Graphical abstract
Abbreviations: KRHB, Krebs-Ringer HEPES Buffer; WME, Williams medium E; SOD, superoxide dismutase; ROS, reactive oxygen species; RNS, reactive nitrogen species; KCl, potassium chloride; NADH, nicotinamide adenine dinucleotide; TBA, thiobarbituric acid; EDTA, Ethylenediaminetetraacetic acid; TCA, trichloroacetic acid; DMSO, Dimethylsulfoxide; BSA, Bovine serum albumin; NaCl, sodium chloride; CaCl2, calcium chloride; MgSO4, magnesium sulfate; CuSO4, copper sulphate; NaOH, sodium hydroxide and MDA Malonaldialdehyde
Keywords: Carbofuran, Oxidative stress, Antioxidant, In vitro, Hepatotoxicity
Highlights
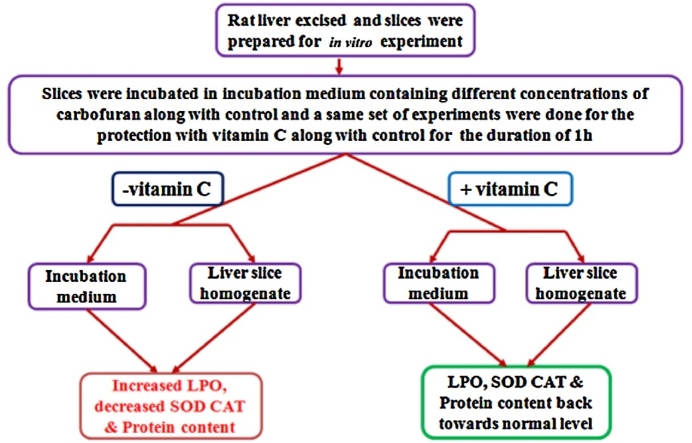
The observations of liver slices when treated with different concentrations of carbofuran were as follows:-
-
1.
increased LPO
-
2.
decreased SOD, CAT, & protein content in all the treatments
The observations of liver slices when treated with different concentrations of carbofuran along with vitamin C were as follows:-
-
1.
the levels of LPO, SOD, CAT & total protein content reinstated towards normal level only in liver slices treated with low concentration
-
2.
at higher concentration of carbofuran treatment Vitamin C does not ameliorate the hepatic toxicity induced by carbofuran
The in vitro liver slice culture may prove to be a useful model for hepatotoxicological studies and Vitamin C, as a hepatoprotectant in mammalian system.
Abstract
Carbamates, most commonly used pesticides in agricultural practices, have been reported to produce free radicals causing deleterious effects in animals. The present study was designed to assess the carbofuran induced oxidative stress in rat liver slices in vitro and also to evaluate protective role of vitamin C by incubating them in Krebs-Ringer HEPES Buffer (KRHB) containing incubation media (Williams medium E (WME) supplemented with glucose and antibiotics) with different concentrations of carbofuran. The results demonstrated that carbofuran caused significant increase in lipid peroxidation and inhibition in the activity of hepatic superoxide dismutase (SOD) in concentration dependent manner. The data with incubation medium reflected that carbofuran at lowest concentration caused an increase in SOD activity followed by its inhibition at higher concentration. Carbofuran treatment caused inhibition in the activity of catalase in liver slices and WME incubation medium. Pre-incubation of liver slices and the WME media with vitamin C restored the values of biochemical indices tested. The results indicated that carbofuran might induce oxidative stress in hepatocytes. The pretreatment with vitamin C may offer hepatoprotection from toxicity of pesticide at low concentration only.
1. Introduction
The use of pesticides in agriculture remains the most effective method for protection of plants and animals from a large number of pests. The carbamates are chemicals mainly used in agriculture as insecticides, fungicides, herbicides, nematocides, and/or sprout inhibitors. The application of carbamates has been preferred over organophosphates and organochlorines as these compounds were extremely toxic and possessed delayed neurotoxic effects [1], [2]. Organocarbamates share with organophosphates in their abilities to inhibit cholinesterases and therefore exhibit similar symptomatology during acute and chronic exposures [3]. Positive correlations have been reported between acetylcholine accumulation and oxidative stress [4], [5].
Carbofuran (C12H15NO3; 2,3-dihydro-2,2-dimethyl-7-benzofuranol methylcarbamate), commonly known as Furadan, is a carbamate pesticide and used in the farm practices in order to increase crop productivity. It is also used as an insecticide, nematicide and acaricide due to its short half life in the environment [6]. The adverse effects of carbofuran like myopathy have been reported to be mediated by oxidative stress and the excessive generation of free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) [2], [7], [5]. The production of ROS is caused by a mechanism in which xenobiotics, toxicants and pathological conditions generate oxidative stress and induce tissue damage in liver, kidney and brain [8]. The protein oxidation oxidative damage to nucleic acids and peroxidation of lipids mediated by free radicals are considered to be crucial events of the cytotoxic actions of ROS [9]. Studies by Kaur and Sandhir [10] have shown an increased lipid peroxidation and a decrease in glutathione level in the liver of carbofuran exposed rats. In another study, we have reported the increased oxidative stress and its amelioration by vitamin C in the different tissues of carbofuran treated rats [11], [12], [13], [14]. Vitamin E [15] and Curcumin [14] have also been reported to be potent ameliorative antioxidants against carbofuran induced toxicity.
In the present study, we have used rat liver slices which have been widely exploited by many researchers as an in vitro model of the organ for toxicological assessment. Such tissue slices contain natural and intact environment in every possible manner including their intercellular and cell-matrix interactions. Previously, the preparation of slices from several mammalian tissues such as liver [16], [17], [18], [19], lung [20], kidney [21], intestine [22], spleen [23], brain [24], heart [25], prostate [26] and several tumor types [27], [28] and their corresponding applications in many different studies have been demonstrated by several researchers [29]. These workers have suggested the use of these tissues slices for many biochemical and toxicological studies including xenobiotics metabolism and their transport, testing the safety and efficacy of drugs using diseased tissues, ischemia/reperfusion damage [30], [31] and specificity of viruses as carriers for gene therapy agents [32], [33].
Several other workers have used liver slices for the analysis of the metabolic rates and fates of physiological substrates [34] as well as xenobiotics [17], modulation of hepatic enzymes [35], [36], [37], uptake of transporter-mediated drugs [38], [39]. In addition, liver slices have also been used to study the biochemical and molecular bases of drug toxicity [40], [41], [42] and to investigate the early toxicological markers [43], [44], [45]. This system has been preferably used because of it is easy and cheap to study with numerous slices. However, the applications of none of these organ slices have been utilized to demonstrate xenobiotics induced production of oxidative stress, the impact on biochemical parameters of liver slices and amelioration by antioxidants.
In this research article we have presented the carbofuran induced production of oxidative stress in rat liver slices in terms of alterations in lipid peroxidation and activities of antioxidative enzymes. The hepatoprotective effect of vitamin C from carbofuran toxicity has also been demonstrated upon pretreatment of rat liver slices in vitro.
