Graphical abstract
Keywords: ZnO, FeO, Cu, Nanopowders, MTT, in vitro cytotoxicity
Highlights
-
•
In vitro cyototoxicity of green synthesized copper, iron oxide and zinc oxide nanopowders were assessed.
-
•
Vero, PK 15 and MDBK cells used for in vitro study for nanopowders use in animal applications.
-
•
Effect of various concentrations (10–50 μg/100 μl) and exposure time of nanopowders were evaluated in the current study.
-
•
It suggested that the activity of green synthesized NPs were highly dependent on concentration, exposure time and type of cells.
-
•
Present study revealed that the all the three selected metallic nanoparticles were found non-toxic.
-
•
The synthesized nanoparticles may be used for in vivo application.
Abstract
In vitro cytotoxic effects of ZnO, FeO and Cu metallic nanopowders (NPs) on Vero (African green monkey kidney cell line), PK 15 (Pig kidney cell line) and Madin Darby Bovine Kidney (MDBK) cell lines were investigated at different time intervals (24 and 48 h). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to determine the cytotoxic effects of green synthesized (plant based) nanopowders. The comparative effects of exposure period and concentration of nanopowders on cell viability were studied. Green synthesized nanopowders showed varying activity on different type of cells and the effect was generally based on the concentration and exposure time. In MDBK cells, only ZnO nanopowder (NP) showed significant effect on cell viability. The ZnO NP showed improved cell viability at lower concentration (10 μg/100 μl) in all type of cells (Vero, PK 15 and MDBK cells). In contrast, FeO NP showed better activity at the concentration of 10 μg/100 μl, 50 μg/100 μl and 40 μg/100 μl after 24 h exposure time in Vero, PK 15 and MDBK cells respectively. However better cell viability was observed in Cu NP treated Vero, PK 15 and MDBK cells at 40 μg/100 μl, 20 μg/100 μl and 10 μg/100 μl correspondingly. These studies suggested that the activity of green synthesized NPs were highly dependent on concentration, exposure time and type of cells.
1. Introduction
Nanotechnology is one of the emerged technology in innovative research because of its numerous applications in science and technology [1]. In recent times, use of nanoparticles to replace bulk materials has been rapidly increasing [2]. Interaction of nanoparticles with biomolecules are influenced by various factors [3] and nanoparticles offer markedly increased surface area in relation to mass and have the ability to change size in different media [4]. The reduction in size of inorganic materials into its nanoform assists numerous properties and technological applications of that particular material; however, the large surface area and density reduction of the nanoparticles makes them a potential candidate in various sectors when compared with bulk materials [5]. In nanotechnology, the synthesis of nanomaterials is a crucial step and involves various physical, chemical and biological methods (plant based-green synthesis and microbe based). Among these methods, green synthesis of nanoparticles using plant is promising for the reason of being environmentally and economically reliable. In modern days, metallic nanoparticles have gained attention because of its biological, biomedical, environmental and commercial significance [6]. Predominantly zinc oxide, iron oxide and copper nanoparticles are currently under investigation due to their applicability in several fields, including drug and gene delivery, biosensors, cancer treatment and diagnostics. It also plays a pivotal role in animal production and health [7] thereby improving the feeding efficiency and nutrition of animals. Trace elements such as copper, zinc and iron in nano-forms, have been included as feed additive and as growth promoters in diets of livestock [8] to enhance their growth performance. Still, the potential toxicity of these nanoparticles if any, has not been well understood. Numerous in vitro studies have been performed to assess the toxicity of several nanoparticles using different assay [9]. With the ever increasing applications of nanoparticles, possible adverse effects of nanoparticles exposure is a growing concern both rationally and publicly [10]. To understand the all aspects of nanoparticles safety is the major consequence [11]. Few nanoparticles can able generate free radicals even under dark conditions which have attributed to surface defects resulting in increase in surface reactivity [12]. In this perspective, it becomes necessary that the toxicity of nanoparticles if any has to be studied extensively in both in vitro and in vivo.
