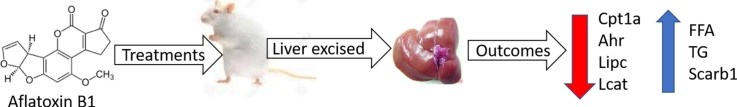
Graphical abstract
Abbreviations: Ahr, aryl hydrocarbon receptor; Cpt1a, carnitine palmitoyl transferase 1A; Lipc, hepatic lipoprotein lipase; Lcat, lecithin – cholesterol acyltransferase; Scarb1, scavenger receptor class B member 1
Keywords: Aflatoxin B1, Liver, Lipid, Lipoprotein, Gene expression
Highlights
-
•
Aflatoxin B1 is a natural food toxicant that induces hepatotoxicity.
-
•
Acute exposure to aflatoxin B1 increased plasma and liver lipids, and downregulated CPT1 while SCARB was upregulated.
-
•
The dysregulation of lipid and lipoprotein metabolizing genes may be associated with increasing the risk of CHD.
Abstract
In this study, alterations in lipid metabolism associated with acute aflatoxin B1 (AFB1) induced hepatotoxicity and gene expression changes underlying these effects were investigated. Rats were orally administered three doses (0.25 mg/kg, 0.5 mg/kg and 1.0 mg/kg) of AFB1 for seven days; after which blood was collected and liver excised. Lipid profiles of plasma and liver were determined spectrophotometrically while the expression of genes associated with lipid and lipoprotein metabolism was assayed by reverse transcriptase polymerase chain reaction. Acute exposure to AFB1 increased the levels of plasma and liver cholesterol, triglycerides and phospholipids. AFB1 at 0.5 mg/kg and 1.0 mg/kg resulted in a dose-dependent (1.2 and 1.5 fold, respectively) downregulation of hepatic Cpt1a with a concomitant 1.2 and 1.5 fold increase in the level of plasma FFA, respectively. A similar observation of 1.2 and 1.3 fold increase was also observed in plasma triglyceride concentration, at both respective doses. AFB1 also decreased the relative expression of Ahr, Lipc and Lcat whereas, it upregulated Scarb1 in a dose dependent manner. AFB1-induced dysregulation of the expression of lipid and lipoprotein metabolizing genes may be one mechanism linking AFB1 to altered lipid metabolism and ultimately risk for coronary heart disease.
1. Introduction
Aflatoxins are secondary metabolites synthesized by Aspergillus fungi particularly Aspergillus flavus and Aspergillus parasiticus [1], [2]. They contaminate food and feedstuff most especially grains and nuts during pre - or post-harvest conditions in tropical regions specifically sub-Saharan Africa and Southeast Asia [1], [3]. Among the naturally occurring aflatoxins that contaminate food significantly (aflatoxin B1, aflatoxin B2, aflatoxin G1, aflatoxin G2), aflatoxin B1 (AFB1) is the most common and most toxic, and the liver is its key target organ [4], [5], [6]. In the liver, AFB1 is biotransformed by microsomal cytochrome P450 to a highly reactive intermediate, AFB1-8, 9-epoxide which binds to nucleic acids to form adducts [4], [7], [8]. These adducts could block transcription and translation, thereby affecting the regulation of functional gene expression and ultimately causing hepatotoxicity [5]. AFB1-induced hepatotoxicity also results from accumulation of reactive oxygen species, which are precursors of hydroxyl radicals that interact with DNA and lead to mutations [9], [10]. AFB1 also induces apoptosis, cytotoxicity and genotoxicity in human hepatocytes (HepG2 cells) [11], [12].
Acute aflatoxicosis resulting from exposure to high doses of AFB1 through the diet over a short period causes hepatotoxicity while chronic aflatoxicosis resulting from exposure to low doses of AFB1 through the diet over a long period of time has been implicated in hepatocellular carcinoma [1], [13]. Although acute aflatoxicosis is less common compared with chronic aflatoxicosis it occurs occasionally and such outbreaks have been reported in Kenya [14], [15], [16]. In an attempt to search for potential biomarkers- using transcriptomics and metabolomics- for earlier detection of AFB1 induced acute hepatotoxicity, Lu et al. [17] reported that gluconeogenesis and lipid metabolism disorders are major metabolic effects following acute AFB1 exposure.
Lipids are molecules that play key roles in metabolic pathways and the lipids of clinical and physiological significance are fatty acids, triglycerides, cholesterol and phospholipids [18]. These lipids are transported in the blood as lipoproteins which are made of a hydrophobic core surrounded by a hydrophilic layer [18], [19]. Disturbances in the homeostasis of these lipids and lipoproteins resulting in dyslipidemia characterized by hypertriglyceridemia, low HDL-cholesterol and elevated LDL- cholesterol are associated with various diseases including cardiovascular disease [20], [21], [22], [23], [24], [25]. Specifically abnormalities in lipoprotein levels and oxidation of LDL have been found to play a role in the development of cardiovascular diseases in humans [26], [27].
AFB1 has been reported to cause alterations in plasma and liver lipid levels [2], [4], [17]. However, the doses at which these effects occur and the mechanisms underlying these alterations need further exploration. Lu et al. [17] reported that gene expression analysis and metabolite profiling are more sensitive than general toxicity studies for the detection of earlier hepatotoxicity induced by AFB1. The LD50 of AFB1 has been reported to be 2.71 mg/kg and the authors reported that no mortality was observed in rats treated with 1.0 mg/kg AFB1 [28]. Therefore, this study investigated the effects of acute oral exposure to three doses (0.25, 0.5 and 1.0 mg/kg) of AFB1, the doses were selected to ensure there was no death during the experimental period, on lipid and lipoprotein metabolism in rats and assessed expression of genes in pathways relevant to lipid metabolism.