2. Materials and methods
2.1. Chemicals and reagents
Technically pure (99.6%) carbofuran (2,3-dihydro-2,2-dimethyl-7-benzofuranyl N-methylcarbamate) in powder form was generously gifted by Rallis India Limited (Bangalore, India) as a gift. Tris HCl, potassium chloride (KCl), sodium-pyruvate, nicotinamide adenine dinucleotide (NADH), Pyrogallol, tris base, EDTA, succinic acid, H2O2, thiobarbituric acid (TBA) and trichloroacetic acid (TCA) were purchased from E. Merck, Darmstadt, Germany and Dimethylsulfoxide (DMSO) from (Qualigens). Bovine serum albumin (BSA), were purchased from Loba Chemie Pvt. Ltd. India. Diethyl ether [(CH3CH2)2O] solution was procured from Sigma-Aldrich, India. β-Hydroxy butyrate, sodium chloride (NaCl), calcium chloride (CaCl2), magnesium sulfate (MgSO4), glucose, Folin-ciocalteu reagent, copper sulphate (CuSO4), vitamin C and sodium hydroxide (NaOH) were purchased from SISCO research laboratory Pvt. Ltd, Mumbai, India.
2.2. Animals
Male albino Wistar rats (body weight between 100 and 130 g and 8–10 weeks of age) were selected for all the experiments. Animals obtained from Central Drug Research Institute, Lucknow, India, were housed in polypropylene cages at an ambient temperature of 25 °C-30 °C and 45–55% relative humidity with 12 h each of dark and light cycle. Animals were fed standard rat chow (Golden feed, New Delhi, India) and drinking water ad libitum. The protocols used in the study were according to the guidelines for use and care of laboratory animals and were approved by the Institutional Ethics Committee of the University.
2.3. Preparation of KRHB
KRHB is used for slicing and storage of liver slices. This buffer is prepared in 1 liters of a 10 X concentrated KRHB stock solution (10 X KRHB) by dissolving 3.67 g CaCl2·2H2O in 500 ml of ultrapure water (solution 1). Solution 2 is prepared by dissolving 3.73 g KCl, 69.0 g, NaCl, 2.71 g, MgSO4·7H2O and 1.63 g KH2PO4 in ultrapure water to a volume of 500 ml. Then solutions 1 and 2 were mixed and filtered through a 0.45 μm filter. This 10 X KRHB can be stored at 4° C for about 6 months. The KRHB was freshly prepared by dissolving 1.05 g NaHCO3, 2.475 g D-glucose monohydrate and 1.19 g HEPES in about 200 ml of ultrapure water at 4° C. Into this solution, 50 ml of 10 X KHB is mixed followed by addition of 250 ml of ultrapure water at 4° C. This solution may be stored at 4° C. The pH of this solution was maintained to be 7.4 by slow addition of 5 N NaOH solution.
2.4. Preparation of WME slice incubation medium
Freshly 500 ml WME slice incubation medium was prepared by adding 1.375 g D-glucose monohydrate and 500 μl gentamicin (50 mg ml−1) to WME containing L-gluamine.
2.5. Development of liver slice in vitro
Rats were sacrificed by mild anesthesia using Diethyl ether [(CH3CH2)2O] solution and followed by the cervical dislocation. The anesthetic used according to the AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Liver lobes were removed and transferred to pre-warmed Krebs-Ringer HEPES Buffer (KRHB) (Hepes 2.5 mM pH 7.4, NaCl 118 mM, KCl 2.85 mM, CaCl2 0.5 mM, KH2PO4 1.5 mM, MgSO4 1.18 mM and glucose 4.0 mM) solution. Liver slice culture was maintained following the protocol developed by Wormser et al. [46] and Invittox Protocol No. 42 (1992). A small piece of liver was cut into thin slices. Each culture tube contained 20–22 slices with about 0.5 mm of thickness and the wet weight of each slice was about 10–12 mg. These slices were washed with 10 ml KRHB medium, every 10 min over a period of 1 h. They were then incubated for 60 min in small plugged beakers containing 2 ml KRHB on a shaker water bath at 37 °C. At the end of incubation, the medium was replaced by fresh 2 ml KRHB and incubated for 1 h at 37 °C with ethanol (1372 mM; Invittox Protocol No. 42, 1992). The liver slices were then treated with different final concentrations of carbofuran (0.025, 0.25, 0.5, 1.0, 2.5, 5.0, 10, 25, and 50 μM) for the same time interval i.e. 1 h. The treated liver slice supernatants were taken separately in different centrifuge tubes (2 ml) and used for biochemical estimations. To evaluate the effect of vitamin C on carbofuran treated liver slices, the liver slices were pre-incubated for 10 min in the media with vitamin C at 567.76 μM concentration. For the time dependent study the media was replaced by fresh 2 ml KRHB solution containing 2.5 μM carbofuran in the KRHB solution. The slices were removed at the different time interval (15, 30, 60, 90 and 120 min). The treated livers slices were homogenized (10%, w/v) in 0.25 M sucrose solution and centrifuged at 9000 x g for 30 min at 4 °C. The supernatants were separated by gentle decantation of centrifuged homogenates and used for estimation of lipid peroxidation, activities of superoxide dismutase, catalase and quantification of protein. The control liver slices (untreated) were also processed accordingly parallel to the experimental liver slices.
2.6. Measurement of MDA level
Lipid peroxidation was measured in the cytosolic fraction of hepatic tissues as well as in the KRHB incubation media by following the method of Niehaus and Samuelsson [47] and the results were expressed as nmol MDA/mg protein using the extinction coefficient of 1.56 × 105 M−1 cm−1.
2.7. Estimation of the activities of antioxidant enzymes
The activity of superoxide dismutase (SOD, E.C. 1.15.1.1) was measured by following the method of Marklund and Marklund [48]. It is a spectrophotometric measurement of optical density of colored complex involving pyrogallol auto-oxidation at 412 nm for 3 min at the interval of 30 s with or without the enzyme protein. One unit of the enzyme activity was expressed as 50% inhibition of auto- oxidation of pyrogallol per min.
Catalase (CAT, E.C.1.11.1.6) activity was measured according to the method of Beers and Sizer [49] by measuring the decrease in the absorbance for H2O2 consumption at 240 nm at the interval of 30 s for 3 min. One unit of CAT activity was defined as micromoles of H2O2 decomposed per min using molar extinction coefficient of H2O2 (43.6 M−1cm−1).
2.8. Estimation of total protein content
The protein content in the sample was measured by the modified Lowry et al. [50] using BSA as a standard. The amount of protein was calculated from the standard curve.
2.9. Statistical analysis
Each sample was run in triplicate. Values were expressed as mean ± standard deviation (SD) tissue, for n = 5 to 6 rats. Values between groups were compared using Dunnet’s comparison tests. Values were considered significant if P < 0.05. Statistical analysis was performed by means of In-Stat package for personal computers version 5 (GraphPad_Software, Inc., San Diego, USA).
