Graphical abstract
Abbreviations: ANOVA, analysis of variance; HCT, hematocrit; Hb, hemoglobin; LC, leukocyte count; WBCs, white blood cells; RBCs, red blood cells; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean Corpuscular Volume; PCV, Packed Cell Volume; PLT, platelets count; ROS, reactive oxygen species; NaF, Sodium Fluoride; SEM, standard error of mean; TLC, total leukocyte count
Keywords: Fluoride intoxication, Hypochromic anemia, Hematological, Parameters, Leukocyte alterations, Fluorosis
Highlights
-
•
Sodium fluoride toxicity in Oryctolagus cunniculus and resulting fluctuations in hematological profile.
-
•
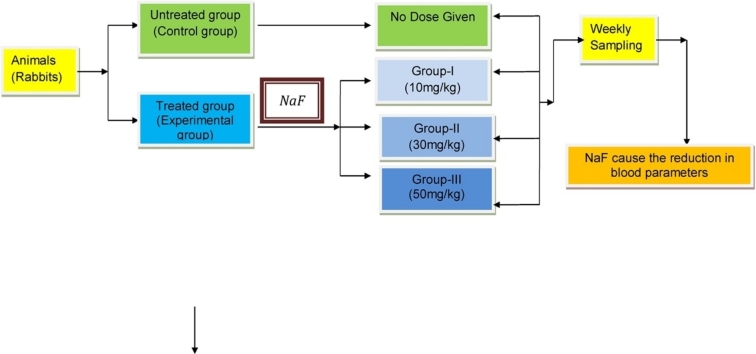
Dose pattern for 5 rabbits in each group 1. Control group without dose, Experimental group I with 10 mg/kg, Experimental group II with 30 mg/kg, Experimental group III with 50 mg/kg.
-
•
Leucocytosis and anemia was observed in affected animals.
Abstract
Blood is promptly affected by environmental pollutants and toxicants that can cause many metabolic disorders. The high level of fluoride acts as a potential pollutant, insecticide and rodenticide with very high toxicity, associated with the hematological damage. This study aimed to determine the toxicity of Sodium Fluoride on hematological parameters in Oryctolagus cunniculus. Twenty rabbits were acclimatized and divided in to control group and three experimental groups.Experimental group-I, II and III were treated with 10, 30 and 50 mg/kg body weight doses of Sodium Fluoride orally. Various blood parameters such as TEC, Hb, HCT, MCV, MCH, MCHC, TLC and PLT count were investigated. Result findings showed that values of blood indices in experimental groups were significantly lower than the control group. Oneway ANOVA was applied for statistical analysis. The outcomes of the current studies indicated the reduction in RBC counts (anemia), leukocyte count (leukocytopenia), monocytosis, eosinopenia, neutrophilia and thrombocytosis on fluoride intoxication. Hematological disruptions like microcytic hypochromic anemia and decreased leukocyte count may be linked to the inflammatory effects of Sodium Fluoride on lymphatic organs.
1. Introduction
Sodium fluoride is registered for use as an insecticide and anti-helmintic drug and is a major environmental pollutant, entering the biosphere from such sources as iron and steel operations, coal power production, aluminum smelting, and phosphate fertilizer manufacturing [1], [2]. Fluoride can be consumed from soil, water, and vegetation. Skeletal and dental Fluorosis, stiffness of the joints and emaciation usually result from chronic fluoride toxicity [3], [4], [5], [6]. Like other Hemotoxic agents fluoride also shows deleterious effects on reproductive capacity of animals and their milk production [7], [8], [9], [10]. The deleterious effects of excess fluoride on human health are: damage to kidney tubules due to continuous filtration of the ingested fluoride, dysfunction of the reproductive system and ageing [11].
The deleterious effects of fluoride on blood have been studied well in different experimental models [3], [12], [13], [14], [15], [16], [17]. Fluoride affects the formation of blood forming cells i.e., hematopoietic cells in cavities of bone marrow and inhibits the transport of K+/Cl− ions [17], [18]. Moreover, it also causes the generation of superoxide radicals (O2–), lipid peroxidation in polymorphonuclear leucocytes [19], [20] and affect the neutrophil along with decreased phagocytic activity [20].
