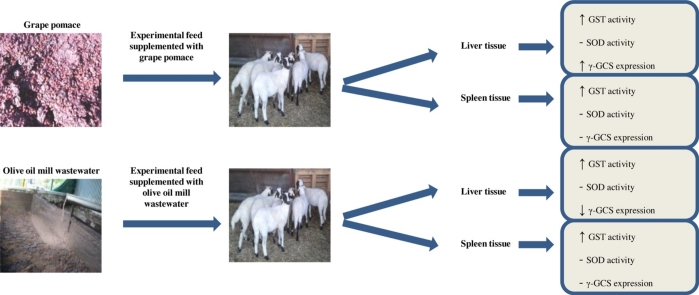
Graphical abstract
Abbreviations: AREs, antioxidant response elements; CDNB, 1 chloro-2,4-dinitrobenzene; DETAPAC, diethylenetriaminepentaacetic acid; DTT, dithiothreitol; GAPDH, glyceraldehyde 3-phosphatedehydrogenase; γ-GCS, γ-synthase glutamyl cysteine; GP, grape pomace; GS, glutathione synthase; GSH, glutathione; GST, glutathione-s-transferase; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; Keap1, kelch-like ECH-associated protein 1; KCl, potassium chloride; KOH, potassium hydroxide; MgCl2, magnesium chloride; NaCl, sodium chloride; NBT, nitroblue tetrazolium; Nrf2, nuclear factor-like 2; OMW, olive oil mill wastewater; PVDF, polyvinylidene difluoride membranes; ROS, reactive oxygen species; SDS, sodium dodecyl sulfate; SOD, superoxide dismutase; XO, xanthine oxidase
Keywords: Grape pomace (GP), Olive oil mill wastewater (OMW), Glutathione-s-transferase (GST), Superoxide dismutase (SOD), γ-synthase glutamyl custeine (γ-GCS)
Highlights
-
•
Feed supplemented with GP or OMW increased GST activity in liver and spleen tissue.
-
•
Feed supplemented with GP increased γ-GCS expression in liver tissue.
-
•
Dietary administration with OMW decreased γ-GCS expression in liver tissue.
-
•
Experimental diet with GP or OMW did not affect SOD activity in both tissues.
-
•
The beneficial effects of the feeds were tissue- and developmental stage-specific.
Abstract
The aim of the present study was to investigate the effects of livestock feed supplemented with grape pomace (GP) or olive oil mill wastewater (OMW) byproducts on the enzymatic activity and protein expression of antioxidants enzymes, in liver and spleen tissue of sheep. Thus, 36 male sheep of Chios breed were divided into 3 homogeneous groups, control group (n = 12), GP group (n = 12) and OMW group (n = 12), receiving standard or experimental feed. Liver and spleen tissues were collected at 42 and 70 days post-birth. The enzymatic activity of superoxide dismutase (SOD) and glutathione-s-transferase (GST) and also the protein expression of γ-synthase glutamyl custeine (γ-GCS) were determined in these tissues. The results showed GP group exhibited increased enzymatic activity of GST and protein expression of γ-GCS in liver compared to control group. In GP group’s spleen, GST activity was increased compared to control but γ-GCS expression was not affected. In OMW group’s liver, GST activity was increased and γ-GCS expression was reduced compared to control. In OMW group’s spleen, GST activity was increased but GCS expression was not affected. SOD activity was not affected in both tissues either in GP or OMW group.
1. Introduction
Oxidative stress is characterized as “the disturbance between prooxidants and antioxidants in favor of the formeivr”. Oxidate stress occurs when the production of free radicals in a system exceeds its antioxidant defence mechanisms [1]. Free radicals can damage cellular biomolecules such as proteins, lipids and DNA and eventually lead to a number of diseases [2], [3]. Oxidative stress has been involved in several diseases including atherosclerosis, cancer, diabetes, rheumatoid arthritis, Parkinson's disease, Alzheimer's disease and Huntington's disease [4], [5].
In veterinary medicine and especially in ruminant health, oxidative stress is an active field of research. A number of factors such as inflammation, dietary imbalances, heat stress, high metabolic load, respiratory diseases and parasites can lead to the formation of free radicals [6]. Recent studies have showed that young animals due to stress factors (weaning stress) have reduced antioxidant mechanisms compared to adults [7]. For example, weaning is a stressful situation for pigs, causing pathological conditions (gastrointestinal disorders) that are directly associated with reduced antioxidant mechanisms in that period [8], [9]. In ruminant animals, oxidative stress is involved in a number of pathological conditions including sepsis, mastitis, enteritis, pneumonia, respiratory and joint diseases [10]. Animal diseases, decrease livestock production and are an important barrier to improved productivity and poverty reduction. According to the World Organisation for Animal Health, morbidness and lethality due to animal diseases cause the loss of at least 20% of livestock production worldwide. In order to enhance animal's antioxidant defense system and prevent oxidative stress, antioxidant supplementation through nutrition is an alternative prevention.
There is a great interest for natural sources of antioxidants in order to enhance the antioxidant defense and prevent the harmful effects of oxidative stress. In recent years, a large number of surveys have directed in the study of bioactive compounds that are present in foods and possess properties which give them the ability to protect against chronic diseases [11], [12]. Polyphenols are the main bioactive phytochemical compounds in foods and the most studied for its biological activities [11]. They are found in fruits, cereals, vegetables and beverages [13], [14]. Polyphenols are classified into four categories: phenolic acids, flavonoids, stilbenes and lignans. Polyphenols can act as antioxidants, by scavenging free radicals and disrupting oxidative reactions, protect from oxidative damage and reduce the danger of diseases associated with oxidative stress [13]. Diets rich in polyphenols protect against a number of diseases such as osteoporosis, cancer, diabetes, cardiovascular and neurodegenerative diseases [15], [16], [17].