3. Results
3.1. Effect of carbofuran on the activities of antioxidant enzymes in rat liver slices
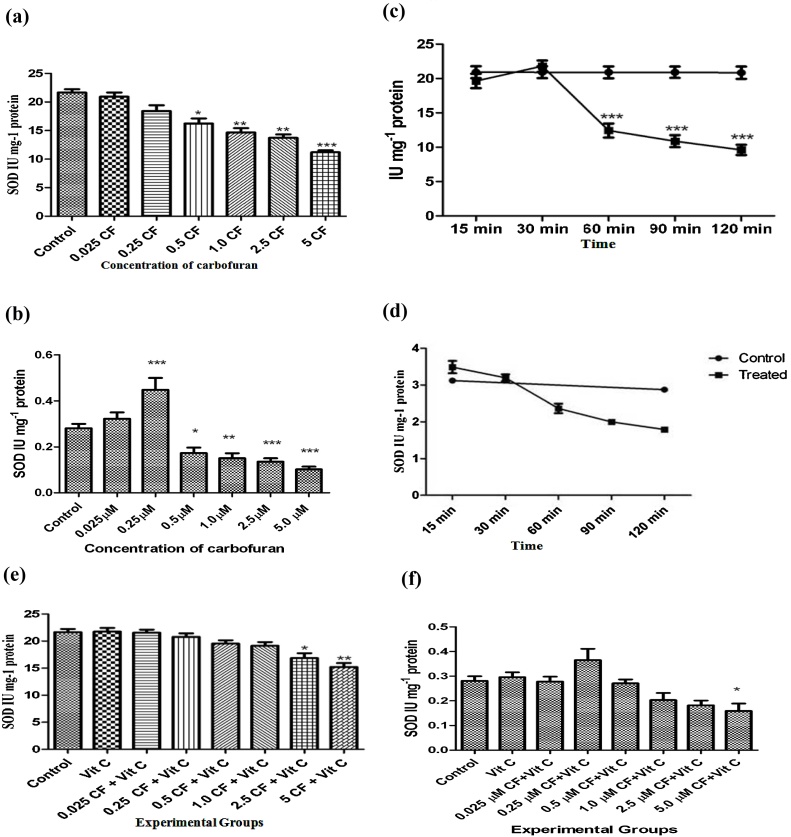
Superoxide dismutases (SODs) form the first line of defense against reactive oxygen species. Fig. 1(a) and (b) show that carbofuran caused a significant inhibition of SOD in the liver tissue and incubation medium from a concentration of 0.5 and 0.25 μM, respectively. Similarly, Fig. 1(c) and (d) demonstrate that carbofuran exhibited a parallel inhibitory effect on SOD in the liver tissue homogenate and the secretary products present in the incubation medium from 60 min of post incubation which continued up to 120 min of incubation. However, vitamin C reversed the SOD inhibition to near normal levels except at a carbofuran concentration of 2.5, 5 and 5 μM in the liver tissue and medium, respectively (Fig. 1(e) and (f)). Inhibition of SOD by increasing concentration and/or incubation time of carbofuran may be due to inhibitory effect of superoxide anions on SOD (***p < 0.001) generated by carbofuran [51].
Fig. 1.
(a): Effect of various concentrations of carbofuran (0.025, 0.25, 0.50, 1.00, 2.50, 5.00 (M) on the levels of SOD activity in the supernatant of homogenate (10%, w/v) prepared in 0.25 M sucrose solution rat liver slices in vitro. Control: control group had not treated with any treatment. Level of SOD activity was determined as described in Materials and Methods. The unit of level of SOD activity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (*), (**), and (***) indicates values significantly decreased the activity from control at (P<0.05). (b): Effect of various concentrations of carbofuran (0.025, 0.25, 0.50, 1.00, 2.50, 5.00 μM) on SOD in the supernatant of the incubation medium of rat liver slices in vitro. Control: control group had not treated with any treatment. Level of SOD activity was determined as described in Materials and Methods. The unit of level of SOD activity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (*), (**), and (***) indicates values significantly decreased the activity from control at (P < 0.05). (c): Time dependent alterations on SOD activity in the supernatant of homogenate (10%, w/v) prepared in 0.25 M sucrose solution of rat liver slices after incubation with 2.5 μ moles of carbofuran in vitro. Control: control group had not treated with any treatment. Level of SOD activity was determined as described in Materials and Methods. The unit of level of SOD activity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (***) indicates values significantly increased from control at (P < 0.05). (d): Time dependent alterations on SOD activity in the supernatant of the incubation medium of rat liver slices after incubation with 2.5 μ moles of carbofuran in vitro. Control: control group had not treated with any treatment. Level of SOD activity was determined as described in Materials and Methods. The unit of level of SOD activity is IU mg−1 Protein. The values were expressed as Mean ± SD. (e): Effect of vitamin C on carbofuran induced changes in SOD activity level in the supernatant of homogenate (10%, w/v) prepared in 0.25 M sucrose solution of rat liver slices in vitro. Control: control group had not treated with any treatment. The experimental groups of liver slices were treated with different conditions as described in materials and methods. The level of SOD activity was determined as described in Materials and Methods. The unit of level of SOD is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (*) and (**) indicates values significant from control at (P < 0.05). (f): Effect of vitamin C on carbofuran induced changes in SOD activity level in the supernatant of the incubation medium of rat liver slices in vitro. Control: control group had not treated with any treatment. The experimental groups of liver slices were treated with different conditions as described in materials and methods. The level of SOD activity was determined as described in Materials and Methods. The unit of level of SOD is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (*) and (**) indicates values significant from control at (P < 0.05).
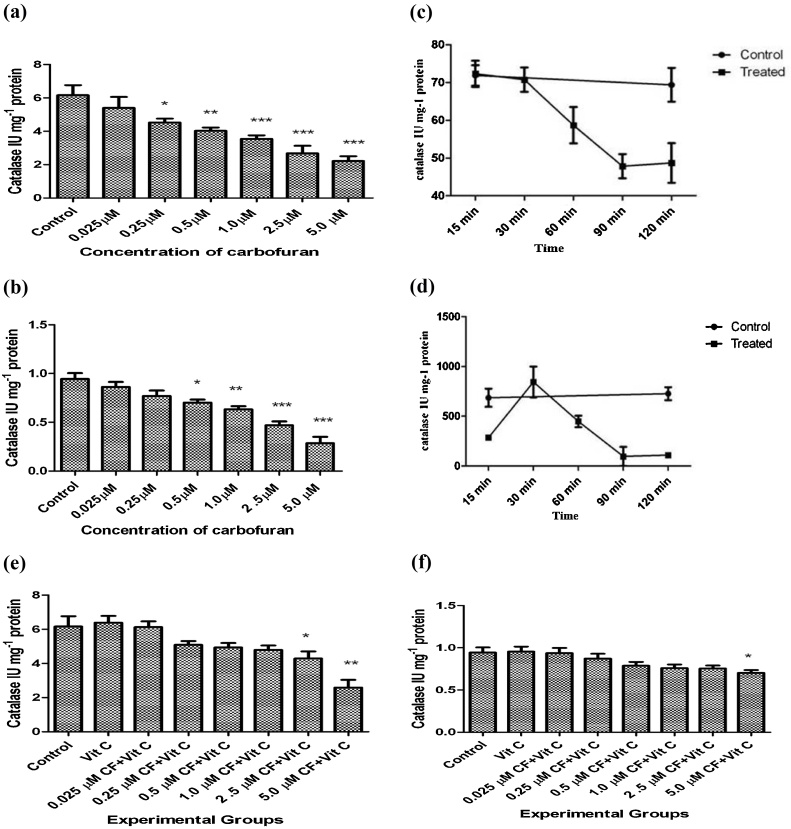
Catalase forms the second stage defense antioxidant enzyme that dissipates H2O2 formed from dismutation of superoxide anions by superoxide dismutases or those formed from other sources. Fig. 2(a) and (b) show that carbofuran from a concentration of 0.25 μM and 0.5 μM till 5.0 μM significantly inhibited catalase in the liver tissue and the secretary products present in the incubation medium, respectively. Hepatic catalase was inhibited by carbofuran from 60 min of incubation till 120 min (Fig. 2(c) and (d)). In the incubation medium, however, there was an increase in catalase activity after 30 min followed by significant inhibition of its activity from 60 min till 120 min of incubation. Vitamin C was able to reverse carbofuran induced catalase inhibition up to 2.5 μM of carbofuran concentration in liver (Fig. 2(e)) and 5.0 μM in medium (Fig. 2(f)).
Fig. 2.
