Graphical abstract
Keywords: Taurine, Dichlorvos, Malondialdehyde, Antioxidant enzymes, Oxidative stress
Highlights
-
•
Subacute dichlorvos exposure elicited oxidative stress in male Wistar rats.
-
•
Taurine protected male Wistar rats from oxidative stress induced by dichlorvos.
-
•
Taurine may be suitable for prophylaxis against dichlorvos toxicity.
Abstract
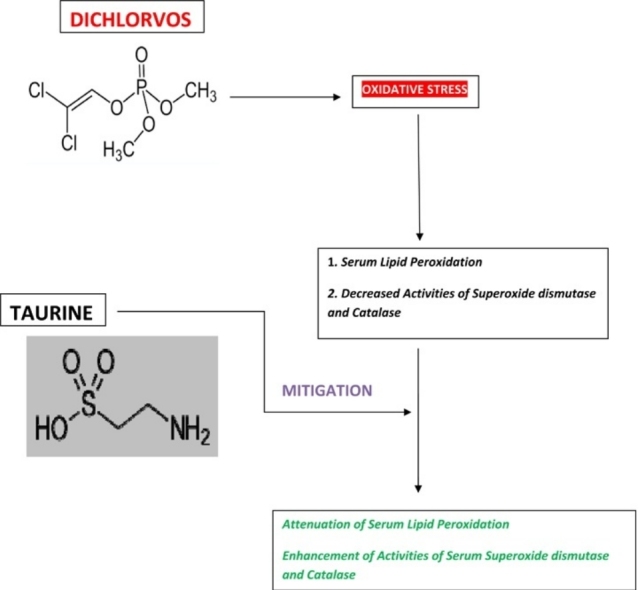
The aim of the study was to determine the effects of taurine in rats exposed to subacute dichlorvos toxicity. Fifty rats were weighed and assigned into five groups of ten rats each. The groups received: distilled water, soya oil (1 ml/kg), taurine (50 mg/kg), dichlorvos (10 mg/kg) and the combination treatment group received taurine first and then dichlorvos 30 min later. The treatments were administered once daily by oral gavage for 4 weeks. The rats were euthanized and blood samples were collected after the termination of the study. Serum samples were analysed for malondialdehyde concentration and activities of antioxidant enzymes (superoxide dismutase and catalase). Dichlorvos increased malondialdehyde concentration and reduced the activities of superoxide dismutase and catalase. There was attenuation of malondialdehyde concentration and improvement of activities of superoxide dismutase (P = 0.0273) and catalase (P < 0.0001) in rats treated with taurine. It is postulated that taurine ameliorated dichlorvos-induced oxidative stress through the reduction of malondialdehyde concentration and the enhancement of activities of antioxidant enzymes.
1. Introduction
Pesticides are chemicals that are widely used in agricultural, domestic and public health settings for the control of pests. There are evidences of the link between exposure to pesticides and the incidence of cancer, Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, diabetes, aging, cardiovascular and chronic renal disease [1], [2]. Dichlorvos (2,2-dichloro-vinyldimethyl phosphate, DP) is an organophosphate insecticide that is used for the protection of greenhouse plants, fruits and vegetables against mushroom flies, aphids, spiders, mites, caterpillars, thrips and white flies [3]. The insecticide is commonly used on cattle, sheep, poultry and around livestock edifices for pest control [4]. It is applied as an anthelminthic in dogs, swine and horses [5]. In Nigeria, DP is widely used by farmers for agricultural pest control [6]. The routes of exposure to DP include ingestion, inhalation and dermal exposure [7]. It is known that organophosphorus compounds, including DP, elicit neurotoxicity in mammals by inhibiting acetylcholinesterase activity [8].
Oxidative stress has been identified as an important molecular mechanism of toxicity of DP [3]. It is defined as an imbalance between the production of free radicals and the antioxidant system of the body [9]. Enzymatic and non-enzymatic antioxidants scavenge free radicals and reactive oxygen species (ROS) in biological systems [10]. Taurine (2-aminoethanesulphonic acid, TA) is a conditionally essential amino acid with high water solubility and it is an end product of cysteine and methionine metabolism [11]. TA is an efficacious non-enzymatic antioxidant that protects many organs in the body against toxicity, oxidative stress [12], and injury [13].