2. Materials and methods
2.1. Chemicals
AFB1 was a product of Sigma-Aldrich (St. Louis, MO). Reagent diagnostic kits were products of BioSino Biotechnology & Science Inc. (Beijing, China). RNAlater® and RNA extraction spin column kit were products of Aidlab Biotechnologies Co. Ltd (Beijing, China) while TransGen EasyScript® one-step RT-PCR kit was a product of TransGen Biotech Co. Ltd (Beijing, China). All other chemicals used in this study, unless otherwise stated, were products of Sigma-Aldrich (St. Louis, MO).
2.2. Animals
Twenty 10-week old inbred male albino rats weighing between 100 and 150 g were used for this research. The rats were housed in clean cages, subjected to standard 12-h light and dark cycles and had access to feed and clean tap water ad libitum. The animals were allowed to acclimatize to their environment for one week before the experiment started. The experiment was conducted, and animals cared for in accordance with the declaration of Helsinki.
2.3. Treatment protocol and tissue collection
The rats were randomly distributed into four treatment groups of five rats each and treated with 0, 0.25, 0.5 or 1.0 mg/kg body weight AFB1. AFB1 in olive oil was administered by oral gavage for seven days while the control rats received equal volume of olive oil alone. The rats were sacrificed on the eighth day, after an overnight fast, under anesthesia and blood collected by cardiac puncture. Blood and liver were processed as previously described by Rotimi et al. [29] while a portion of the left lobe was preserved in RNAlater® and another portion kept in 10% formalin for histological studies. Plasma was obtained from whole blood by centrifugation at 3000 rpm for 15 min.
2.4. Biochemical analysis
2.4.1. Plasma lipid profiles
Total cholesterol and triglyceride concentrations in the plasma were determined using commercially available kits according to the manufacturer’s instructions. Total HDL and HDL3 were recovered from the plasma using dextran sulfate-MgCl2 precipitation at the final concentration of 10 mg/mL dextran sulfate, 0.5 M MgCl2, 0.05% NaN3 and 19.1 mg/mL dextran sulfate, 1.95 M MgCl2, 0.05% NaN3, respectively [30], [31]. The supernatant, containing the lipoprotein, was recovered after centrifugation at 1500 g for 30 min. Cholesterol and triglyceride concentrations were determined in this supernatant with the same commercial kits used for total cholesterol and triglyceride.
Plasma phospholipids were determined as described by Rifai et al. [30], using 1-amino-2-naptho-4-sulphonic acid reagent, while free fatty acids (FFA) were determined spectrophotometrically at 620 nm as described by Rotimi et al. [32].
2.4.2. Liver lipid profiles
Lipids were extracted from the liver according to the method of Folch et al. [33] and aliquots of the extract was used for determining cholesterol, triglycerides and phospholipids as earlier described by Rotimi et al. [34].
2.5. RNA extraction
RNA was extracted from RNAlater® – stabilized liver using the Aidlab spin column RNA extraction kit according to the instructions of the manufacturer. Concentration and purity of extracted RNA was determined at 260 nm and 280 nm using a NanoDrop® 2000 spectrophotometer (Thermo Scientific). RNA samples were kept at −80 °C until gene expression analysis.
2.6. Expression of lipid metabolizing genes
The levels of expression of some lipid metabolizing genes were assessed in the liver using semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR) as previously described by Rotimi et al. [35]. Briefly, the RT-PCR was carried out with 500 ng RNA template using TranGen EasyScript one-step RT-PCR kit according to manufacturer’s instructions. The RNA samples were subjected to an initial 30 min incubation at 45 °C for cDNA synthesis after which PCR amplification was carried out, using gene specific primers (GSP) (Table 1), at 94 °C for 5 min followed by 40 cycles of 94 °C for 30 s, 5 min at the annealing temperature of GSP, and 1 min at 72 °C. All amplifications were carried out in C1000 Touch™ Thermal Cycler (Bio-Rad Laboratories, Hercules, CA). After PCR, amplicons were visualized on 1.2% agarose gel in 1X Tris Borate EDTA buffer using UVP BioDoc-It™ Imaging system (Upland, CA, USA). The intensity of the bands were analyzed using Image J software [36]. Results were presented as relative expression (ratio of intensity of each gene to that of β-actin, Actb) of each gene in comparison with a housekeeping (β-actin, Actb) gene. There were no changes in the expression of the housekeeping gene across the treatment groups.
Table 1.
Sequences of gene – specific primers.
| Gene | Sequence (5′–3′) | Template |
|---|---|---|
| Ahr | Forward: GGGCCAAGAGCTTCTTTGATG | NM_001308255.1 |
| Reverse: GCAAGTCCTGCCAGTCTCTGA | ||
| Lipc | Forward: GAGCCCAGTCCCCCTTCA | NM_012597.2 |
| Reverse: ATGTCATTCTTTGCTGCGTCTC | ||
| Scarb1 | Forward: GGCAAATTTGGCCTGTTCGT | NM_031541.1 |
| Reverse: CCACAGCAATGGCAGGACTA | ||
| Lcat | Forward: AACTGGCTGTGCTACCGAAA | NM_017024.2 |
| Reverse: TAGGTCTTGCCAAAGCCAGG | ||
| Cpt1a | Forward: AAGTCAACGGCAGAGCAGAG | NM_031559.2 |
| Reverse: ACGCCCAAGTATTCACAGGG | ||
| Actb | Forward: GTCAGGTCATCACTATCGGCAAT | NM_031144.3 |
| Reverse: AGAGGTCTTTACGGATGTCAACGT |
2.7. Histopathology
A portion of liver samples were fixed in 10% formalin immediately after harvesting. The tissues were processed by cutting pieces in tissue cassettes with an automated tissue processor. They were then embedded in paraffin and thereafter were sectioned with microtome. The slides were then stained with haematoxylin and eosin.