(a): Effect of various concentrations of carbofuran (0.025, 0.25, 0.50, 1.00, 2.50, 5.00 μM) on the levels of activity of catalase in the supernatant of homogenate (10%, w/v) in 0.25 M sucrose solution of rat liver slices in vitro. Control: control group had not treated with any treatment. Level of catalase activity was determined as described in Materials and Methods. The unit of level of catalase activity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (*), (**), and (***) indicates values significantly decreased from control at (P < 0.05). (b): Effect of various concentrations of carbofuran (0.025, 0.25, 0.50, 1.00, 2.50, 5.00 μM) on the levels of catalase activity in the supernatant of the incubation medium of rat liver slices in vitro. Control: control group had not treated with any treatment. Level of catalase activity was determined as described in Materials and Methods. The unit of level of catalase actvuity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (*), (**), and (***) indicates values significantly decreased from control at (P < 0.05). (c): Time dependent alterations on the levels of catalase activity form the supernatant of homogenate (10%, w/v) prepared in 0.25 M sucrose solution of rat liver slices after incubation with 2.5 μmoles of carbofuran in vitro. Control: control group had not treated with any treatment. Level of catalase was determined as described in Materials and Methods. The unit of level of catalase activity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (■) and (●) indicates values of Experimental and control. (d): Time dependent alterations on the levels of catalase activity from the supernatant of the incubation medium of rat liver slices after incubation with 2.5 μmoles of carbofuran in vitro. Control: control group had not treated with any treatment. Level of Catalase was determined as described in Materials and Methods. The unit of level of catalase activity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (■) and (●) indicates values of Experimental and control, respectively. (e): Effect of vitamin C on carbofuran induced changes on the levels of catalase activity from the supernatant of homogenate (10%, w/v) prepared in 0.25 M sucrose solution of rat liver slices in vitro. Control: control group had not treated with any treatment. The experimental groups of liver slices were treated with different conditions as described in materials and methods. The level of catalase activity was determined as described in Materials and Methods. The unit of level of catalase activity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (*) and (**) indicates values are significant from control at (P < 0.05). (f): Effect of vitamin C on carbofuran induced changes on the levels of catalase activity from the supernatant of incubation medium of rat liver slices in vitro. Control: control group had not treated with any treatment. The experimental groups of liver slices were treated with different conditions as described in materials and methods. The level of catalase activity was determined as described in Materials and Methods. The unit of level of Catalase activity is IU mg−1 Protein. The values were expressed as Mean ± SD. The sign (*) indicates values are significant from control at (P < 0.05).
3.2. Effect of carbofuran on the level of MDA in rat liver slices
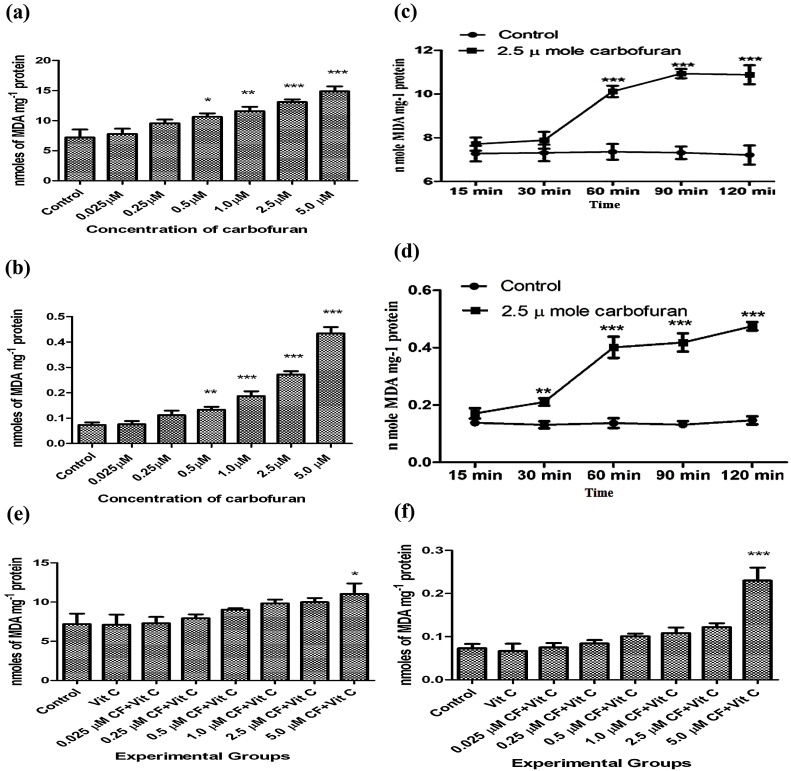
The results presented in Fig. 3(a) show the effect of different concentrations of carbofuran on lipid peroxidation in liver slices. Carbofuran at doses of 0.5, 1.0, 2.5 and 5.0 μM caused significant increase (P < 0.05) in lipid peroxidation in the slices. When extent of lipid peroxidation under this condition was monitored in the incubation medium which contained the liver slices, it was found that carbofuran at doses of 0.5, 1.0, 2.5 and 5.0 μM caused significant increases (P < 0.05) in lipid peroxidation in the secretary products present in the incubation medium the WME incubation medium. The results are shown in Fig. 3(b).
Fig. 3.
(a): Effect of various concentrations of carbofuran (0.025, 0.25, 0.50, 1.00, 2.50, 5.00 μM) on lipid peroxidation in the supernatant of homogenate (10%, w/v) prepared in 0.25 M sucrose solution from rat liver slices in vitro. Control: control group had not treated with any treatment. Level of MDA was determined as described in Materials and Methods. The unit of level of MDA is mg−1 Protein. The values were expressed as Mean ± SD. The sign (*), (**), and (***) indicates values significantly increased from control at (P < 0.05). Carbofuran at doses of 0.5, 1.0, 2.5 and 5.0 μM has been observed to be significantly increased. (b): Effect of various concentrations of carbofuran (0.025, 0.25, 0.50, 1.00, 2.50, 5.00 μM) on lipid peroxidation in the supernatant of incubation medium of rat liver slices in vitro. Control: control group had not treated with any treatment. Level of MDA was determined as described in Materials and Methods. The unit of level of MDA is mg−1 Protein. The values were expressed as Mean ± SD. The sign (*), (**), and (***) indicates values significantly increased from control at (P < 0.05). Carbofuran at doses of 0.5, 1.0, 2.5 and 5.0 μM has been observed to be significantly increased. (c): Time dependent alterations in MDA levels in the supernatant of homogenate (10%, w/v) prepared in 0.25 M sucrose solution from rat liver slices after incubation with 2.5 μ moles of carbofuran in vitro. Control: control group had not treated with any treatment. Level of MDA was determined as described in Materials and Methods. The unit of level of MDA is mg−1 Protein. The values were expressed as Mean ± SD. The sign (***) indicates values significantly increased from control at (P < 0.05). (d): Time dependent alterations in MDA levels in the supernatant of homogenate (10%, w/v) in 0.25 M sucrose solution of the supernatant from the incubation medium of rat liver slices after incubation with 2.5 μ moles of carbofuran in vitro. Control: control group had not treated with any treatment. Level of MDA was determined as described in Materials and Methods. The unit of level of MDA is mg−1 Protein. The values were expressed as Mean ± SD. The sign (***) indicates values significantly increased from control at (P < 0.05). (e): Effect of vitamin C on carbofuran induced changes on the level of lipid peroxidation from the supernatant of homogenate (10%, w/v) prepared in 0.25 M sucrose solution of rat liver slices in vitro. Control: control group had not treated with any treatment. The experimental groups of liver slices were treated with different conditions as described in materials and methods. The level of MDA was determined as described in Materials and Methods. The unit of level of MDA is mg−1 Protein. The values were expressed as Mean ± SD. The sign (*) indicates values significantly increased from control at (P < 0.05). (f): Effect of vitamin C on carbofuran induced changes on the levels of lipid peroxidation in the supernatant of the incubation medium in vitro. Control: control group had not treated with any treatment. The experimental groups of liver slices were treated with different conditions as described in materials and methods. The level of MDA was determined as described in Materials and Methods. The unit of level of MDA is mg−1 Protein. The values were expressed as Mean ± SD. The sign (*) indicates values significantly increased from control at (P < 0.05).