In the present study we examined the effects of wine-making and olive mill waste (OMW) by-products, as additives in the livestock feeding, on the levels of antioxidant enzymes. The main by-product of winemaking is grape pomace. Grape pomace is the residue left after pressing grapes during the procedure of wine-making. During wine-making a high amount of the phenolic content of the grapes remains in the by-products and especially in grape pomace [18]. The phenolic compounds of grape pomace include gallic acid, coumaric acid, catechin, epicatechin, epicatechin-3-O-gallate, tannins and anthocyanins [19]. Based on the polyphenol content, many studies in vitro and in vivo have highlighted the antioxidant effect of this by-product, suggesting grape pomace as a natural source of antioxidants [20], [21], [22], [23], [24], [25]. The OMW by-products are produced mainly from the water that is used during the various stages of oil production. Oleuropein, tyrosol and hydroxytyrosol are the main phenolic compounds found in OMW. Other phenolic compounds that are found in olive oil are caffeic acid, vanillic acid, coumaric acid, ferulic acid, gallic acid, hydroxybenzoic acid, kaempherol, apigenin and quercetin [26]. Various studies in animals and humans have shown that polyphenol components of OMW exhibit important biological activities that can prevent oxidative stress associated diseases [27]. Our research group found that feed supplemented with byproducts from OMW increased antioxidant capacity in blood and tissues of piglets and broiler chickens [28], [29]. Furthermore, the harnessing of GP and OMW byproducts contributes to reduced environmental pollution, as they are pollutants with high organic load.
Thus, the aim of the present study was to assess the effects of feed supplemented with GP or OMW byproducts on the enzymatic activity and protein expression of antioxidant enzymes in sheep’s liver and spleen tissue. This was made within the context of our effort to unravel the effects of feeds fortified with antioxidants on enzymes playing a key role in tissues’ redox capacity.
2. Materials and methods
2.1. Winery by-product
Red grape pomace (Vitis vinifera L. var. Moschato) was obtained from a winery in Tyrnavos (Larissa, Greece) in September 2014.
2.2. Olive oil mill waste (OMW)
Liquid olive mill wastes were obtained from a local Olive Oil Mill in Larissa perfecture (Greece) in September 2014.
2.3. Silage preparation
GP and OMW were added in the livestock feed of growing sheep as corn silage. Based on a previous study, the proportion of the ingredients was such that the final silage contained 60% solids and 40% liquids [29]. In particular, for GP silage, corn was made containing 51% solids, 9% GP and 40% water. For the OMW silage, corn was made containing 52.5% solids, 7.5% OMW and 20% water. Then, standard commercial formulation of lactic acid bacteria was used for the lactic fermentation of corn and the preparation of corn silage (11CFT Pioneer, Buxtehude, Germany). The lactic acid bacteria had been dissolved in water (10% w/v) by stirring and warmed to 40 °C in order to be activated prior to their mixing with corn. After activation, lactic acid bacteria were mixed with corn (1 g of bacteria with 100 kg of corn). The resulting silage was placed into vacuum bags and just before feed administration was mixed with other ingredients for making the final sheep’s feed.
2.4. Animals and diets
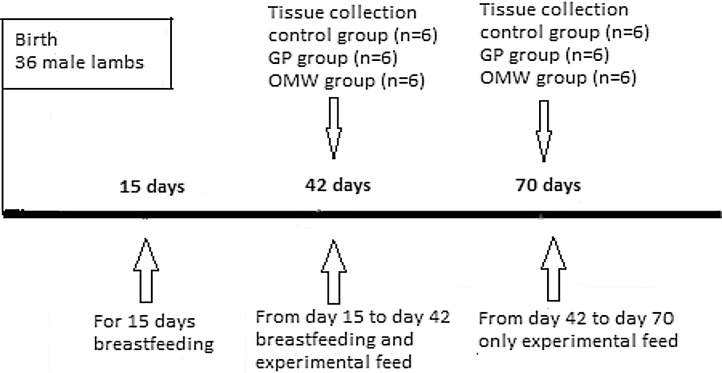
Thirty six male sheep of Chios breed were selected from the flock of ‘Research Institute of Animal Science’ (Paralimni Giannitson, Greece/Hellenic Agricultural Organization − Demeter). When the feeding started at 15 day after birth, the lambs weighed on average 7.99 ± 1.80 kg and were divided into three homogeneous groups [control group (n = 12), GP group (n = 12) and OMW group (n = 12)]. During the age of weaning (from 15 to 42 day), sheep remained in three separated stalls (one for each group) along with the ewes for breastfeeding and also had access to feed (either to standard or to experimental ration), alfalfa hay and water for consumption ad libitum. The control group was fed with standard ration, while the GP group was fed with ration containing silage with polyphenolic additives from red grape pomace and the OMW group was fed with ration containing silage with additives from OMW (Table 1). After weaning, sheep were separated from the ewes and were fed with experimental feed and alfalfa hay for twenty eight days (from 42 to 70 days after birth) (Table 2) (Fig. 1). All procedures were in accordance with the European Union guidelines for the care and use of laboratory animals and with the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Table 1.
Composition (% w/w) of experimental diets before weaning.
| Ingredients | Control group | GP group | OMW group |
|---|---|---|---|
| Corn | 45.0a | – | – |
| Silage | – | 45.0b | 45.0c |
| Wheat bran | 9.0 | 9.0 | 9.0 |
| Soybean meal 44%CP | 21.0 | 21.0 | 21.0 |
| Milk replacer | 20.0 | 20.0 | 20.0 |
| Vitamin and mineral premix (2.5%) | 2.5 | 2.5 | 2.5 |
| Salt | 0.5 | 0.5 | 0.5 |
| Limestone | 1.2 | 1.2 | 1.2 |
| Monocalcium Phosphate | 0.8 | 0.8 | 0.8 |
GP=grape pomace; OMW=olive oil mill wastewater.
Corn contains 60% solids and 40% water in control feed.
GP silage contains 51% solids, 9% grape pomace and 40% water.
OMW silage contains 52.5% solids, 7.5% olive oil mill wastewater and 40% water.
Table 2.
Composition (% w/w) of experimental diets after weaning.
| Ingredients | Control group | GP group | OMW group |
|---|---|---|---|
| Corn | 45.0a | – | – |
| Silage | – | 45.0b | 45.0c |
| Wheat bran | 15.0 | 15.0 | 15.0 |
| Wheat meal | 13.0 | 13.0 | 13.0 |
| Soybean meal 44%CP | 18.0 | 18.0 | 18.0 |
| Milk replacer | 2.5 | 2.5 | 2.5 |
| Vitamin and mineral premix (2.5%) | 4.0 | 4.0 | 4.0 |
| Salt | 0.5 | 0.5 | 0.5 |
| Limestone | 1.2 | 1.2 | 1.2 |
| Monocalcium Phosphate | 0.8 | 0.8 | 0.8 |
GP = grape pomace; OMW = olive oil mill wastewater.
Corn contains 60% solids and 40% water in control feed.
GP silage contains 51% solids, 9% grape pomace and 40% water.
OMW silage contains 52.5% solids, 7.5% OMW and 40% water.