Fluoride usually enters in blood circulation by absorption from duodenal and gastric mucosa via passive diffusion. Sodium fluoride causes gastrointestinal damage, intermittent diarrhea, anorexia, flatulence, abdominal pain, constipation, nausea, and loss of appetite [21], [22], [23], [24], [25], [26]. Susheela reported that fluoride intoxication of the human causes anemia or premature erythrocyte deaths i.e. life span of RBCs decreases due membrane degeneration that turns them into echinocytes [27], [28]. In humans, hematologic disorders are hypochromic anemia, variation in the size and shape of erythrocytes, presence of Heinz bodies, eosinophilic leukocytosis, lymphopenia, increase in the amount of methemoglobin, and alterations in Hematocrit. [29], [30].
The main objective of this investigation is to figure out the hematological effects of Sodium fluoride toxicity in Oryctolagus cunniculus and to observe the induced modifications in the values of Mean Corpuscular Hemoglobin Concentration (MCHC), Mean Corpuscular Hemoglobin (MCH), Hemoglobin (Hg), Mean Corpuscular Volume (MCV), Platelet Count (PLT), Total Leukocytes Count (TLC), Total Erythrocytes Count (TEC), Hematocrit (Hct).
2. Materials & methods
Twenty adult rabbits of the species Oryctolagus cunniculus weighing (800–1000 g) were selected as experimental animals. All the rabbits were healthy and were acclimatized for 7 days in steel cages with proper light and humidity. The animals fed with Berseem fodder (Trifolium alexandrium), carrots, vegetables, fruit pulp & drinking water ad libitum. Rabbits were divided into control group and experimental groups. Sodium fluoride (NaF) solution selected as hemotoxic agent to be administered orally according to dose pattern used by Shashi et al. [31]. The concentration of NaF dose in experimental group I, II and III was 10 mg/kg, 30 mg/kg and 50 mg/kg, respectively. The doses are designed according to the body weight in 5 ml distilled water. Phlebotomy (blood drawing) from rabbit was done from the marginal vein of the ear and transported into heparinized tubes to avoid any contamination of sample [32]. Hematological counter (Hemacount, Gesellschaft fur Biochemica, Germany) was used to assess the effect of Sodium fluoride on hematological parameters i.e., Mean Corpuscular Hemoglobin Concentration (MCHC), Mean Corpuscular Hemoglobin (MCH), Hemoglobin (Hg), Mean Corpuscular Volume (MCV), Platelet Count (PLT), Total Leukocytes Count (TLC), Total Erythrocytes Count (TEC), Hematocrit (Hct) in experimental and control blood group samples.
The obtained values were analyzed by comparing means using one way analysis of variance, ANOVA [33]. The p-values less than 0.05 were considered to be significant. The values of various hematological parameters were represented by Mean ± S.E.M. The data was analyzed by commercially available software package SPSS (SPSS Inc, Chicago, USA). The values considered significant were represented by an asterisk.
3. Results and discussion
Fluoride being the most damaging environmental pollutant, disturbs the normal metabolic pathways of an organism at elevated levels [34], [35]. Taking in account the existing controversies in literature regarding the effect of Sodium Fluoride on hematological parameters, this study was operated to scrutinize the blood cell count and accessory parameters in Oryctolagus cunniculus following fluoride intoxication in four weeks of experimental period. The study was started by treating experimental groups of rabbits with a selected dose of Sodium Fluoride. Hematological parameters such as: MCHC, MCH, Hg, MCV, PLT, TLC, TEC, Hct were studied [36], [37].
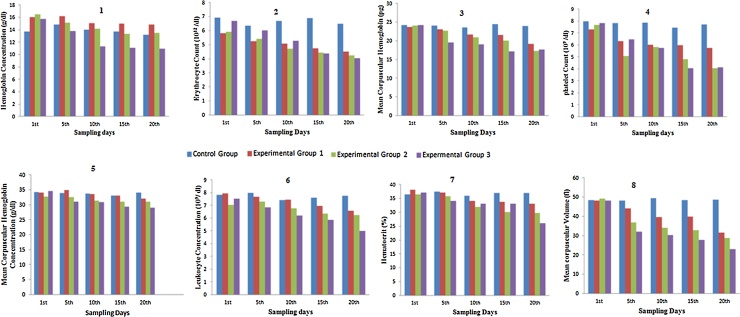
The results of present study indicate that there is a significant decrease in erythrocyte count (Fig. 1) and associated parameters including Hematocrit number (Fig. 1), Mean corpuscular hemoglobin (Fig. 1), Mean corpuscular volume (Fig. 1), Hemoglobin concentration (Fig. 1), thus an overall decreasing trend was observed during the treatment with the Sodium Fluoride doses (Table 1).