2.8. Statistical analysis
Data were expressed as Mean ± SEM of five replicates. Analysis of variance (ANOVA) was carried out to test for level of homogeneity at p < 0.05 among the groups while Duncan multiple range test was used to separate the heterogeneous groups. The ANOVA results was subjected to contrast analysis to determine the linear dose-response (trend analysis) among the groups using linear polynomial.
3. Results
3.1. Histology results
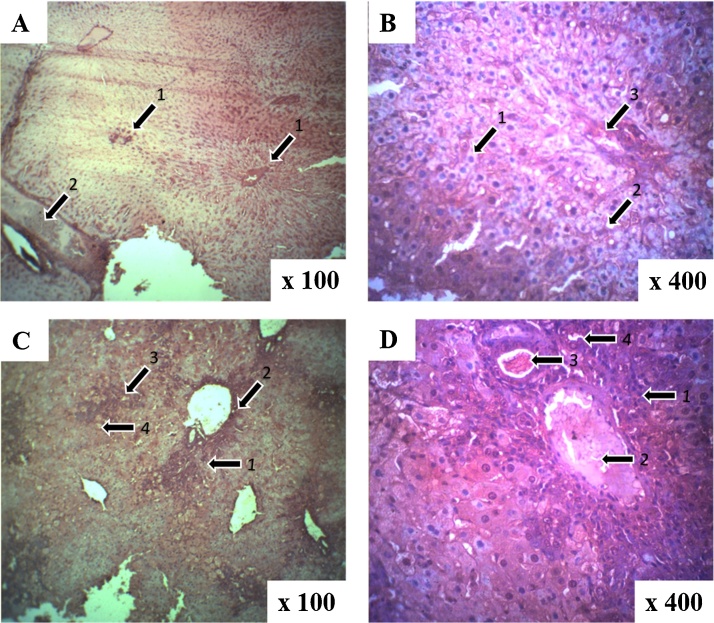
A comparison of the sections of the liver of control and AFB1 treated animals showed that 0.25 mg/kg AFB1 resulted in minimal congestion of hepatic lobules and microvesicular degeneration with few macrovesicles. Meanwhile the liver of rats treated with 0.50 mg/kg AFB1 indicated additional damages like infiltration by inflammatory cells around the porta triad as well as congestion of the sinusoid and the vessels. Further damage to the liver was observed at 1.0 mg/kg AFB1 treatment which included central vein (porto-central) inflammation, numerous macrovesicles with inflammatory infiltrates on the portal triad extending and reaching the contiguous portal triad (porto-porto), and associated proliferation of the bile ductules (Fig. 1).
Fig. 1.
Photomicrograph of liver tissues after aflatoxin B1 treatments with hematoxylin and eosin staining. Representative slides are shown for each of the four exposure groups: (A) control showing: (1) portal triad and (2) central vein (B) 0.25 mg/kg AFB1 showing: (1) microvesicles, (2) macrovesicles and (3) portal triad (C) 0.50 mg/kg AFB1 showing: (1) inflammatory cells, (2) portal triad, (3) central vein and (4) microvesicles and (D) 1.0 mg/kg AFB1 showing (1) inflammatory cells, (2) hepatic artery, (3) portal vein and (4) bile duct. Compared to the control group, pathology of the 0.25 mg/kg was minimal. In the 0.5 mg/kg group, sinusoid congestion was evident along with inflammatory infiltration around the portal triad. In the highest dose group, inflammation extended to the central vein and the portal triad along with profileration of the bile ductules.
3.2. Lipid profiles
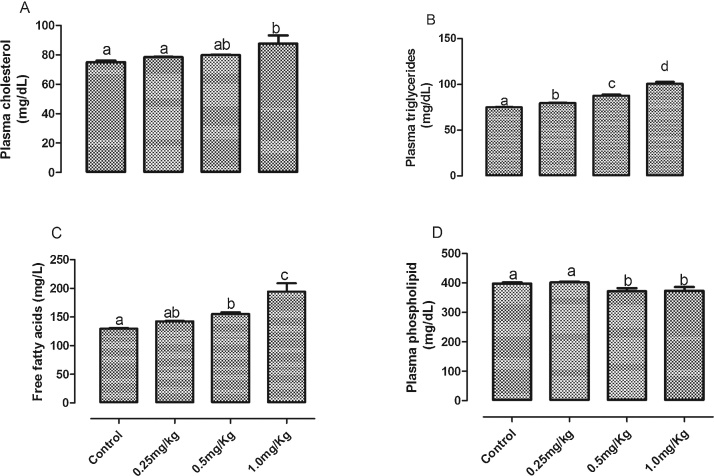
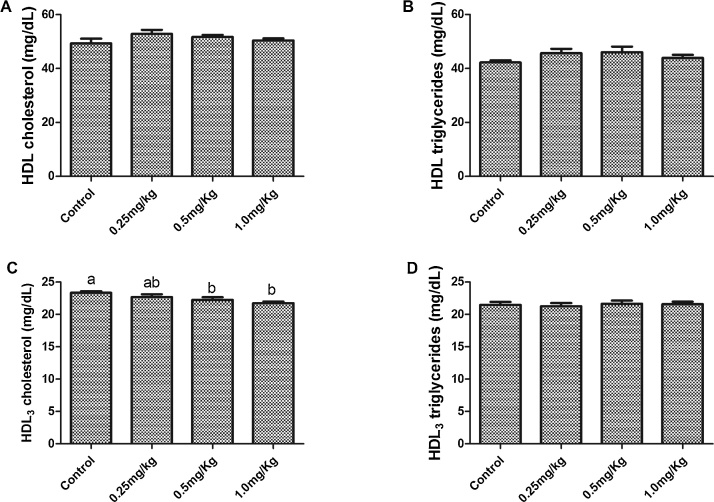
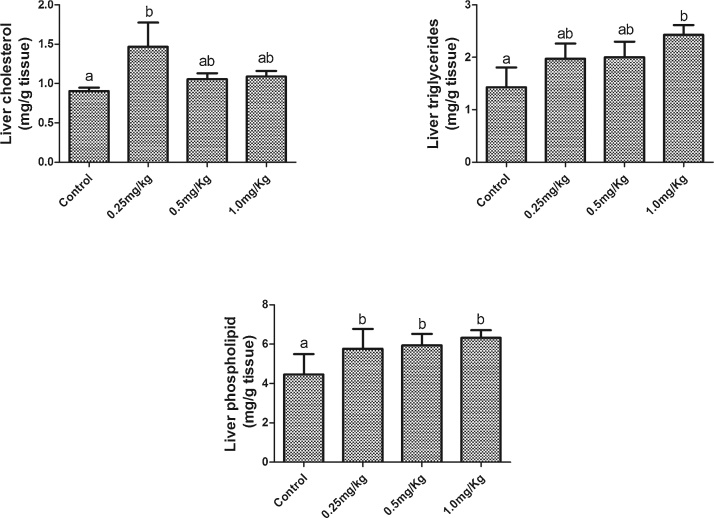
Acute exposure to AFB1 significantly (p < 0.05) increased plasma triglyceride (Fig. 2A) and FFA (Fig. 2B) in a dose dependent manner with a significant (p < 0.001) linear trend. While only the highest dose (1.0 mg/kg) significantly (p < 0.05) increased plasma total cholesterol compared to the control group, there was a significant (p = 0.006) trend for a dose-dependent increase across groups. However, plasma phospholipid was