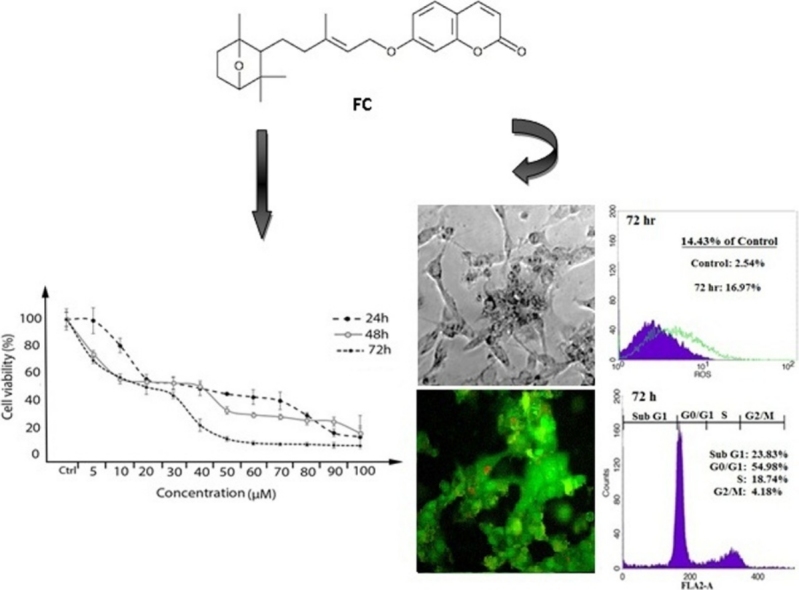
Graphical abstract
Keywords: Apoptosis, Farnesiferol C, Catalase, Superoxide dismutase, Malondialdehyde, MCF-7 cell line
Highlights
-
•
Farnesiferol C (FC) reduced viability in MCF-7 cells.
-
•
This compound causes G0/G1 cell cycle arrest in the cells.
-
•
The FC leads to cellular oxidative stress and induction of apoptosis in MCF-7 cells.
Abstract
Farnesiferol C is one of the major compounds, isolated from Ferula asafoetida (a type of coumarins) and used for cancer treatment as a folk remedy. Treatment of many cancers depends on oxidative stress situation. In this study, we sought the hypothesis that oxidative stress induced by Farnesiferol C contribute to anticancer property and induce apoptosis in MCF-7, human breast cancer cell line. We investigated the effect of Farnesiferol C on oxidative stress by measurement of some enzymes activity including catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), as well as some parameters such as total thiol and ROS levels. Also we evaluated Farnesiferol C effects on the cell cycle and apoptosis induction by using flow cytometry analysis. Our findings demonstrated that Farnesiferol C significantly induced apoptosis mediated by increasing in the cellular ROS levels. This compound increased cellular SOD and CAT activities in 24 and 48 h and reduced activity of these enzymes after 72 h exposure. Furthermore, MDA and total thiol levels were increased and decreased, respectively in the cells treated with Farnesiferol C after 24–72 h. G0/G1 phase cell cycle arrest followed by induction of apoptosis was also observed in MCF-7 cells after treatment with Farnesiferol C. According to these data, Farnesiferol C has a therapeutic effect on MCF-7 cells and can be suitable candidate for breast cancer treatment; however it is necessary for further experiments.
1. Introduction
Plants are considered as therapeutic approaches in the treatment of many diseases in folk remedy for decades [1], [2], [3], [4]. Among these, a large group of natural drugs such as sesquiterpene coumarins extracted from plants owing wide range of pharmacological activity. Types of coumarins (2H-1-benzopyran-2-one) comprising a large group of phenolic compound exist in plants and are formed of fused α-pyrone and benzene rings [5], [6], [7].
It has been recently reported antitumor activity of coumarin and its some metabolites against several human tumor cell lines. Both coumarin and coumarin derivatives have shown promise as potential inhibitors of cellular proliferation in various carcinoma cell lines [8], [9]. Additionally, studies on dicumarol from coumarin family have demonstrated a decreased metastasis in vivo situations [10], [11]. Ferula species are known in folk medicine, although there is sporadic documents on the chemosensitizing and chemopreventive characteristic of some coumarins isolated from the genus Ferula (Apiaceae), have a variety distribution throughout central Asia, Northern Africa and Mediterranean region [12]. Rutaceae and Asteraceae from Ferula species that grows in Iran, are also considered to be important [4]. Ferula species is considered very effective in the treatment of different diseases such as inflammations, headache, neurological diseases, arthritis, dysentery, digestive disorders, rheumatism and dizziness [13], and have been demonstrated both of the growth inhibitory and cytotoxic property in various cancer cell lines [6].
Farnesiferol C belongs to the family of coumarins is a polycyclic aromatic compound containing a 1-benzopyran moiety with a ketone group at the C2 carbon atom (Scheme. 1). This compound with various anti-tumor characteristic is a natural product extracted from Ferula asafoetida speciesis that used as a food spice in many Asian countries. It has been used for the treatment of asthma, bronchitis, ulcer, kidney stone, pain, and cancer in folk remedy. Recently, it was reported two coumarins, Farnesiferol C and Umbelliprenin separated from the chloroform extract of the F. asafoetida with anti-cancer, anti-angiogenic, anti-mutagenic, and antiviral properties [14]. Treatment of some cancers depends on oxidative stress situation. Some chemotherapy drugs used in cancer treatment exert their effectiveness by production of free radicals and reactive oxygen species (ROS), which are part of the metabolic activity of these drugs [15]. Some bioactive agents such as piperlongumine from long peppers can also induce pancreatic cancer cell death in cell culture and animal models by enhancing reactive oxygen species and DNA damage [16].
