Graphical abstract
Keywords: Tetraodontiformes, Tetraodontidae, Edible puffer fishes, Heavy metals and proximate analysis
Highlights
-
•
The proximate composition and distribution of heavy metals in different edible marine puffer fishes were evaluated.
-
•
The results showed puffer fishes are rich in nutrients and high level of non-essential metals (Pb & Cd) than essential metals (Cu & Zn).
-
•
Multivariate statistical analyses accessed to understand the distribution pattern of metals in different organs of fishes.
-
•
This is the first report on the distribution of heavy metals and proximate analysis in marine edible puffer fishes from South India.
-
•
The concentration of toxic metals (Pb & Cd) in puffer fishes showed higher concentration than the WHO recommended values.
Abstract
In the present study, the heavy metal concentration in different organs (skin, tissue, liver, kidney, gill, intestine, and ovary) and muscle proximate composition were studied in marine edible puffer fishes Takifugu oblongus, Lagocephalus guentheri, Arothron hispidus, Chelonodon patoca and Arothron immaculatus collected from Mandapam fish landing centre, South east coast of India. Heavy metals (Cd, Cu, Pb & Zn) were analyzed in different organs for the above mentioned species. The heavy metals concentration ranges in fish organs of all the five species were Cu (0.42 –6.31 mg/kg), Cd (0.01–0.79 mg/kg), Pb (5.80–19.87 mg/kg), and Zn (6.75–65.08 mg/kg). Zn was detected higher in all the samples followed by Pb, Cu and Cd. The proximate composition was determined in edible muscle tissues of all the five species. The highest and lowest protein contents were observed in T. oblongus (20.6 ± 0.6%) and C. patoca (17.9 ± 0.3%). In the present study, heavy metal concentrations were found very high in all the internal organs when compared to muscle tissues. Further, this is the first report on distribution of heavy metals and proximate compositions of commercialized important edible puffer fishes from Mandapam coast of Gulf of Mannar, Southeast coast of India.
1. Introduction
Heavy metals are natural components in the earth's crust that cannot be degraded or destroyed. They are dangerous substances because of their bioaccumulation and toxicity can threaten aquatic living organisms [33]. The Industrial wastes and mining of metals are the potential sources of heavy metals accumulation in the aquatic environment [32]. Permatasari [51] stated that the metal could not be ignored from daily life as it is actively used in agriculture, medicine, and industry. Recent increase of industries and power plants in the Tuticorin and Mandapam coast of Gulf of Mannar region discharges the heavy metal contaminants [47]. The research must be focused on heavy metals because of their environmental promise, toxicity at low concentration and capability to incorporate into food chain of marine organism [25]. The extensive persistence of heavy metals infectivity in bioaccumulation and biomagnifications are a serious threat of the food chain [9] and these heavy metals will automatically transfer into the body while consumed these seafood by humans [19].
Fishes are widely used to monitor the variations in marine environment of anthropogenic pollutants [4]. Fishes, crabs and shrimps form an important link in transferring the media to humans. Information on the level of heavy metal pollution in coastal origin is important because they cause serious environmental health hazards [62]. The estimation of heavy metals in the food-chain will be used to know the heavy metal transfer to the human body through sea-food [6]. The possible ways of heavy metal accumulation in fishes are through the direct uptake of water and food on the heavy metal polluted environment [50]. The heavy metals entering to the fish through gills and other organs have a chance to get accumulated in different parts of the body tissues and the excessive amount can build up to a toxic level [6]. Puffer fishes (Family: Tetraodontidae, order: Tetraodontiformes), which are carnivorous, slow swimming fish and they can live in different ecosystem such as open sea, estuaries and freshwater areas [66]. Totally 19 genera including 130 species of puffer fishes were recorded in Indian waters [43] and 28 genera with 189 species in all over the world water were recorded within the Tetraodontidae family [45]. Around 50 species were reported as poisonous to humans for certain reasons [57]. Yedukondala and Rukminisirisha [68] stated that consumption of puffer fishes as an alternative food to meet the increasing food demand due to growing population and day-by-day collapse of natural resources. Hence, it is the time to study the proximate composition and to calculate the nutritional significance of the puffer fishes or else it can be exploited rationally.
Puffer fishes are non-target species and it is caught by incidentally or accidentally by trawl net and it is considered as trash fishes. Although these species are known to cause potential risk to humans, they contain potent and multifaceted neurotoxin called tetrodotoxin (TTX) that has been rarely detected in the muscles of fishes and it is also considered as a delicious food in few countries, particularly in China, Korea, Japan and Taiwan [38]. Chunfai & Hoifu [10] stated that puffer fishes contained rich amount of nutritional values in muscle. Recently, some of the Puffer fishes have been cultured due to high demand has been increased for human consumption [31]. In this present study, the bioaccumulations of heavy metals (Cd, Cu, Pb & Zn) in different organs of commercially important puffer fishes namely Takifugu oblongus, Lagocephalus guentheri, Arothron hispidus, Chelodan patoca and Arothron immaculatus and proximate composition of muscle tissues were analyzed and the permissible limit of these heavy metals via fish consumption were also discussed.
2. Materials and methods
2.1. Sample collection and preservation
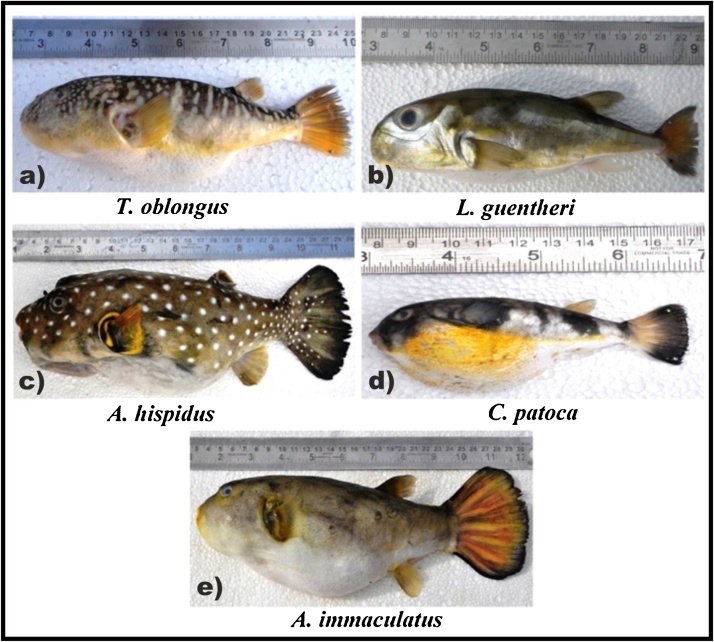
Five species of marine puffer fishes from Tetraodontidae family namely Takifugu oblongus, Lagocephalus guentheri, Arothron hispidus, Chelanodon patoca and Arothron immaculatus (Fig. 2a-e) were collected from Mandapam (latitude 9°16′14”N; longitude 79°7′10”E) fish landing centre, Gulf of Mannar Biosphere Reserve, Southeast coast of India during the period of March to June 2015 (Fig. 1). In total, 10 specimens of each species were collected and the samples were transported to the laboratory within 5–6 h in ice packed condition in a storage box. The specimen was thawed to room temperature for morphometric study and weight was examined for all the collected specimens. All the specimens were measured to the nearest mm, whereas weights were recorded with the use of electronic balance to the nearest 0.01 g and stored in −20°C for further heavy metal and proximate analysis. The specimens were identified using the standard works [43,http://www.fishbase.org/]
Fig. 2.