Fig. 1.
Effect of Sodium Fluoride on Blood Parameters i.e (1) Hb (2) RBCs (3) MCH (4) Pt (5) MCHC (6) WBCs (7) Hct (8) MCV of Rabbits (Oryctolagus cunniculus).
Table 1.
Different parameters of blood in control and experimental groups with Sodium Fluoride intoxication.
| Sampling Days | Total Erythrocyte Count (g/dl) | Hemoglobin concentration (g/dl) | Total Leukocyte Count (109/dl) | Hematocrit Number (%) | Mean Corpuscular Volume (fL) | Mean Corpuscular Hemoglobin (pg/dl) | Platelet Count (103/dl) | Mean Corpuscular Hemoglobin Concentration (g/dl) | |
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Control group | ±0.37 | 13.76 ± 0.50 | 7.81 ± 0.20 | 36.37 ± 0.65 | 48.27 ± 0.65 | 24.19 ± 0.33 | 7.99 ± 0.41 | 34.3 ± 0.11 |
| Experimental group 1 | 5.81 ± 0.84* | 16.01 ± 0.20* | 7.92 ± 0.11* | 38.09 ± 0.04* | 48.11 ± 0.22* | 23.72 ± 0.02* | 7.31 ± 0.36* | 34.1 ± 0.63* | |
| Experimental group 2 | 5.92 ± 0.75* | 16.48 ± 0.12* | 7.03 ± 0.32* | 36.42 ± 0.65* | 49.11 ± 0.13* | 24.06 ± 0.47* | 7.68 ± 0.32* | 32.7 ± 0.14* | |
| Experimental group 3 | 6.69 ± 0.33* | 15.70 ± 0.43* | 7.52 ± 0.71* | 37.03 ± 0.39* | 48.06 ± 0.48* | 24.18 ± 0.05* | 7.81 ± 0.207* | 34.5 ± 0.49* | |
| Day 10 | Control group | 6.86 ± 0.86 | 13.99 ± 0.99 | 7.41 ± 0.16 | 36.01 ± 0.34 | 49.28 ± 0.32 | 23.57 ± 0.09 | 7.84 ± 0.44 | 33.7 ± 0.33 |
| Experimental group 1 | 5.06 ± 0.04* | 15.06 ± 0.24* | 7.45 ± 0.55* | 34.05 ± 0.07* | 39.64 ± 0.49* | 21.72 ± 0.32* | 6.02 ± 0.54* | 33.6 ± 0.10* | |
| Experimental group 2 | 4.71 ± 1.01* | 14.17 ± 0.71* | 6.78 ± 0.18* | 31.97 ± 0.63* | 34.11 ± 0.23* | 20.91 ± 0.13* | 5.81 ± 0.64* | 31.3 ± 0.81* | |
| Experimental group 3 | 5.27 ± 0.26* | 11.29 ± 0.32* | 6.19 ± 0.43* | 33.09 ± 0.77* | 30.21 ± 0.01* | 19.05 ± 0.07* | 5.76 ± 0.45* | 30.78 ± 0.32* | |
| Day 20 | Control group | 6.47 ± 6.47 | 13.21 ± 0.18 | 7.76 ± 0.36 | 37.01 ± 0.12 | 48.54 ± 0.22 | 23.93 ± 0.16 | 7.72 ± 0.15 | 34.1 ± 0.03 |
| Experimental group 1 | 4.51 ± 0.75* | 14.81 ± 0.59* | 6.58 ± 0.84* | 33.14 ± 0.27* | 31.51 ± 0.08* | 19.16± 0.01* | 5.73 ± 0.51* | 32.01 ± 0.84* | |
| Experimental group 2 | 4.22 ± 0.07* | 13.48 ± 0.11* | 6.23 ± 0.37* | 29.71 ± 0.53* | 28.86 ± 0.37* | 17.23 ± 0.09* | 4.06 ± 0.67* | 29.8 ± 0.11* | |
| Experimental group 3 | 4.02 ± 0.10* | 10.96 ± 0.04* | 5.01 ± 0.96* | 26.11 ± 0.11* | 23.05 ± 0.03* | 17.64 ± 0.78* | 4.13 ± 0.20* | 29.04 ± 0.46* | |
(Mean ± S.E.M, p < 0.05, n = 5).