Scheme 1.
Chemical structure of Farnesiferol C.
Breast cancer is the most common cancer in women aged between 40–44 years old [17]. It is necessary to develop safe and effective drugs to treatment of this disease. Due to the expansive effects and outcomes from various in vitro investigations on coumarins especially Farnesiferol C, the mechanisms of their action is not yet obvious in human breast cancer. Also, correlation between chemical structures and ferula’s effects is not conclusive at the moment. As well as, because of the importance of breast cancer treatment and for preventing of some chemotherapy side effects, we sought to test the hypothesis that Farnesiferol C might exert oxidative stress situations and induce apoptosis in MCF-7 cell line. The purpose of the current research was to evaluate the cytotoxic and apoptosis induction activity of Farnesiferol C against MCF-7 human breast cancer cell line.
2. Materials and methods
2.1. Material
The fetal bovine serum (FBS), penicillin-streptomycin and cell culture medium (RPMI-1640) were obtained from Gibco BRL Life Technologies (Paisley, Scotland). The culture plates were acquired from SPL (Korea). MTT reagent [3 0-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide], dimethylsulfoxide (DMSO) and of Acridine orange and Ethidium bromide (AO/EtBr) was purchased from Sigma Aldrich (Germany). Annexin-V/PI (propidium Iodide) staining kit was acquired from Roche Corporation (Germany). The MCF-7 cells were acquired from Pasture Research Institute (Iran).
2.2. Farnesiferol C isolation and purification
Farnesiferol C (FC) has been isolated and purified from Ferula asafoetida according to the previously described methods [13].
2.3. Cell viability assay
For detection of the MCF-7 cell viability, we used the tetrazolium dye (MTT, Sigma) as previously described [18]. Briefly, the MDF-7 cells (1 × 104 cells/well) were seeded into 96-well plates in triplicate and cultured in the course of the night. After 24 h incubation, when the cells obtained standard confluency, the supernatant medium replaced with different concentrations of Farnesiferol C in complete medium plus DMSO (5–100 μM) for various times (24 h, 48 h and 72 h). The end of each treatment, the wells medium was eliminated and fresh medium including MTT reagent (last concentration, 0.5 mg/ml) was added to each well. Then, the plates were maintained for 4 h at 37 °C. The intracellular formazan blue crystals were dissolved in DMSO and the optical density of the solution was measured at 570 nm using ELISA reader (Bio-Tek, USA). The viability of each group was presented as percentage of the control absorbance [19].
2.4. Cell cycle analysis by flow cytometry
The cells (1 × 105) were seeded in 24 well plates and treated with 20 μM concentrations of Farnesiferol C for 24–72 h. At the end time, the cells were detached by trypsin-EDTA solution at 37 °C for 5 min. Next, the trypsin activity was stopped with adding 10% FBS–RPMI 1640 medium. Both adherent and detached cells were collected, washed in cold PBS twice, fixed by ice-cold ethanol (70% w/w) and then incubated in PBS containing 0.1%, Triton X-100,0.1% sodium citrate, RNase A (50 μg/ml; Fermentas), and PI (50 μg/ml; Sigma) at 4 °C for 30 min. The percent of calculated cells in the sub-G1, G0/G1, S, and G2/M phases were analyzed by flow cytometry (BD FACSCalibur flow cytometer, USA).
2.5. Annexin V/propidium iodide staining
This assay is based on the efficacy of the protein annexin V/PI to bind the apoptotic and necrotic cells. Firstly the cells were collected and washed with 100 μl prediluted binding buffer (10x) twice and resuspended in 100 μl binding buffer. Afterward, 2 μl annexin V-FITC (Ref No: 11–8005-74, Ebioscience company) were added and incubated for 10 min in the dark at room temperature. Then, the cells were centrifuged, resuspended in 200 μl binding buffer and finally 2 μl PI was added and examined by flow cytometry (BD FACSCalibur flow cytometer, USA).
2.6. Acridine orange/Ethidium bromide (AO/EtBr) double staining
This is an accurate and easy technique for detection of cell apoptosis and necrosis. The cells (2 × 104 cells/well) were seeded in cell culture palte (SPL, Korea) and treated with Farnesiferol C (at IC50 value) for 24–72 h. Then, cells were washed with PBS and stained by 20 μl of a mixture of AO/EB (1:1, 100 μg/ml) for 5 min. Finally, the photos were imaged by a fluorescence microscope (Olympus BX41, Germany). The viable cells appear uniformly green with acridine orange (AO) and ethidium bromide (EtBr) staining. Cells in the early stages of apoptosis are observed light green and orange to red color were detected in the late stage apoptotic cells and necrotic cells.
2.7. Measurement of oxidative stress markers in MCF-7 cells
24 h after seeding of MCF-7 cells in 12 well plates, cells exposed with 20 μM Farnesiferol C for 24, 48 and 72 h. Then collected samples were lysed for analyzing of antioxidant enzymes, Malondialdehyde (MDA) and total thiol content.