Plate showing edible Puffer fishes. a) Takifugu oblongus, b) Lagocephalus guentheri, c) Arothron hispidus, d) Chelonodon patoca and e) Arothron immaculatus.
Fig. 1.
Mandapam fish landing from Gulf of Mannar Marine Biosphere Reserve, South India.
2.2. Analysis of heavy metal concentration in edible marine puffer fishes
Throughout the study, all acids and chemicals used were analytical grade. For acid digestion, various parts namely, Skin, Tissue, Liver, Kidney, Gill, Intestine, and Ovary of fish samples were dissected using sterile stainless knife and scissor. One gram (wet weight) of the each sample were kept in a 100 ml glass beaker with 10 ml of mixed reagent (Con H2SO4: HNO3: HCLO4 ratio of 1:5:2) and heated on a hot plate at 60 °C until the tissue gets partial digestion, followed by 5 ml of 2N HCL was added to the samples and allowed few minutes for outright digest to reduce the volume at least 1 ml. Finally, digested suspension was filtered and made up to 25 ml with double distilled water and stored in acid cleaned fresh polypropylene containers at room temperature until analysis by slightly modified method of Olusola and Festus [46]. Blanks were also prepared using above procedure without adding fish samples. The samples were analyzed for Cd, Cu, Pb and Zn by Atomic Absorption Spectrophotometer (AA7000-Shimadzu, Japan) using air-acetylene lame with digital read out system, deuterium lamp background corrector, and automatic zero to compensate the blank. Settings were followed as recommended by the manufacturer. Calibrations using standard solutions were made by stepwise dilution of the stock solution. The absorption wavelength and detection limits were 228.8 nm for Cd, 324.7 nm for Cu, 217.0 nm for Pb and 213.9 nm for Zn. Quality assurance and Quality control testing was relied on the control of blanks and yield for chemical procedure. For precious quantification triplicates of the samples, blanks and standard reference were used throughout the analysis. The metal standards was obtained from Himedia laboratories, Mumbai as 1000 ppm and serially diluted with deionized water to make different concentration of metal standards (0.5, 1.0, 2.0 and 5 ppm) and they were run to check the accuracy of the instrument throughout the analysis. The metal concentrations were expressed as micrograms per gram wet weight of each specimen.
2.3. Multivariate statistical analysis
Multivariate statistical method is used to reduce the dimensionality of data set consisting of a large number of inter related variables. The PCA is a typical multivariate statistical method used to access various environmental studies, primarily for the identification of potent source of pollution neither natural nor anthropogenic activity [53], [40]. Cluster analysis was performed to elucidate similarities among metal concentration with respect to different tissues of fishes. Ward's method is applied to make hierarchical clustering. The classification of the samples into clusters is based on a visual observation of the dendrogram (SPSS 20 Windows). In the present study both PCA (PAST 3.0 Windows) and CA was used to identify the relationship between the metal concentrations in different part of fish tissues based on the correlation matrix.
2.4. Analysis of proximate compositions
The proximate analysis, skin and bone removed muscle tissues alone were manually homogenized. Then the sample was transferred to desiccators after the complete removal of moisture by using hot air oven for 24 h at 60 °C. Finally weighed sample were grinded using mortar and pestle for further biochemical analysis using standard methods. For moisture analysis, known quantities of fresh tissue were taken and excess moisture was removed using filter paper and dried and the final weight was calculated with% of pre weighed sample [54]. The total protein content was analyzed by Biuret method [35]. Carbohydrates were estimated according to Dubois et al. [15]. Total lipids were estimated by chloroform: methanol method as described by Folch et al. [22]. The ash content was determined in burning oven-dried sample by muffle furnace at 550°C [5].
3. Results and discussion
3.1. Phenotypic features of edible puffer fishes
The Phenotypic features are the valid method to identify the specimen with their systematic morphology. The level of bioaccumulation of metals depends on age, sex and size of the fishes. Among the five species of puffer fishes (Takifugu oblongus, Lagocephalus guentheri, Arothron hispidus, Chelonodon patoca and Arothron immaculatus), 10 speciemens from each species were used in this study (Table 1 & Fig. 2a–e)
Table 1.
Phenotypic features of 10 specimens of each puffer fish species.