significantly decreased in the groups exposed to 0.5 mg/kg and 1.0 mg/kg AFB1. Plasma phospholipid of the group that received the lowest dose was not significantly (p > 0.05) different from the control group. A significant decrease in HDL3 cholesterol was observed in groups exposed to 0.5 mg/kg and 1.0 mg, there was a significant (p = 0.004) trend for a dose-dependent decrease across groups. However, there was no significant difference in HDL cholesterol (Fig. 3A), HDL-triglyceride (Fig. 3B) and HDL3-triglyceride (Fig. 3D) among all the groups. The levels of hepatic cholesterol (Fig. 4A), triglycerides (Fig. 4B) and phospholipids (Fig. 4C) were significantly (p < 0.05) increased by all the doses of AFB1. However, only the increase in liver triglycerides was dose dependent, with a significant (p = 0.036) linear trend.
Fig. 2.
Effects of Aflatoxin B1 on (A) plasma cholesterol (B) plasma triglycerides (C) plasma free fatty acids and (D) plasma phospholipid concentrations.
Bars represent the means ± SEM (n = 5). Bars with different alphabets are significantly (p < 0.05) different from each other.
Fig. 3.
Effects of aflatoxin B1 on (A) HDL cholesterol, (B) HDL triglycerides, (C) HDL3 cholesterol and (D) HDL3 triglycerides concentrations.
Bars represent the means ± SEM (n = 5). Bars with different alphabets are significantly (p < 0.05) different from each other.
Fig. 4.
Effects of Aflatoxin B1 on (A) hepatic cholesterol, (B) hepatic triglycerides and (C) hepatic phospholipids.
Bars represent the means ± SEM (n = 5). Bars with different alphabets are significantly (p < 0.05) different from each other.
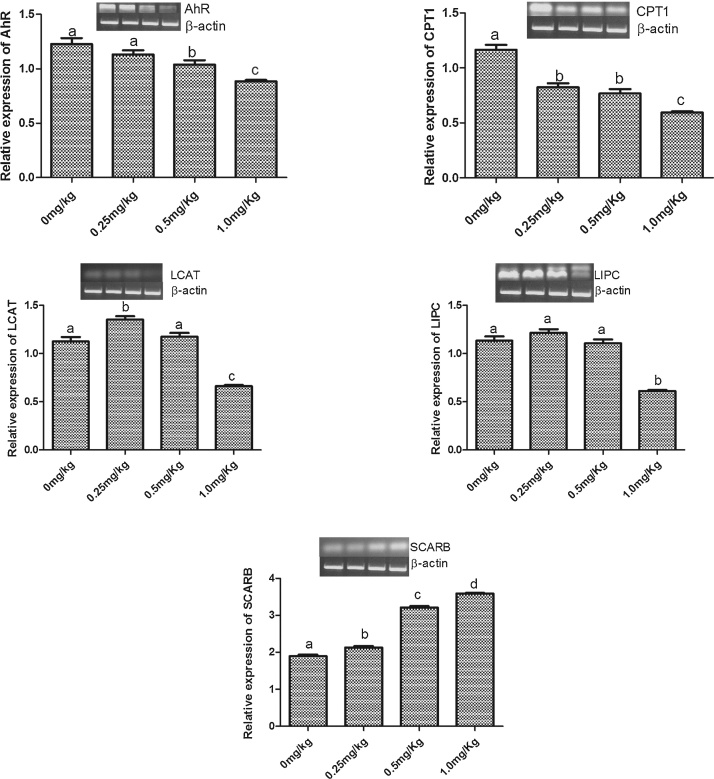
3.3. Relative expression of lipid metabolizing genes
The expression of five genes was assessed in liver samples from all treatment groups. The relative expression of aryl hydrocarbon receptor (Ahr) was significantly (p < 0.05) decreased by only 0.5 mg/kg and 1.0 mg/kg AFB1 (Fig. 5A). All three doses used in this experiment significantly (p < 0.05) decreased, compared to control, the relative expression of carnitine palmitoyl transferase 1A (Cpt1a) (Fig. 5B). 0.5 mg/kg AFB1 significantly increased the relative expression of lecithin – cholesterol acyltransferase (Lcat) whereas 1.0 mg/kg AFB1 significantly decreased the relative expression of Lcat compared with the control (Fig. 5C). The relative expression of hepatic lipoprotein lipase (Lipc) was significantly (p < 0.05) decreased in only the group given 1.0 mg/kg AFB1 (Fig. 5D). However, 0.25, 0.5 and 1.0 mg/kg AFB1 resulted in 15.8, 63.2, and 84.2% increases respectively in the relative expression of scavenger receptor class B member 1 (Scarb1) (Fig. 5E).