The time dependent alterations in the levels of MDA in liver slices were also evaluated after incubation with 2.5 μM of carbofuran. The results presented in Fig. 3(c) indicated that carbofuran caused a significant increase (P < 0.001) in malonaldehyde formation after 60 min of incubation which continued to increase up to 120 min. Fig. 3(d) shows a parallel increase in MDA liver in the secretary products present in the incubation media after incubation with 2.5 μM of carbofuran. An earlier significant increase (P < 0.01) in lipid peroxidation which started at 30 min after incubation was observed which continued up to 120 min. Fig. 3(e) and (f) showed the effects of vitamin C on carbofuran induced lipid peroxidation in the hepatic tissues and the secretary products present in the incubation medium incubation medium, respectively. Vitamin C was able to reverse increased lipid peroxidation induced by all the doses of carbofuran tested except 5 μM carbofuran in the liver and incubation medium to near normal levels. At a higher carbofuran concentration of 5 μM vitamin C failed to neutralize increased lipid peroxidation both in the liver slices as well as in the incubation medium which could be possibly due to saturation of its antioxidant capacity.
4. Discussion
4.1. Perturbations in the activities of antioxidant enzymes due to carbofuran treatment of rat liver slices
Superoxide dismutases (SODs) dissipate superoxide to H2O2 and O2, which are the most important parts of antioxidant enzyme defense against ROS, particularly superoxide anion radicals [52]. Catalase dissipates H2O2 formed from dismutation of superoxide anions by superoxide dismutases. The restoration of catalase activity after vitamin C treatment may be attributed to the supporting role of this vitamin in improving the rate of removal of H2O2 in metabolically normal animals [53].
Vitamin C is a water soluble antioxidant, has been reported to ameliorate the free radical induced damage by carbofuran [54], [55]. Since ascorbic acid is water soluble, it can work both inside and outside the cells to combat free radical damage [55]. There are several transport mechanisms to get it inside: Glucose-dependent transporters like GLUT1 and 3 as well as more specialized sodium-dependent transporters for ascorbic acid, SVCT1 and 2 [56]. Vitamin C can “donate electrons to free radicals such as hydroxyl and superoxide radicals and quench their reactivity” [57], [58]. Vitamin C reported to be an antioxidant [59], has been shown to mitigate lead induced oxidative stress toxicity [60]. The extract of C. limon fruit, which is rich in vitamin C has also been reported to play a protective role against free radical mediated carbofuran induced hepatotoxicity in rats [13]. Therefore, the present study confirms the protective role of vitamin C on carbufuran induced alterations in enzyme activities only up to a certain concentration of carbofuran. At a higher concentration of carbofuran (5 mM), vitamin C was not able to completely restore activities of antioxidant enzymes to normal level. This may be due to fixed concentration of vitamin C used for all concentrations of carbofuran tested. At higher carbofuran concentrations, the antioxidant effect of vitamin C may be limited due to saturation of its antioxidant capacity.
4.2. Carbofuran induces alterations in rat liver slices
The lipid peroxidation of biomembranes results in production of several compounds that are routinely used as molecular markers of oxidative stress. Malonaldehyde is one of the most widely used indicators of the cellular redox state [61]. In present study elevated lipid peroxidation caused by increased doses of Carbofuran and increased incubation time may be due to the damage of membrane lipids by free radicals causing the membranes to lose their fluidity and release of some of their altered macromolecules into the incubation medium. These molecules released into the medium could contribute to a parallel increase in lipid peroxidation in the medium. Earlier studies have described increased lipid peroxidation in the brain and liver of carbofuran treated rats [62]. Malathion, β-cypermethrin and avermectin along with phoxim (one of the organophosphorus pesticides) and Cypermethrin have been reported to induce oxidative stress in various insects [63], [64]. Due to high concentration of polyunsaturated fatty acids in cells, lipid peroxidation is a major outcome of free radical-mediated injury [65], [66]. A critically important aspect of lipid peroxidation is that it proceeds until oxidizable substrate is consumed or termination occurs, making this fundamentally different from many other forms of free radical injury in that the self-sustaining nature of the process may cause extensive tissue damage [67]. Two broad outcomes of lipid peroxidation are structural damage to cellular membranes and generation of oxidized products, some of which are chemically reactive and may covalently modify cellular macromolecules [68]. Carbofuran has been reported to cause injury by inhibiting acetylcholinesterase which coincides with increased lipid peroxidation [7]. The lipophilic nature of carbofuran has been reported to cause oxidative injury resulting alterations in membrane structure and function [62], [54].
Ladurner et al. [69] have studied the uptake of ascorbate into cultured endothelial cells. They observed that the uptake of ascorbate increases with increasing time. The ascorbate (100 μM) has been reported to exhibit time dependent effect up to 24 h on total eNOS activity level and epithelial BH4 levels. Some workers have reported that vitamin C may exhibit dose- and time-dependent protective effect on NaF mediated alterations in the levels of 3β-HSD and 17β-HSD, SOD, CAT, GPx, GST, γ-GT, and GSH, as well as the activities of sertoli cells in vitro. They observed that the enzymes when incubated for 24 and 48 h, maximum protection of them were achieved after longer duration of incubation i.e. 48 h [70].
Our study bears some important limitations such as (i) along with the experimental results with Vitamin C there should have been a parallel result obtained with carbofuran treated group. This data was essential to conclude that Vit C actually decreased the effect of Carbofuran; (ii) in Fig. 2F and G, as well as in Fig. 3E and F, to have conditions without vitamin C seems to be important; (iii) There are two main variables that influence the oxidative stress indicators in our experiments: time and dose response. The best experimental design would have beeen to observe if the dose response and time course curves move to the right or not in the presence of constant amount of Vit C. This design would clearly show how Vit C acts as antioxidant to neutralize some of the oxidative molecules and shift the dose and time response curves to the right; (iv) the data for Control, Vit C, and Carbofuran ± Vit C for both time and dose response curves would have been beneficial. However, in separate experiments we have shown that carbofuran alone can produce serious adverse effects at lower doses. The Fig. 2F and G as well As Fig. 3E and F lack the data without vitamin C but it could be indirectly correlated with the results of other experiments done separately as shown in Figures.
5. Conclusions
The use of rat liver slices cultures offers the unique opportunity to investigate the direct toxicological responses in a system exposed in vitro (A combined system that more closely mimics the in vivo situation). Therefore it allows more realistic and accurate data and their conclusions to be obtained. The results indicated that rat liver slices when treated in vitro with different concentrations of carbofuran displayed significant alterations at the levels of antioxidative indices in both the tissues as well as the secretary products present in the incubation medium, thereby indicating its effects being mediated via generation of oxidative stress, which may be significantly ameliorated by application of vitamin C. Vitamin C being a potential antioxidant was useful in amelioration of pesticide toxicity but failed to offer protection at higher concentration of the pesticide.