Blood being most sensitive act as an indicator of countless metabolic disorders. The Current study elucidated the significant decrease in the number of RBCs through the comparison of the RBCs count of all the three experimental groups with the control groups. The total erythrocyte count on 1st day of the experiment was 6.92 ± 0.37 in the control group which on exposure to different concentrations of sodium fluoride was decreased to 4.51 ± 0.75*, 4.22 ± 0.07*and 4.02 ± 0.10* in experimental group I, II and III at 20th day. The findings on TEC is in agreement with the work of Mohiuddin and Reddy [3], [38] who reported a reduction of RBC count in sheep and cattle. The decrease in total erythrocyte count (TEC) in present study and in other studies was dose dependent. However, Gujarathi and colleagues [39] reported increase in TEC in buffalo calves.
Through hematological examination, the decreased level of total leukocytes counts in all the experimental groups is also observed. The total leukocyte count (TLC) on 1st day of the experiment was 7.81 ± 0.20 in control group. But in experimental groups I, II and III the count decreased to 6.58 ± 0.84*, 6.23 ± 0.37* and 5.01 ± 0.96* respectively at 20th day of the experiment. It was suggested that the TLC is decreased in fluoride fed groups, which might be the result of under production from the germinal center of lymphoid organs by cause of necrosis [40]. Leukopenia and lymphopenia was also reported in higher dosed groups which might be due to direct toxic action of sodium fluoride on leucopoiesis in lymphoid organs (Fig. 1). Similar findings are also reported in cow and buffalo [41], [42].
It has been previously reported that approximately 2–4 weeks vulnerability to 10, 30 and 50 mg NaF/kg body weight led to anemia, a considerable decrease in total nucleated cell counts and hypoplasia of bone marrow [43].The differential count of WBC shows decrease in polyneutrophils and an increase in lymphocytes in the fluoride treated groups as compared to the control animals. This increase in WBC suggests that fluoride as a foreign body may evoke the immune response through the lymphocytes, which is more prone to the fluoride and comes in action as its rapid increasing number suggests. Maheswaran et al., reported that the increased in WBC count might be due to the tissue damage i.e. stimulation of the immune system caused by sodium fluoride [44].
The tested chemicals also affected differential leukocyte counts, significantly increasing the percentage of neutrophil and monocytes which is a first order immune response of the animals to the tested chemicals. In contrast Das et al. [45] reported significant reduction in Neutrophil and Monocytes in rats exposed to fluorides for 28 days, and concluded that these effects would lead to lowered cellular immunity. Our results suggest that the animals attempted to defend themselves from the pollutants during short term exposure.
The hematological examination in current study also elucidated a decrease of total hemoglobin level as compared to the control group. The hemoglobin levels on 1st day was 13.76 ± 0.50 in the control group where as in experimental group I, II and III, it was decreased as 14.81 ± 0.59*, 13.48 ± 0.11*and 10.96 ± 0.04* respectively at 20th day of experiment. The possible explanation for this decrease is the lysis and lower production of RBCs due to decrease production of bone marrow result in anemia and low hemoglobin production [46].
Some studies in rats also reported the decrease in hemoglobin percentage after receiving the sodium fluoride treatment. The light microscopic findings in similar studies depicted the presence of macrophages in the spleen causing more damage to erythrocytes in fluoride treated groups than control groups. This suggests the fluoride induced enhancement in Phagocytosis in spleen localized macrophages which consequently leads to the development of anemia [47], [48]. Moreover, fluoride also retards the normal process of erythropoiesis by interacting with the iron of hemoglobin.
Reduction in blood parameters could also be due to hemolysis and hemorrhages caused by fluoride and bone marrow suppression. The mean corpuscular hemoglobin (MCH) level on 1st day of experiment was 24.19 ± 0.33 in the control group, whereas in experimental group I, II and III, it was decreased as 19.16± 0.01*, 17.23 ± 0.09* and 17.64 ± 0.78* respectively on the 20th day of experiment. It could be collaborated with the reports of other workers in mammals [49].