Fig. 1.
Experimental design to study the effects of livestock feed supplemented with grape pomace (GP) and olive oil mill wastewater (OMW) byproducts on the enzymatic activity of superoxide dismutase (SOD) and glutathione-s-transferase (GST) and on the protein expression of γ-synthase glutamyl cysteine (γ-GCS) in liver and spleen tissue of sheep. The downwards arrows show the timepoints of tissue collection. The upwards arrows show sheep's nutrition.
2.5. Tissue collection
Tissues were collected 42 and 70 days post-birth. For tissue collection, sheep were transported to a slaughterhouse and were immediately stunned prior to slaughtering in order to avoid suffering. All relevant procedures (e.g slaughter, bleeding, gutting, viscera separation and washing) were executed by specialized staff according to industry-accepted procedures. Tissues from liver and spleen were removed as quickly as possible. After cutting the tissues into small pieces with scissors, they were placed in tubes, snap-frozen in liquid nitrogen and storaged at −80 °C until analysis. In preparation for GST and SOD activity assays, 100 mg of tissue were homogenized with 0.5 ml phosphate buffer saline [PBS (0.01 M, pH 7.4, Gibco)] and a cocktail of protease inhibitor tablet (Complete™ mini protease inhibitors, Roche) was added. The homogenate was vigorously vortexed, and a brief sonication treatment on ice was applied for a better homogenization. The homogenate was then centrifuged at 10,000g for 15 min at 4 °C, the supernatant was collected and the protein concentration was measured using the Bradford method. For GCS protein determination, 100 mg of tissue were homogenized with 1 ml cytosolic lysis buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-potassium hydroxide (HEPES, Serva-KOH, Sigma-Aldrich) pH 7.9, 1.5 mM magnesium chloride (MgCl2, Panreac Quimica SA), 10 mM potassium chloride (KCl, Sigma-Aldrich), 0.5 mM dithiothreitol (DTT, Fluka), 0.5% Nonidet P-40 (NP-40, Sigma-Aldrich) to which protease inhibitors were added (Complete™ mini protease inhibitors, Roche). The homogenate was agitated for 40 min, then it was centrifuged at 10,000g for 20 min at 4 °C, the supernatant was collected and the protein concentration was measured using the Bradford method. Samples were stored at–80 °C until biochemical analysis.
2.6. Determination of GST activity
The determination of GST activity in the cytosolic lysate was based on the method of Habig [30]. More specifically, 920 μl of phosphate buffer (100 mM, pH 7.4) were mixed with 50 μl of GSH (1 mM) and 20 μl of 1-chloro-2,4-dinitrobenzene (CDNB, Sigma-Aldrich) and the samples were incubated for 5 min at 30 °C. This was followed by the addition of 10 μl of liver tissue lysate (diluted 1:3) or 20 μl of spleen tissue lysate (the measurement required >10 μg of total amount of protein in the tested sample) and the change in absorbance was measured at 340 nm for 5 min. Upon conjugation of the thiol group of glutathione to the CDNB substrate, there was an increase in the absorbance at 340 nm using a spectrophotometer (HITACHI, U-1900 UV/VIS). Samples containing tissue lysate alone were used as blanks and their absorbance was measured throughout the assay time. GST activity in the tissue lysates was normalized to total cellular protein in each sample. The results were expressed as units (μmol of CDNB conjugate produced min−1 ml−1) per mg of protein.
2.7. Determination of SOD activity
The determination of SOD activity in the whole cell lysate was based on the method of nitroblue tetrazolium salt (NBT, Sigma-Aldrich) according to Oberley & Spitz [31]. More specifically, this assay included a negative control made by mixing 800 μl of SOD buffer [1 mM diethylenetriaminepentaacetic acid (DETAPAC, Sigma-Aldrich) in 0.05 M potassium phosphate buffer (pH 7.8); 1 U catalase (Sigma-Aldrich); 5.6 × 10−5 M NBT; 10−4 M xanthine (Sigma-Aldrich)] with 200 μl of 0.05 M potassium phosphate buffer. Subsequently, ∼60 mU of xanthine oxidase (XO, Sigma-Aldrich) were added and the rate of increase in absorbance was measured at 560 nm for 3.5 min. In the test samples, 100 μl of liver and spleen tissue lysate (diluted 1:3) (the measurement required >10 μg total amount of protein) were added to 800 μl of SOD buffer followed by the addition of ∼60 m U of XO and the rate of increase in absorbance was measured for 3.5 min at 560 nm using a spectrophotometer (HITACHI, U-1900 UV/VIS). Calculation of SOD activity in the test samples was based on the percent inhibition of the rate of increase in absorbance. The rate of increase in absorbance (A) per minute for the negative control and for the test samples was determined by the formula (1) and the percentage inhibition for each sample was calculated using the formula (2):
| ΔΑ560 nm/min = (A560nm final − A560nm initial)/3.5 min | (1) |
| % Inhibition = [(ΔΑ560 nm/minnegativecontrol − ΔΑ560 nm/minsample)/ΔΑ560 nm/minnegativecontrol] x 100 | (2) |
SOD activities in tissue lysates were normalized to total cellular protein in each sample.
2.8. Western blot analysis for GCS protein
In order to determine the expression levels of GCS, immunoblotting was used. In particular, 40 μg of protein were used. Tissue lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using an 8% polyacrylamide gel. Proteins were then transferred onto a polyvinylidene difluoride membrane (PVDF) (Millipore, Bedford, MA, USA). The membranes were blocked overnight with 5% non-fat milk in buffer [13 mM Tris (Serva), 150 mM sodium chloride (NaCl, Sigma-Aldrich), pH 7.5] containing 0.2% Tween-20 (Sigma-Aldrich). Then, they were probed with polyclonal rabbit anti-human/mouse GCS (1:600; Santa Cruz Biotechnology; Cat. No sc-22755) primary antibodies for 1 h at room temperature. Membranes were then incubated with polyclonal horseradish peroxidase conjugated goat anti-rabbit secondary antibody (1:5000, Thermo Scientific, Cat. No 31462) for 30 min at room temperature. Membranes were reprobed with monoclonal anti-turkey/monkey/canine/chicken/human/bovine/rat/mouse/mink/rabbit/hamster glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:10000, Sigma-Aldrich, Cat. No G9295) as internal control. The optical density of protein bands was measured by using the Alpha View quantification software (Alpha Innotech, CA, USA).