Conflict of interest
The authors declare that they do not have any competing interests.
Author contributions
All the authors have contributed equally to this work.
Transparency document
The Transparency document associated with this article can be found in the online version.
Acknowledgements
SKJ and VKG are grateful to University Grant Commission (UGC), New Delhi, India for providing financial support in the form of a Research Fellowship for this work at Department of Biochemistry, University of Allahabad, Allahabad, India. NJS gratefully thanks the Research Center, Female Center for Scientific and Medical Colleges, King Saud University, Riyadh, for the support.
References
- 1.Hour T.C., Chen L., Lin J.K. Comparative investigation on the mutagenicities of organophosphate, phthalimide, pyrethroid and carbamate insecticides by the Ames and lactam tests. Mutagenesis. 1998;13(2):157–166. doi: 10.1093/mutage/13.2.157. (PubMed PMID: 9568589) [DOI] [PubMed] [Google Scholar]
- 2.Bertsias G.K., Katonis P., Tzanakakis G., Tsatsakis A.M. Review of clinical and toxicological features of acute pesticide poisonings in Crete (Greece) during the period 1991–2001. Med. Sci. Monit. 2004;10(11) (CR622-7. PubMed PMID: 15507854) [PubMed] [Google Scholar]
- 3.Soloneski S., Larramendy M.L. Genetic toxicological profile of carbofuran and pirimicarb carbamic insecticides Farzana Perveen. Insecticides-Pest Engineering. 2012 Available from: http://www.intechopen.com/books/insecticides-pest-engineering/genetic-toxicological-profile-of-carbofuran-and-pirimicarb-carbamic-insecticides. [Google Scholar]
- 4.Yang Z.P., Dettbarn W.D. Diisopropylphosphorofluoridate-induced cholinergic hyperactivity and lipid peroxidation. Toxicol. Appl. Pharmacol. 1996;138(1):48–53. doi: 10.1006/taap.1996.0096. (PubMed PMID: 8658512) [DOI] [PubMed] [Google Scholar]
- 5.Agrawal A., Sharma B. Pesticides induced oxidative stress in mammalian systems: a review. Int. J. Biol. Med. Res. 2010;1(3):90–104. [Google Scholar]
- 6.Gupta R.C. Carbofuran toxicity. J. Toxicol. Environ. Health. 1994;43(4):383–418. doi: 10.1080/15287399409531931. (PubMed PMID: 7990167) [DOI] [PubMed] [Google Scholar]
- 7.Milatovic D., Gupta R.C., Dekundy A., Montine T.J., Dettbarn W.D. Carbofuran-induced oxidative stress in slow and fast skeletal muscles: prevention by memantine and atropine. Toxicology. 2005;208(1):13–24. doi: 10.1016/j.tox.2004.11.004. (PubMed PMID: 15664429) [DOI] [PubMed] [Google Scholar]
- 8.Yu F., Wang Z., Ju B., Wang Y., Wang J., Bai D. Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Exp. Toxicol. Pathol. 2008;59(6):415–423. doi: 10.1016/j.etp.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Juránek I., Nikitovic D., Kouretas D., Hayes A.W., Tsatsakis A.M. Biological importance of reactive oxygen species in relation to difficulties of treating pathologies involving oxidative stress by exogenous antioxidants. Food Chem. Toxicol. 2013;61:240–247. doi: 10.1016/j.fct.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 10.Kaur M., Sandhir R. Comparative effects of acute and chronic carbofuran exposure on oxidative stress and drug-metabolizing enzymes in liver. Drug Chem. Toxicol. 2006;29(4):415–421. doi: 10.1080/01480540600837969. (PubMed PMID: 16931442) [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal S.K., Siddiqi N.J., Sharma B. Carbofuran induced oxidative stress in rat heart: ameliorative effect of vitamin C. SRN Oxid. Med. 2013:2013. (Article ID 824102, 10 pages) [Google Scholar]
- 12.Jaiswal S.K., Siddiqi N.J., Sharma B. Carbofuran imbalances the redox status in rat brain: amelioraton by vitamin C. J. Biochem. Res. 2013;1(4):36–43. http://www.peakjournals.org/sub-journals-JBR.html (ISSN: 2329-2717) [Google Scholar]
- 13.Jaiswal S.K., Gupta V.K., Siddiqi N.J., Pandey R.S., Sharma B. Hepatoprotective effect of citrus limon fruit extract against carbofuran induced toxicity in wistar rats. Chin J. Biol. 2015:2015. (Article ID 686071, 10 pages) [Google Scholar]
- 14.Jaiswal S.K., Sharma A., Gupta V.K., Singh R.K., Sharma B. Curcumin mediated attenuation of carbofuran induced oxidative stress in rat brain. Biochem. Res. Int. 2016 doi: 10.1155/2016/7637931. (Article ID 7637931, 7 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal S.K., Siddiqi N.J., Sharma B. Carbofuran induced oxidative stress mediated alterations in Na+-K+-ATPase activity in rat brain: amelioration by vitamin e. J. Biochem. Mol. Toxicol. 2014;28:320–327. doi: 10.1002/jbt.21568. [DOI] [PubMed] [Google Scholar]
- 16.Fisher R.L., Hasal S.J., Sipes I.G., Gandolfi A.J., Brendel K. Comparative metabolism and toxicity of dichlorobenzenes in Sprague-Dawley, Fischer-344 and human liver slices. Hum. Exp. Toxicol. 1995;14(5):414–421. doi: 10.1177/096032719501400505. (PubMed PMID: 7612303) [DOI] [PubMed] [Google Scholar]
- 17.Lerche-Langrand C., Toutain H.J. Precision-cut liver slices: characteristics and use for in vitro pharmaco-toxicology. Toxicol. 2000;153(1–3):221–253. doi: 10.1016/s0300-483x(00)00316-4. (PubMed PMID: 11090959) [DOI] [PubMed] [Google Scholar]
- 18.de Kanter R., Monshouwer M., Meijer D.K., Groothuis G.M. Precision-cut organ slices as a tool to study toxicity and metabolism of xenobiotics with special reference to non-hepatic tissues. Curr. Drug Metab. 2002;3(1):39–59. doi: 10.2174/1389200023338071. (PubMed PMID: 11878310) [DOI] [PubMed] [Google Scholar]
- 19.de Graaf I.A., Groothuis G.M., Olinga P. Precision-cut tissue slices as a tool to predict metabolism of novel drugs. Expert Opin. Drug Metab. Toxicol. 2007;3(6):879–898. doi: 10.1517/17425255.3.6.879. (PubMed PMID: 18028031) [DOI] [PubMed] [Google Scholar]
- 20.Moreno L., Perez-Vizcaino F., Harrington L., Faro R., Sturton G., Barnes P.J., Mitchell J.A. Pharmacology of airways and vessels in lung slices in situ: role of endogenous dilator hormones. Respir. Res. 2006;7(111) doi: 10.1186/1465-9921-7-111. (PubMed PMID:16923180; PubMed Central PMCID: PMC1592489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vickers A.E., Fisher R.L. Precision-cut organ slices to investigate target organ injury. Expert Opin. Drug Metab. Toxicol. 2005;1(4):687–699. doi: 10.1517/17425255.1.4.687. (PubMed PMID: 16863433) [DOI] [PubMed] [Google Scholar]