The Hematocrit also recognized as an erythrocyte volume fraction (EVF) or packed cell volume (PCV) is the volume percentage (%) of RBCs in blood. The percentage of Hematocrit in control group at 1st day of the experiment in the control group was 36.37 ± 0.65, whereas in the experimental group I, II and III, it was decreased as 33.14 ± 0.27*, 29.71 ± 0.53* and 26.11 ± 0.11* at 20th day of the experiment. In goats, the repeated administration of fluoride caused the reduction of Hb which may be a indication for the development of anemia [50]. Similarly decrease in PCV was observed by Swarup and Singh [41] and Gujrathi et al, [39] during Fluorosis in cattle and buffaloes. The increase in the clotting time may be due to the fact that NaF is an anticoagulant and also chelates calcium ions from the blood, which is pre-requisite for blood clotting. Similar findings have been reported in buffalo calves [51], [52].
The platelet counts may decrease either due to bone marrow suppression or when they are trapped in the spleen (Fig. 1) [53]. The percentage of platelets in control group at 1st day of experiment was 7.99 ± 0.41, whereas in experimental group I, II and III, it was decreased to 5.73 ± 0.51*, 4.06 ± 0.67* and 4.13 ± 0.20* at 20th day of experiment. Decreased WBC, PLT, and neutrophil counts were probably due to harmful effects of fluoride on bone marrow and hematopoietic organs. The decreased RBC count in present study may have been associated with a decreased rate of erythrogenesis due to the negative effect of fluoride on erythropoiesis or to shortened life span of erythrocytes and membrane degeneration by means of fluoride causing erythrocytes to change into echinocytes [49], [54]. The outcomes of this study also indicated a significant decrease in the number of MCV. The total MCV count on 1st day of experiment was 48.27 ± 0.65 in control group whereas in experimental group I, II and III, it was decreased to 31.51 ± 0.08*, 28.86 ± 0.37* and 23.05 ± 0.03* respectively at 20th day of experiment. The decrease in total erythrocyte count (MCV) was dose dependent.
Some studies reported that fluoride caused changes in blood parameters such as Hb, Hct, MCV, MCH, and MCHC (Fig. 1) [55], [56], [57]. It has been reported that fluoride toxicity causes hematopoietic progenitor cells injury in humans [1], [58]. The values of MCHC show a decrease in treated groups as compared to control group. The value of MCHC in control group was 34.3 ± 0.11, while experimental groups I, II and III shows decrease as 32.01 ± 0.84*, 29.8 ± 0.11*and 29.04 ± 0.46* at last day of experiment.
Our current findings concords with many previous studies, the interaction of toxic substances with red blood cells may affect their Hb-carrying capacity, consequently lowering Hb content Oda et al., [59] reported a small increase in WBC and MCHC values while Khandare et al., [12] observed an opposite trend for WBC, as noted by us. The lack of WBC impairs the body’s ability to fight infections. Packed cell volume values are also important in measuring stress on animal health and are also an indicator of oxygen carrying capacity of the blood [60]. The reduction in WBC and PCV values suggest stress in the treated mice.
4. Conclusion
Thus, it is concluded that the like other toxic agents sodium fluoride toxicity may increase the phagocytic activity of macrophages to engulf more RBC in spleen and may contribute in developing anemia as evident by the increased number of white blood cells [61], [62]. Its toxicity produces hematological alterations characterized by anemia and stress leukogram. With the combined exposure to these chemicals, the alterations are more severe, indicating a positive hemato-toxic interaction between these two chemicals.
Acknowledgement
One of the Author Maryam abbas is grateful to Dr. Muhammad Hassan Siddiqi for his guidance. Authors are also thankful to anonymous reviewers for their valuable comments.