The purpose of the study was to determine whether taurine could protect male Wistar rats from the adverse effects of subacute dichlorvos toxicity. It is important to investigate the attenuation of the toxicity of DP with TA (a putative antioxidant) because oxidative stress has been identified as one of the important molecular mechanisms of DP toxicity. In addition, the protective effects of TA against DP toxicity should be explored because the residues of DP have been detected in food stuffs in several countries including Nigeria [14], [15].
2. Methods
2.1. Experimental animals
The male Wistar rats used for this study were obtained from the Department of Biochemistry, Ahmadu Bello University, Zaria, Kaduna State, Nigeria. They were housed in cages in the Toxicology Laboratory of the Department of Veterinary Pharmacology and Toxicology, University of Abuja, Federal Capital Territory, Nigeria. The animals were housed under standard environmental conditions (25 °C, 12 h light/dark cycle) and had access to standard laboratory ration and tap water ad libitum. The Wistar rats were acclimatized for two weeks before the commencement of the study. The research was approved by the University of Abuja research ethics committee and the animals were handled in accordance with the guidelines of the National Institute of Health Guide for Care and Use of Laboratory animals [16].
2.2. Chemicals
Commercial grade dichlorvos (DP, 100% solution containing 100 g per liter of 2,3-dichlorovinyl dimethylphosphate) marketed as Sniper® was purchased from an agrochemical company in Abuja, Federal Capital Territory, Nigeria. DP was reconstituted in soya oil (SO, Grand Cereals and Oil Mills Limited, Jos, Nigeria) to a 5% stock solution. An analytical grade of taurine (TA, Chemical Abstract Service number: 107-35-7, purity ≥ 99%) was obtained from Sigma Aldrich® (Steinheim, Germany). Prior to daily administration, 100 mg of TA was reconstituted in distilled water (DW) to obtain 100 mg/ml suspension.
2.3. Subacute toxicological study
The Wistar rats were weighed and divided randomly into five groups, with 10 rats in each group. Distilled water was given to the DW group, while the SO group was administered with soya oil (1 ml/kg). The TA group was treated with taurine only (50 mg/kg [17], [18]), while the DP group received dichlorvos only (10 mg/kg, 1/7th LD50). The combination treatment group received TA first (50 mg/kg), and then DP (10 mg/kg) 30 min after the administration of TA. The treatments were administered once daily by oral gavage for 4 weeks. The rats were observed for clinical signs of toxicity during the study. At the end of the study, the rats were euthanized by decapitation [19] and 3 ml of blood samples were collected into centrifuge tubes. The blood samples were incubated at room temperature for 30 min and then centrifuged at 1000 × g for 5 min to obtain sera samples.
2.4. Determination of MDA concentration
The concentration of malondialdehyde (MDA) was evaluated in the serum. The method described by Draper and Hadley [20] was used. The principle of the method was based on the spectrophotometric measurement of the colour developed during the reaction of thiobarbituric acid with MDA. The solutions were cooled under tap water and the absorbance was measured with an ultraviolet (UV) spectrophotometer (T80+ UV/Visible Spectrophotometer®, PG Instruments Ltd., Leicestershire, United Kingdom) at 532 nm. The concentration of MDA in the samples was calculated by using the absorbance coefficient, MDA-TBA complex 1.56 × 105 cm−1 M−1.
2.5. Assays of the activities of serum antioxidant enzymes
Superoxide dismutase (SOD) was assayed with the Northwest Life Science Specialties (NWLSSTM) SOD activity assay kit based on the method described by Martin et al. [21]. Catalase activity was measured by the NWLSSTM CAT activity assay kit according to the method described by Beers and Sizer [22]. The assay kits were purchased from Northwest Life Science Specialties, LLC, Vancouver, Washington, DC, United States of America.