2.7.1. Determination of superoxide dismutase (SOD) activity
SOD activity in the cell lysate, was assayed using a method based on the capability of the enzyme to inhibit the autoxidation of pyrogallol. Briefly, 1 ml of Tris-Hcl (45 mM) buffer containing EDTA was mixed with 25 μl of the cell lysate supernatant. The unit was blanked at 420 nm and then 50 μl of pyrogallol (0.2 mM) was added to the above solution and quickly the absorbance of samples was measured at 420 nm every 15 s, up to two minutes. The inhibition of pyrogallol autoxidation is proportionate to the activity SOD present in the sample. Enzyme inhibitory capacity is defined as one unit of SOD [20]. The enzyme activity was presented as IU/mg of protein.
2.7.2. Catalase (CAT) activity assay
For the measurement of catalase activity, one unit of catalase was required to decompose 1 μM of H2O2 in 1 min. By adding 1.0 ml of 20 mM H2O2 (freshly prepared), the reaction was initiated. Decomposition level of H2O2 was determined by spectrophotometer at 240 nm for 2 min. The enzyme activity was presented as IU/mg of protein [21].
2.7.3. ROS detection
All biological systems constantly produce reactive oxygen species (ROS) during aerobic metabolism. One of the most widely used methods for the investigation of the intracellular ROS generation involve oxidizable fluorescent dyes, such as acetylated forms of 2′,7′-dichlorofluorescein (DCFH-DA). This dye is a stable compound that readily diffuses into cells and is hydrolyzed by intracellular esterase to yield DCFH which is trapped within cells. A wide range of ROS, including H2O2 or low-molecular weight hydroperoxides produced by cells oxidize DCFH to the highly fluorescent compound, 2′,7′-dichlorofluorescein (DCF). Thus, the fluorescence intensity is proportional to the amount of peroxide produced by the cells [22]. In the present study, MCF-7 cells were treated with IC50 value of FC, after 72 h were treated with 10 μM DCFH-DA for 30 min. Then, the treated cells were washed twice with PBS to remove the extracellular compound, and DCFH-DA fluorescence was detected using flow cytometry (BD FACSCalibur, USA).
2.7.4. Determination of total thiol content
Serum thiol groups are indicators of free radical damages. This sensitive factors decreases in oxidative damage situations. Total −SH groups were measured using DTNB (5, 5′-dithiobis- 2- nitrobenzoic acid) as the Ellman’s reagent, in which it reacts with the SH groups to produce a yellow colored complex which has a peak absorbance at 412 nm [23]. Briefly, 1 ml Tris EDTA buffer (pH 8.6) was added to 50 μl cell lysate and absorbance was read at 412 nm against Tris-EDTA buffer alone (A1). Then, 20 μl DTNB reagents (10 mM in methanol) was added to the mixture and after 15 min (stored in laboratory temperature), the sample absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mM) was calculated using the following equation:
| Total thiol concentration (mM) = (A2-A1-B) × 1.07/0.05 × 13.6 |
2.7.5. Measurement of MDA level
Concentration of MDA as a marker of lipid peroxidation was determined via thiobarbituric acid reactive substances (TBARS) assay in the MCF-7 cells lysate. It is a reliable colorimetric test for determining the lipid peroxidation levels. This method is based on the reaction of two molecules of thiobarbituric acid (TBA) with one molecule of MDA. The final soluble contains the substances which are responsible for the pink color [24]. Absorption of the samples was read at 532 nm using a UV–vis spectrophotometer (Jenway 6505, UK). The results are expressed as nmol TBARS per mg of protein.
3. Results
3.1. Cytotoxic effect of farnesiferol C on MCF-7
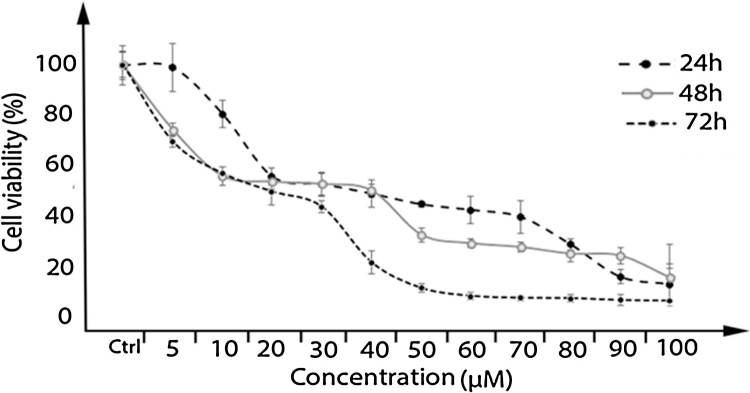
Farnesiferol C induced cell death was determined under MTT method in MCF-7 cell line. Our study results indicated that the cell proliferation was inhibited in a dose and time dependent manner. As shown in Fig. 1, incubation for 24, 48 and 72 h resulted in a dose and time dependent decrease in cell viability, with approximate IC50 of 43, 20 and 14 μM, respectively. Our results indicated that toxicity was mostly related to concentration.
Fig. 1.
The cytotoxicity effect of Farnesiferol C in different concentration (5–100 μM) on MCF-7 cells. MTT assay were done for determination of cell viability after 24, 48 and 72 h treatment with Farnesiferol C, resulted in a dose and time dependent decrease in cell viability, with approximate IC50 of 43, 20 and 14 μM, respectively. Data are presented as means ± S.E.M (n = 8), (p ˂ 0.05) with prism6 software.