| Parameters | Species name |
||||
|---|---|---|---|---|---|
| T. oblongus | A. hispidus | L. guentheri | C. patoca | A. immaculatus | |
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| Total length (cm) | 23.04 ± 12.28 | 19.06 ± 6.59 | 24.68 ± 3.45 | 23.40 ± 5.94 | 19.61 ± 5.78 |
| Standard length (cm) | 19.34 ± 10.29 | 15.81 ± 5.34 | 18.57 ± 2.96 | 17.71 ± 4.75 | 16.57 ± 5.19 |
| Head length (cm) | 4.96 ± 2.06 | 4.57 ± 1.98 | 4.40 ± 1.18 | 4.88 ± 1.30 | 4.31 ± 1.72 |
| Depth of body (cm) | 5.53 ± 2.53 | 5.40 ± 2.65 | 4.94 ± 0.83 | 6.41 ± 0.89 | 5.30 ± 2.17 |
| Weight (gm) | 252.60 ± 216.65 | 331.21 ± 69.64 | 252.60 ± 216.5 | 110.34 ± 6.95 | 104.62 ± 68.14 |
3.2. Heavy metals in puffer fishes
Puffer fishes are bottom living, carnivorous, slow swimmer and non-target fish species. Now-a-days, consumption of puffer fishes as an alternative food to meet out the increasing food demand due to growing population and day-by-day collapse of natural resources. The main reasons for the consumption of puffer fishes are due to easily available nature and low marker price. The concentrations of heavy metals Cu, Cd, Pb and Zn in various tissues of puffer fishes were showed in Fig. 3. As shown, the range and mean concentrations of the heavy metals in the fish tissue were Cu (0.42–6.31 mg/kg; 1.80), Cd (0.01–0.79 mg/kg; 0.24), Pb (5.80–19.87 mg/kg; 8.31), and Zn (6.75–65.08 mg/kg; 43.37) (range; mean) respectively. Among the samples, Zn was detected as higher, followed by Pb, Cu and Cd. The elevated level of metals found in kidney was 75% (Pb, Cu and Cd) while decreased concentration of Zn found in liver. Other organs like skin, gill, ovary, intestine and muscle also has considerable level of metals like kidney and liver. The concentration of Pb in various fish tissues are shown in the following order kidney > skin > intestine > liver > muscle > gill > ovary.
Fig. 3.
Heavy metals content of puffer fishes with reference to different organs. a) Takifugu oblongus, b) Lagocephalus guentheri, c) Arothron hispidus, d) Chelonodon patoca and e) Arothron immaculatus.
The average of Pb concentration in all the fishes was around 8.31 mg/kg and it was several order higher than the permissible limit recommended for human consumption according to FAO (Food and Agriculture Organization) and WHO (World Health Organization) (Table 4). Among the puffer fishes, high level of Pb was found in the kidney of C. patoca (19.87 mg/kg), whereas the lowest level (5.80 mg/kg) was observed in L. guentheri intestine. This study evidenced that the major accumulation of Pb was in kidney of the fishes [1]. Pb is a non-essential element for living organism and also it possess various adverse effects such as neuro and nephro toxicity, rapid behavioral malfunction, and decreases the growth, metabolism, and survival rate, alteration of social behavior in some mammals [23]. Rashed [55] found that elevated Pb level in fishes obtained from fresh water ecosystem affected by extended agriculture, poultry forms, textile, industrial and other activities. So the sediments could be the major sources of Pb contamination and the bottom feeders may directly affects with this deposited element in consequence to their feeding habitat [60]. From the literature survey of Papanikolaou et al. [49] the halflife of lead in blood (3–4 weeks) and soft tissues (40 days) have short period. It may use as a biomarker of resent lead contaminant on polluted environment and it cause longer chronic effect in children.
Table 4.
Standard levels in (1 g wet weight) of metals in fish described in literature and range of concentrations found in organs of commercial puffer fishes from Mandapam.
| Organization/country | Cd | Cu | Pb | Zn | Reference |
|---|---|---|---|---|---|
| European Community | 0.05 | – | 0.2 | – | EC [16] |
| England | 0.2 | 20 | 2.0 | – | MAFF [36] |
| FAO | – | 30 | 0.5 | 30 | FAO [20] |
| FAO/WHO limits | 0.5 | 30 | 0.5 | 150 | FAO/WHO [67] |
| Present study | 0.01–0.79 | 0.42–6.31 | 5.80–19.87 | 6.75–65.08 |
All tissue concentrations are in 1 g wet weight; (–) data were not available or variable was not studied.
EC- European country; MAFF- Ministry of Forestry and Fisheries; FAO − Food and Agriculture Organization; FAO/WHO − Food and Agriculture Organization/World Health Organization.
Fishes have the ability to accumulate Cd in various parts of the body tissues, especially in the kidney and liver, adjacent to other tissues like muscle and skin [17]. The concentration of Cd in various tissues of puffer fishes ranged from 0 (A. hispidus; skin) to 0.79 mg/kg (A. hispidus; kidney), and the order of metal concentration in organs are as follows kidney > intestine > liver > muscle > ovary > gill > skin. The mean concentration (0.24 mg/kg) of Cd was above the critical limit for human consumption suggested by FAO. The amount of cadmium in tissues of various shrimp species (0.05-0.10 mg/kg) is due to anthropogenic activities in the aquatic environment. The considerable variations in cadmium bioaccumulation may be the due to the difference in their feeding habits. Even cadmium concentrations of 5 and 10 g/L have harmful effects on reproduction, fertilization in aquatic animals [60], [29]. Nurjanah et al. [44] reported that the banana puffer fish from West Java having higher concentration of Cd in internal organs than skin and muscle. The cadmium concentration in previous reports were 0.06 ppm in Bighead carp and mangrove snapper, 0.02 ppm in Catfish, Grass carp and Mandarin fish from the Pearl River Delta (PRD), China [34]. Bluefish 0.014 ppm, Grey mullet 0.013 ppm, Mediterranean horse mackerel 0.008 ppm, Shad 0.009 ppm, Atlantic bonito 0.015 ppm, Sprat 0.005 ppm and Goby 0.008 ppm from black sea [37] are several folds lesser than the present study.
Gulf of Mannar region was recently contaminated by heavy metals with respect to various industrial activities along Arumuganeri, Tuticorin and Mandapam coast. Hence, Chloralkali plants, Sterlite copper, thermal power plants and pulp and paper manufacturing units release huge quantities of heavy metals containing effluents in the southern region of Gulf of Mannar [48], [64]. Cd and other metals like Hg, Pb did not show any significant biological activity towards living organism, even when it was toxic at tracer level [56] which damages the kidney, testicular and blood cells [24]. Higher concentration of Zn was found in liver of T. obalongus as 65.08 mg/kg while lowest in kidney of L. guentheri as 6.75 mg/kg. Zn concentration found in the study was within the permissible level [16], [66]. Zn is the essential element for both human and aquatic organisms and they showed productive activity against Cd and Pb toxicity in biological organisms [39]. Coetzee et al. [11] reported that kidney and gills of Clarias gariepinus and Labeo umbratus showed high degree of metal accumulating behavior and the present study was disagreed. Hence, it was confirmed that the metal accumulation behavior of fishes is depending not only the organs and species but also influenced by various environmental parameters.