Fig. 5.
Effects of Aflatoxin B1 on the relative expression of hepatic lipid metabolizing genes: (A) Ahr; (B) Cpt1a; (C) Lcat; (D) Lipc; (E) Scarb1.
Bars represent the means ± SEM (n = 3). Relative expression is ratio of intensity of each gene to that of housekeeping gene (β-actin, Actb). Bars with different alphabets are significantly (p < 0.05) different from each other.
4. Discussion
The results of this study confirm several hallmarks of AFB1-induced hepatotoxicity occur in a dose-dependent manner in a rat model testing three sub-lethal doses and provide new insight into potential mechanisms underlying these effects. The results demonstrate that AFB1 induced hepatic damage with concomitant dyslipidemia, and this may occur in part through the alteration of expression of lipid and lipoprotein metabolising genes. The various degrees of histological damage to the liver include infiltration of inflammatory cells around the portal triad and bile duct proliferation which is consistent with other studies [4], [5], [17]. Plasma dyslipidemia was characterized by increased concentrations of cholesterol, triglyceride and free fatty acids, and decreased concentrations of phospholipids and HDL3–cholesterol while hepatic dyslipidemia was characterized by increased concentrations of cholesterol, triglyceride and phospholipids. Expression of all five lipid-related genes significantly changed by AFB1 exposure. The doses of AFB1 used in this study have been previously shown to induce acute aflatoxicosis in rats without causing mortality [17], [28].
Alterations in lipids and lipoproteins may play a role in the pathogenesis of coronary heart diseases (CHD) [32], [37], [38]. Although, total HDL-cholesterol has been known as “good” cholesterol which protects from these diseases because of its ability to remove cholesterol from peripheral tissues, other studies have indicated the importance of studying the distinct subclasses of this lipoprotein because they remove cholesterol from cells by different mechanisms [39], [40], [41]. Kim and colleagues [42] discovered in their study, that HDL3-cholesterol may be superior to total HDL-cholesterol and HDL2-cholesterol in predicting the risk of coronary heart diseases. The findings of our study, which showed that AFB1 decreased HDL3-cholesterol, coroborates this discovery.
A major finding of this study was that AFB1 increased free fatty acids in a dose dependent manner. This is in agreement with Lu et al. [17] who reported increased concentrations of long chain fatty acids; 9,12-octadecadienoic acid, trans-9-octadecanoic acid and octadecanoic acid, after AFB1 administration. These findings could have resulted from the down-regulation of Cpt1, an enzyme that facilitates the transportation of long chain fatty acids into the mitochondria for β-oxidation and subsequently formation of ATP [43], [44]. Yarru et al. [44] also observed down regulation of Cpt1 in chicks while Chen et al. [43] reported that the reduction in Cpt1 observed in ducklings were not statistically significant. They however attributed this observation to the low dose of AFB1 given to the ducklings, which indicates that down-regulation of Cpt1 by AFB1 is dose dependent. On the other hand, Zhang et al. [5] reported the upregulation of genes associated with promoting fatty acid synthesis and elongation in ducklings. This study confirmed a AFB1 dose-dependent increase in FFA synthesis and supression of Cpt1, which may be a mechanism underlying changes in FFA. The excess FFA could be taken up by the liver and esterified to form triglycerides or be mobilized for the synthesis of phospholipids. The accumulation of phospholipid in tissue has been reported to be induced by several xenobiotics [45]. This xenobiotic-induced phospholipidosis has been reported to be due to enhanced FFA availability and/or increased cholesterogenesis [46]. These two mechanisms might be involved in the elevated level of liver phospholipid observed in this study.
The AFB1-induced increase in both plasma and liver triglycerides observed in this study is consistent with that of El-Nekeety et al. [2]. Over the years, hypertriglyceridemia have been reported to increase the risk of CHD [47] and one enzyme involved in the regulation of plasma triglyceride is hepatic lipase. Hepatic lipase is a lipolytic enzyme synthesized by the hepatocytes [48], and found in the liver, adrenal glands and ovaries [49]. Hepatic lipase, encoded by the gene Lipc, plays a multifunctional role in lipoprotein metabolism, it regulates the phopholipid, triglyceride and cholesterol content of lipoproteins because of its role as a phospholipase and triglyceride lipase [47], [49]. The role of Lipc in atherosclerosis is unclear with some studies suggesting it is pro-atherogenic and others anti-atherogenic [47], [48], [49], [50]. This may be due to complex responses of Lipc to various endogenous and exogenous factors. Our results showed that only the highest dose of AFB1 significantly decreased the expression of Lipc, suggesting other genes are involved in lipoprotein metabolism changes observed in the lower AFB1 doses.
The present investigation shows that AFB1 increased the expression of Scarb1 in a dose dependent manner. Scarb1 is a lipoprotein receptor, belonging to the class B family of receptor, which regulates the selective uptake of HDL-cholesterol esters (HDL-CE) [51], [52], [53]. Apart from this major role, it appears to play a role in cholesterol metabolism, bi-directional cholesterol shuttling and could act as an anti-atherogenic agent [53]. During reverse cholesterol transport, nascent HDL matures into spherical HDL when HDL-free cholesterol is converted to HDL-CE by Lcat [54]. The mature HDL delivers its CE, through a process mediated by Scarb1, to the liver where it is hydrolyzed and excreated as bilary cholesterol [54], [55]. Although, the lowest dose of AFB1 upregulated Lcat, the highest dose caused a down regulation. The increase in the expression of Lcat observed could be as a result of increased need for reverse cholesterol transport. However the highest AFB1 dose could have overwhelmed this need. It is noteworthy that the group administered the lowest dose (0.25 mg/kg) of AFB1 had the highest concentration of cholesterol in the liver as well as the highest expression of Lcat, this could mean that AFB1 might have repressed the reverse cholesterol transport. Scarb1 has been reported to perform the role of a ‘lipid trader’, therefore when the demand for HDL-cholesterol exceeds its supply (due to downregulation of Lcat observed at the highest dose), the trader actively seeks lipids [51]. Therefore, the upregulation of Scarb1 observed in this study could be a response to change in lipid homeostasis as well as protection against atherosclerosis [53].