The present study is aimed to investigate the in vitro cytotoxicity of green synthesized and characterized Zinc oxide, Iron oxide and Copper nanopowders (NPs) for their applications in livestock animal industry. Hence, in vitro cytotoxicity was evaluated in Vero (African green monkey kidney cell line), PK 15 (Pig kidney cell line) and MDBK (Madin-Darby bovine kidney cell line) cells.
2. Materials and methods
Zinc oxide, Iron oxide and Copper NPs were prepared by green synthesis method and characterized by scanning electron microscope, transmission electron microscope, X-ray diffraction spectrophotometer and Fourier transform infra red spectrophotometer (Previous study-Manuscript has been submitted). These nanoparticles were used for in vitro cytotoxicity studies.
2.1. Cell lines
Vero (African green monkey kidney cell line), PK 15 (Pig kidney cell line) and MDBK (Madin-Darby bovine kidney cell line) cells were procured from American Type Culture Collection, USA and National Centre for Cell Sciences, Pune, India and used in this study.
2.2. Green synthesis and characterization of nanopowders
Aqueous leaf extract of Musa ornate and Zea mays were used as reducing and stabilizing agent for the green synthesis of copper (Cu), iron oxide (FeO) and zinc oxide (ZnO) nanoparticles (NPs) using copper chloride, ferrous sulphate and zinc sulphate solution as precursor salt respectively. Further the synthesized nanoparticles were characterized by Scanning electron microscope, transmission electron microscope, X-ray diffraction spectrophotometer analysis, Fourier transform and infra red spectrophotometer, Zeta potential and particle size analysis.
Assessment of in vitro cytotoxic effect of green synthesized nanopowders
Vero, PK 15 and MDBK cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM-Sigma) supplemented with 10% fetal bovine serum (Sigma) and 1X antibiotic and antimycotic solution (Gibcco). Cells (105 cells/ml- Sun et al. [13] were seeded into 96-well plates containing 100 ml of DMEM and maintained in a humidified, 5% CO2 incubator at 37 °C. After 24 h of incubation, the green synthesized nanopowders (Cu, ZnO and FeO) at different concentrations (10 μg/100 μl to 50 μg/100 μl) were dispersed in distilled water and added to each well and incubated for 24 and 48 h, to study the effect of concentration of metallic nanopowders and exposure time in cell viability. Control cells received the same amount of the diluent (distilled water). Each test was performed in triplicate to check the sensitivity. After the completion of the exposure period (24 and 48 h) the medium in each well was replaced by fresh medium (100 ml) containing 5 mg/mL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-Sigma). Subsequent to four hours of incubation at dark, the formazan crystal of MTT reduction was dissolved in DMSO and absorbance was measured using a microtitre plate ELISA reader (BioTek). The effect of ZnO, FeO and Cu nanopowders was quantified as the percentage of control absorbance of reduced MTT dye at 570 nm. All the experiments were repeated for three times to check the sensitivity. Viability of the cells was assessed by the ability of living cells to reduce the yellow dye MTT to a blue formazan crystal. The percentage of cell viability was calculated by using the formula,
| % cell viability = (OD sample/OD control) * 100 |
Where, OD is the optical density value.
2.3. Statistical analysis
Comparisons of multiple group means (Concentration of nanopowders and time) were performed using student T test, so as to determine the statistically significant differences. All statistical analysis was performed using SPSS software, v. 17.0. An alpha level of p < 0.001 was considered as the criterion for statistical significance.
3. Results and discussion
ZnO, FeO and Cu metallic nanopowders were green synthesized and characterized by scanning electron microscope, transmission electron microscope, X-ray diffraction spectrophotometre and Fourier transform infra red spectrophotometre (Results are not shown). There are indications that, the toxicity of nanoparticles is highly dependent on cell type, concentration and exposure time [14]. The cytotoxicity of green synthesized nanopowders (ZnO, FeO and Cu) in various cell lines (Vero, PK 15 and MDBK) was assessed by MTT. This assay was based on the ability of mitochondrial succinate dehydrogenase enzyme to convert yellow tetrazolium dye into formazan crystal. The rate of formazan crystal formation is directly proportional to cell viability [15] which is measured in terms of optical density.
