 was lucidenic acid A [36], [37].
4. Conclusion
The results of our current investigation support the potential role of H. zeylanicum as an antioxidant, anti-diabetic and anti-inflammatory agent. Our approach was to perform some assays regarding reactive species and enzymes with their biological significance (e.g., DPPH, superoxide dismutase, nitric oxide, hydroxyl and metal chelating activities) and the studies revealed that H. zeylanicum had high antioxidant activity in all assays with lower IC50 values. We further investigated the anti-diabetic and anti-inflammatory activities and based on the significant enzymes inhibition of α-amylase and α-glucosidase and denaturation of proteins studies, it may be inferred that the bark of H. zeylanicum may be the best sources of anti-diabetic and anti-inflammatory agent. Lucidenic acid A reported first time in the bark of this plant and produces a significant anti-inflammatory activities in a dose dependent manner. Other specific compounds responsible for biological activities need to be explored and further investigations for the most active compounds will be done in the near future.
Declaration of interest
The authors report no declarations of interest.
Acknowledgement
The authors wish to acknowledge the financial support of Science and Technology, Department of Biotechnology, Government of Odisha, Bhubaneswar for funding the research (1181/ST; Dt.13-03-2014).
References
- 1.Harcourt B.E., Penfold S.A., Forbes J.M. Coming full circle in diabetes mellitus: from complications to initiation. Nat. Rev. Endocrinol. 2013;91:13–123. doi: 10.1038/nrendo.2012.236. [DOI] [PubMed] [Google Scholar]
- 2.Vikram A., Jena G., Ramarao P. Insulin-resistance and benign prostatic hyperplasia: the connection. Eur. J. Pharmacol. 2010;641:75–81. doi: 10.1016/j.ejphar.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Sarah W., Gojka R., Anders G., Richard S., Hilary K. Global prevalence of diabetes. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Wang L.F., Chen J.Y., Xie H.H., Ju X.R., Liu R.H. Phytochemical profiles and antioxidant activity of adlay varieties. J. Agric. Food Chem. 2013;615:103–5113. doi: 10.1021/jf400556s. [DOI] [PubMed] [Google Scholar]
- 5.Mursu J., Virtanen J.K., Tuomainen T.P., Nurmi T., Voutilainen S. Intake of fruit, berries, and vegetables and risk of type2 diabetes in Finnish men: the Kuopio ischaemic heart disease risk factor study. Am. J. Clin Nutr. 2014;9:328–333. doi: 10.3945/ajcn.113.069641. [DOI] [PubMed] [Google Scholar]
- 6.Hassan B.A.R. Overview on diabetes mellitus (Type2) J. Chromatogr. Sep. Tech. 2013;4:2. [Google Scholar]
- 7.Sandhya S., Sai K.P., Vinod K.R., David B., Kumar K. Plants as potent anti-diabetic and wound healing agents-a review. Hygeia J. Drugs Med. 2011;3:11–19. [Google Scholar]
- 8.Madhavachetty K., Sivaj K., Tulashi R.K. Student Offset Printers; Tirupati: 2008. Flowering Plants of Chittoor District. 45 p. [Google Scholar]
- 9.Mahapatra A.K., Pani S.S., Sahoo A.K. Free radical scavenging activities of Homalium species-An endangered medicinal plant of Eastern Ghats of India. Nat. Prod. Res. 2013;29:2112–2116. doi: 10.1080/14786419.2014.987142. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi C., Franceschini J. Experimental observations on Haffner's method for testing analgesic drugs. Br. J. Pharmacol Chemother. 1956;9:280–284. doi: 10.1111/j.1476-5381.1954.tb01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misbah H., Abdul A.A., Aminudin N. Antidiabetic and antioxidant properties of Ficus deltoidea fruit extracts and fractions. BMC Complement. Altern. Med. 2013;13:118. doi: 10.1186/1472-6882-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan J.B.L., Yap J.W., Tan S.Y., Lim Y.Y. Antioxidant content, antioxidant activity, and antibacterial activity of five plants from the Commelinaceae family. Antioxidants. 2014;3:758–769. doi: 10.3390/antiox3040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sreejayan N., Rao M.N.A. Nitric oxide scavenging by cucuminoids. J. Pharm. Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 14.Klein S.M., Cohen G., Cederbaum A.I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry. 1981;20:6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- 15.Yen G.C., Chen H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995;43:27–32. [Google Scholar]