References
- 1.Machalinski B. The influence of sodium fluoride on the clonogenecity of human hematopoietic progenitor cells: preliminary report. Fluoride. 2000;33(4):168–173. [Google Scholar]
- 2.Pain G.N. 2017. Fluoride Is a Developmental Nephrotoxin–coming to a Kidney near You. [Google Scholar]
- 3.Kant V. Haematological profile of subacute oral toxicity of fluoride and ameliorative efficacy of aluminium sulphate in goats. Toxicol. Int. 2009:31. doi: 10.4103/0971-6580.72676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kant V. Experimental osteo-fluorosis in goats of Jammu and Kashmir. Feedback. 2009;4(2):45. [Google Scholar]
- 5.Meena C. Assessment of Non-skeletal fluorosis in children of Jaipur district of Rajasthan, India. Int. Jo. Sci. Res. 2017;5(12) [Google Scholar]
- 6.Pereira A.G. Effects of fluoride on insulin signaling and bone metabolism in ovariectomized rats. J. Trace Elem. Med. Biol. 2017;39:140–146. doi: 10.1016/j.jtemb.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 7.McCune D., Weinstein L. 9 Effects of Fluorides. Air Pollut. Plant Life. 2002:163. [Google Scholar]
- 8.Shupe J., Fluorides L. Paragon Press Inc; 1983. Effects on Vegetation, Animals and Humans. [Google Scholar]
- 9.Cronin S. Fluoride: A review of its fate, bioavailability, and risks of fluorosis in grazed‐pasture systems in New Zealand. N. Z. J. Agric. Res. 2000;43(3):295–321. [Google Scholar]
- 10.Moemeni H. A comparison between serum Lead status in elderly osteopenic and osteoporotic patients versus. Healthy Control. 2015 [Google Scholar]
- 11.Sharma J., Solanki M., Solanki D. Sodium fluoride toxicity on reproductive organs of female albino rats. Asian J. Exp. Sci. 2007;21(2):359–364. [Google Scholar]
- 12.Khandare A.L., Kumar P.U., Lakshmaiah N. Beneficial effect of tamarind ingestion on fluoride toxicity in dogs. Fluoride. 2000;33(1):33–38. [Google Scholar]
- 13.Cetin N. Effect of fluoride application on some blood parameters in rabbits. EU J. Health Sci. 2004;13:46–50. [Google Scholar]
- 14.Eren E. Fluorosis and its hematological effects. Toxicol. Ind. Health. 2005;21(9):255–258. doi: 10.1191/0748233705th236oa. [DOI] [PubMed] [Google Scholar]
- 15.Karadeniz A., Altintas L. Effects of panax ginseng on fluoride-induced haematological pattern changes in mice. Fluoride. 2008;41(1):67. [Google Scholar]
- 16.Uslu B. Effect of fluoride on hemoglobin and hematocrit. Fluoride. 1981;14(1):38–41. [Google Scholar]
- 17.Choubisa S. Prevalence of fluorosis in some villages of Dungarpur district of Rajasthan. Indian J. Environ. Health. 1996;38(2):119–126. [Google Scholar]
- 18.Santoyo-Sanchez M.P. Effects of acute sodium fluoride exposure on kidney function: water homeostasis, and renal handling of calcium and inorganic phosphate. Biol. Trace Elem. Res. 2013;152(3):367–372. doi: 10.1007/s12011-013-9622-y. [DOI] [PubMed] [Google Scholar]
- 19.Kessabi M. Experimental acute sodium fluoride poisoning in sheep: renal, hepatic, and metabolic effects. Fundam. Appl. Toxicol. 1985;5(6):1025–1033. doi: 10.1016/0272-0590(85)90139-3. [DOI] [PubMed] [Google Scholar]
- 20.Elferink J.G. Fluoride-induced superoxide production in rabbit polymorphonuclear leukocytes. Biochem. Pharmacol. 1981;30(14) doi: 10.1016/0006-2952(81)90209-4. [DOI] [PubMed] [Google Scholar]
- 21.Sondhi H., Gupta M., Gupta G. Intestinal effects of sodium fluoride in Swiss albino mice. Fluoride. 1995;28(1):21–24. [Google Scholar]
- 22.Shashi A. Gastric lesions in experimental fluorosis. Asian J. Microbiol. Biotech. Environ. Sci. 1999;1:171–175. [Google Scholar]
- 23.Shashi A., Sharma N., Bhardwaj M. Pathological evaluation of pancreatic exocrine glands in experimental fluorosis. Asian Pac. J. Trop. Med. 2010;3(1):36–40. [Google Scholar]
- 24.Waldbott, G., GASTRIC-ULCER AND FLUORIDE, 1977, INT SOC FLUORIDE RESEARCH 216 ATKINSON RD, TITIRANGI, AUCKLAND 7, NEW ZEALAND.