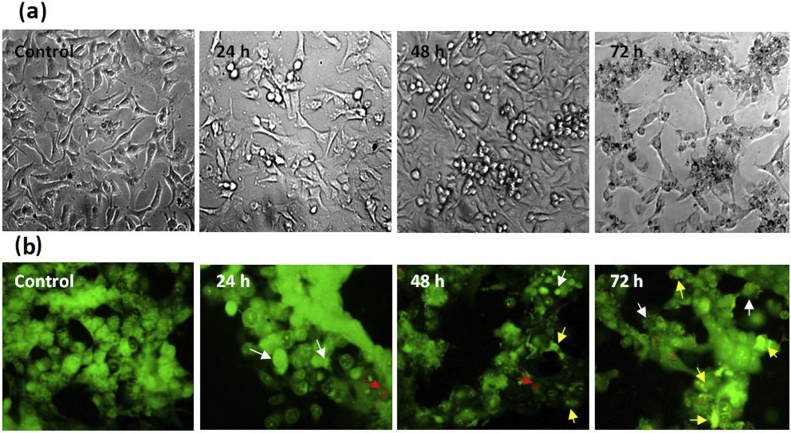
3.2. Morphological changes in MCF-7 cells treated with farnesiferol C
2 × 104 of MCF-7 cells were cultured in 96 wells plate. After 24 h, cells were exposed with 20 μM Farnesiferol C for 24, 48 and 72 h. Morphological changes of treated cells and control group were analyzed by inverted and fluorescence microscopy. Cytoplasmic vacuolization and shrinkage of treated cells was observed in comparison of control group by inverted microscope (Fig. 2a). For evaluation of apoptosis induction, MCF-7 cells were cultured in chamber slide. Then, the cells were exposed with Farnesiferol C (20 μM) for 24, 48 and 72 h and following AO/EtBr staining analyzed by fluorescence microscope. The obtained results showed the apoptotic effect of the FC, qualitatively. The arrows shown on Fig. 2b represent the condensed chromatin content and the fragmented nuclei during apoptosis.
Fig. 2.
Morphological changes in MCF-7 cells after 24–72 h treatment with Farnesiferol C. (a) Changes in the cells morphology was observed by inverted microscope. Cell death increased time-dependently from 24 to 72 h, as well as disruption of cell morphology was observed at 72 h. (b) Evaluation of the apoptotic cells by fluorescent microscope. White arrows indicate early apoptotic cells; yellow arrows indicate late apoptotic cells; red arrows indicate necrotic cells.
3.3. Farnesiferol C arrest cell cycle in MCF-7 cells
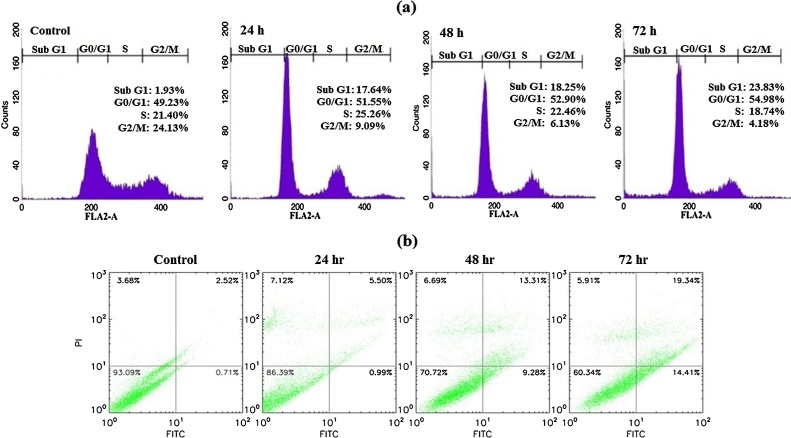
After treatment of cells for 24, 48 and 72 h with Farnesiferol C (20 μM), collected samples were analyzed by flow cytometry assay for determining of cell cycle phase. Detection of most treated cells in sub-G1 phase showed the strong evidence of inducing apoptosis. Farnesiferol C
induced a time-dependent increase in sub-G1 peak (apoptotic cells), so those accumulations in sub-G1 peak were nearly 15.71%, 16.32% and 21.9% of control cells after 24, 48 and 72 h treatment, respectively (Fig. 3a). Investigation of sub-G1 peak of cell cycle in treated MCF-7 cell line demonstrated an increase in apoptosis event during 24–72 h. Our results demonstrated that treated cells compared with the control group showed arrest at G0/G1. In general, an increase in G0/G1 phase population was observed after 24 h treatment and longer exposure (48 h) to the Farnesiferol C led to a further increase in the proportion of G0/G1 cells. This was accompanied by a slightly decrease in the proportion of S and a significant decrease in G2/M phase percentage. According to our data, percentage of untreated cells in the sub-G1, G0/G1, S and G2/M phases were calculated 1.93%, 49.23%, 21.40% and 24.13%, respectively. While these proportion for the cells treated with Farnesiferol C obtained 17.64%, 51.55%, 25.26% and 9.09% (for 24 h), 18.25%, 52.90%, 22.46% and 6.13% (for 48 h), 23.83%, 54.98%, 18.74% and 4.18% (for 72 h), respectively (Fig. 3a).
Fig. 3.