The mean concentration of Cu in the tissue samples of fishes were varied between 0.42–6.31 mg/kg. The mean Cu concentration present in this study was exceeded several folds than the available literature [13] but not exceeding the permissible level recommended by WHO, FAO (Table 4) for human consumption. The highest concentration of Cu was recorded in kidney of A. immaculatus and lowest was found in skin of C. patoca. The highest concentration of Cu is mainly due to increased boating activities, recurrent usage of antifouling paint, oil dropping from boats and commercial fishing activity in the study area. Cu showed wild array of essential role in haemoglobin biosynthesis [63] and also it causes adverse effects of liver and kidney damage [28]. Concentration of Cu found in various literatures are as follows: Bighead carp (2.06 ppm), Mandarin fish (0.79 ppm) from Pearl River Delta (PRD), China [34], Channa striata (0.24 ppm), Catla catla (6.45 ppm), Oreochromis mossambicus (3.69 ppm), Etroplus suratensis (3.55 ppm), Mystus vittatus (1.80 ppm) and Cirrhinus mrigala (0.46 ppm) from Cauvery delta region, India [13].
The metals Cu, Pb and Cd exceeded the maximum limit recommended by international agencies because of the uncontrolled anthropogenic activities takes place in the study area. There are numerous literatures reported that the sediment and water from south east coast has elevated level of heavy metals when compared to permissible limit [3]. Developmental activities along the coastal area are the prime source for elevated level of heavy metals including large scale industries, thermal power plants, chemical and fertilizer industries, textile mills [59] and also attributed by municipal wastes, mining wastes, aquaculture and agricultural discharges [14]. The source for Zn is mainly influenced by effluents from the metal processing, paint and pigment industries, Cd from both point and non-point source [39], Cu from number of deteriorated boats in the boatyard [52], copper smelter industry, petrochemicals and Tuticorin port trust are the major source of metals like Pb, Cu and Zn [2].
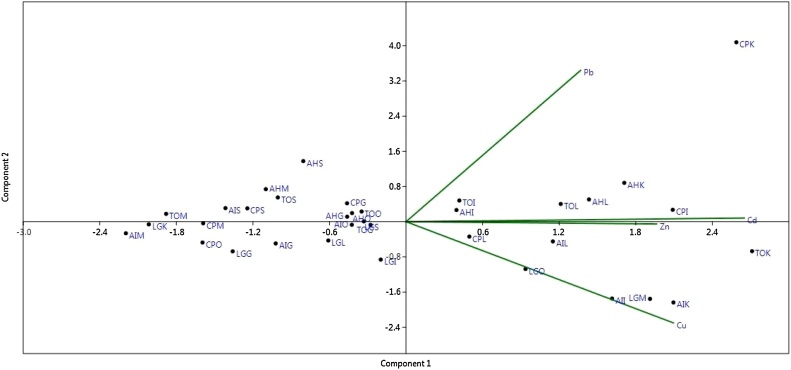
Principal components were extracted based on correlation matrix (Fig. 4). There are four PCs were extracted from available dataset with respect to Eigen values. The PC which has Eigen values greater than 0.6 was taken into the account. Among 4 PCs, PC1 (49.63%), PC2 (74.64%) and PC3 (93.03%), respectively and possessed Eigen value greater. PC1, PC2 and PC3 were strongly influenced with Cd, Pb and Zn respectively. Moreover, there was strong correlation of PC 2 with Pb, PC 3 with Zn and there was no correlation for PC4. The results suggested that source of PC 1 and PC 2 can be considered as anthropogenic inputs particularly from manmade activities in the study area (Table 2).
Fig. 4.
Biplot of PC1 and PC2 for heavy metal concentration in fish tissues. AI − Arothron immaculatus, AH − Arothron hispidus, TO − Takifugu oblongus, LG − Lagocephalus guentheri,CP − Chelonodon patoca/S- Skin, M- Muscle, O-Ovary, L-Liver, I- Intestine, G- Gill, K- Kidney.
Table 2.
Results of Principal Component Analysis (PCA).
| Metals | PC 1 | PC 2 | PC 3 | PC 4 |
|---|---|---|---|---|
| Zn | 0.47 | −0.01 | 0.87 | 0.12 |
| Pb | 0.33 | 0.83 | −0.22 | 0.39 |
| Cd | 0.64 | 0.02 | −0.25 | −0.73 |
| Cu | 0.51 | −0.56 | −0.36 | 0.55 |
| Eigenvalue | 1.96 | 1.02 | 0.73 | 0.29 |
| % variance | 48.98 | 25.61 | 18.24 | 7.17 |
| Cumulative | 49.63% | 74.64% | 93.03% | 100.00% |
Bolded values indicated that eigen values > 0.6.
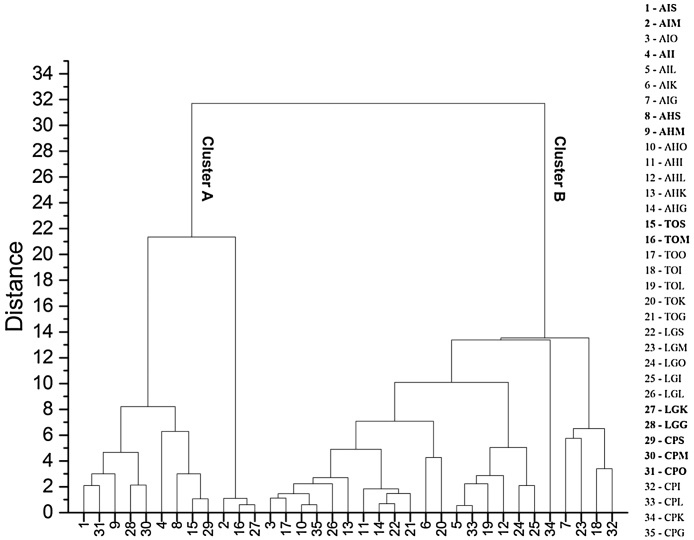
PC 3 can be considered as either anthropogenic or lithogenic source. The PCA results suggested that the concentration of heavy metals in the fish tissues was related to natural and manmade activities. Cluster analysis was performed to access the similarities in heavy metal concentration among different tissues. The generated dendrogram showed (Fig. 5) that the dataset has separated into two main clusters namely Cluster A and B. Cluster A consisted of 40% sub clusters with mostly muscles from 80% of fishes followed by skin, whereas cluster B consisted of 60% sub clusters with 90% of other internal organs like kidney, ovary, liver, intestine, gills and skin. Strong similarities found in most of the internal organs of fishes showed that the internal organs preferably having enriched heavy metals compared with muscle tissue. The dendrogram results also correlated with experimental data and also there was no similarity found in heavy metals concentration in fish muscle and internal organs.