2.6. Statistical analysis
The data obtained were expressed as mean ± standard error of the mean. The biochemical parameters were analysed with one-way analysis of variance followed by Tukey’s multiple comparism post hoc test. The statistical package used was Graphpad Prism version 4.00 for Windows (Graphpad software, San Diego, California, USA). Values of P < 0.05 were considered statistically significant.
3. Results
3.1. Effects of treatments on serum malondialdehyde concentration
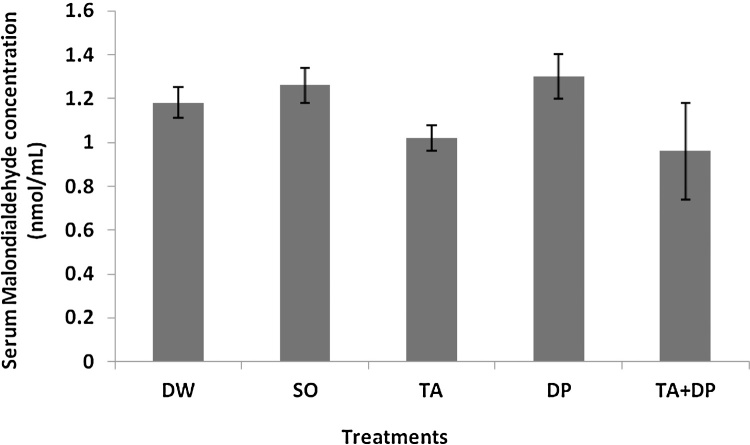
The effects of the treatments on serum malondialdehyde (MDA) concentration are shown in Fig. 1. The highest serum MDA concentration was recorded in the DP group, while the lowest serum MDA concentration was observed in the TA + DP group. F4,45 = 1.506; P = 0.2164.
Fig. 1.
Effects of treatments on serum malondialdehyde concentration.
Data are expressed as mean ± standard error of the mean, n = 10 animals/group.
DW: Distilled Water; SO: Soya Oil (1 ml/kg); TA: Taurine (50 mg/kg); DP: Dichlorvos (10 mg/kg); TA + DP: Taurine (50 mg/kg) + Dichlorvos (10 mg/kg).
F4,45 = 1.506; P = 0.2164.
3.2. Effects of treatments on serum superoxide dismutase activity
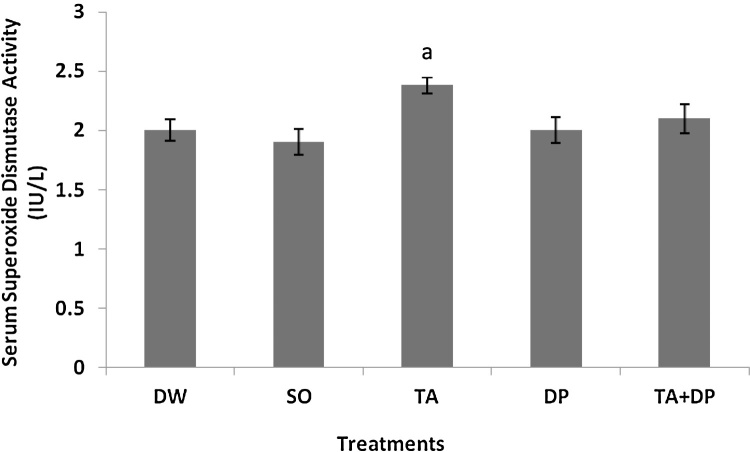
There was a significant increase (P < 0.05) in the serum superoxide dismutase activity in the TA group compared to the SO group. This is illustrated in Fig. 2, where a = P < 0.05; TA group versus SO group. F4,45 = 3.283; P = 0.01.
Fig. 2.
Effects of treatments on serum superoxide dismutase activity.
Data are expressed as mean ± standard error of the mean, n = 10 animals/group.