The Aryl hydrocarbon receptor (Ahr) is a ligand- activated transcription factor which plays a major role in xenobiotic metabolism [56] and other biological processes including lipid metabolism [57], [58], [59]. Specifically, this receptor regulates fatty acid and cholesterol biosynthesis because its activation represses fatty acid synthesis and cholesterol secretion in the liver [58], [59]. In this study AFB1 repressed the expression of Ahr which could account for the increase in lipids observed in this study. Our results are consistent with that of Mary et al. [60] who observed a decrease in the expression of Ahr mRNA levels in spleen mononuclear cells of rats after 24 h of AFB1 treatment. Wada et al. [57] reported upregulation of genes associated with lipogenesis in high fat diet-induced hepatic steatosis using Ahr deficient mice, they concluded that Ahr may also play a role in protecting the liver against lipotoxicity.
5. Conclusion
Acute exposure to high doses of AFB1 for seven days, induced liver damage and dysregulation of lipid and lipoprotein metabolism through altered expression of Cpt1a, Lipc, Lcat Scarb1 and Ahr genes which are associated with lipid and lipoprotein metabolism. Notably, Cpt1 and Scarb1 may be more sensitive indicators of aflatoxicosis as dose-dependent expression changes occurred in these genes starting with the lowest dose. Overall, the changes observed in this study may be associated with increased risk of CHD and may play a critical role in acute aflatoxicosis.
Disclosure statement
The authors declare no conflict of interest.
Acknowledgement
The authors are grateful to Dr. Jaclyn Goodrich for reviewing and editing this manuscript.
References
- 1.Gong Y.Y., Watson S., Routledge M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016;4:14–27. doi: 10.14252/foodsafetyfscj.2015026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Nekeety A.A., Abdel-Azeim S.H., Hassan A.M., Hassan N.S., Aly S.E., Abdel-Wahhab M.A. Quercetin inhibits the cytotoxicity and oxidative stress in liver of rats fed aflatoxin-contaminated diet. Toxicol. Rep. 2014;1:319–329. doi: 10.1016/j.toxrep.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wild C.P., Gong Y.Y. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., Ye Y., An Y., Tian Y., Wang Y., Tang H. Systems responses of rats to aflatoxin b1 exposure revealed with metabonomic changes in multiple biological matrices. J. Proteome Res. 2010;10:614–623. doi: 10.1021/pr100792q. [DOI] [PubMed] [Google Scholar]
- 5.Zhang N.-Y., Qi M., Gao X., Zhao L., Liu J., Gu C.-Q., Song W.-J., Krumm C.S., Sun L.-H., Qi D.-S. Response of the hepatic transcriptome to aflatoxin b1 in ducklings. Toxicon. 2016;111:69–76. doi: 10.1016/j.toxicon.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Sun L.-H., Lei M.-y., Zhang N.-Y., Gao X., Li C., Krumm C.S., Qi D.-S. Individual and combined cytotoxic effects of aflatoxin b1, zearalenone, deoxynivalenol and fumonisin b1 on brl 3a rat liver cells. Toxicon. 2015;95:6–12. doi: 10.1016/j.toxicon.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Iyer R.S., Coles B.F., Raney K.D., Thier R., Guengerich F.P., Harris T.M. DNA adduction by the potent carcinogen aflatoxin b1: mechanistic studies. J. Am. Chem. Soc. 1994;116:1603–1609. [Google Scholar]
- 8.Kamdem L.K., Meineke I., Gödtel-Armbrust U., Brockmöller J., Wojnowski L. Dominant contribution of p450 3a4 to the hepatic carcinogenic activation of aflatoxin b1. Chem. Res. Toxicol. 2006;19:577–586. doi: 10.1021/tx050358e. [DOI] [PubMed] [Google Scholar]
- 9.Blankson G.K., Mill-Robertson F.C. Aflatoxin contamination and exposure in processed cereal-based complementary foods for infants and young children in greater accra, ghana. Food Control. 2016;64:212–217. [Google Scholar]
- 10.Abdel-Wahhab M.A., Aljawish A., El-Nekeety A.A., Abdel-Aiezm S.H., Abdel-Kader H.A.M., Rihn B.H., Joubert O. Chitosan nanoparticles and quercetin modulate gene expression and prevent the genotoxicity of aflatoxin b1 in rat liver. Toxicol. Rep. 2015;2:737–747. doi: 10.1016/j.toxrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Du M., Zhang G. Proapoptotic activity of aflatoxin b 1 and sterigmatocystin in hepg2 cells. Toxicol. Rep. 2014;1:1076–1086. doi: 10.1016/j.toxrep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Lv Y., Huang K., Luo Y., Xu W. Zinc inhibits aflatoxin b1-induced cytotoxicity and genotoxicity in human hepatocytes (hepg2 cells) Food Chem. Toxicol. 2016;92:17–25. doi: 10.1016/j.fct.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Ingawale D.K., Mandlik S.K., Naik S.R. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): a critical discussion. Environ. Toxicol. Pharmacol. 2014;37:118–133. doi: 10.1016/j.etap.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Lewis L., Onsongo M., Njapau H., Schurz-Rogers H., Luber G., Kieszak S., Nyamongo J., Backer L., Dahiye A.M., Misore A. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central kenya. Environ. Health Perspect. 2005:1763–1767. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azziz-Baumgartner E., Lindblade K., Gieseker K., Rogers H.S., Kieszak S., Njapau H., Schleicher R., McCoy L.F., Misore A., DeCock K. Case-control study of an acute aflatoxicosis outbreak, Kenya. Environ. Health Perspect. 2004;2005:1779–1783. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngindu A., Kenya P., Ocheng D., Omondi T., Ngare W., Gatei D., Johnson B., Ngira J., Nandwa H., Jansen A. Originally published as volume 1, issue 8285 of acute hepatitis caused by aflatoxin poisoning in kenya. Lancet. 1982;319:1346–1348. doi: 10.1016/s0140-6736(82)92411-4. [DOI] [PubMed] [Google Scholar]