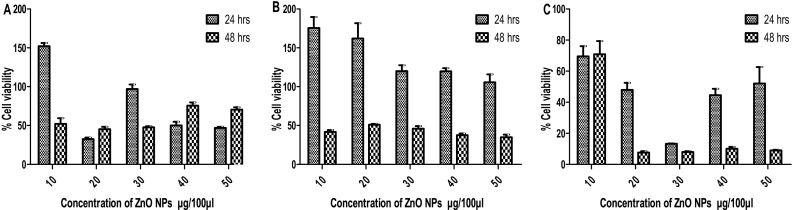
The results of ZnO nanopowders effect on Vero, PK 15 and MDBK cells are presented in Fig. 1. The results indicated that in Vero cells, the cell viability decreased with increase in concentration and exposure time of ZnO NPs. This may be due to the capability of reactive oxygen species (ROS) generation of the nanoparticles. Elevated ROS levels induce significant damage to the DNA of the cells, resulting in the arrest of cell-cycle and subsequently cell death [16]. Similar results were also observed with ZnO NPs treated PK 15 cells. On the contrary, in MDBK cells maximum cell viability was observed after 48 h of exposure but the cell viability got reduced when the concentration of ZnO NPs was increased. Vero and PK 15 cells exhibited superior cell viability at lower concentration of ZnO NPs (10 μg/100 μl) after 24 h treatment. Whereas in case of MDBK cells, improved cell viability was observed upto 48 h exposure. ZnO NPs did not cause any significant (p > 0.05) toxicity in all the concentrations at both 24 h and 48 h in PK 15 and MDBK cells whereas significant difference (p < 0.001) was observed in Vero cells. All these findings clearly suggested that the cytotoxicity of ZnO nanopowder was dependent on concentration, exposure time, cell type and proliferation as has been observed in previous studies [17], [2], [18], [19] irrespective of the type of synthesis.
Fig. 1.
Cell viability (%) observation. Each cell line was treated with indicated amount of ZnO NPs and viability was measured after 24, 48 and 72 h of exposure. The data is presented as the mean ± SD of three independent experiments. A. Vero, B. PK 15 and C. MDBK cells.
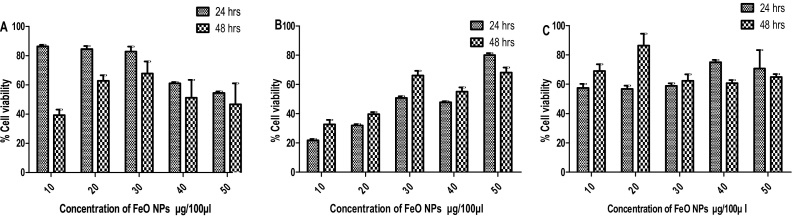
Evaluation of cytotoxic effect of FeO NPs on Vero, PK 15 and MDBK cells are presented in Fig. 2. Similar to ZnO NPs, FeO NPs exhibited cell viability, in concentration and time dependent manner in Vero cells. Decreased cell viability was observed when the concentration and exposure time of the FeO NPs increased. Interestingly in PK 15 cells, improved cell viability was observed when the concentration of FeO NPs increased because of the type of cells and proliferation. Maximum cell viability was observed at 50 μg/100 μl concentration of FeO NPs after 24 h of exposure period. Similar kind of result was also observed by Coricovac et al. [20]. In their study, they used different concentrations (5, 10, and 25 μg·mL−1) of magnetic iron oxide nanoparticles to stimulate HaCat cells. After 24 h treatment with magnetic iron oxide nanoparticles they observed that there was increase in cell viability as compared to control cells, which indicated that the iron nanoparticles did not affect cells viability (maximum viability was obtained at higher concentration (50 μg mL−1)).