Evaluation of cell cycle and induction of apoptosis in MCF-7 cells treated with Farnesiferol C (20 μM) after 24–72 h. (a) Farnesiferol C caused an increase in sub G1 phase, as well as cell cycle arrest in G0/G1 phase in a time dependent manner. Investigation of sub-G1 peak of cell cycle in treated MCF7 cells demonstrated an increase in apoptosis event during 24–72 h. Our results demonstrated that treated cells compared with the control group showed arrest at G0/G1. In general, an increase in G0/G1 phase population was observed after 24 h treatment and longer exposure (48 h) to the Farnesiferol C led to a further increase in the proportion of G0/G1 cells. (b) Annexin V/PI double staining assay showed that the rate of early apoptosis (Annx+/PI−) and late apoptosis (Annx+/PI+) in the cells treated with Farnesiferol C increased in a time dependent manner.
3.4. Farnesiferol C induces apoptosis in MCF-7 cells
To quantitative assessment of apoptosis, we investigated redistribution of the plasma membrane of cells (as hallmark of apoptosis) to visible phosphatidyl serine (PS) after double staining by annexin-V/PI. After 24–72 h treatment of the cells with 20 μM FC, the cells were investigated for the detection of early, late apoptosis and necrosis by flow cytometry. As illustrated in Fig. 3b, the translocation of phosphatidyl serine (Annexin V positive) was increased after 24, 48 and 72 h. The rate of early apoptosis (Annx+/PI−) and late apoptosis (Annx+/PI+) in the cells treated with Farnesiferol C was calculated 0.99%, 9.28% and 14.4%1 percent and 5.50%, 13.31%, 19.34% percent after 24, 48 and 72 h, respectively. These findings represent an increase in rate of apoptosis in a time dependent manner (Fig. 3b).
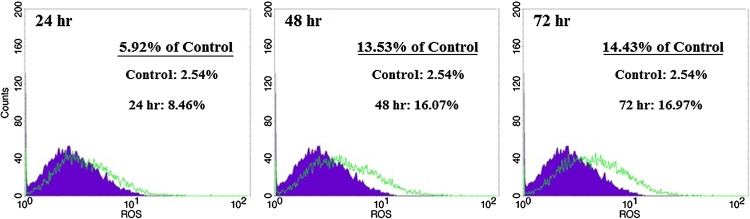
3.5. ROS evaluation
It has been recently shown that chemotherapy agents can induce apoptosis in cancer cells via increase in ROS generation or decrease in ROS scavenging capacity [25]. So, we decided to assay stress oxidative factors in the present tested compound. To determine the effect of Farnesiferol C on production of intracellular ROS, we used DCFH-DA staining. Fig. 4 demonstrated that ROS levels in the cells treated with Farnesiferol C (20 μM), significantly have been increased. Amount of ROS levels were calculated by flow cytometry 8.46%, 16.07% and 16.97%, after 24, 48 and 72 h of treatment, respectively. It was observed a time dependently (24–72 h) right shift in MCF-7 cells treated with Farnesiferol C in comparison with untreated cells (Fig. 4). It seems that increase in cell death is related to the oxidative stress situation.
Fig. 4.
Investigation of ROS levels in MCF-7 cells treated with Farnesiferol C (IC50 value) after 24–72hr. After 24–72 h treatment of the MCF-7 cells with 20 μM Farnesiferol C, the cells were incubated with DCHF-DA and detected by flow-cytometry. A right shift as a percentage in ROS level was observed in comparison with control group. It demonstrated that ROS level increased 5.92%, 13.53% and 14.43% after 24, 48 and 72 h exposure, respectively.
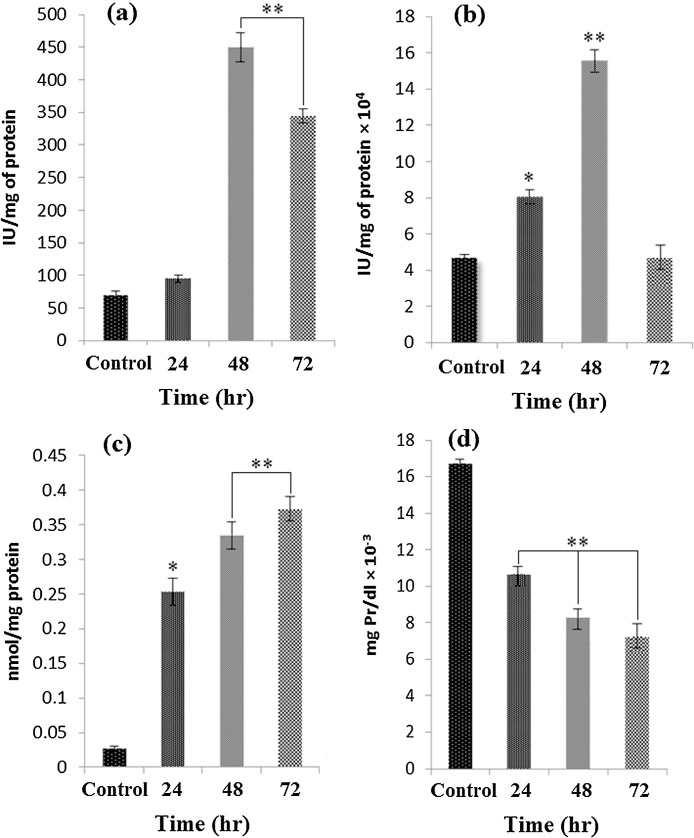
3.6. Evaluation of stress oxidative parameters
In order to detection the effect of Farnesiferol C on the cellular redox status in MCF-7 cells, antioxidant defense system capabilities, lipid peroxidation and protein oxidation were evaluated according to appropriate methods reported in materials and methods. SOD and CAT play vital role in the cellular antioxidant defense mechanism. The activities of these enzymes (IU/mg of protein) in MCF-7 cells line were increased significantly in Farnesiferol C treated cells for 24 and 48 h compared to controls (P < 0.001). However, it was observed a significant decrease in the activity of both enzymes after 72 h (Fig. 5a and b). In general, Farnesiferol C led to reduce the level of antioxidant enzymes after 72 h of treatment. Lipid peroxidation (LPO) is refers to the oxidation of lipids by free radicals and it is one of the main manifestations of oxidative damage in tissues and cells [26]. Our results indicated that the formation of TBARS levels as indicator of lipid peroxidation increased in the cells treated with 20 μM Farnesiferol C after 24, 48 and 72 h compared to control groups (Fig. 5c). Reduction in total thiol levels in cells is a vital marker of oxidative stress [27]. As illustrated in Fig. 5d, the cells treated with Farnesiferol C (20 μM) significantly decreased total thiol time dependently after 24–72 h. 72 h after exposure of the cells to 20 μM FC, the amount of total thiol reduced to 7.2. μmol/mg Pr which was about 2.5 fold lower than untreated cells (16.7.μmol/mg Pr). It seems Farnesiferol C has a critical role in induction of oxidative stress situation.