DW: Distilled Water; SO: Soya Oil (1 ml/kg); TA: Taurine (50 mg/kg); DP: Dichlorvos (10 mg/kg); TA + DP: Taurine (50 mg/kg) + Dichlorvos (10 mg/kg).
a = P < .05 TA group versus SO group.
F4,45 = 3.283; P = 0.0191.
3.3. Effects of treatments on serum catalase activity
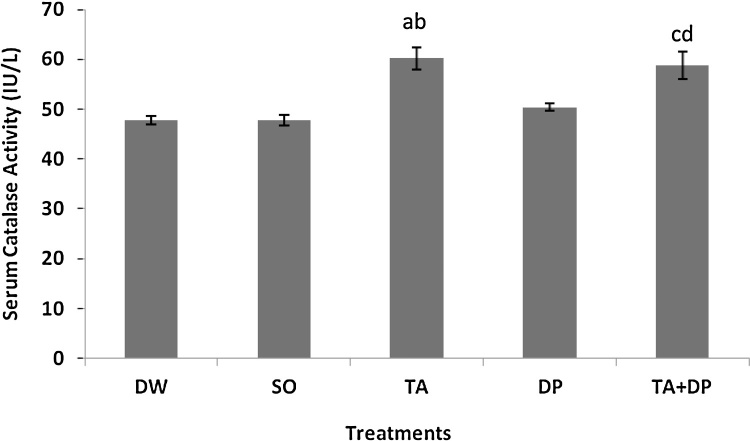
The serum catalase (CAT) activity was significantly increased (P < 0.001) in the TA group compared to those in the DW and SO groups respectively. This is depicted in Fig. 3, where a = p < 0.001, TA group versus DW and SO groups respectively. In addition, the serum CAT activity in the TA group was improved (P < 0.01) relative to that in the DP group; b = p < 0.01 TA group versus DP group. The serum CAT activity in the TA + DP group was elevated (P < 0.001) compared to those in the DW and SO groups respectively; c = p < 0.001 TA + DP group versus DW and SO groups respectively (Fig. 3). The serum CAT activity in the TA + DP group was enhanced (P < 0.05) compared to that in the DP group; d = p < 0.05 TA + DP group versus DP group. F 4,45 = 11.93; P < 0.0001.
Fig. 3.
Effects of treatments on serum catalase activity.
Data are expressed as mean ± standard error of the mean, n = 10 animals/group
DW: Distilled Water; SO: Soya Oil (1 ml/kg); TA: Taurine (50 mg/kg); DP: Dichlorvos (10 mg/kg); TA + DP: Taurine (50 mg/kg) + Dichlorvos (10 mg/kg)
a = p < 0.001 TA group versus DW and SO groups respectively.
b = p < 0.01 TA group versus DP group.
c = p < 0.001 TA + DP group versus DW and SO groups respectively.
d = p < 0.05 TA + DP group versus DP group.
F4,45 = 11.93; P < 0.0001.
4. Discussion
In this study, the highest serum MDA concentration was recorded in the DP group and this suggested that DP elicited lipid peroxidation. Lipid peroxidation is the process of oxidative disintegration of polyunsaturated fatty acids and its occurrence in biological membranes is characterized by impairment of membrane fluidity and inactivation of several membrane bound enzymes [23]. Eroğlu et al. [24] reported that treatment with DP caused an increase in the level of MDA and decreased the activities of antioxidant enzymes in human erythrocytes in vitro. This suggested that DP evoked lipid peroxidation in the human erythrocytes. In a research conducted by Ojo et al. [25], DP increased serum lipid peroxidation in male Wistar rats. Moreover, DP elicited oxidative stress in mice serum and kidneys in studies conducted by Agarwal et al. [26] and Agarwal et al. [27] respectively. In the current investigation, the increase in the serum MDA concentration in the DP group was not statistically significant. This might be attributed to the short duration of the study (4 weeks) and the low dose of DP that was administered to the experimental animals.