- 22.Khan A.A., Chow E.C., van Loenen-Weemaes A.M., Porte R.J., Pang K.S., Groothuis G.M. Comparison of effects of VDR versus PXR, FXR and GR ligands on the regulation of CYP3A isozymes in rat and human intestine and liver. Eur. J. Pharm. Sci. 2009;37(2):115–125. doi: 10.1016/j.ejps.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann A.F., Zakko S.F., Lira M., Clerici C., Hagey L.R., Lambert K.K., Steinbach J.H., Schteingart C.D., Olinga P., Groothuis G.M. Novel biotransformation and physiological properties of norursodeoxycholic acid in humans. Hepatology. 2005;42(6):1391–1398. doi: 10.1002/hep.20943. (PubMed PMID: 16317695) [DOI] [PubMed] [Google Scholar]
- 24.Gähwiler B.H., Capogna M., Debanne D., McKinney R.A., Thompson S.M. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20(10):471–477. doi: 10.1016/s0166-2236(97)01122-3. (PubMed PMID: 9347615) [DOI] [PubMed] [Google Scholar]
- 25.Parrish A.R., Gandolfi A.J., Brendel K. Precision-cut tissue slices: applications in pharmacology and toxicology. Life Sci. 1995;57(21):1887–1901. doi: 10.1016/0024-3205(95)02176-j. (PubMed PMID: 7475939) [DOI] [PubMed] [Google Scholar]
- 26.Parrish A.R., Sallam K., Nyman D.W., Orozco J., Cress A.E., Dalkin B.L., Nagle R.B., Gandolfi A.J. Culturing precision-cut human prostate slices as an in vitro model of prostate pathobiology. Cell Biol. Toxicol. 2002;18:205–219. doi: 10.1023/a:1015567805460. [DOI] [PubMed] [Google Scholar]
- 27.Kern M.A., Haugg A.M., Eiteneuer E., Konze E., Drebber U., Dienes H.P., Breuhahn K., Schirmacher P., Kasper H.U. Ex vivo analysis of antineoplastic agents in precision-cut tissue slices of human origin: effects of cyclooxygenase-2 inhibition in hepatocellular carcinoma. Liver Int. 2006;26(5):604–612. doi: 10.1111/j.1478-3231.2006.01268.x. (PubMed PMID: 16762006) [DOI] [PubMed] [Google Scholar]
- 28.Parajuli N., Doppler W. Precision-cut slice cultures of tumors from MMTV-neu mice for the study of the ex vivo response to cytokines and cytotoxic drugs. In Vitro Cell Dev. Biol. Anim. 2009;45(8):442–450. doi: 10.1007/s11626-009-9212-7. [DOI] [PubMed] [Google Scholar]
- 29.de Graaf I.A., Olinga P., de Jager M.H., Merema M.T., de Kanter R., van de Kerkhof E.G., Groothuis G.M. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 2010;5(9):1540–1551. doi: 10.1038/nprot.2010.111. [DOI] [PubMed] [Google Scholar]
- 30.Olinga P., van der Hoeven J.A., Merema M.T., Freund R.L., Ploeg R.J., Groothuis G.M. The influence of brain death on liver function. Liver Int. 2005;25(1):109–116. doi: 10.1111/j.1478-3231.2005.01035.x. (PubMed PMID: 15698407) [DOI] [PubMed] [Google Scholar]
- 31.Lee S.H., Culberson C., Korneszczuk K., Clemens M.G. Differential mechanisms of hepatic vascular dysregulation with mild vs. moderate ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294(5):G1219–26. doi: 10.1152/ajpgi.00527.2007. [DOI] [PubMed] [Google Scholar]
- 32.Stoff-Khalili M.A., Stoff A., Rivera A.A., Banerjee N.S., Everts M., Young S., Siegal G.P., Richter D.F., Wang M., Dall P., Mathis J.M., Zhu Z.B., Curiel D.T. Preclinical evaluation of transcriptional targeting strategies for carcinoma of the breast in a tissue slice model system. Breast Can. Res. 2005;7(6):R1141–R1152. doi: 10.1186/bcr1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann M., Armeanu S., Smirnow I., Kupka S., Wagner S., Wehrmann M., Rots M.G., Groothuis G.M., Weiss T.S., Königsrainer A., Gregor M., Bitzer M., Lauer U.M. Human precision-cut liver tumor slices as a tumor patient-individual predictive test system for oncolytic measles vaccine viruses. Int. J. Oncol. 2009;34(5):1247–1256. (PubMed PMID: 19360338) [PubMed] [Google Scholar]
- 34.Wang S., Rijk J.C.W., Riethoff-Poortman J.H., Van Kuijk S., Peijnenburg A.A.C.M., Bovee T.F.H. Bovine liver slices combined with an androgen transcriptional activation assay: an in-vitro model to study the metabolism and bioactivity of steroids. Anal. Bioanal. Chem. 2010;397(2):631–641. doi: 10.1007/s00216-010-3605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pushparajah D.S., Umachandran M., Plant K.E., Plant N., Ioannides C. Differential response of human and rat epoxide hydrolase to polycyclic aromatic hydrocarbon exposure: studies using precision-cut tissue slices. Mutat. Res. 2008;640(1–2):153–161. doi: 10.1016/j.mrfmmm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Pushparajah D.S., Umachandran M., Plant K.E., Plant N., Ioannides C. Up-regulation of the glutathione S-transferase system in human liver by polycyclic aromatic hydrocarbons; comparison with rat liver and lung. Mutagenesis. 2008;23(4):299–308. doi: 10.1093/mutage/gen012. [DOI] [PubMed] [Google Scholar]
- 37.Palamanda J.R., Kumari P., Murgolo N., Benbow L., Lin X., Nomeir A.A. Evaluation of CYP1A1 and CYP2B1/2 m-RNA induction in rat liver slices using the Nano String technology: a novel tool for drug discovery lead optimization. Drug Metab. Lett. 2009;3(3):171–175. doi: 10.2174/187231209789352094. (PubMed PMID: 19702544) [DOI] [PubMed] [Google Scholar]
- 38.Olinga P., Hof I.H., Merema M.T., Smit M., de Jager M.H., Swart P.J., Slooff M.J., Meijer D.K., Groothuis G.M. The applicability of rat and human liver slices to the study of mechanisms of hepatic drug uptake. J. Pharmacol. Toxicol. Methods. 2001;45(1):55–63. doi: 10.1016/s1056-8719(01)00127-7. (PubMed PMID: 11489665) [DOI] [PubMed] [Google Scholar]
- 39.Olinga P., Elferink M.G., Draaisma A.L., Merema M.T., Castell J.V., Pérez G., Groothuis G.M. Coordinated induction of drug transporters and phase I and II metabolism in human liver slices. Eur. J. Pharm. Sci. 2008;33(4–5):380–389. doi: 10.1016/j.ejps.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 40.van de Bovenkamp M., Groothuis G.M., Meijer D.K., Olinga P. a. Precision-cut fibrotic rat liver slices as a new model to test the effects of anti-fibrotic drugs in vitro. J. Hepatol. 2006;45(5):696–703. doi: 10.1016/j.jhep.2006.04.009. (PubMed PMID: 16828918) [DOI] [PubMed] [Google Scholar]
- 41.van de Bovenkamp M., Groothuis G.M., Meijer D.K., Slooff M.J., Olinga P. Human liver slices as an in vitro model to study toxicity-induced hepatic stellate cell activation in a multicellular milieu. Chem. Biol. Interact. 2006;162(1):62–69. doi: 10.1016/j.cbi.2006.05.006. (PubMed PMID: 16815347) [DOI] [PubMed] [Google Scholar]