Fig. 5.
Effect of Farnesiferol C on the activities of SOD (a), CAT (b), MDA (c) and total thiol (d) levels in MCF-7 cells after 24–72 h. The cells were exposed to 20 μM Farnesiferol C for 24, 48 and 72 h. Then, stress oxidative parameters were measured. Increase in intracellular SOD and CAT activities was observed 24 and 48 h of treatment with Farnesiferol C. In addition, increase in amount of MDA level and decrease in total thiol content were seen in the treated cells after 24–72 h. Values are mean ± SEM (n = 6), (* p < 0.01, **p < 0.001).
4. Discussion
Coumarins have attracted intense interest in recent decades because of their different pharmacological and biological properties. Among these characteristic, their oxidative stress situation, cytotoxic and anticancer effects have been featured [6]. According to previous studies, coumarins have potential to inhibit the dynamic growth of tumor from lymphocytes, stomach, colon and liver tissues in a dose-dependent manner [28].
Different species isolated from the genus Ferula (Apiaceae) have had various therapeutic applications for many centuries. Many biological property of this genus such as cytotoxicity, antiviral, antibacterial, P-glycoprotein (P-gp) inhibitory and anti-inflammatory activity have been established until yet [13], [29]. Ferula asafoetida in gastro intestinal tract is an effective remedy for stomach diseases and it is applied as a best remedies in the digestive powders [30]. Some studies have been shown that Farnesiferol C as a constituent of Ferula asafoetida has anti-angiogenesis feature [31]. This compound has showed potent cytotoxic and anti-proliferative activities in various human tumors, including A549, SKOV-3 (human ovary cancer cell line), SK-MEL-2 (human melanoma cancer cell line) [28]. In this study, we hypothesized that Farnesiferol C might exert oxidative stress situations-induced apoptosis in MCF-7 human breast cancer cell line. There are several documents that exhibit coumarins and some subfamilies of them such as ferula’s genus might be efficient in the treatment of some diseases such as cancers. For example a series of novel coumarin-3-carboxamides act as a potent antioxidant and anti-inflammatory factors [32]. Furthermore, some ingredient of assafoetida has a significant inhibition on lipid peroxidation in the liver of rats [30]. Our study showed that Farnesiferol C significantly increased MDA levels in MCF-7 cells (Fig. 5c). It demonstrated that Farnesiferol C might be appropriate candidate for breast cancer treatment for the reason of inducing oxidative stress situation in MCF-7 cells. Poonam Mahendra and coworker concluded that assafoetida genus significantly reduce the levels of cytochrome P450 and cytochrome b5 in rats. There also was an enhancement in the activities of glutathione-S-transferase, deoxythymidine- diaphoresis, superoxide dismutase (SOD), catalase (CAT), and reduced glutathione [30]. Differential activation of proteins involved in oxidative stress and cell damage during progression of carcinogenesis in rats have also been reported [33]. Our investigation exhibited that FC, as a constituent of F. assafoetida, increased cellular ROS, SOD and CAT activities in 24 and 48 h and reduced activity of these enzymes after 72 h exposure (Fig. 5). Reduce the activity of these enzymes (after 72 h) may be due to increase in amount of apoptotic cells [24]. On the other words, increase in free radicals at earlier stages (24 and 48 h) (Fig. 4) lead to increase in activity and level of the antioxidant enzymes (SOD and CAT) (Fig. 5a and b). Followed by a further increase in oxidative stress with a subsequent increase in apoptotic cells (after 72 h), the activities of enzymes were reduced. Total thiol level also decreased time dependently after 24, 48 and 72 h. According to these findings, Farnesiferol C may be a good candidate for breast cancer treatment by reducing in anti-oxidant enzymes activity and increase in oxidative stress levels. Although recent study showed that coumarin had just a mild anticancer property against the allogeneic Sarcoma-180 with toxic side effect [34]. Another investigation have demonstrated that galbanic acid (GBA), a major compound of Ferula assafoetida, was known to have cytotoxic, anti-angiogenic and apoptotic effects in prostate cancer and cytotoxicity in A549 and wild EGFR type H460 cells. Also, GBA meaningfully activated caspase-9 and Bax and reduced the expression of Bcl-2, Bcl-xL in H460 cells [35]. In our study, Farnesiferol C induced apoptosis via enhancing intracellular ROS generation and induction of oxidative stress (Fig. 4, Fig. 5). However, it has been recently reported anti-proliferative and pro-apoptotic properties of dendrosomal Farnesiferol C (DFC) with increase of Bax/Bcl-2 ratio as a potent indicator of apoptosis on the AGS gastric cancer cells [36]. In addition, anti-angiogenic characteristic of Farnesiferol C contribute to anticancer efficacy. In human umbilical vein endothelial cells (HUVEC), exposure to the Farnesiferol C inhibited vascular endothelial growth factor (VEGF), so inhibited cell proliferation, migration, metastasis, and the expression of matrix metalloproteinase-2 that has a critical role in metastasis [31].