- 16.Dinis T.C.P., Madeira V.M.C., Almeida M.L.M. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 17.Brueggeman G.P., Hollingsworth R.I. A preparation and screening strategy for glucosidase inhibitors. Tetrahedron. 2001;57:8773–8778. [Google Scholar]
- 18.Ali H., Houghton P.J., Soumyanath A. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006;107:449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Bisswanger H. WILEY-VCH Verlag; GmbH, Weinheim: 2002. Enzyme Kinetic, Principle and Method; pp. 51–130. [Google Scholar]
- 20.Kwon Y.I., Apostolidis E., Kim Y.C., Shetty K. Health benefits of traditional corn, beans and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Food. 2007;10:266–275. doi: 10.1089/jmf.2006.234. [DOI] [PubMed] [Google Scholar]
- 21.Elias G., Rao M.N. Inhibition of albumin denaturation and anti-inflammatory activity of dehydrozingerone and its analogs. Indian J. Exp. Biol. 1988;26:540–542. [PubMed] [Google Scholar]
- 22.Hajhashemi V., Sadeghi H., Minaiyan M., Movahedian A., Talebi A. The role of central mechanisms in the anti-inflammatory effect of amitriptyline on carrageenan-induced paw edema in rats. Clinics. 2010;65:1183–1187. doi: 10.1590/S1807-59322010001100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes P.X., Silva S.F., Guedes R.J., Almeida S. Biological oxidations and antioxidant activity of natural products Phytochemicals as nutraceuticals-global approaches to their role in nutrition and health. BMC Complement. Altern. Med. 2012;12:215. [Google Scholar]
- 24.Umamaheswari M., Chatterjee T.K. In vitro antioxidant activities of the fractions of Coccinnia grandis L. leaf extract. Afr. J. Tradit. Complement. Altern. Med. 2008;5:61–73. [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy M.P. Nitric oxide and cell death. Biochim. Biophys. Acta. 1999;1411:401–414. doi: 10.1016/s0005-2728(99)00029-8. [DOI] [PubMed] [Google Scholar]
- 26.Hausladen A., Stamler J.S. Nitrosative stress. Methods Enzymol. 1999;300:389–395. doi: 10.1016/s0076-6879(99)00143-3. [DOI] [PubMed] [Google Scholar]
- 27.Tsai C.H., Stern A., Chiou J.F., Chern C.L., Liu T.Z. Rapid and specific detection of hydroxyl radical using an ultraweak chemiluminescence analyzer and a low-level chemiluminescence emitter: application to hydroxyl radical-scavenging ability of aqueous extracts of Food constituents. J. Agric. Food Chem. 2001;49:2137–2141. doi: 10.1021/jf001071k. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr. Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H., Wallace D.C., Epstein C.J. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 30.Končić M.Z., Barbarić M., Perković I., Zorc B. Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules. 2011;16:6232–6242. doi: 10.3390/molecules16086232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shihabudeen H.M.S., Priscilla D.H., Thirumurugan K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr. Metab. 2011;8:46. doi: 10.1186/1743-7075-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried M., Abramson S., Meyer J.H. Passage of salivary amylase through the stomach in humans. Dig. Dis. Sci. 1987;32:1097–1103. doi: 10.1007/BF01300195. [DOI] [PubMed] [Google Scholar]
- 33.Apostolidis E., Kwon Y.I., Shetty K. Inhibitory potential of herb fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Tech. 2007;8:46–54. [Google Scholar]
- 34.Matsuda H., Morikawa T., Yoshikawa M. Antidiabetogenic constituents from several natural medicines. Pure Appl. Chem. 2002;74:1301–1308. [Google Scholar]
- 35.Mizushima Y., Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 1968;20:169–173. doi: 10.1111/j.2042-7158.1968.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 36.Min Y., Xiaoming W., Shuhong G., Jiameng X. Analysis of triterpenoids in Ganoderma lucidum using liquid chromatography coupled with electrospray ionization Mass spectrometry. J. Am. Soc. Mass Spectrom. 2007;28:927–939. doi: 10.1016/j.jasms.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Nishitoba T., Hiroji S., Takanori K., Hirokazu K., Sadao S. New bitter C27 and C30 terpenoids from the fungus Ganoderma lucidum (Reishi) Agric. Biol. Chem. 1985;49:1793–1798. [Google Scholar]