Fig. 5.
Dendrogram of heavy metal concentration in different tissues of fishes. AI − Arothron immaculatus, AH − Arothron hispidus, TO − Takifugu oblongus, LG − Lagocephalus guentheri,CP − Chelonodon patoca/S- Skin, M- Muscle, O-Ovary, L-Liver, I- Intestine, G- Gill, K- Kidney.
3.3. Proximate compositions of puffer fishes
Proximate analysis includes the estimation of moisture content, protein, carbohydrates, lipid and ash in muscle tissues of five marine edible puffer fishes, A. hispidus, T. Oblongus, C. patoca, A. immaculatus and L. guentheri were presented in Table 3. Among these species, high amount of moisture content was recorded in A. immaculatus and lowest content was observed in T. oblongus. Aydın et al. [7] reported that highest and lowest values of moisture contents i.e. 78.47% and 76.48%, respectively, were found in marine puffer fish, Lagocephalus sceleratus. However, Eswar et al. [18] reported the range of moisture content from 80.32% to 86.05% of two puffer fishes Lagocephalus lunaris and L. inermis from Parangipettai, Southeast coast of India. The moisture level is beneficially influenced in locomotory stabilization of the organism.
Table 3.
Percentage of proximate compositions of muscles of puffer fishes.
| Proximate Composition (%) | ||||||
|---|---|---|---|---|---|---|
| Sl.No | Name of Species | Moisture | Protein | Carbohydrates | Lipid | Ash |
| 1 | Arothron hispidus | 70.4 ± 2.5 | 20.1 ± 0.4 | 1.3 ± 0.2 | 7.1 ± 0.9 | 1.1 ± 0.1 |
| 2 | Takifugu oblongus | 70.5 ± 2.6 | 20.6 ± 0.6 | 1.3 ± 0.2 | 6.3 ± 0.7 | 1.3 ± 0.1 |
| 3 | Chelonodon patoca | 73.5 ± 2.0 | 17.9 ± 0.3 | 0.9 ± 0.2 | 6.9 ± 0.7 | 0.8 ± 0.2 |
| 4 | Arothron immaculatus | 75.0 ± 3.0 | 18.3 ± 0.3 | 0.7 ± 0.3 | 4.9 ± 0.2 | 1.1 ± 0.2 |
| 5 | Lagocephalus guentheri | 71.1± 0.4 | 18.8 ± 0.4 | 1.3 ± 0.2 | 7.7 ± 0.4 | 1.1 ± 0.1 |
The highest protein content was found in T. oblongus (20.6 ± 0.6%) and the lowest in C. patoca (17.9 ± 0.3%). Nair and Mathew [41] stated that protein is the most essential biochemical part in fish meat presents between 15 and 21%, whereas in India the values ranging between 8% and 22% in several common fishes. Aydın et al. [7] estimated that higher concentration of protein found in L. sceleratus especially in September (21.62%) and July (19.30%) due to food and feeding habitats of the fish. Jakhar et al. [30] reported that the protein content of fishes in Rohu, Catla, Pangas and Magur were 9.53%, 10.11%, 13.60% and 14.87% respectively. They also discussed that this difference is due to ecological condition and availability of food source. Carbohydrates content were recorded in the range of 0.7 ± 0.3% to 1.3 ± 0.2%. .A. hispidus, T. oblongus and L. guentheri were having equally high carbohydrate content and the lowest in A. immaculatus. Azrina and Ismail [8] reported that 3.07% carbohydrate concentration in marine fish Fringescale sardinella from Peninsular Malaysia. Fapohunda and Ogunkoya [21] determined the carbohydrates level in fresh, dried and deteriorating fish sample of various species like Parachanna obscura 7.62%, 6.93%, 1.92%, Clarias gariepinus 11.89%, 10.25%, 17.35% and Tilapia zillii 4.72%, 6.93%, 16.19% respectively.
The above results were comparatively higher than the present study. Glycogen in marine animals may be the reason for low values of carbohydrates recorded in the present study and carbohydrates does not contribute much to the reserves in the body like lipids [12]. Nath et al. [42] stated, flexibility of lipid content was affected by external (environment, culturing method, tropic effects) and internal factors (feeding regime, life cycle, topographical origin and parts of muscle tissue). In the present study, the lipid content varies from 4.9% to 7.7%. The highest amount of lipid recorded in L. guentheri and lowest amount was observed in A. immaculatus. The lipid content of Lagocephalus lagocephalus in Mediterranean Sea was 0.78% [47] and cultured puffer fish Takifugu rubripes contain (0.84–0.96%) [31]. Hazra et al. [26] studied the influence of seasonal variation in lipid concentration of fish and recorded maximum on monsoon season (40.1–48.8%) in puffer fishes internal organs of Chelonodon patoca, Lagocephalus lunaris, L. inermis and Sphaeroides oblongus of Indian coastal waters. Saito and Kunisaki [58] reported that the lipid content was 0.7% in wild and 0.9% in cultured Takifugu rubripes. The above reports are comparatively lower than the present study. Eswar et al. [18] reported that the lipid content was very high in two marine puffer fishes L. lunaris and L. inermis (11.25% and 11.98%). Further, they reported that the territorial, primary and secondary producer may rational to lipid concentration. Suvitha et al. [65] has reported utmost elevation in lipid concentration variation in marine edible fishes (Sardinella longiceps and Plotosus lineatus) from 66.53 to 79.93 mg/g in reflection of territorial food and feeding habit. The recorded ash content in the current study was 1.1 ± 0.1%, 1.3 ± 0.1%, 0.8 ± 0.2%, 1.1 ± 0.2%, and 1.1 ± 0.1% (Table 3). The maximum was observed in T. oblongus and minimum in C. patoca. Eswar et al. [18] estimated ash content on L. lunaris and L. inermisas with 0.96% and 1.27% respectively. Holma et al. [27] was summoned ash content in four different fish samples like fresh fish (6.00%), traditionally smoked fish (6.07%), fried fish (30.00%), and salted red fish (6.00%). These divergences may be mainly linked with species size, habitat and/or seasonal circumstances. The overall differences in proximate compositions of puffer fishes may be depend on species type, seasonal changes, food availability and geographical location [7].