2.9. Statistical analysis
All results are expressed as mean ± sem. Kolmogorov-Smirnof test was used for testing the distribution of the samples. For the statistical analysis of GP group, one-way ANOVA was applied followed by Tukey’s test for post hoc analysis in order to compare the means between the different groups. For the statistical analysis of OMWW group, Kruskal-Wallis was applied in order to compare the means between the different groups. Differences were considered significant at p < 0.05. All statistical analyses were performed using the SPSS software (version 14.0; SPSS).
3. Results
3.1. Assessment of GST activity
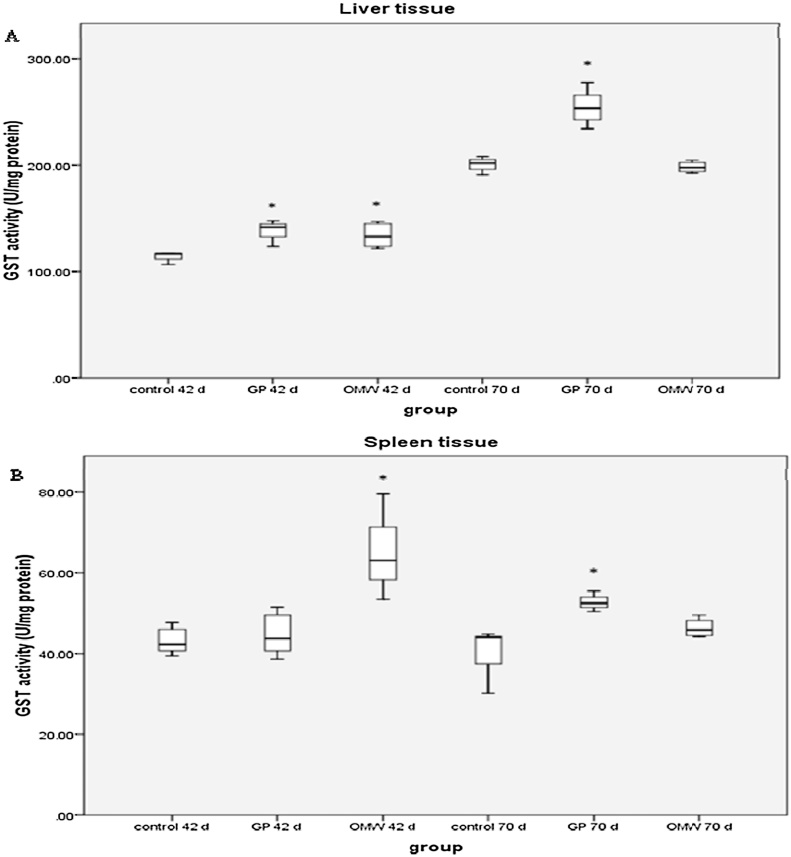
In liver tissue, GP group exhibited significantly increased GST activity at 42 and 70 day of the experiment by 21 and 30.7% compared to controls, respectively (Fig. 2A). The OMW group exhibited significantly increased GST activity at the 42 day of the experiment by 17.8% compared to control (Fig. 2A). In spleen tissue, GST activity in GP group increased significantly at the 70 day of the experiment by 29% compared to control (Fig. 2B) while in OMW group increased significantly at the 42 day of the experiment by 37.7% compared to control (Fig. 2B).
Fig. 2.
Effects of grape pomace (GP) and olive oil mill wastewater (OMW) byproducts on the enzymatic activity of glutathione-s-transferase (GST) in (A) liver and (B) spleen tissue at 42 and 70 days. *Statistically significant difference compared to control (p < 0.05). Results are presented as mean ± SEM.
3.2. Assessment of SOD activity
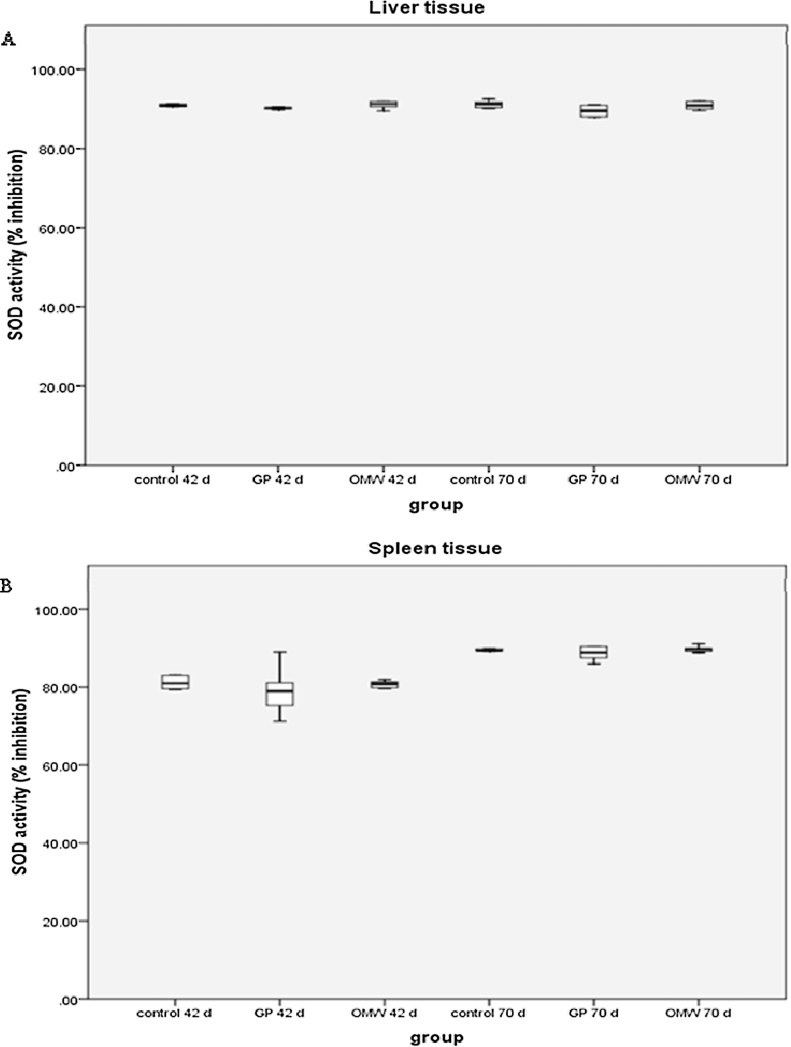
The SOD activity has not been affected neither in liver nor in spleen tissue by the administration of GP and OMW silages (Fig. 3A, B).