Another investigation revealed that the combination of low level doses of Farnesiferol C with doxorubicin increased in intracellular accumulation of Rhodamine 123 in MCF-7/Adr cells [13]. It has been demonstrated that coumarin compounds might inhibit cell proliferation by intervening with mitotic spindle microtubule structure and function [6]. Some class of coumarins display anti-proliferative property on M4Beu cells (human metastatic pigmented malignant melanoma cell line) through cell cycle arrest in G1 phase and apoptosis inducing features on A549 (human lung cancer cell line) through mitochondrial dependent mechanisms [36]. We also observed G0/G1 cell cycle arrest in MCF-7 cells treated with Farnesiferol C (Fig. 3a). Based on these data, Farnesiferol C possesses significant anticancer activity through regulation of free radicals generation and can be proposed as effective agents for more investigation in future.
5. Conclusion
Herein, we evaluated apoptosis induction activity of Farnesiferol C depends on oxidative stress in MCF-7 human breast cancer cell line. Annexin V/PI method and florescent microscopy showed that Farnesiferol C induces apoptosis in MCF-7 cells in a time dependent manner. Farnesiferol C increased cellular SOD and CAT activities and also ROS and MDA levels. According to change in these oxidative stress parameters and induction of apoptosis in MCF-7 cells, we can conclude that Farnesiferol C has potential role as a therapeutic factor to inducing oxidative stress, however further experiments are needed to evaluate.
Conflict of interests
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
The authors appreciate support of this investigation by the research council of University of Tabriz, Tabriz, Iran. We would like to thank Dr. Iranshahi for providing Farnesiferol C and also thanks Dr. Reza Rahbarghazi, Narges Seyfizadeh and Nayer Seyfizadeh to help us during the work.
References
- 1.Chin Y.-W., Balunas M.J., Chai H.B., Kinghorn A.D. Drug discovery from natural sources. AAPS J. 2006;8:E239–E253. doi: 10.1007/BF02854894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. [Google Scholar]
- 3.Holetz F.B., Pessini G.L., Sanches N.R., Cortez D.A.G., Nakamura C.V., Dias Filho B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memórias do Instituto Oswaldo Cruz. 2002;97:1027–1031. doi: 10.1590/s0074-02762002000700017. [DOI] [PubMed] [Google Scholar]
- 4.Gliszczyńska A., Brodelius P.E. Sesquiterpene coumarins. Phytochem. Rev. 2012;11:77–96. [Google Scholar]
- 5.Allaker R.P., Douglas C.I. Novel anti-microbial therapies for dental plaque-related diseases. Int. J. Antimicrob. Agents. 2009;33:8–13. doi: 10.1016/j.ijantimicag.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Kostova I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem.-Anti-Cancer Agents. 2005;5:29–46. doi: 10.2174/1568011053352550. [DOI] [PubMed] [Google Scholar]
- 7.Hoult J., Paya M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen. Pharmacol.: Vasc. Syst. 1996;27:713–722. doi: 10.1016/0306-3623(95)02112-4. [DOI] [PubMed] [Google Scholar]
- 8.Jain P., Joshi H. Coumarin: chemical and pharmacological profile. J. Appl. Pharm.Sci. 2012;2:236–240. [Google Scholar]
- 9.Madari H., Panda D., Wilson L., Jacobs R.S. Dicoumarol A Unique Microtubule Stabilizing Natural Product that Is Synergistic with Taxol. Cancer Res. 2003;63:1214–1220. [PubMed] [Google Scholar]
- 10.Smith G.F., Neubauer B.L., Sundboom J.L., Best K.L., Goode R.L., Tanzer L.R., Merriman R.L., Frank J., Herrmann R.G. Correlation of the in vivo anticoagulant, antithrombotic, and antimetastatic efficacy of warfarin in the rat. Thromb. Res. 1988;50:163–174. doi: 10.1016/0049-3848(88)90184-3. [DOI] [PubMed] [Google Scholar]
- 11.Lewis A., Ough M., Li L., Hinkhouse M.M., Ritchie J.M., Spitz D.R., Cullen J.J. Treatment of pancreatic cancer cells with dicumarol induces cytotoxicity and oxidative stress. Clin. Cancer Res. 2004;10:4550–4558. doi: 10.1158/1078-0432.CCR-03-0667. [DOI] [PubMed] [Google Scholar]
- 12.Valiahdi S.M., Iranshahi M., Sahebkar A. Cytotoxic activities of phytochemicals from Ferula species. DARU J. Pharm. Sci. 2013;21:39. doi: 10.1186/2008-2231-21-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazari Z.E., Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother. Res. 2011;25:315–323. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 14.Lee C.-L., Chiang L.-C., Cheng L.-H., Liaw C.-C., Abd El-Razek M.H., Chang F.-R., Wu Y.-C. Influenza A (H1N1) antiviral and cytotoxic agents from Ferula assa-foetida. J. Nat. Prod. 2009;72:1568–1572. doi: 10.1021/np900158f. [DOI] [PubMed] [Google Scholar]