In contrast, the serum MDA concentration was reduced in the male Wistar rats that received TA. TA acts as a direct antioxidant by scavenging free radicals and by inhibiting lipid peroxidation [28]. Also, TA mitigates the production of MDA and consequently stabilizes biological membranes against lipid peroxidation [29]. It is known that TA may impede lipid peroxidation by increasing the activities of GPx and SOD in biological systems [30]. It has been postulated that TA decreased serum MDA concentration in male Wistar rats co-administered with chlorpyrifos and lead probably through its antioxidant properties [18]. To the best of our knowledge, this is the first investigation in which TA was reported to mitigate subacute DP toxicity through the alleviation of oxidative stress.
Furthermore, the activities of the serum antioxidant enzymes (SOD and CAT) were reduced in the male Wistar rats administered with DP. The endogenous antioxidant enzymes (SOD and CAT) are the first line of defense against oxidative stress induced by various xenobiotics [31]. SOD catalyzes the transformation of superoxide anion radical to hydrogen peroxide, while CAT changes hydrogen peroxide into water [32]. It has been posited that DP decreased SOD and CAT activities in rats through the induction of oxidative stress [33].
On the contrary, the activities of the serum antioxidant enzymes were augmented in the rats that were treated with TA. It has been demonstrated that TA enhanced the activities of serum antioxidant enzymes in male Wistar rats exposed to environmental toxicants [18]. According to Eppler and Dawson [34], TA elicits its antioxidant effects by improvement of the antioxidant system, production of chloramines with hypochlorous acid and restoration of GSH during oxidative stress. In our study, the enhancement of the activities of the serum antioxidant enzymes by TA might be ascribed to its antioxidant property.
5. Conclusion
The results of this study indicated that subacute DP toxicity induced oxidative stress in male Wistar rats and this was manifested by increased serum malondialdehyde concentration and reduction in the activities of serum antioxidant enzymes (superoxide dismutase and catalase). However, TA attenuated serum lipid peroxidation and enhanced the activities of serum antioxidant enzymes in the rats. It is hypothesized that TA reduced oxidative stress evoked by subacute DP toxicity through the reduction of serum malondialdehyde concentration and augmentation of the activities of serum antioxidant enzymes.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors are grateful to the members of staff of the Department of Veterinary Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Abuja, Nigeria for their support in the course of conducting this research.
References
- 1.de Souza A., Medeiros A.R., de Souza A.C., Wink M., Siqueira I.R., Ferreira M.B., Fernandes L., Loayza H.M.P., Torres I.L. Evaluation of the impact of exposure to pesticides on the health of the rural population: Valedo Taquari, State of Rio Grande do Sul (Brazil) Cien. Saude Colet. 2011;16:3519–3528. doi: 10.1590/s1413-81232011000900020. [DOI] [PubMed] [Google Scholar]
- 2.Mostafalou S., Abdollahi M. Concerns of environmental persistence of pesticides and human chronic diseases. Clin. Exp. Pharmacol. 2012;(S5):e002. [Google Scholar]
- 3.Sharma P., Singh R. Dichlorvos and lindane induced oxidative stress in rat brain: protective effects of ginger. Pharmacogn. Res. 2012;4:27–32. doi: 10.4103/0974-8490.91031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agency for Toxic Substances and Disease Registry (ATSDR) 1997. Toxicological Profile for Dichlorvos.https://www.atsdr.cdc.gov/toxprofiles/tp88.pdf [PubMed] [Google Scholar]
- 5.United States Environmental Protection Agency (USEPA), https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0151_summary.pdf (1994).