Fig. 3.
Effects of grape pomace (GP) and olive oil mill wastewater (OMW) by-products on the enzymatic activity of superoxide dismutase (SOD) in (A) liver and (B) spleen tissue at 42 and 70 days. Results are presented as mean ± SEM.
3.3. Western blot analysis for GCS expression
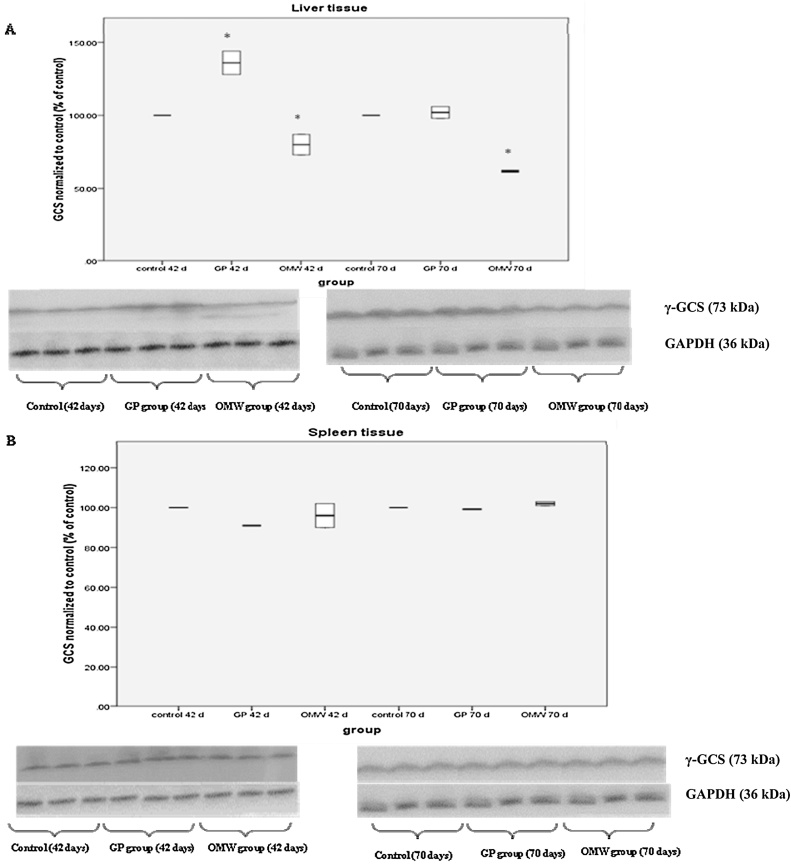
In liver tissue, GCS expression in GP group increased significantly at the 42nd day of the experiment by 36% compared to control (Fig. 4A). In OMW group, GCS expression reduced significantly at 42 and 70 day of the experiment by 20% and 37.5% compared to controls, respectively (Fig. 4A). In spleen tissue, GCS activity has not been affected neither in GP group nor in OMW group (Fig. 4B).
Fig. 4.
Representative Western blots showing the effects of grape pomace (GP) and olive oil mill wastewater (OMW) by-products on the expression of γ-synthase glutamyl cysteine (γ-GCS) in (A) liver and (B) spleen tissue at 42 and 70 days. Densitometric quantification for all enzymes is also reported. The expression of GAPDH was used as loading control for normalization. *Statistically significant difference compared to control (p < 0.05). The results are presented as mean ± SEM.
4. Discussion
Several studies have shown that in farm animals, oxidative stress is involved in a number of pathological conditions (e.g. sepsis, mastitis, enteritis, pneumonia, joint diseases), related to the livestock production and welfare [32]. Thus, it has been suggested that the administration of antioxidants for the treatment of pathological conditions related with oxidative stress in farm animals. Several studies have focused on the beneficial properties of GP and OMW byproducts on animal health, due mainly to their antioxidant activity based on their high polyphenolic content [33], [34], [35]. In a previous study, we have found that feeds supplemented with GP [21] and OMW (unpublished data) improved sheep's redox status. Therefore, the purpose of the present study was to investigate the molecular mechanisms through which feeds supplemented with GP and OMW byproducts exerted beneficial effects on sheep's liver and spleen tissue by determining the enzymatic activity of SOD and GST as well as the protein expression of γ-GCS. Thus, 36 male sheep were divided into three groups according to the kind of feed (control group, GP group and OMW group). After feeding, GST and SOD enzymatic activity as well as γ-GCS protein expression in liver and spleen tissue were determined in all animals at 42 and 70 days post-birth.
The above two mentioned tissues were selected, since liver is a vital organ, as it has a wide range of functions including detoxification and protein synthesis. Liver is also one of the primary organs affected by reactive oxygen species (ROS). The parenchymal cells, Kupffer cells, hepatic asteroid cells and endothelial cells are potentially more susceptible or sensitive to oxidative stress. When ROS production is excessive, redox homeostasis is disturbed resulting in oxidative stress, which plays a critical role in liver diseases and other chronic and degenerative disorders. A number of cytokines can be induced in response to oxidative stress resulting in the occurrence of inflammation and/or apoptosis. Spleen is a lymphoid organ of animals and the largest of the lymphatic tissue. It constitutes a hematopoietic organ during fetal life, acts as a filter that purifies blood from abnormal blood cells, antigens and microorganisms and plays an important role in non-specific and specific immunity. Its role in mediating oxidative damage in response to stress factors has underestimated importance [36].
In the GP group, the results showed that, in liver tissue, at 42 days post-birth, GST activity and γ-GCS expression were increased significantly compared to control. GST is a phase II metabolic enzyme and responsible for the detoxification of xenobiotic and electrophile compounds in living organisms by catalyzing the conjugation of thiol group of reduced glutathione (GSH) [37]. GCS is the first enzyme involved in the biosynthesis pathway of GSH. The synthesis of GSH from its amino acids involves two enzymatic steps: formation of γ-glutamylcysteine from glutamate and cysteine, a rate limiting step that is catalyzed by GCS, and formation of GSH from γ-glutamylcysteine and glycine, that is catalyzed by glutathione synthetase (GS) [38]. In one of our previous studies on the same samples, it was shown that GSH levels were not affected after GP administration at 42 days post-birth in liver tissue [21]. The observed increase in GST activity could lead to a reduction in GSH levels due to its conjugation to electrophiles. However, the increased γ-GCS levels may attribute to the de novo synthesis of GSH in order to compensate for its possible reduction due to the increased GST activity. In another study, it was also found that GP increased GST activity in rabbit liver tissues [39]. Our results are also consistent with one of our previous studies showing that GP extract increased γ-GCS levels and GST activity in muscle and endothelial cells [24]. At 70 days post-birth, GP administration led to increased GST activity levels compared to control, while γ-GCS expression levels were not affected. These results are in accordance with our findings showing decreased GSH levels at 70 days post-birth after GP administration in liver tissue [21]. For example, Bergelson et al. [40] have reported that activation of the rat GST-Ya gene acts through a common mechanism involving the depletion of GSH. In another study it was also found that the introduction of GST-π cDNA into KB/BSO3 cells resulted in a decrease in GSH levels [41]. Bioactive compounds of GP did not affect SOD activity, neither at 42 nor at 70 days post-birth. SOD is an enzyme that eliminates intracellular superoxide radicals and plays an important role in the defense system against oxidative stress [42], [43].