- 42.van de Bovenkamp M., Groothuis G.M., Meijer D.K., Olinga P. Liver slices as a model to study fibrogenesis and test the effects of anti-fibrotic drugs on fibrogenic cells in human liver. Toxicol. In Vitro. 2008;22(3):771–778. doi: 10.1016/j.tiv.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Elferink M.G., Olinga P., Draaisma A.L., Merema M.T., Bauerschmidt S., Polman J., Schoonen W.G., Groothuis G.M. Microarray analysis in rat liver slices correctly predicts in vivo hepatotoxicity. Toxicol. Appl. Pharmacol. 2008;229(3):300–309. doi: 10.1016/j.taap.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 44.Plazar J., Hreljac I., Pirih P., Filipic M., Groothuis G.M. Detection of xenobiotic-induced DNA damage by the comet assay applied to human and rat precision-cut liver slices. Toxicol. In Vitro. 2007;21(6):1134–1142. doi: 10.1016/j.tiv.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Plazar J., Filipic M., Groothuis G.M. Antigenotoxic effect of Xanthohumol in rat liver slices. Toxicol. In Vitro. 2008;22(2):318–327. doi: 10.1016/j.tiv.2007.09.009. (PubMed PMID: 17981005) [DOI] [PubMed] [Google Scholar]
- 46.Wormser U., Ben Zakine S., Stivelband E., Eizen O., Nyska A. The liver slice system: a rapid in vitro acute toxicity test for primary screening of hepatotoxic agents. Toxicol. In Vitro. 1990;4(6):783–789. doi: 10.1016/0887-2333(90)90049-y. (PubMed PMID: 20702166) [DOI] [PubMed] [Google Scholar]
- 47.Niehaus W.G., Jr., Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968;6(1):126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. (PubMed PMID: 4387188) [DOI] [PubMed] [Google Scholar]
- 48.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. (PubMed PMID: 4215654) [DOI] [PubMed] [Google Scholar]
- 49.Beers R.F., Jr., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. (PubMed PMID: 14938361) [PubMed] [Google Scholar]
- 50.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. (PubMed PMID: 14907713) [PubMed] [Google Scholar]
- 51.Kono Y., Fridovich I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982;257(10):5751–5754. (PubMed PMID: 6279612) [PubMed] [Google Scholar]
- 52.Gao X.M., Jia F.X., Shen G.M., Jiang H.Q., Dou W., Wang J.J. Involvement of superoxide dismutase in oxidative stress in the oriental fruit fly, Bactrocera dorsalis:molecular cloning and expression profiles. Pest Manag. Sci. 2013;69:1315–1325. doi: 10.1002/ps.3503. [DOI] [PubMed] [Google Scholar]
- 53.Caetano A.C., da Veiga L.F., Capaldi F.R., de Alencar S.M., Azevedo R.A., Bezerra R.M.N. The antioxidant response of the liver of male Swiss mice raised on a AIN 93 or commercial diet. BMC Physiol. 2013;13:3. doi: 10.1186/1472-6793-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rai D.K., Rai P.K., Rizvi S.I., Watal G., Sharma B. Carbofuran-induced toxicity in rats: protective role of vitamin C. Exp. Toxicol. Pathol. 2009;61(6):531–535. doi: 10.1016/j.etp.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Costa C., Ozcagli E., Gangemi S., Schembri F., Giambò F., Androutsopoulos V., Tsatsakis A., Fenga C. Molecular biomarkers of oxidative stress and role of dietary factors in gasoline station attendants. Food Chem. Toxicol. 2016;90:30–35. doi: 10.1016/j.fct.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Sotiriou S., Gispert S., Cheng J., Wang Y., Chen A., Hoogstraten-Miller S., Miller G.F., Kwon O., Levine M., Guttentag S.H., Nussbaum R.L. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 2002;8(5):514–517. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- 57.Bendich A. Antioxidant micronutrients and immune responses. Ann. N. Y. Acad. Sci. 1990;587:168–180. doi: 10.1111/j.1749-6632.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 58.Bindhumol V., Chitra K.C., Mathur P.P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188:117–124. doi: 10.1016/s0300-483x(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 59.Padayatty S.J., Katz A., Wang Y., Eck P., Kwon O., Lee J.H., Chen S., Corpe C., Dutta A., Dutta S.K., Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. (PubMed PMID:12569111) [DOI] [PubMed] [Google Scholar]
- 60.Rendón-Ramírez A.L., Maldonado-Vega M., Quintanar-Escorza M.A., Hernández G., Arévalo-Rivas B.I., Zentella-Dehesa A., Calderón-Salinas J.V. Effect of vitamin E and C supplementation on oxidative damage and total antioxidant capacity in lead-exposed workers. Environ. Toxicol. Pharmacol. 2014;37(1):45–54. doi: 10.1016/j.etap.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Vincent H.K., Taylor A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. (Lond) 2006;30(3):400–418. doi: 10.1038/sj.ijo.0803177. (PubMed PMID: 16302012) [DOI] [PubMed] [Google Scholar]
- 62.Rai D.K., Sharma B. Carbofuran-induced oxidative stress in mammalian brain. Mol. Biotechnol. 2007;37(1):66–71. doi: 10.1007/s12033-007-0046-9. (PubMed PMID: 17914167) [DOI] [PubMed] [Google Scholar]
- 63.Buyukguzel K. Malathion-induced oxidative stress in a parasitoid wasp: effect on adult emergence, longevity, fecundity, and oxidative and antioxidative response of Pimpla turionellae (Hymenoptera:Ichneumonidae) J. Eco. Entom. 2006;99:1225–1234. [PubMed] [Google Scholar]
- 64.Yu Q.Y., Fang S.M., Zuo W.D., Dai F.Y., Zhang Z., Lu C. Effect of organophosphate phoxim exposure on certain oxidative stress biomarkers in the silkworm. J. Econ. Entomol. 2011;104(1):101–106. doi: 10.1603/ec10260. (PubMed PMID: 21404846) [DOI] [PubMed] [Google Scholar]
- 65.Montine T.J., Quinn J.F., Milatovic D., Silbert L.C., Dang T., Sanchez S., Terry E., Roberts L.J., Kaye J.A., Morrow J.D. Peripheral F2-isoprostanes and F4-neuroprostanes are not increased in Alzheimer's disease. Ann. Neurol. 2002;52(2):175–179. doi: 10.1002/ana.10272. (PubMed PMID: 12210787) [DOI] [PubMed] [Google Scholar]
- 66.Montine T.J., Neely M.D., Quinn J.F., Beal M.F., Markesbery W.R., Roberts L.J., Morrow J.D. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic. Biol. Med. 2002;33(5):620–626. doi: 10.1016/s0891-5849(02)00807-9. (PubMed PMID: 12208348) [DOI] [PubMed] [Google Scholar]
- 67.Porter N.A., Caldwell S.E., Mills K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30(4):277–290. doi: 10.1007/BF02536034. (PubMed PMID: 7609594) [DOI] [PubMed] [Google Scholar]
- 68.Milatovic D., Gupta R.C., Aschner M. Anticholinesterase toxicity and oxidative stress. Scient. W. J. 2006;6:295–310. doi: 10.1100/tsw.2006.38. (PubMed PMID:16518518.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ladurner A., Schmitt C.A., Schachner D., Atanasov A.G., Werner E.R., Dirsch V.M., Heiss E.H. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic. Biol. Med. 2012;52(10):2082–2090. doi: 10.1016/j.freeradbiomed.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orta B., Erkana M. Effects of vitamin c on antioxidant systems and Steroidogenic enzymes in sodium fluoride exposed Tm4 sertoli cells. Fluoride. 2014;47(2):139–151. [Google Scholar]