4. Conclusion
Fishes are the majour source of food utilized in wide spectrum. The long term assessment of heavy metal level in edible fish is important safety measure for fish consumer. Tuticorin is the main coastal and industrial city present in Gulf of mannar region. The heavy metal elucidation in consequence to the effluent discharge like increased industrialization, urbanization and aquaculture practices of anthropogenic activities [48], [64] along the Gulf of Mannar. There are plenty of reports are available about the heavy metals contamination in Gulf of Mannar in particular to water, sediment and biological samples. But there is no study on heavy metals contamination in puffer fishes. The present study reports the heavy metal accumulation in different organs of puffer fishes and proximate composition in muscle of the above fishes. The proximate composition was found to be high and nearby equal to other edible fishes. At the same time, the heavy metal accumulation is eventually higher than the permissible limit given by WHO/FAO. This study showed that the five species contain high level of heavy metal accumulation in all organs when compare to the muscles. The high accumulation of heavy metals in puffer fishes is due to its carnivorous feeding nature and bottom habitat. Even though the puffer fishes are non-target species, peoples are started to consume because of their huge quantity or extended by-catch, low market price and high nutrient value. The present study concluded that the long term consumption of these fishes may leads to potential risk to humans in future. So regular monitoring of marine resources is essential to improve the quality of sea-food against contaminants. However it may leads to establish the effect of increased bioaccumulation factor and the heavy metal tolerance of fish in further [61]. This is first report of heavy metal and proximate analysis on recently commercialized marine puffer fishes on Gulf of Mannar region and this finding may lead new insight for further research.
Acknowledgments
The authors would like to thank the authorities of Bharathidasan University for facilities provided. Authors also thank to University Grants Commission - Rajiv Gandhi National Fellowship, New Delhi, Government of India (F1-17.1/2016-17/RGNF-2015-17-SC-TAM-19298/(SA-III Website) for financial assistance through manpower. Authors also acknowledge the AAS facility availed through Ministry of Earth Science, New Delhi, Government of India (Ref. No.: MoES/36/OOIS/Extra./9/2013) funded scheme.
References
- 1.Abdel-Baki A.S., Dkhil M.A., Al-Quraishy S. Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of WadiHanifah Saudi Arabia. Afr. J. Biotechnol. 2011;10:2541–2547. [Google Scholar]
- 2.Abukashim E., Mohamed A.F., Omar A.A., Pragatheeswaran V., Yogeshkumar J.S. Distribution of heavy metal pollution in vaipar coastal sediments, southeast coast of India. Int. J. Adv. Res. 2014;2:886–891. [Google Scholar]
- 3.Ajeeshkumar K.K., Vishnu K.V., Kumari K.R., Navaneethan R., Asha K.K., Ganesan B., Suseela M. Biochemical composition and heavy metal content of selected marine fish from the gulf of mannar. India Fishery Technol. 2015;52 [Google Scholar]
- 4.Alemdaroglu T., Erkakan F. Trace metal levels In surfıcıal sedıments of lake manyas, Turkey and trıbutary Rıvers. Intern. J. Environ. Stud. 2003;60:287–298. [Google Scholar]
- 5.AOAC . 16th ed. AOAC International; 1995. Official Methods of Analysis of AOAC International. [Google Scholar]
- 6.Arun Kumar K. Hema Achyuthan, Heavy metal accumulation in certain marine animals along the East Coast of Chennai Tamil Nadu. India J. Env. Biol. 2005;28:637–643. [PubMed] [Google Scholar]
- 7.Aydın M., Tufan B., Sevgili H., Kose S. Seasonal changes in proximate composition and fatty acid profile of puffer fish (Lagocephalus sceleratus Gmelin, 1789) from the Mediterranean Sea of Turkey. J. Aquat. Food Prod. Technol. 2013;22:178–191. [Google Scholar]
- 8.Azrina A., Ismail A. Proximate composition and energetic value of selected marine fish and shellfish from the West coast of Peninsular Malaysia. Int. Food. Res. J. 2011;18:137–148. [Google Scholar]
- 9.Chourpagar A.R., Kulkarn G.K. Heavy metal toxicity to a freshwater crab, Barytelphus acunicularis (westwood) from Aurangabad region. Rec. Res. Sci. Tech. 2011;3:1–5. [Google Scholar]
- 10.Chunfai Yu., Peter HoifuYu. A preliminary study of puffer fishes and their toxins found in Hong Kong waters. J. Food. Hyg. Soc. (Japan) 1997;38:460–463. [Google Scholar]
- 11.Coetzee L., du Preez H.H., Vuren J.H.J. Metal concentrations in Clariasgariepinus and Labeoumbratus from the Olifants and Klein Olifants River, Mpumalanga, South Africa: zinc, copper, manganese,lead, chromium, nickel, aluminium and iron. Water S.A. 2002;28:433–448. [Google Scholar]
- 12.Das A.V.S., Sahu B.K. Biochemical composition and calorific content of fishes and shellfishes from Rushikulya estuary, south Orissa coast of India. Indian. J. Fish. 2001;48:297–302. [Google Scholar]
- 13.Dhanakumar S., Solaraj G., Mohanraj R. Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region India, Ecotoxicol. Environ. Saf. 2015;113:145–151. doi: 10.1016/j.ecoenv.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Dhinesh P., Rajaram R., Mathivanan K., Vinothkumar S., Ramalingam V. Heavy metal concentration in water and sediment samples of highly polluted cuddalore coast, southeastern Iindia. Int. J. Curr. Res. 2014;6:8692–8700. [Google Scholar]
- 15.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal.Chem. 1956;28:350–356. [Google Scholar]
- 16.EC. (European Commission).Commission of Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs.L 364/5, 20.12.2006.