In spleen tissue of GP group, at 42 days post-birth, GST activity and γ-GCS expression were not affected after GP administration. This lack of effects conforms to the unaffected GSH levels at the same time-point observed in another of our studies [21]. At 70 days post-birth, GP administration led to increased GST activity compared to control, while γ-GCS expression levels were not affected. These effects would normally lead to decreased GSH levels. However, GSH levels were increased, in spleen tissue, at 70 days post-birth [21]. This contradiction may be explained if GSH formation in spleen tissue, at 70 days post-birth, follows another pathway which is not based on the de novo synthesis. For example, GSH can be regenerated from oxidized glutathione (GSSG) by glutathione reductase (GR). GR is the key enzyme of glutathione metabolism and is conserved between species. GR is essential for the glutathione redox cycle that maintains adequate levels of reduced cellular GSH. Other studies have shown that polyphenols led to increased GR activity. Specifically, grape extracts from different cultivars increased GST and GR activity as well as GSH levels in liver tissue of goats [44]. Furthermore, another study has reported that quercetin, a known flavonoid present in grapes, induced the enzymatic activity of GR in the liver of mice treated with chronic doses of ethanol [45]. In addition, administration of polyphenolic extracts from other plants (e.g. green tea) to mice with CCL4-induced liver damage led to significantly increased GR activity [46]. Skrzydlewska et al. [47] have also shown that oral administration of green tea increased significantly GR activity in rats' liver. Like liver, SOD activity was not affected, neither at 42 nor at 70 days post-birth in spleen. Furthermore, the feed supplemented with GP had beneficial effects on animal health. In particular, the experimental feed decreased the population of pathogenic bacteria in fecal microbiota such as Enterobacteriaceae and E. coli in sheep. Also, it has been found that administration of feed supplemented with GP decreased lipid and protein oxidation (both of them are caused by oxidative stress). Specifically, in liver tissue, at 42 days, protein carbonyls levels were decreased. Moreover, in liver and spleen tissue, at 70 days, TBARS levels (lipid peroxidation marker) were decreased. Thus, the administration of feed supplemented with GP decreased oxidant levels [21].
In the OMW group, the results showed that, in liver tissue, at 42 days post-birth, GST activity was increased compared to control. However, γ-GCS expression was decreased compared to control at 42 and 70 days post birth, which was in contrast with the result found in liver tissue. Since GSH levels were not changed, in both OMW (unpublished data) and GP [21] groups at 42 days post-birth, we hypothesize that different antioxidant enzymes involved in GSH metabolism may be activated by the phenolic compounds of OMW than by these of GP. A possible enzyme may be GR regenerating GSH from GSSG. Interestingly, at the same time point the levels of thiobarbituric acid reactive substances (TBARS), a marker of lipid peroxidation, were decreased significantly compared to control in liver tissue (unpublished data). This could be attributed to the increase in GST activity. Since, in addition to its ability to catalyze the formation of conjugates, GST also exhibits glutathione peroxidase activity and catalyzes the reduction of organic hydroperoxides such as lipid peroxides to their corresponding alcohols [48]. Likewise for GP group, SOD activity was not affected neither at 42 nor at 70 days post-birth in liver of OMW group.
At 42 days post-birth, OMW administration led to increased GST activity levels in spleen tissue compared to control, while γ-GCS expression levels were not affected. Thus, GSH levels would be expected to be decreased. In contrary, it was shown that GSH levels were increased (unpublished data). As mentioned above, the activity of other enzymes (e.g. GR) involved in GSH metabolism may be enhanced by the feed supplemented with OMW, thus contributing to GSH regeneration. At 70 days post-birth, in spleen tissue, the feed supplemented with OMW did not affect the activity of GST and the expression of γ-GCS, although GSH levels were increased (unpublished data). In that case, the enhancement of other antioxidant mechanisms may rescue GSH from reaction with free radicals. Similarly to GP group, feed supplemented with OMW did not affect SOD activity neither at 42 nor at 70 days post-birth in spleen.
The above beneficial effects of GP and OMW feeds on the redox status of tissues were possibly attributed to their polyphenolic content [19], [26]. Polyphenols have been shown to modulate the activity of phase I and II enzymes, in particular GSH-related enzymes [49]. For example, administration of quercetin and curcumin to rats had protective effects against paracetamol-induced oxidative injury by increasing GST activity in liver and kidney tissues [50]. Also, the effect of certain flavonoids, such as rutin and silymarin, increased GST activity in the liver of diabetic rats [51]. Furthermore, hydroxytyrosol from olive oil induced GST gene and protein expression in HepG2 cells via Nrf2 pathway [52]. In addition, polyphenols from green tea increased cytosolic GST activity in the liver of wistar rats [53], while the administration of polyphenolic extract from Pisonia aculeata L increased GST activity in rat' liver [54]. Also, dietary treatment with resveratrol, a major polyphenol of grapes, enhanced GST gene expression and enzymatic activity in rat liver [55]. The activity of GST was also increased with animal age, probably as a compensatory mechanism against increased formation of oxidative stress products (e.g., organic hydroperoxides) accompanying process of aging [56], [57].
A possible mechanism that can lead to the activation of GST and γ-GCS enzymes is the signaling pathway of nuclear factor-like 2 (Nrf2), one of the most important defensive mechanisms against oxidative stress [58]. Under normal conditions, Nrf2 is anchored with kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm. Antioxidant agents or oxidative stress can lead to the translocation of Nrf2 to the nucleus where it activates the antioxidant response elements (AREs) of antioxidant enzymes (e.g. γ-GCS and GST) [59], [60], [61]. A number of studies in cell cultures and animals have demonstrated that polyphenolic compounds induce Nrf2 activation [32]. Polyphenols can oxidize or modify the cysteine thiol groups of Keap1 resulting in dissociation of Nrf2 and its subsequent translocation to the nucleus [62].