Fig. 2.
Cell viability (%) observation. Each cell line was treated with indicated amount of FeO NPs and measured after 24, 48 and 72 h of exposure period. Data is presented as mean ± SD of three independent experiments. A. Vero, B. PK 15 and C. MDBK cells.
In MDBK cells, enhanced cell viability was observed at 50 μg/100 μl concentration of FeO NPs after 48 h. As in case of ZnO NPs, increased cell viability was observed in MDBK cells after 48 h treatment. There was no significant difference (p > 0.05) between the concentration and exposure time in FeO NPs treated cells. However, when the exposure time was increased from 24 h to 48 h nanoparticles had a substantial toxic impact on Vero cells. This time dependent result could be standard dose-response behaviour. These results are supported by previous published results that indicated that longer (3 day) exposure generated a greater toxicity to Mouse embryonic stem cells (Royan B1) by MTT assay [21]. In contrast, MDBK cells exhibited improved cell viability at 48 h after the exposure period. This consequence may due to the type of cell because toxicity of NPs also dependent on cell type.
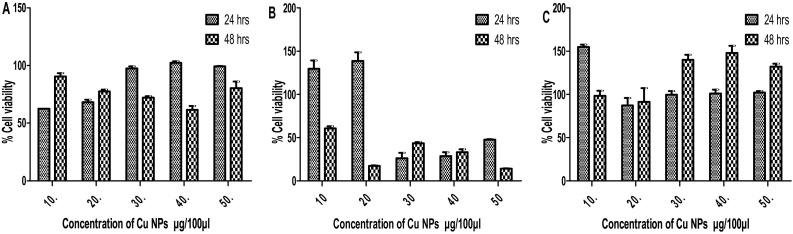
In Cu NPs, it was observed that the cell viability of Vero, PK 15 and MDBK cells were not statistically significant (p > 0.05) between the concentrations and exposure time (Fig. 3). The MTT results indicated varying results with regard to concentration and exposure time. In Vero cells, maximum cell viability was observed at 40 μg/100 μl concentration of FeO NPs after 24 h of exposure. In PK 15 and MBDK cells maximum cell viability was observed at 20 and 10 μg/100 μl concentrations respectively. These results concurred with the results of Badanavalu et al. [22]. In their study, when the DRG neurons were exposed to copper nanoparticles, cell viability was reduced significantly in a concentration- and size-dependent manner by the results of MTS (a tetrazolium dye-based) assay. The cytotoxic effect of Cu, ZnO and FeO nanopowders on Vero, PK 15 and MDBK cell line were showed a contradiction. This may due to the MTT reduction process was greatly dependent on activity of metabolic cycles associated with NAD(P)H streams. As a result, cells with low metabolic activity reduce lower amounts of MTT. It must be brought up that under certain culture conditions the changes in metabolic activity can be responsible for the low rate cellular reduction of MTT and not the decrease in cell viability [23]. It is concluded that, the activity of green synthesized NPs were highly dependent on concentration, exposure time and type of cells.
Fig. 3.
Cell viability (%) observation. Each cell line was treated with indicated amount of Cu NPs and measured after 24, 48 and 72 h of exposure period. Data is presented as the mean ± SD of three independent experiments. A. Vero, PK 15 and C. MDBK cells.