- 17.Lu X., Hu B., Shao L., Tian Y., Jin T., Jin Y., Ji S., Fan X. Integrated analysis of transcriptomics and metabonomics profiles in aflatoxin b1-induced hepatotoxicity in rat. Food Chem. Toxicol. 2013;55:444–455. doi: 10.1016/j.fct.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Rolim A.E.H., Henrique-Araújo R., Ferraz E.G., de Araújo Alves Dultra F.K., Fernandez L.G. Lipidomics in the study of lipid metabolism: current perspectives in the omic sciences. Gene. 2015;554:131–139. doi: 10.1016/j.gene.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Lee C.-H., Olson P., Evans R.M. Minireview lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 20.Wu L., Parhofer K.G. Diabetic dyslipidemia. Metabolism. 2014;63:1469–1479. doi: 10.1016/j.metabol.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Kelesidis T., Currier J.S. Dyslipidemia and cardiovascular risk in human immunodeficiency virus infection. Endocrinol. Metab. Clin. North Am. 2014;43:665–684. doi: 10.1016/j.ecl.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsiki N., Mikhailidis D.P., Mantzoros C.S. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65:1109–1123. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Franssen R., Monajemi H., Stroes E.S.G., Kastelein J.J.P. Obesity and dyslipidemia. Endocrinol. Metab. Clin. North Am. 2008;37:623–633. doi: 10.1016/j.ecl.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Diamanti-Kandarakis E., Papavassiliou A.G., Kandarakis S.A., Chrousos G.P. Pathophysiology and types of dyslipidemia in pcos. Trends Endocrinol. Metab. 2007;18:280–285. doi: 10.1016/j.tem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Grundy S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016;26:364–373. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Ungurianu A., Margină D., Grădinaru D., Băcanu C., Ilie M., Tsitsimpikou C., Tsarouhas K., Spandidos D.A., Tsatsakis A.M. Lipoprotein redox status evaluation as a marker of cardiovascular disease risk in patients with inflammatory disease. Mol. Med. Rep. 2017;15:256–262. doi: 10.3892/mmr.2016.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Hegele R.A., Krauss R.M., Raal F.J., Schunkert H. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the european atherosclerosis society consensus panel. Eur. Heart J. 2017:ehx144. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKean C., Tang L., Tang M., Billam M., Wang Z., Theodorakis C.W., Kendall R.J., Wang J.S. Comparative acute and combinative toxicity of aflatoxin b1 and fumonisin b1 in animals and human cells. Food Chem. Toxicol. 2006;44:868–876. doi: 10.1016/j.fct.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Rotimi O.A., Rotimi S.O., Oluwafemi F., Ademuyiwa O., Balogun E.A. Coexistence of aflatoxicosis with protein malnutrition worsens hepatic oxidative damage in rats. J. Biochem. Mol. Toxicol. 2016;30:269–276. doi: 10.1002/jbt.21787. [DOI] [PubMed] [Google Scholar]
- 30.Rifai N., Warnick G.R., Dominiczak M.H. Am. Assoc. Clinical Chemistry; 2000. Handbook of Lipoprotein Testing. [Google Scholar]
- 31.Warnick G.R., Benderson J., Albers J.J. Dextran sulfate-mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 32.Rotimi S.O., Ojo D.A., Talabi A.O., Balogun E.A., Ademuyiwa O. Tissue dyslipidemia in salmonella-infected rats treated with amoxillin and pefloxacin. Lipids Health Dis. 2012;11 doi: 10.1186/1476-511X-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957:226. [PubMed] [Google Scholar]
- 34.Rotimi O.A., Olayiwola I.O., Ademuyiwa O., Balogun E.A. Effects of fibre-enriched diets on tissue lipid profiles of msg obese rats. Food Chem. Toxicol. 2012;50:4062–4067. doi: 10.1016/j.fct.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Rotimi S.O., Bankole G.E., Adelani I.B., Rotimi O.A. Hesperidin prevents lipopolysaccharide-induced endotoxicity in rats. Immunopharmacol. Immunotoxicol. 2016:1–8. doi: 10.1080/08923973.2016.1214142. [DOI] [PubMed] [Google Scholar]
- 36.Abràmoff M.D., Magalhães P.J., Ram S.J. Image processing with imagej. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 37.Ademuyiwa O., Ugbaja R.N., Rotimi S.O. Plasma lipid profile, atherogenic and coronary risk indices in some residents of abeokuta in south-western nigeria. Biokemistri. 2008:20. [Google Scholar]
- 38.Hirakawa Y., Lam T.-H., Welborn T., Kim H.C., Ho S., Fang X., Ueshima H., Suh I., Giles G., Woodward M. The impact of body mass index on the associations of lipids with the risk of coronary heart disease in the Asia Pacific region. Prev. Med. Rep. 2016;3:79–82. doi: 10.1016/j.pmedr.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buring J., O'Connor G., Goldhaber S., Rosner B., Herbert P., Blum C., Breslow J., Hennekens C. Decreased hdl2 and hdl3 cholesterol, apo ai and apo a-ii, and increased risk of myocardial infarction. Circulation. 1992;85:22–29. doi: 10.1161/01.cir.85.1.22. [DOI] [PubMed] [Google Scholar]