In conclusion, the present study showed for the first time that biofunctional feeds for sheep, supplemented with either GP or OMW byproducts increased the enzymatic activity of the detoxification and antioxidant GST enzyme as well as the protein expression of γ-GCS, a key enzyme for the synthesis of the antioxidant molecule GSH, in liver and spleen tissues. However, the beneficial effects of the feeds were tissue- and developmental stage-specific, probably due to physiological differences as well as to different presence of antioxidant molecules in each case. The findings also present interest because the literature concerning the redox status of different tissues and its changes due to diet is limited. Also, the elucidation of the molecular mechanisms involved in the improvement of animal redox status after administration of feeds supplemented with either GP or OMW byproducts would help developing a low-cost intervention for pathological conditions associated with oxidative stress, while at the same time reducing the risk of environmental pollution from their deposition on soil.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The present work was funded by the MSc program “Toxicology” in the Department of Biochemistry and Biotechnology at the University of Thessaly.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2017.06.007.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed. Res. Int. 2014 doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mc Cord J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000;108:652–659. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 3.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- 5.Lobo V., Patil A., Phatak A., Chandra N. Free radicals: antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celi P., Gabai G. Oxidant/Antioxidant balance in animal nutrition and health: the role of protein oxidation. Front. Vet. Sci. 2015;2:48. doi: 10.3389/fvets.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain A., Flora S.J. Dose related effects of nicotine on oxidative injury in young, adult and old rats. J. Environ. Biol. 2012;33:233–238. [PubMed] [Google Scholar]
- 8.Boudry G., Péron V., Le Huërou-Luron I., Lallès J.P., Sève B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 2004;134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- 9.Zhu L.H., Zhao K.L., Chen X.L., Xu J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012;90:2581–2589. doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]
- 10.Lykkesfeldt J., Svendsen O. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet. J. 2007;173:502–511. doi: 10.1016/j.tvjl.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Bidlack W.R. Technomic; Lancaster: 2000. Phytochemicals as Bioactive Agents. [Google Scholar]
- 12.Kris-Etherton P.M., Hecker K.D., Bonanome A., Coval S.M., Binkoski A.E., Hilpert K.F., Griel A.E., Etherton T.D. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002;30:71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 13.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain T., Tan B., Yin Y. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scalbert A., Johnson I.A., Saltmarsh M. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 2005;81:215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 16.Arts I.C., Hollman P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 17.Lima G.P., Vianello F., Correa C.R., Campos R.A., Borguini M.G. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 2014;5:1065–1082. [Google Scholar]
- 18.Ribeiro L.F., Ribani R.H., Francisco T.M., Soares A.A., Pontarolo R., Haminiuk C.W. Profile of bioactive compounds from grape pomace (Vitis vinifera and Vitis labrusca) by spectrophotometric, chromatographic and spectral analyses. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015;1007:72–80. doi: 10.1016/j.jchromb.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Pinelo M., Laurie V.F., Waterhouse A.L. A simple method to separate red wine nonpolymeric and polymeric phenols by solid-phase extraction. J. Agric. Food Chem. 2006;54:2839–2844. doi: 10.1021/jf052814a. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira A., Baenas N., Dominguez-Perles R., Barros A., Rosa E., Moreno D.A., Garcis-Viguera C. Natural bioactive compounds from winery by-products as health promoters. Int. J. Mol. Sci. 2014;15:15638–15678. doi: 10.3390/ijms150915638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kafantaris I., Kotsampasi B., Christodoulou V., Kokka E., Kouka P., Terzopoulou Z., Gerasopoulos K., Stagos D., Mitsagga C., Giavasis I., Makri S., Petrotos K., Kouretas D. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. 2016 doi: 10.1111/jpn.12569. [DOI] [PubMed] [Google Scholar]
- 22.Goutzourelas N., Stagos D., Demertzis N., Mavridou P., Karterolioti H., Georgadakis S., Kerasioti E., Aligiannis N., Skaltsounis L., Statiri A., Tsioutsiouliti A., Tsatsakis A.M., Hayes A.W., Kouretas D. Effects of polyphenolic grape extract on the oxidative status of muscle and endothelial cells. Hum. Exp. Toxicol. 2014;33:1099–1112. doi: 10.1177/0960327114533575. [DOI] [PubMed] [Google Scholar]
- 23.Goutzourelas N., Stagos D., Housmekeridou A., Karapouliou C., Kerasioti E., Aligiannis N., Skaltsounis A.L., Spandidos D.A., Tsatsakis A.M., Kouretas D. Grape pomace extract exerts antioxidant effects through an increase in GCS levels and GST activity in muscle and endothelial cells. Int. J. Mol. Sci. 2015;36:433–441. doi: 10.3892/ijmm.2015.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goutzourelas N., Stagos D., Spanidis Y., Liosi M., Apostolou A., Priftis A., Haroutounian S., Tsatsakis D.A., Kouretas D. Polyphenolic composition of grape stem extracts affects antioxidant activity in endothelial and muscle cells. Mol. Med. Rep. 2015;12:5846–5856. doi: 10.3892/mmr.2015.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makri S., Kafantaris I., Stagos D., Chamokeridou T., Petrotos K., Gerasopoulos K., Mpesios A., Goutzourelas N., Kokkas S., Goulas P., Komiotis D., Kouretas D. Novel feed including bioactive compounds from winery wastes improved broilers' redox status in blood and tissues of vital organs. Food Chem. Toxicol. 2017;102:24–31. doi: 10.1016/j.fct.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Frankel E., Bakhouche A., Lozano-Sánchez J., Segura-Carretero A., Fernández-Gutiérrez A. Literature review on production process to obtain extra virgin olive oil enriched in bioactive compounds. Potential use of byproducts as alternative sources of polyphenols. J. Agric. Food Chem. 2013;61:5179–5188. doi: 10.1021/jf400806z. [DOI] [PubMed] [Google Scholar]