4. Conclusion
The green synthesized ZnO, FeO and Cu nanopowders were assessed for their toxicity in Vero, PK 15 and MDBK cell lines. The results of this study indicated a direct relationship between cell toxicity and the exposure time, cell type and concentrations of nanopowders. Particularly concentration and exposure time played the predominant role in the cytotoxicity of cells. The exposure time of nanopowders (ZnO, FeO and Cu) might have increased the production of oxygen free radicals within the cells which causes cell death. The ZnO NPs exhibited superior cell viability at lower concentration (10 μg/100 μl) in all the cell types. It indicated that, the concentration and exposure time had direct effect on cell viability. In FeO NPs treated cells, quite the opposite results were observed. The cell viability was greatly depending on the type of cells. In Vero cells viability was depended on the concentration and exposure time. Whereas in case of PK 15 cells, increased cell viability was observed while increasing the concentration of NPs and exposure time. In MDBK cells there was no significant difference between the concentration and exposure time. The maximum cell viability was observed at 20 μg/100 μl concentration after 48 h post exposure. Cu NPs also exhibited the cell viability based on cell type. For the reason that, in Vero and MDBK cells, there was no direct effect of concentration and exposure time of NPs were observed. While in PK 15 cells, it was observed that the increase in the concentration and exposure time leads to the decrease in the cell viability.
Acknowledgements
The authors thank the Government of India and Government of Tamil Nadu for supporting this work under the National Agricultural Development Programme (RKVY). The authors also thank the Director of Research and Dean, Faculty of Basic Sciences, Tamil Nadu Veterinary and Animal Sciences University, Dean, Madras Veterinary College, Chennai, Professor and Head, Department of Animal Biotechnology, Madras Veterinary College and the Professor and Head, University Research Farm, Madhavaram for the facilities provided.
References
- 1.Sudeep S., Alka J., Vikas A., Raj Kumar S., Neha B., Jain V.K. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015;2:27–39. doi: 10.1016/j.toxrep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fin D., Amanda H., Shahnaz B., Lucky J., Chris W. In vitro cytotoxicity assessment of selected nanoparticles using human skin fibroblasts. Japanese Society for Alternatives to Animal Experiments; AATEX 14, Special Issue, 397–400 Proc. 6th World Congress on Alternatives & Animal Use in the Life Sciences August 21–25, 2007, Tokyo, Japan; 2008. [Google Scholar]
- 3.Neagu M., Piperigkou Z., Karamanou K., Engin A.B., Docea A.O., Constantin C., Negrei C., Nikitovic D., Tsatsakis A. Protein bio-corona: critical issue in immune nanotoxicology. Arch. Toxicol. 2016;91(March (3)):1031–1048. doi: 10.1007/s00204-016-1797-5. (Epub 2016 Jul 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piperigkou Z., Karamanou K., Engin A.B., Gialeli C., Docea A.O., Vynios D.H., Pavão M.S., Golokhvast K.S., Shtilman M.I., Argiris A., Shishatskaya E., Tsatsakis A.M. Emerging aspects of nanotoxicology in health and disease: from agriculture and food sector to cancer therapeutics. Food Chem. Toxicol. 2016;91(May 42–57) doi: 10.1016/j.fct.2016.03.003. (Epub 2016 Mar 8) [DOI] [PubMed] [Google Scholar]
- 5.Raman V., Suresh S., Savarimuth P.A., Raman T., Tsatsakis A.M., Golokhvast K.S., Vadivel V.K. Synthesis of Co3O4 nanoparticles with block and sphere morphology, and investigation into the influence of morphology on biological toxicity. Exp. Ther. Med. 2016;11:553–560. doi: 10.3892/etm.2015.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ankamwar B., Chaudhary M., Sastry M. Gold nanotriangles biologically synthe- sized using tamarind leaf extract and potential application in vapour sensing. Synth. React. Inorg. Met. Org. Nano. Met. Chem. 2005;35:19–26. [Google Scholar]
- 7.Kuzma J. Nanotechnology in animal production −upstream assessment of applications. Livestock Sci. 2010;130:14–24. [Google Scholar]