- 17.El-Nemr A. Concentrations of certain heavy metals in imported frozen fish in Egypt. Egpyt. J. Aquat. Biol. Fish. 2003;7:139–154. [Google Scholar]
- 18.Eswar A., Kathirvel K., Anbarasu R., Ramamoorthy K., Sankar G., Suvitha S., Manikandarajan T. Proximate composition and fatty acid analysis of puffer fish, lagocephalus inermis (Temminck and schlegel, 1850) and lagocephalus lunaris (Bloch and schneider, 1801) from parangipettai, southeast coast of India. ILNS. 2014;12:21–29. [Google Scholar]
- 19.Ezejiofor T.I.N., Udebuani A.C., Ezeji E.U., Ayalogbu E.A., Azuwuike C.O., Ezejiofor A.N., Ngwogu K.O. Environmental metals pollutants load of a densely populated and heavily industrialized commercial city of Aba Nigeria. J. Toxicol. Environ. Health. Sci. 2013;5:1–11. [Google Scholar]
- 20.FAO . Food and Agriculture Organization; 1983. Compilation of Legal Limits for Hazardous Substances in Fish and Fishery Products. FAO Fishery Circular No. 464; p. 5e100. [Google Scholar]
- 21.Fapohunda O.O., Ogunkoya M. Effect of smoke-drying on the proximate composition of Tilapia zillii, Parachanna obscura and Clarias gariepinus obtained from Akure, Ondo-State, Nigeria. Anim. Res. Int. 2006;3 [Google Scholar]
- 22.Folch J., Lees M., Sloane-Stanley G.H.A. Simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1956;226:497–509. [PubMed] [Google Scholar]
- 23.Garcia-Leston J., Mendez J., Pasaro E., Laffon B. Genotoxic effects of lead: an updated review. Environ. Int. 2010;36:623–636. doi: 10.1016/j.envint.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Gupta B.N., Mathur A.K. Toxicity of heavy metals. Ind. J. Med. Sci. 1983;37:236–240. [PubMed] [Google Scholar]
- 25.Harte J., Holdren C., Schneider R., Shirley C. University of California Press; Oxford England: 1991. Toxics A to Z A Guide to Everyday Pollution Hazards. [Google Scholar]
- 26.Hazra A.K., Ghosh S., Banerjee S., Mukherjee B. Studies on lipid and fatty acid compositions of puffer livers from Indian coastal waters with seasonal variation. J. Am. Oil Chem. Soc. 1998;75:1673–1678. [Google Scholar]
- 27.Holma K.A., Maalekuu B.K. Effect of traditional fish processing methods on the proximate composition of red fish stored under ambient room conditions. Am. J. Food. Nutr. 2013;3:73–82. [Google Scholar]
- 28.Ikem A., Egiebor N.O. Assessment of trace elements in canned fishes (mackerel tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America) J. Food. Comp. Anal. 2005;18:771–787. [Google Scholar]
- 29.Acosta Izani Bonel, Sergio Varela Antonio, Junior, Fernandes e Silva Estela, Figueiredo Cardoso Tainã, Caldas Jôsie Schwartz, Jardim Rodrigo Desessards, Corcini Carine Dahl. Effects of exposure to cadmium in sperm cells of zebrafish, Danio rerio. Toxicol. Rep. 2016;3:696–700. doi: 10.1016/j.toxrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakhar K.A., Pal A.K., Reddy D.A., Sahu N.P., Venkateshwarlu G., Vardia H.K. Fatty acids composition of some selected Indian fishes. Afr. J. Basic. Appl. Sci. 2012;4:155–160. [Google Scholar]
- 31.Koizumi K., Hiratsuka S. Fatty acid compositions in muscles of wild and cultured ocellate puffer Takifugurubripes. Fish. Sci. 2009;75:1323–1328. [Google Scholar]
- 32.Lee Y.H., Stuebing R.B. Heavy metal contamination in the River Toad Bufojuxtasper (Inger), near a copper mine in East Malaysia. Bull. Environ. Contam. Toxicol. 1990;45:272–279. doi: 10.1007/BF01700195. [DOI] [PubMed] [Google Scholar]
- 33.Lenntech Water Treatment and Air Purification . Lenntech; Rotterdamseweg Netherlands: 2004. Water Treatment. (www.excelwater.com/thp/filters/Water-Purification.htm) [Google Scholar]
- 34.Leung H.M., Leung A.O.W., Wang H.S., Ma K.K., Liang Y., Ho K.C., Cheung K.C., Tohidi F., Yung K.K.L. Assessment of heavy metals/metalloid (As, Pb Cd, Ni, Zn, Cr, Cu, Mn) concentrations in edible fish species tissue in the pearl river delta (PRD), China. Mar. Pollut. Bull. 2014;78:235–245. doi: 10.1016/j.marpolbul.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.MAFF (Ministry of Agriculture, Fisheries and Food) Aquatic Environment Monitoring Report No. 52. Center for Environment, Fisheries and Aquaculture Science; Lowestoft, UK: 2000. Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea, 1997. [Google Scholar]
- 37.Makedonski L., Peycheva K., Stancheva M. Determination of some heavy metal of selected black sea fish species. Food Control. 2015;72:313–318. [Google Scholar]
- 38.Makoto O., Yoshimichi F., Fumio T., Shingo I. Fatty acid composition of total lipids in puffer fish meat. Food. Preservation Sci. 2000;26:333–338. [Google Scholar]
- 39.Malik N., Biswas A.K., Qureshi T.A., Borana K., Virha R. Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environ. Monit. Assess. 2010;160:267. doi: 10.1007/s10661-008-0693-8. (267) [DOI] [PubMed] [Google Scholar]
- 40.Mathivanan K., Rajaram R. Anthropogenic influences on toxic metals in water and sediment samples collected from industrially polluted Cuddalore coast, Southeast coast of India. Environ. Earth. Sci. 2014;72:997–1010. [Google Scholar]
- 41.Nair P.V., Mathew S. CIFT; Cochin: 2000. Biochemical Composition of Fish and Shellfish.CIFT-Technology Advisory Services; pp. 281–289. [Google Scholar]
- 42.Nath A.K., Patra A., Sen B., Dey D., Das I., Mukherjee I., Paul S. ;1 Fatty acid compositions of four edible fishes of Hooghly Estuary, West Bengal. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:208–218. [Google Scholar]
- 43.Nelson J.S. Family tetraodontidae (509)-puffers. In: Nelson J.S., editor. Fishes of the World. John Wiley and Sons; Hoboken, New Jersey: 2006. pp. 456–457. [Google Scholar]