- 27.Cicerale S., Lucas L., Keast R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010;11:458–479. doi: 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerasopoulos K., Stagos D., Petrotos K., Kokkas S., Kantas D., Goulas P., Kouretas D. Feed supplemented with polyphenolic byproduct from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015;86:319–327. doi: 10.1016/j.fct.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Gerasopoulos K., Stagos D., Kokkas S., Petrotos K., Kantas D., Goulas P., Kouretas D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015;82:42–49. doi: 10.1016/j.fct.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 31.Oberley L.W., Spitz D.R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984;105:457–464. doi: 10.1016/s0076-6879(84)05064-3. [DOI] [PubMed] [Google Scholar]
- 32.Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2016 doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- 33.Dermeche S., Nadour M., Larroche C., Moulti-Mati F., Michaud P. Olive mill wastes: biochemical characterizations and valorization strategies. Process Biochem. 2013;48:1532–1552. [Google Scholar]
- 34.Apostolou A., Stagos D., Galitsiou E., Spyrou A., Haroutounian S., Portesis N., Trizoglou I., Hayes A.W., Tsatsakis A.M., Kouretas D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013;61:60–68. doi: 10.1016/j.fct.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y., Burton S., Kim C., Sismour E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016;4:125–133. doi: 10.1002/fsn3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mebius R.E., Kraal G. Structure and function of the spleen. Nat. Rev. Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 37.Singhal S.S., Singh S.P., Singhal P. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal: a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015;289:361–370. doi: 10.1016/j.taap.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Choi C.S., Chung H.K., Choi M.K., Kang M.H. Effects of grape pomace on the antioxidant defense system in diet-induced hypercholesterolemic rabbits. Nutr. Res. Pract. 2010;4:114–120. doi: 10.4162/nrp.2010.4.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergelson S., Pinkus R., Daniel V. Intracellular glutathione levels regulate Fos/Jun induction and activation of glutathione S-transferase gene expression. Cancer Res. 1994;54:36–40. [PubMed] [Google Scholar]
- 41.Yokomizo A., Kohno K., Wada M., Ono M., Morrow C.S., Cowan K.H., Kuwano M. Markedly decreased expression of glutathione S-transferase pi gene in human cancer cell lines resistant to buthionine sulfoximine, an inhibitor of cellular glutathione synthesis. J. Biol. Chem. 1995;33:19451–19457. doi: 10.1074/jbc.270.33.19451. [DOI] [PubMed] [Google Scholar]
- 42.Carlioz A., Touati D. Isolation of superoxide dismutase mutants in E. coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fridovich I. Superoxide dismutases. Adv. Enzymol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- 44.Singha I., Subir Kumar Das S.K. Scavenging and antioxidant properties of different grape cultivars against ionizing radiation-induced liver damage ex vivo. Indian J. Exp. Biol. 2016;54:280–285. [PubMed] [Google Scholar]
- 45.Molina M.F., Sanchez-Reus I., Iglesias I., Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol. Pharm. Bull. 2003;26:1398–1402. doi: 10.1248/bpb.26.1398. [DOI] [PubMed] [Google Scholar]
- 46.Tsai C.F., Hsu Y.W., Ting H.C., Huang C.F., Yen C.C. The in vivo antioxidant and antifibrotic properties of green tea (Camellia sinensis, Theaceae) Food Chem. 2013;136:1337–1344. doi: 10.1016/j.foodchem.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 47.Skrzydlewska E., Ostrowska J., Farbiszewski R., Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002;9:232–238. doi: 10.1078/0944-7113-00119. [DOI] [PubMed] [Google Scholar]
- 48.Hayes J., Pulford D. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isozymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 49.Steele V.E., Kelloff G.J., Balentine D., Boone C.W., Mehta R., Bagheri D., Sigman C.C., Zhu S., Sharma S. Comparative chemoprotective mechanisms of green tea: black tea and selected polyphenol extracts measured by in vitro bioassays. Carcinogenesis. 2000;21:63–67. doi: 10.1093/carcin/21.1.63. [DOI] [PubMed] [Google Scholar]
- 50.Yousef M.I., Omar S.A., El-Guendi M.I., Abdelmegid L.A. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem.Toxicol. 2010;48:3246–3261. doi: 10.1016/j.fct.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 51.Al-Enazi M.M. Combined therapy of rutin and silymarin has more protective effects on streptozotocin-induced oxidative stress in rats. J. Appl. Pharm. Sci. 2014;4:021–028. [Google Scholar]
- 52.Martín M.A., Ramos S., Granado-Serrano A.B., Rodríguez-Ramiro I., Trujillo M., Bravo L., Goya L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010;54:956–966. doi: 10.1002/mnfr.200900159. [DOI] [PubMed] [Google Scholar]
- 53.Maliakal P.P., Coville P.F., Wanwimolruk S. Tea consumption modulates hepatic drug metabolizing enzymes in Wistar rats. J. Pharm. Pharmacol. 2001;53:569–577. doi: 10.1211/0022357011775695. [DOI] [PubMed] [Google Scholar]
- 54.Palanivel M.G., Rajkapoor B., Kumar R.S., Einstein J.W., Kumar E.P., Kumar M.R., Kavitha K., Kumar M.P., Jayakar B. Hepatoprotective and antioxidant effect of pisonia aculeata L. against CCl4- induced hepatic damage in rats sci. Sci. Pharm. 2008;76:203–215. [Google Scholar]
- 55.Sadi G., Baloğlu M.C., Pektaş M.B. Differential gene expression in liver tissues of streptozotocin-induced diabetic rats in response to resveratrol treatment. PLoS One. 2015;10:e0124968. doi: 10.1371/journal.pone.0124968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maurya P.K., Rizvi S.I. Age-dependent changes in glutathione-S-transferase: correlation with total plasma antioxidant potential and red cell intracellular glutathione. Indian J. Clin. Biochem. 2010;25:398–400. doi: 10.1007/s12291-010-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.VyskoIilová E., Szotáková B., Skálová L., Bártíková H., HlaváIová J., Boušová I. Age-related changes in hepatic activity and expression of detoxification enzymes in male rats. Biomed Res. Int. 2013 doi: 10.1155/2013/408573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakagami Y. Nrf2 is an attractive therapeutic target for retinal diseases. Oxid. Med. Cell. Longev. 2016;74 doi: 10.1155/2016/7469326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osburn W.O., Kensler T.W. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mut. Res. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giudice A., Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28:169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 61.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sanchez-Perez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bensasson R.V., Zoete V., Dinkova-Kostova A.T., Talalay P. Two-step mechanism of induction of the gene expression of a prototypic cancer-protective enzyme by diphenols. Chem. Res. Toxicol. 2008;21:805–812. doi: 10.1021/tx7002883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.