- 8.Hahn J.D., Baker D.H. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J. Anim. Sci. 1993;71:3020–3024. doi: 10.2527/1993.71113020x. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson K., Aitken R., Tran L., Stone V., Duffin R., Forrest G., Alexander A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006;92:5e22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 10.Lewinski N., Colvin V., Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 11.Bostan H.B., Rezaee R., Valokala M.G., Tsarouhas K., Golokhvast K., Tsatsakis A.M., Karimi G. Cardiotoxicity of nano-particles. Life Sci. 2016;165:91–99. doi: 10.1016/j.lfs.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Balmuri S.R., Selvaraj U., Kumar V.V., Anthony S.P., Tsatsakis A.M., Golokhvast K.S., Raman T. Effect of surfactant in mitigating cadmium oxide nanoparticle toxicity: implications for mitigating cadmium toxicity in environment. Environ. Res. 2017;152:141–149. doi: 10.1016/j.envres.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Sun T., Yan Y., Zhao Y., Guo F., Jiang C. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS One. 2012;7(8):e43442. doi: 10.1371/journal.pone.0043442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz B., Sanchez-Espinel C., Arruebo M. Assessing methods for blood cell cytotoxic responses to inorganic nanoparticles and nanoparticle aggregates. Small. 2008;4:2025–2034. doi: 10.1002/smll.200800199. (Recent research article that deals with the toxicity of various nanoparticles on numerous types of cells and effect of nanoparticle aggregates. PubMed: 18855973]) [DOI] [PubMed] [Google Scholar]
- 15.Hussain R.F., Nouri A.M.E., Oliver R.T.D. A new approach for measurement of cytotoxicity using colorimetric assay. J. Immunol. Methods. 1993;160:89–96. doi: 10.1016/0022-1759(93)90012-v. [DOI] [PubMed] [Google Scholar]
- 16.Singh N., Manshian B., Jenkins G., Griffiths S.M., Williams P.M., Maffeis T. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials? Biomaterials. 2009;30(23–24):3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Sathyabama S., Sankaranarayanan S. An In-vitro biosynthesis of zinc oxide nanoparticles using rich flavonoid extract from the petals of Delonix regia and evaluation of their antioxidant and anticancer properties. Int. J. Pharm. Phytochem. Res. 2015;7(5):1112–1119. [Google Scholar]
- 18.Orazizadeh M., Khodadadi A., Bayati V., Saremy S., Farasat M., Khorsandi L. In vitro toxic effects of zinc oxide nanoparticles on rat adipose tissue-derived mesenchymal stem cells. Cell J. 2015;17(3):412–421. doi: 10.22074/cellj.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prashanth G.K., Prashanth P.A., Utpal B., Manoj G., Nagabhushana B.M., Ananda S., Krishnaiah G.M., Sathyananda H.M. In vitro antibacterial and cytotoxicity studies of ZnO nanopowders prepared by combustion assisted facile green synthesis. Karbala Int. J. Mod. Sci. 2015:1–67e77. [Google Scholar]
- 20.Coricovac D.E., Moaca E.A., Pinzaru I., Citu C., Soica C., Mihali C.V., Pacurariu C., Tutelyan V.A., Tsatsakis A., Dehelean C.A. Biocompatible colloidal suspensions based on magnetic iron oxide nanoparticles: synthesis, characterization and toxicological profile. Front. Pharmacol. 2017;8:154. doi: 10.3389/fphar.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homa M.K., Sahar K., Ali A.R., Rahman F. Cytotoxic effect of iron oxide nanoparticles on mouse embryonic stem cells by MTT assay. Iranian J. Toxicol. 2013;Vol. 7 (No 21) [Google Scholar]
- 22.Badanavalu M.P., Ali S.F., Murdock R.C., Hussain S.M., Malathi S. Copper nanoparticles exert size and concentration dependent toxicity on somato sensory neurons of rat. Nanotoxicology. 2010;4(2):150–160. doi: 10.3109/17435390903337693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shishatskaya E.I., Nikitovic D., Vasilievich A.S., Tzanakakis G.N., Tsatsakis A.M., Menzianova N.G. Short-term culture of monocytes as an in vitro evaluation system for bionanomaterials designated for medical use. Food Chem. Toxicol. 2016;96:302–308. doi: 10.1016/j.fct.2016.08.025. [DOI] [PubMed] [Google Scholar]