- 6.Musa U., Hati S.S., Mustapha A., Magaji G. Dichlorvos concentrations in locally formulated pesticide (Ota-piapia) utilized in northeastern Nigeria. Sci. Res. Essay. 2010;5:49–54. [Google Scholar]
- 7.Koutros S., Mahajan R., Zheng T., Hoppin J.A., Ma X., Lynch C.F., Blair A., Alavanja M.C. Dichlorvos exposure and human cancer risk: results from the agricultural health study. Cancer Causes Control. 2008;19:59–65. doi: 10.1007/s10552-007-9070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazarika A., Sarkar S.N., Hajare S., Kataria M., Malik J.K. Influence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction study. Toxicology. 2003;185:1–8. doi: 10.1016/s0300-483x(02)00574-7. PMID:12505439. [DOI] [PubMed] [Google Scholar]
- 9.Verma R.S., Mehta A., Srivastava N. Comparative studies on chlorpyrifos and methyl parathion induced oxidative stress in different parts of rat brain: attenuation by antioxidant vitamins. Pestic. Biochem. Physiol. 2009;95:152–158. [Google Scholar]
- 10.Gultekin F., Delibas N., Yasar S., Kilinc I. In vivo changes in antioxidant systems and protective role of melatonin and a combination of vitamin C and vitamin E on oxidative damage in erythrocytes induced by chlorpyrifos-ethyl in rats. Arch. Toxicol. 2001;75:88–96. doi: 10.1007/s002040100219. [DOI] [PubMed] [Google Scholar]
- 11.Waters E., Wang J.H., Redmond H.P., Wu Q.D., Kay E., Bouchier-Hayes D. Role of taurine in preventing acetaminophen-induced hepatic injury in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:1274–1279. doi: 10.1152/ajpgi.2001.280.6.G1274. PMID:11352821. [DOI] [PubMed] [Google Scholar]
- 12.Parildar-Karpuzoğlu H., Mehmetçik G., Ozdemirler-Erata G., Doğru-Abbasoğlu S., Koçak-Toker N., Uysal M. Effect of taurine treatment on pro-oxidant-antioxidant balance in livers and brains of old rats. Pharmacol. Rep. 2008;60:673–678. PMID:19066413. [PubMed] [Google Scholar]
- 13.Heidari R., Jamshidzadeh A., Niknahad H., Mardani E., Ommati M.M., Azarpira N., Khodaei F., Zarei A., Ayarzadeh M., Mousavi S., Abdoli N., Yeganeh B.S., Saeedi A., Najibi A. Effect of taurine on chronic and acute liver injury: focus on blood and brain ammonia. Toxicol. Rep. 2016;3:870–879. doi: 10.1016/j.toxrep.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.1999. International Agency for Research on Cancer (IARC) Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: Some Halogenated Hydrocarbons.https://monographs.iarc.fr/ENG/Monographs/vol71/mono71.pdf [Google Scholar]
- 15.Obida M.G., Hati S.S., Dimari G.A., Ogugbuaja V.O. Pesticide residues in bean samples from Northeastern Nigeria. ARPN J. Sci. Technol. 2012;2:79–84. [Google Scholar]
- 16.Garber J.C., Barbee R.W., Bielitzki J.T., Clayton L.A., Donovan J.C., Hendriksen C.F.M., Kohn D.F., Lipman N.S., Locke P.A., Melcher J., Quimby F.W., Turner P.V., Wood G.A., Würbel H., editors. Guide for the Care and Use of Laboratory Animals. 8th edn. National Academies Press; Washington: 2011. [Google Scholar]
- 17.Cetiner M., Sener G., Sehirli A.O., Eksioglu-Demiralp E., Ercan F., Sirvanci S., Gedik N., Akpulat S., Tecimer T., Yegen B.C. Taurine protects against methotrexate-induced toxicity and inhibits leucocyte death. Toxicol. Appl. Pharmacol. 2005;209(1):39–50. doi: 10.1016/j.taap.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Akande M.G., Aliu Y.O., Ambali S.F., Ayo J.O. Co-treatment of chlorpyrifos and lead induce serum lipid disorders in rats: alleviation by taurine. Toxicol. Ind. Health. 2014;32:1328–1334. doi: 10.1177/0748233714560394. [DOI] [PubMed] [Google Scholar]