- 44.Nurjanah N., Jacoeb M., Asren S., Hidayat T. Minerals and heavy metals of banana puffer fish from sea of region gebang, cirebon west java. J. Agric. Sci. Eng. 2015;1:28–33. [Google Scholar]
- 45.Oliveira J.S.1, Fernandes S.C., Schwartz C.A., Bloch C. Jr., Melo J.A., Rodrigues Pires O. Jr., de Freitas J.C. Toxicity and toxin identification in Colomesusasellus, an Amazonian (Brazil) freshwater puffer fish. Toxicon. 2006;48(2006):55–63. doi: 10.1016/j.toxicon.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Olusola J.O., Festus A.A. Assessment of heavy metals in some marine fish species relevant to their concentration in water and sediment from coastal waters of ondo state, Nigeria. J Marine. Sci. Res. Dev. 2015;5:163. [Google Scholar]
- 47.Ozogul Y., Özogul F.H., Çiçek E., Polat A. E., Kuley Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int. J. Food. Sci. Nutr. 2009;60:464–475. doi: 10.1080/09637480701838175. [DOI] [PubMed] [Google Scholar]
- 48.Palanichamy S., Rajendran A. Heavy metal concentrations in seawater and sediments of Gulf of Mannar and Palk Bay, Southeast coast of India. Indian J. Mar Sci. 2000;29:116–119. [Google Scholar]
- 49.Papanikolaou N.C., Hatzidaki E.G., Belivanis S., Tzanakakis G.N., Tsatsakis A.M. Lead toxicity update. Brief Rev. Med. Sci. Monit. 2005;10:329–336. [PubMed] [Google Scholar]
- 50.Paquin R.R., Farley K., Santore R.C., Kavvadas C.D., Mooney K.G., Winfield R.P., Wu K.B., Di Toro D.M. SETAC; 2003. Metals in Aquatic Systems: a Review of Exposure, Bioaccumulation, and Toxicity Models; pp. 61–90. [Google Scholar]
- 51.Permatasari S.I. Departemen Kimia, Fakultas Matematikadan Ilmu Pengetahuan Alam, Institut (Pertanian Bogor); Bogor (ID): 2006. Penentuan K and unganLogam Hg dan AspadaIk andengan Metode Analisis Pengaktifan Neutron [skripsi] [Google Scholar]
- 52.Rajaram R., SumithaBanu J., Mathivanan K. Biosorption of Cu (II) ions by indigenous copper-resistant bacteria isolated from polluted coastal environment. Toxicol. Environ. Chem. 2013;95:590–604. [Google Scholar]
- 53.Rajaram R., Ganeshkumar A., Vinothkumar S., Rameshkumar S. Multivariate statistical and GIS-based approaches for toxic metals in tropical mangrove ecosystem, Southeast coast of India. Environ. Monit. Asses. 2017 doi: 10.1007/s10661-017-5980-9. [DOI] [PubMed] [Google Scholar]
- 54.Rajendran M. Vol. 1. Madurai Kamaraj University; India: 1973. pp. 1–86. (A Guide to the Study of 56 Fresh Water Calanoids). [Google Scholar]
- 55.Rashed M.N. Monitoring of environmental heavy metals in fish from Nasser Lake. Environ. Int. 2001;27:27–33. doi: 10.1016/s0160-4120(01)00050-2. [DOI] [PubMed] [Google Scholar]
- 56.Robert G. Casarett and Doull’stoxicology. Pergamon Press; 1991. Toxic effects of metals; pp. 662–672. [Google Scholar]
- 57.Russell F.E. Marine toxins and venomous and poisonous marine animals. Adv. Mar. Biol. 1965;3:255–384. [Google Scholar]
- 58.Saito M., Kunisaki N. Proximate composition fatty acid composition, free amino acid contents, mineral contents, and hardness of muscle from wild and cultured puffer fish Takifugurubripes. Bull. Jpn. Soc. Sci. Fish. 1998;64:116–120. [Google Scholar]
- 59.Sankar R., Ramkumar L., Rajkumar M., Sun J., Ananthan G. Seasonal variations in physico-chemical parameters and heavy metals in water and sediments of Uppanar estuary Nagapattinam. India J. Environ. Biol. 2010;31:681–686. [PubMed] [Google Scholar]
- 60.Sarkar T., Masihul Alam M., Parvin N., Fardous Z., Alamgir Z.Chowdhury, Hossain S., Haque M.E., Biswas N. Assessment of heavy metals contamination and human health risk in shrimp collected from different farms and rivers at Khulna-Satkhira region, Bangladesh. Toxicol. Rep. 2016;3:346–350. doi: 10.1016/j.toxrep.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sfakianakis D.G., Renieri E., Kentouri M., Tsatsakis A.M. Effect of heavy metals on fish larvae deformities: a review. Environ. Res. 2015;137:246–255. doi: 10.1016/j.envres.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 62.Shukla V., Rathi P., Sastry K.V. Effect of cadmium individually and in combination with other metals on the nutritive value of fresh water fish, Channa punctatus. J. Environ. Biol. 2007;23:105–110. [PubMed] [Google Scholar]
- 63.Sivaperumal P., Sankar T.V., Nair P.V. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food. Chem. 2007;102:612–620. [Google Scholar]
- 64.Sulochanan B., Krishnakumar P.K., Prema D., Kaladharan P., Valsala K.K., Bhat G., Muniyandi S.K. Trace metal contamination of the marine environment in Palk Bay and Gulf of Mannar. J. Mar. Biol. Assoc. India. 2007;49:12–18. [Google Scholar]
- 65.Suvitha S., Eswar A., Anbarasu R., Ramamoorthy K., Sankar G. Proximate Amino acid and Fatty acid profile of selected two Marine fish from Parangipettai Coast. Asian J. Biomed. Pharm. Sci. 2015;5:38. [Google Scholar]
- 66.Wahyuni Sulistiono T., Affandi R. Kebiasaan makanan ikan buntal pisang (Tetraodon lunaris) di Perairan Mayang. Jawa Barat. Jurnal Iktiologi Indonesia. 2004;4:25–30. [Google Scholar]
- 67.WHO/FAO (World Health Organization/Food and Agriculture Organization of the United Nations); Geneva, Switzerland: 1989. Heavy Metals −Environmental Aspects. Environment Health Criteria.No.85. [Google Scholar]
- 68.Yedukondala P., Rukminisirisha I. Changes in the muscle biochemical composition of Lagocephalus spadiceus (Richardson, 845) and Lagocephalus lunaris (Bloch and schneider 1801) off visakhapatnam, east coast of India. Int. J. Sci. Res. Publ. 2013;3:2250–3153. [Google Scholar]