- 25.Susheela A. Fluorosis Research and Rural Development Foundation; 2001. Fluorosis Indian Scienario: A Treatise on Fluorosis. [Google Scholar]
- 26.Gharzouli K., Senator A. Fluoride absorption in vitro by the gastrointestinal tract of the rat. Fluoride. 1994;27(4):185–188. [Google Scholar]
- 27.Agalakova N.I., Gusev G. Diverse effects of fluoride on Na+ and K+ transport across the rat erythrocyte membrane. Fluoride. 2008;41(1):28–39. [Google Scholar]
- 28.Zhan Y. The role of platelets in inflammatory immune responses in generalized aggressive periodontitis. J. Clin. Periodontol. 2017 doi: 10.1111/jcpe.12657. [DOI] [PubMed] [Google Scholar]
- 29.Schenk G. Crystal structures of a purple acid phosphatase, representing different steps of this enzyme's catalytic cycle. BMC Struct. Biol. 2008;8(1):1. doi: 10.1186/1472-6807-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braceland M. Technical pre-analytical effects on the clinical biochemistry of Atlantic salmon (Salmo salar L.) J. Fish Dis. 2017;40(1):29–40. doi: 10.1111/jfd.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shashi A., Singh J., Thapar S. Toxic effects of fluoride on rabbit kidney. Fluoride. 2002;35(1):38–50. [Google Scholar]
- 32.Liu S. Effects of estrogen on renal function of pregnant rabbits with hemorrhagic shock. Nan fang yi ke da xue bao = J. South. Med. Univ. 2014;34(2):232–235. [PubMed] [Google Scholar]
- 33.Crawford J.R. Inferential methods for comparing a single case with a control sample: modified t‐tests versus mycroft et al. 's (2002) modified anova. Cogn. Neuropsychol. 2004;21(7):750–755. doi: 10.1080/02643290342000276. [DOI] [PubMed] [Google Scholar]
- 34.Azmat R., Talat R., Ahmed K. The length-weight relationship: condition factor and impact of fluoride concentration in Johnius belangerii of Arabian Sea. Res. J. Environ. Toxicol. 2007;1(3):138–143. [Google Scholar]
- 35.Masoud, M., et al. The effect of fluoride and other ions on algae and fish of coastal water of Mediterranean Sea, Egypt . 2006.
- 36.Jena C.K., Gupta A.R., Patra R.C. Protective effect of moringa oleifera on haematological and biochemical parameters of cattle from industrial fluoride polluted area. J. Anim. Res. 2016;6(1):91. [Google Scholar]
- 37.Nunes R.d.C.A. Effect of sodium fluoride on bone biomechanical and histomorphometric parameters and on insulin signaling and insulin sensitivity in ovariectomized rats. Biol.Trace Element Res. 2016;173(1):144–153. doi: 10.1007/s12011-016-0642-2. [DOI] [PubMed] [Google Scholar]
- 38.Mohiuddin S., Reddy V. Haematological and biochemical studies on fluoride toxicity in sheep. Indian Vet. J.l. 1989;66(11):1089–1091. [Google Scholar]
- 39.Gujarathi S., Bhoop S., Bhikane A. Effect of acute experimental fluorine poisoning on hematological and biochemical indices in buffalo calves (Buhalus huhalis) Indian J. Vet. Med. 1991;11:80–82. [Google Scholar]
- 40.Bely M. Lymphoid depletion of spleen due to experimental fluorosis in rats. Fluoride. 2000;33(1):S1–S2. [Google Scholar]
- 41.Swarup D., Singh Y. Bovine fluorosis in a brick kiln congested zone. Indian J. Vet. Med. 1989;9(1):12–14. [Google Scholar]
- 42.Choe J. Correlations among various blood parameters at exsanguination and their relationships to pork quality traits. Anim. Prod. Sci. 2015;55(5):672–679. [Google Scholar]
-
43.L.H. Alol, The Prophylactic Role of Flavonoids of Black Grape (Vitis vinifera L.) on Some Biochemical Parameters in Adult females Rats Treated with Sodium Fluoride,
 Kufa J. Vet. Med. Sci., 6 (2) (2016).
Kufa J. Vet. Med. Sci., 6 (2) (2016).