- 19.Leary S., Underwood W., Anthony R., Cartner S., Corey D., Grandin T., Greenacre C., Gwaltney-Brant S., McCrackin M.A., Meyer R., Miller D., Shearer J., Yanong R. 2013 edition. 2013. AVMA Guidelines for the Euthanasia of Animals. 978-1-882691-21-0. [Google Scholar]
- 20.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. PMID: 2233309. [DOI] [PubMed] [Google Scholar]
- 21.Martin Jr J.P., Dailey M., Sugarman E. Negative and positive assays of superoxide dismutase based on haematoxylin autoxidation. Arch. Biochem. Biophys. 1987;255:329–336. doi: 10.1016/0003-9861(87)90400-0. PMID: 3036004. [DOI] [PubMed] [Google Scholar]
- 22.Beers Jr R.F., Sizer I.W. A spectrophotometry method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. PMID: 14938361. [PubMed] [Google Scholar]
- 23.Goel A., Dani V., Dhawan D.K. Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos induced toxicity. Chemico-Biol. Interact. 2005;156:131–140. doi: 10.1016/j.cbi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Eroğlu S., Pandir D., Uzun F.G., Bas H. Protective role of vitamins C and E in dichlorvos induced oxidative stress in human erythrocytes in vitro. Biol. Res. 2013;46:33–38. doi: 10.4067/S0716-97602013000100005. [DOI] [PubMed] [Google Scholar]
- 25.Ojo O.A., Oyinloye B.E., Ajiboye B.O., Ojo A.B., Musa H., Olanrewaju O.I. Dichlorvos-induced oxidative stress in rat brain: protective effects of the ethanolic extract of Alstonia boonei stem bark. Asian J. Pharm. 2014;8:216–221. [Google Scholar]
- 26.Agarwal S., Chaudhary B., Bist R. Protective propensity of bacoside A and bromelain on renal cholinesterases, γ-aminobutyric acid and serotonin level of Mus musculus intoxicated with dichlorvos. Chemico-Biol. Interact. 2017;261:139–144. doi: 10.1016/j.cbi.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal S., Chaudhary B., Bist R. Bacoside A and bromelain relieve dichlorvos induced changes in oxidative responses in mice serum. Chemico-Biol. Interact. 2016;254:173–178. doi: 10.1016/j.cbi.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 28.El-Sayed W.M., Al-Kahtani M.A., Abdel-Moneim A.M. Prophylactic and therapeutic effects of taurine against aluminium-induced acute hepatotoxicity in mice. J. Hazard. Mater. 2011;192:880–886. doi: 10.1016/j.jhazmat.2011.05.100. [DOI] [PubMed] [Google Scholar]
- 29.Manna P., Sinha M., Sil P.C. Amelioration of cadmium-induced cardiac impairment by taurine. Chemico-Biol. Interact. 2008;174:88–97. doi: 10.1016/j.cbi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Kilic N., Yildirim Z. Effects of taurine and age on liver antioxidant status and protein.oxidation. Turk. J. Biochem. 2008;33:169–174. [Google Scholar]
- 31.Ojha A., Yaduvanshi S.K., Srivastava N. Effect of combined exposure of commonly used organophosphate pesticides on lipid peroxidation and antioxidant enzymes in rat tissues. Pestic. Biochem. Physiol. 2011;99:148–156. [Google Scholar]
- 32.El-Demerdash F. Lipid peroxidation, oxidative stress and acetylcholinesterase in rat brain exposed to organophosphate and pyrethroid insecticides. Food Chem. Toxicol. 2011;49:1346–1352. doi: 10.1016/j.fct.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Yadav P., Jadhav S.E., Kumar V., Kaul K.K., Pant S.C., Flora S.J.S. Protective efficacy of 2-PAMCl, atropine and curcumin against dichlorvos induced toxicity in rats. Interdiscip. Toxicol. 2012;5:1–8. doi: 10.2478/v10102-012-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eppler B., Dawson Jr R. Cytoprotective role of taurine in a renal epithelial cell culture model. Biochem. Pharmacol. 2002;63:1051–1060. doi: 10.1016/s0006-2952(02)00843-2. PMID: 11931837. [DOI] [PubMed] [Google Scholar]