- 40.Sweetnam P.M., Bolton C.H., Yarnell J., Bainton D., Baker I.A., Elwood P.C., Miller N.E. Associations of the hdl2 and hdl3 cholesterol subfractions with the development of ischemic heart disease in British men. The caerphilly and speedwell collaborative heart disease studies. Circulation. 1994;90:769–774. doi: 10.1161/01.cir.90.2.769. [DOI] [PubMed] [Google Scholar]
- 41.Pirillo A., Uboldi P., Pappalardo G., Kuhn H., Catapano A.L. Modification of hdl3 by mild oxidative stress increases atp-binding cassette transporter 1-mediated cholesterol efflux. Cardiovasc. Res. 2007;75:566–574. doi: 10.1016/j.cardiores.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 42.Kim D.S., Burt A.A., Rosenthal E.A., Ranchalis J.E., Eintracht J.F., Hatsukami T.S., Furlong C.E., Marcovina S., Albers J.J., Jarvik G.P. Hdl-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. J. Am. Heart Assoc. 2014;3:e000902. doi: 10.1161/JAHA.114.000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Horn N., Cotter P., Applegate T. Growth, serum biochemistry, complement activity, and liver gene expression responses of pekin ducklings to graded levels of cultured aflatoxin b1. Poult. Sci. 2014;93:2028–2036. doi: 10.3382/ps.2014-03904. [DOI] [PubMed] [Google Scholar]
- 44.Yarru L., Settivari R., Antoniou E., Ledoux D., Rottinghaus G. Toxicological and gene expression analysis of the impact of aflatoxin b1 on hepatic function of male broiler chicks. Poult. Sci. 2009;88:360–371. doi: 10.3382/ps.2008-00258. [DOI] [PubMed] [Google Scholar]
- 45.Rotimi S.O., Ojo D.A., Talabi O.A., Ugbaja R.N., Balogun E.A., Ademuyiwa O. Amoxillin- and pefloxacin-induced cholesterogenesis and phospholipidosis in rat tissues. Lipids Health Dis. 2015;14:13. doi: 10.1186/s12944-015-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawada H., Takami K., Asahi S. A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol. Sci. 2005;83 doi: 10.1093/toxsci/kfh264. [DOI] [PubMed] [Google Scholar]
- 47.Chatterjee C., Sparks D.L. Hepatic lipase, high density lipoproteins, and hypertriglyceridemia. Am. J. Pathol. 2011;178:1429–1433. doi: 10.1016/j.ajpath.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perret B., Mabile L., Martinez L., Tercé F., Barbaras R., Collet X. Hepatic lipase structure/function relationship, synthesis, and regulation. J. Lipid Res. 2002;43:1163–1169. [PubMed] [Google Scholar]
- 49.Jansen H., Verhoeven A.J., Sijbrands E.J. Hepatic lipase a pro-or anti-atherogenic protein? J. Lipid Res. 2002;43:1352–1362. doi: 10.1194/jlr.r200008-jlr200. [DOI] [PubMed] [Google Scholar]
- 50.Santamarina-Fojo S., González-Navarro H., Freeman L., Wagner E., Nong Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2004;24:1750–1754. doi: 10.1161/01.ATV.0000140818.00570.2d. [DOI] [PubMed] [Google Scholar]
- 51.Rhainds D., Brissette L. The role of scavenger receptor class b type i (sr-bi) in lipid trafficking: defining the rules for lipid traders. Int. J. Biochem. Cell Biol. 2004;36:39–77. doi: 10.1016/s1357-2725(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 52.Xin P., Han H., Gao D., Cui W., Yang X., Ying C., Sun X., Hao L. Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class b type i gene expressions in rats. Food Chem. Toxicol. 2013;52:12–18. doi: 10.1016/j.fct.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Shen W.-J., Hu J., Hu Z., Kraemer F.B., Azhar S. Scavenger receptor class b type i (sr-bi): A versatile receptor with multiple functions and actions. Metabolism. 2014;63:875–886. doi: 10.1016/j.metabol.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunnen S., Van Eck M. Lecithin cholesterol acyltransferase: old friend or foe in atherosclerosis? J. Lipid Res. 2012;53:1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaur S.C. Vol. 1. Springer Science & Business Media; 2006. (Biochemistry of Atherosclerosis). [Google Scholar]
- 56.Köhle C., Bock K.W. Coordinate regulation of phase i and ii xenobiotic metabolisms by the ah receptor and nrf2. Biochem. Pharmacol. 2007;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 57.Wada T., Sunaga H., Miyata K., Shirasaki H., Uchiyama Y., Shimba S. Aryl hydrocarbon receptor plays protective roles against high fat diet (hfd)-induced hepatic steatosis and the subsequent lipotoxicity via direct transcriptional regulation of socs3 gene expression. J. Biol. Chem. 2016;291:7004–7016. doi: 10.1074/jbc.M115.693655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanos R., Murray I.A., Smith P.B., Patterson A., Perdew G.H. Role of the ah receptor in homeostatic control of fatty acid synthesis in the liver. Toxicol. Sci. 2012;129:372–379. doi: 10.1093/toxsci/kfs204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanos R., Patel R.D., Murray I.A., Smith P.B., Patterson A.D., Perdew G.H. Aryl hydrocarbon receptor regulates the cholesterol biosynthetic pathway in a dioxin response element-independent manner. Hepatology. 2012;55:1994–2004. doi: 10.1002/hep.25571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mary V.S., Valdehita A., Navas J.M., Rubinstein H.R., Fernández-Cruz M.L. Effects of aflatoxin b1, fumonisin b1 and their mixture on the aryl hydrocarbon receptor and cytochrome p450 1a induction. Food Chem. Toxicol. 2015;75:104–111. doi: 10.1016/j.fct.2014.10.030. [DOI] [PubMed] [Google Scholar]