- 44.Maheswaran R. Haematological studies of freshwater fish: clarias batrachus (L.) exposed to mercuric chloride. Int. J. Integr. Biol. 2008;2(1):49–54. [Google Scholar]
- 45.Das S., Maiti R., Ghosh D. Fluoride-induced immunotoxicity in adult male albino rat: a correlative approach to oxidative stress. J. Immunotoxicol. 2006;3(2):49–55. doi: 10.1080/15476910600631587. [DOI] [PubMed] [Google Scholar]
- 46.SAYYED M.A., KHAN S.A. Effect of acute fluoride intoxication on some hematological changes in chicken (Gallus domesticus) Biol (PAKISTAN) 2010;56(1&2):123–127. [Google Scholar]
- 47.Kahl S., Wojcik K., Ewy Z. Effect of fluoride on some hematological indices and 59Fe distribution in the blood and iron-storing tissues in rats. Bulletin Serie des sciences biologiques. 1973 [PubMed] [Google Scholar]
- 48.Danilov V., Kasyanova V. Experimental data on the effect of hydrofluoric acid on embryogenesis of white rats. Gig. Tr. Prof. Zabol. 1975;1:57–58. [PubMed] [Google Scholar]
- 49.Atmaca N. Effect of resveratrol on hematological and biochemical alterations in rats exposed to fluoride. BioMed. Res. Int. 2014 doi: 10.1155/2014/698628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heyroth F.F. Toxicological evidence for the safety of the fluoridation of public water supplies*. Am. J. Public Health Nations Health. 1952;42(12):1568–1575. doi: 10.2105/ajph.42.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill K.K., Dumka V.K. Antioxidant status in oral subchronic toxicity of fipronil and fluoride co-exposure in buffalo calves. Toxicol. Ind. Health. 2016;32(2):251–259. doi: 10.1177/0748233713500376. [DOI] [PubMed] [Google Scholar]
- 52.Mandal K.D.et al. Effect of Moringa oleifera on hematological parameters of calves reared in industrial fluorotic area. Vet. world. 2015;8(11):1364. doi: 10.14202/vetworld.2015.1364-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma S. Comparative study on acute toxicity of fluoride: aluminium and aluminium fluoride to swiss albino mice. Australas. J. Ecotoxicol. 2010;16(1):41–47. [Google Scholar]
- 54.Ozsvath D.L. Fluoride and environmental health: a review. Rev. Environ. Sci. Bio/Technol. 2009;8(1):59–79. [Google Scholar]
- 55.Agha F.E. Role of vitamin E in combination with methionine and L-carnosine against sodium fluoride-induced hematological: biochemical, DNA damage, histological and immunohistochemical changes in pancreas of albino rats. Life Sci. J. 2012;9(2):1260–1275. [Google Scholar]
- 56.Khan A.M. Toxic effects of deltamethrin and fluoride on hematological parameters in rats. Fluoride. 2013;46(1):34–38. [Google Scholar]
- 57.Dubey N., Raina R., Khan A.M. Toxic effects of deltamethrin and fluoride on antioxidant parameters in rats. Fluoride. 2012;45(3 Pt 2):242–246. [Google Scholar]
- 58.Machalinska A. In vivo effects of sodium fluoride on bone marrow transplantation in lethally irradiated mice. Fluoride. 2002;35(2):81–89. [Google Scholar]
- 59.Oda H., Nogami H., Nakajima T. Reaction of hemoglobin with nitric oxide and nitrogen dioxide in mice. J. Toxicol. Environ. Health. 1980;6(3):673–678. doi: 10.1080/15287398009529884. (Part A Current Issues) [DOI] [PubMed] [Google Scholar]
- 60.Larsson Å., Haux C., Sjöbeck M.-L. Fish physiology and metal pollution: results and experiences from laboratory and field studies. Ecotoxicol. Environ. Saf. 1985;9(3):250–281. doi: 10.1016/0147-6513(85)90045-4. [DOI] [PubMed] [Google Scholar]
- 61.Kumar R., Banerjee T. Arsenic induced hematological and biochemical responses in nutritionally important catfish Clarias batrachus (L.) Toxicol. Rep. 2016;3:148–152. doi: 10.1016/j.toxrep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsatsakis A. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Human Exp. Toxicol. 2017;36(6):554–564. doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]