- 15.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J. Pharm. Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 16.Dhillon H., Chikara S., Reindl K.M. Piperlongumine induces pancreatic cancer cell death by enhancing reactive oxygen species and DNA damage. Toxicol. Rep. 2014;1:309–318. doi: 10.1016/j.toxrep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousavi S.M., Montazeri A., Mohagheghi M.A., Jarrahi A.M., Harirchi I., Najafi M., Ebrahimi M. Breast cancer in Iran: an epidemiological review. Breast J. 2007;13:383–391. doi: 10.1111/j.1524-4741.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 18.Rahimi R., Mahdavi M., Pejman S., Zare P., Balalaei S. Inhibition of cell proliferation and induction of apoptosis in K562 human leukemia cells by the derivative (3-NpC) from dihydro-pyranochromenes family. Acta Biochim. Pol. 2015;62:83–88. doi: 10.18388/abp.2014_825. [DOI] [PubMed] [Google Scholar]
- 19.Gerlier D., Thomasset N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 20.Flohe L. [10] Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- 21.Chance B., Maehly A. [136] Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- 22.Eruslanov E., Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry, Advanced protocols in oxidative stress II. Methods Mol. Biol. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 23.Patel B.P., Rawal U.M., Dave T.K., Rawal R.M., Shukla S.N., Shah P.M., Patel P.S. Lipid peroxidation, total antioxidant status, and total thiol levels predict overall survival in patients with oral squamous cell carcinoma. Integr. Cancer Ther. 2007;6:365–372. doi: 10.1177/1534735407309760. [DOI] [PubMed] [Google Scholar]
- 24.Buege J.A., Aust S.D. [30] microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 25.Khoshtabiat L., Mahdavi M., Dehghan G., Rashidi M. Oxidative stress-Induced apoptosis in chronic myelogenous leukemia K562Cells by an active compound from the dithio-Carbamate family. Asian Pac. J. Cancer Prev.: APJCP. 2016;17:4267. [PubMed] [Google Scholar]
- 26.Hodges D.M., DeLong J.M., Forney C.F., Prange R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- 27.Anwar M.M., Meki A.-R.M. Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp. Biochem. Physiol. Part A: Mol. Integ. Physiol. 2003;135:539–547. doi: 10.1016/s1095-6433(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 28.Weber U., Steffen B., Siegers C. Antitumor-activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res. Commun. Mol. Pathol. Pharmacol. 1998;99:193–206. [PubMed] [Google Scholar]
- 29.Kasaian J., Iranshahy M., Iranshahi M. Synthesis, biosynthesis and biological activities of galbanic acid–a review. Pharm. Biol. 2014;52:524–531. doi: 10.3109/13880209.2013.846916. [DOI] [PubMed] [Google Scholar]
- 30.Mahendra P., Bisht S. Ferula asafoetida: traditional uses and pharmacological activity. Pharmacogn. Rev. 2012;6:141. doi: 10.4103/0973-7847.99948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J.-H., Choi S., Lee Y., Lee H.-J., Kim K.-H., Ahn K.-S., Bae H., Lee H.-J., Lee E.-O., Ahn K.-S. Herbal compound farnesiferol C exerts antiangiogenic and antitumor activity and targets multiple aspects of VEGFR1 (Flt1) or VEGFR2 (Flk1) signaling cascades. Mol. Cancer Ther. 2010;9:389–399. doi: 10.1158/1535-7163.MCT-09-0775. [DOI] [PubMed] [Google Scholar]
- 32.Melagraki G., Afantitis A., Igglessi-Markopoulou O., Detsi A., Koufaki M., Kontogiorgis C., Hadjipavlou-Litina D.J. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur. J. Med. Chem. 2009;44:3020–3026. doi: 10.1016/j.ejmech.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Moreira A.J., Rodrigues G., Bona S., Cerski C.T., Marroni C.A., Mauriz J.L., González-Gallego J., Marroni N.P. Oxidative stress and cell damage in a model of precancerous lesions and advanced hepatocellular carcinoma in rats. Toxicol. Rep. 2015;2:333–340. doi: 10.1016/j.toxrep.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosskopf F., Kraus J., Franz G. Immunological and antitumor effects of coumarin and some derivatives. Die Pharmazie. 1992;47:139–142. [PubMed] [Google Scholar]
- 35.Unnikrishnan M., Kuttan R. Tumour reducing and anticarcinogenic activity of selected spices. Cancer Lett. 1990;51:85–89. doi: 10.1016/0304-3835(90)90235-p. [DOI] [PubMed] [Google Scholar]
- 36.Aas Z., Babaei E., Hosseinpour Feizi M., Dehghan G. Anti-proliferative and apoptotic effects of dendrosomal farnesiferol C on gastric cancer cells. Asian Pac. J. Cancer Prev. 2015;16:5325–5329. doi: 10.7314/apjcp.2015.16.13.5325. [DOI] [PubMed] [Google Scholar]